In-Depth Method Investigation for Determination of Boron in Silicate Samples Using an Improved Boron–Mannitol Complex Digestion Method by Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Assessment of Boron Recovery of the Boron–Mannitol Complex Strategy
2.1.1. Temperature Effect on Boron Recovery
2.1.2. Effects of Ultrasonic Treatment on Boron Recovery
2.1.3. Sample Boron Concentration Effect on Boron Recovery
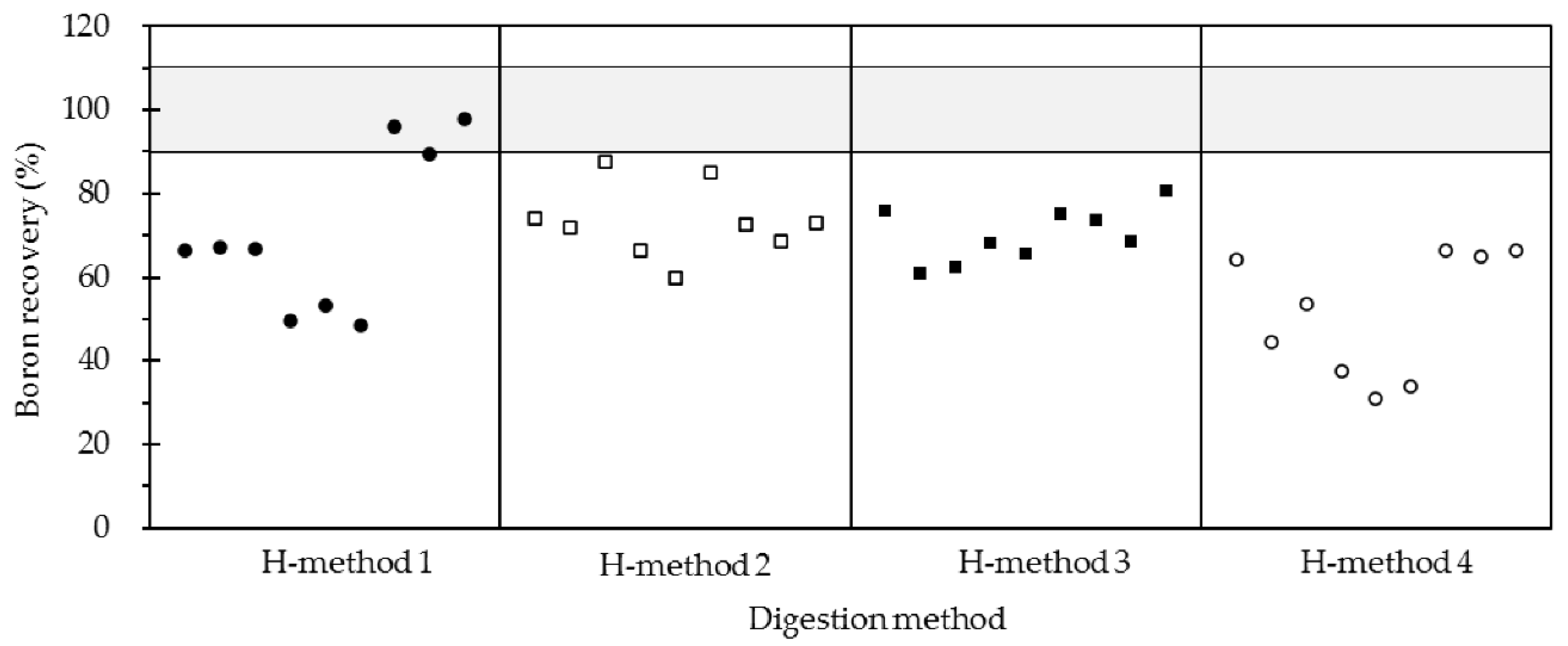
2.2. A Comparison of Boron Recovery to High-Pressure Closed Acid Digestion Method
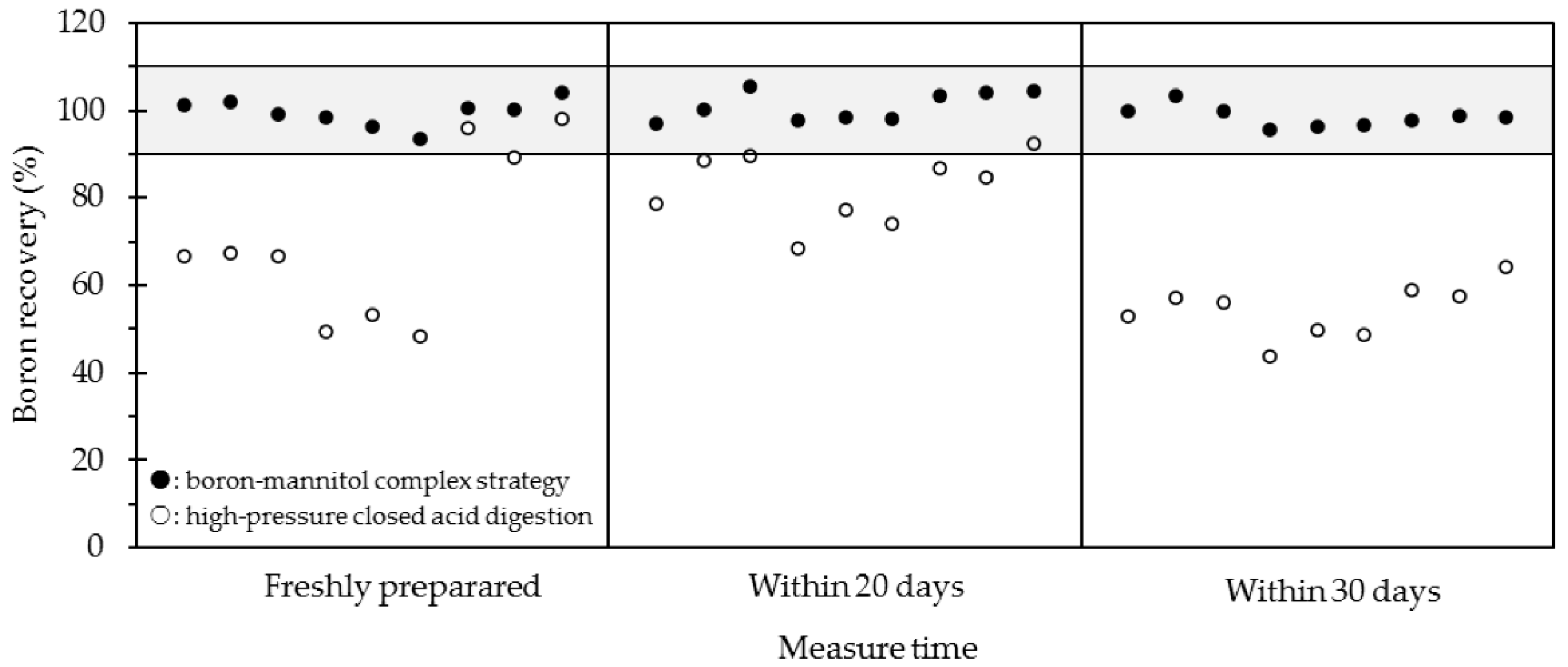
2.3. Long-Term Stability Study of Boron Quantification
3. Materials and Methods
3.1. Instrumental Apparatus and Operating Conditions of ICP-MS
3.2. Reagents and Chemicals
3.3. Silicate Standard Materials
3.4. Digestion Method Description
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Palmer, M.R.; Swihart, G.H. Boron isotope geochemistry; an overview. Rev. Mineral. Geochem. 1996, 33, 709–740. [Google Scholar]
- Chaussidon, M.; Marty, B. Primitive boron isotope composition of the mantle. Science 1995, 269, 383–386. [Google Scholar] [CrossRef]
- Ottolini, L.; Laporte, D.; Raffone, N.; Devidal, J.L.; Fèvre, B.L. New experimental determination of Li and B partition coefficients during upper mantle melting. Contrib. Mineral. Petr. 2009, 157, 313–325. [Google Scholar] [CrossRef]
- Chaussidon, M.; Jambon, A. Boron content and isotopic composition of oceanic basalts: Geochemical and cosmochemical implications. Earth Planet Sci. Lett. 1994, 121, 277–291. [Google Scholar] [CrossRef]
- Grew, E.S. Boron: From cosmic scarcity to 300 minerals. Elements 2017, 13, 225–229. [Google Scholar] [CrossRef]
- Leeman, W.P.; Sisson, V.B. Geochemistry of boron and its implications for crustal and mantle processes. Rev. Mineral. Geochem. 1996, 33, 645–707. [Google Scholar]
- Konrad-Schmolke, M.; Halamam, R. Combined thermodynamic–geochemical modeling in metamorphic geology: Boron as tracer of fluid-rock interaction. Lithos 2014, 208–209, 393–414. [Google Scholar] [CrossRef]
- Palmer, M.R. Boron cycling in subduction zones. Elements 2017, 13, 237–342. [Google Scholar] [CrossRef]
- Farzaneh, A.; Troll, G.; Neubauer, W. Rapid determination of boron traces in silicate materials. Fresenius’ Z. Anal. Chem. 1979, 296, 383–385. [Google Scholar] [CrossRef]
- Woods, W.G. An introduction to boron: History, sources, uses, and chemistry. Environ. Health Persp. 1994, 102, 5–11. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 91st ed.; CRC Press Inc.: Boca Raton, FL, USA, 2010; pp. 4–53. [Google Scholar]
- Sah, R.N.; Brown, P.H. Boron determination—A review of analytical methods. Microchem. J. 1997, 56, 285–304. [Google Scholar] [CrossRef]
- Musashi, M.; Oi, T.; Ossaka, T.; Kakihana, H. Extraction of boron from GSJ rock reference samples and determination of their boron isotopic ratios. Anal. Chim. Acta 1990, 231, 147–150. [Google Scholar] [CrossRef]
- Ryan, J.G.; Langmuir, C.H. The systematics of boron abundances in young volcanic rocks. Geochim. Cosmochim. Acta 1993, 57, 1489–1498. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, S.Y.; Wei, H.Z.; Yan, Y.; Wu, H.P.; Pu, W. Extraction and determination of boron isotopic composition in tourmalines. Chin. J. Anal. Chem. 2012, 40, 1654–1660. [Google Scholar] [CrossRef]
- Leeman, W.P.; Tonarini, S. Boron isotopic analysis of proposed borosilicate mineral reference samples. Geostand. Newsl. 2001, 25, 309–403. [Google Scholar] [CrossRef]
- Smith, G.; Wiederin, D.R.; Houk, R.S.; Egan, C.B.; Surfass, R.E. Measurement of boron concentration and isotope ratios in biological samples by inductively coupled plasma mass spectrometry with direct injection nebulization. Anal. Chim. Acta 1991, 248, 229–234. [Google Scholar] [CrossRef]
- San, R.N.; Brown, P.H. Isotope ratio determination in boron analysis. Biol. Trace Elem. Res. 1998, 66, 39–52. [Google Scholar]
- Ishikawa, T.; Nakamura, E. Suppression of boron volatilization from a hydrofluoric acid solution using a boron-mannitol complex. Anal. Chem. 1990, 62, 2612–2616. [Google Scholar] [CrossRef]
- Nakai, S. Boron determinations of silicate reference rocks by the isotope dilution method in a high-background environment. Geochem. J. 2021, 55, 27–32. [Google Scholar] [CrossRef]
- Nakamura, E.; Ishikawa, T.; Birck, J.L.; Allegre, C.J. Precise boron isotopic analysis of natural rock samples using a boron-mannitol complex. Chem. Geol. 1992, 94, 193–204. [Google Scholar] [CrossRef]
- Makishima, A.; Nakamura, E.; Nakano, T. Determination of boron in silicate samples by direct aspiration of sample HF solutions into ICPMS. Anal. Chem. 1997, 69, 3754–3759. [Google Scholar] [CrossRef]
- D’Orazio, M. Boron determination in twenty one silicate rock reference materials by isotope dilution ICP-MS. Geostand. Newsl. 1999, 23, 21–29. [Google Scholar] [CrossRef]
- Liu, Y.H.; Huang, K.F.; Lee, D.C. Precise and accurate boron and lithium isotopic determinations for small sample-size geological materials by MC-ICP-MS. J. Anal. At. Spectrom. 2018, 33, 846–855. [Google Scholar] [CrossRef]
- Tan, X.J.; Wang, Z.M. General high-pressure closed acidic decomposition method of rock samples for trace element determination using inductively coupled plasma mass spectrometry. J. Anal. Chem. 2020, 75, 1295–1303. [Google Scholar]
- Makishima, A. Thermal Ionization Mass Spectrometry (TIMS): Silicate Digestion, Separation, and Measurement; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Wei, G.J.; Wei, J.X.; Liu, Y.; Ke, T.; Ren, Z.Y.; Ma, J.L.; Xu, Y.G. Measurement on high-precision boron isotope of silicate materials by a single column purification method and MC-ICP-MS. J. Anal. At. Spectrom. 2013, 28, 606–612. [Google Scholar]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A new geochemical database for reference materials and isotopic standards. Geostand. Geoanal. Res. 2005, 9, 333–338. [Google Scholar] [CrossRef]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. 1994 compilation of analytical data for minor and trace elements in seventeen GSJ geochemical reference samples, “Igneous rock series”. Geostand. Geoanal. Res. 2007, 19, 135–213. [Google Scholar] [CrossRef]
- Wilke, T.; Wildner, H.; Wünsch, G. Ester generation for the determination of ultratrace amounts of boron in volatile high-purity process chemicals by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1997, 12, 1083–1086. [Google Scholar] [CrossRef]
| Procedure | Method | |||
|---|---|---|---|---|
| M-Method 1 | M-Method 2 | M-Method 3 | M-Method 4 | |
| Ultrasonic pretreatment | Add 0.6 mL of HF, 30 μL of HNO3, and 50 μL of 2% mannitol into 25–50 mg of sample | |||
| 4 h | 4 h | 4 h | – | |
| Hotplate digestion | 65 °C, overnight | 100 °C, overnight | 140 °C, overnight | 65 °C, overnight |
| Fluoride formation prevention | Dry at 65 °C, then add 0.6 mL of 8% HNO3 and flux overnight | Dry at 100 °C, then add 0.6 mL of 8% HNO3 and flux overnight | Dry at 140 °C, then add 0.6 mL of 8% HNO3 and flux overnight | Dry at 65 °C, then add 0.6 mL of 8% HNO3 and flux overnight |
| Fluoride decomposition | Dry at 65 °C, then add 0.6 mL of 6% HCl and flux overnight | Dry at 100 °C, then add 0.6 mL of 6% HCl and flux overnight | Dry at 140 °C, then add 0.6 mL of 6% HCl and flux overnight | Dry at 65 °C, then add 0.6 mL of 6% HCl and flux overnight |
| Hotplate redissolution | Dry at 65 °C, then add 2.0 mL of 40% HNO3 and flux 4 h | Dry at 100 °C, then add 2.0 mL of 40% HNO3 and flux 4 h | Dry at 140 °C, then add 2.0 mL of 40% HNO3 and flux 4 h | Dry at 65 °C, then add 2.0 mL of 40% HNO3 and flux 4 h |
| Sample solution for ICP-MS | Age overnight, and dilute 1000-fold using 2% HNO3 | |||
| Method | Sample | 10B | 11B | ||||
|---|---|---|---|---|---|---|---|
| Content 1 μg/g | 2σ | Recovery 2 % | Content μg/g | 2σ | Recovery % | ||
| M-method 1 | W-2_1 | 11.94 | 0.20 | 95.5 | 12.11 | 0.12 | 96.9 |
| W-2_2 | 12.33 | 0.18 | 98.7 | 12.51 | 0.17 | 100.1 | |
| W-2_3 | 13.10 | 0.20 | 104.8 | 13.19 | 0.12 | 105.5 | |
| JB-2a_1 | 29.46 | 0.16 | 98.3 | 29.29 | 0.16 | 97.7 | |
| JB-2a_2 | 29.92 | 0.39 | 99.8 | 29.54 | 0.20 | 98.5 | |
| JB-2a_3 | 29.32 | 0.21 | 97.8 | 29.41 | 0.19 | 98.1 | |
| JR-2_1 | 148.1 | 0.6 | 102.2 | 149.8 | 1.0 | 103.3 | |
| JR-2_2 | 149.4 | 5.3 | 103.1 | 150.9 | 5.7 | 104.0 | |
| JR-2_3 | 149.6 | 0.8 | 103.2 | 151.4 | 0.7 | 104.4 | |
| M-method 2 | W-2_1 | 13.07 | 0.33 | 104.6 | 13.11 | 0.16 | 104.9 |
| W-2_2 | 12.81 | 0.20 | 102.5 | 12.89 | 0.12 | 103.1 | |
| W-2_3 | 12.78 | 0.11 | 102.2 | 12.81 | 0.12 | 102.5 | |
| JB-2a_1 | 29.74 | 0.26 | 99.2 | 28.84 | 0.28 | 96.2 | |
| JB-2a_2 | 29.61 | 0.42 | 98.8 | 28.71 | 0.11 | 95.8 | |
| JB-2a_3 | 29.37 | 0.13 | 98.0 | 28.77 | 0.17 | 96.0 | |
| JR-2_1 | 145.2 | 1.1 | 100.1 | 144.4 | 1.5 | 99.6 | |
| JR-2_2 | 146.1 | 1.3 | 100.8 | 144.7 | 1.0 | 99.8 | |
| JR-2_3 | 145.8 | 1.4 | 100.5 | 145.8 | 0.4 | 100.5 | |
| M-method 3 | W-2_1 | 12.31 | 0.21 | 98.5 | 12.46 | 0.19 | 99.6 |
| W-2_2 | 12.20 | 0.20 | 97.6 | 12.60 | 0.08 | 100.8 | |
| W-2_3 | 12.25 | 0.17 | 98.0 | 12.43 | 0.08 | 99.5 | |
| JB-2a_1 | 29.30 | 0.39 | 97.7 | 29.55 | 0.26 | 98.6 | |
| JB-2a_2 | 29.23 | 0.31 | 97.5 | 29.51 | 0.14 | 98.4 | |
| JB-2a_3 | 29.51 | 0.37 | 98.4 | 29.90 | 0.24 | 99.7 | |
| JR-2_1 | 143.8 | 0.8 | 99.2 | 144.5 | 0.4 | 99.7 | |
| JR-2_2 | 145.5 | 0.7 | 100.4 | 146.7 | 0.6 | 101.2 | |
| JR-2_3 | 144.8 | 1.0 | 99.9 | 146.1 | 0.9 | 100.8 | |
| M-method 4 | W-2_1 | 12.39 | 0.21 | 99.1 | 12.48 | 0.09 | 99.9 |
| W-2_2 | 13.01 | 0.14 | 104.1 | 12.95 | 0.06 | 103.6 | |
| W-2_3 | 13.28 | 0.17 | 106.3 | 13.27 | 0.07 | 106.1 | |
| JB-2a_1 | 29.94 | 0.28 | 99.9 | 29.58 | 0.24 | 98.7 | |
| JB-2a_2 | 30.08 | 0.21 | 100.3 | 29.75 | 0.20 | 99.2 | |
| JB-2a_3 | 29.95 | 0.25 | 99.9 | 29.82 | 0.15 | 99.5 | |
| JR-2_1 | 143.6 | 1.1 | 99.1 | 167.4 | 2.5 | 115.5 | |
| JR-2_2 | 145.6 | 1.3 | 100.4 | 172.5 | 1.8 | 118.9 | |
| JR-2_3 | 148.9 | 1.0 | 102.7 | 173.8 | 1.1 | 119.8 | |
| Method | Specific Procedures | Sample | Content 1 μg/g | 2σ | Recovery % | |
|---|---|---|---|---|---|---|
| H-method 1 | Hotplate pressure relief at 140 °C | Evaporation at 140 °C | W-2_1 | 8.32 | 0.05 | 66.6 |
| W-2_2 | 8.41 | 0.13 | 67.2 | |||
| W-2_3 | 8.33 | 0.12 | 66.7 | |||
| JB-2a_1 | 14.84 | 0.11 | 49.5 | |||
| JB-2a_2 | 15.96 | 0.12 | 53.2 | |||
| JB-2a_3 | 14.54 | 0.17 | 48.5 | |||
| JR-2_1 | 139.0 | 0.6 | 95.9 | |||
| JR-2_2 | 129.5 | 0.6 | 89.3 | |||
| JR-2_3 | 142.1 | 1.2 | 98.0 | |||
| H-method 2 | Hotplate pressure relief at 100 °C | Evaporation at 100 °C | W-2_1 | 9.28 | 0.08 | 74.2 |
| W-2_2 | 8.96 | 0.10 | 71.7 | |||
| W-2_3 | 10.94 | 0.19 | 87.5 | |||
| JB-2a_1 | 19.91 | 0.16 | 66.4 | |||
| JB-2a_2 | 17.97 | 0.10 | 60.0 | |||
| JB-2a_3 | 25.54 | 0.28 | 85.2 | |||
| JR-2_1 | 105.2 | 1.0 | 72.6 | |||
| JR-2_2 | 99.71 | 0.52 | 68.8 | |||
| JR-2_3 | 105.6 | 0.5 | 72.8 | |||
| H-method 3 | Hotplate pressure relief at 60 °C | Evaporation at 60 °C | W-2_1 | 9.48 | 0.07 | 75.8 |
| W-2_2 | 7.60 | 0.29 | 60.8 | |||
| W-2_3 | 7.81 | 0.03 | 62.4 | |||
| JB-2a_1 | 20.44 | 0.05 | 68.2 | |||
| JB-2a_2 | 19.69 | 0.04 | 65.7 | |||
| JB-2a_3 | 22.55 | 0.08 | 75.2 | |||
| JR-2_1 | 107.0 | 1.1 | 73.8 | |||
| JR-2_2 | 99.60 | 1.34 | 68.7 | |||
| JR-2_3 | 116.7 | 1.0 | 80.5 | |||
| H-method 4 | – | Evaporation at 140 °C | W-2_1 | 8.02 | 0.10 | 64.1 |
| W-2_2 | 5.57 | 0.07 | 44.6 | |||
| W-2_3 | 6.69 | 0.09 | 53.5 | |||
| JB-2a_1 | 11.22 | 0.08 | 37.4 | |||
| JB-2a_2 | 9.31 | 0.08 | 31.1 | |||
| JB-2a_3 | 10.18 | 0.08 | 34.0 | |||
| JR-2_1 | 96.24 | 0.79 | 66.4 | |||
| JR-2_2 | 94.00 | 1.20 | 64.8 | |||
| JR-2_3 | 96.25 | 0.53 | 66.4 | |||
| Method 1 | Proposed boron–mannitol complex digestion method | ||||||||
| Sample | Freshly prepared | Within 20 days | Within 30 days | ||||||
| Content 2 μg/g | 2σ | Recovery % | Content μg/g | 2σ | Recovery % | Content μg/g | 2σ | Recovery % | |
| W-2_1 | 12.66 | 0.24 | 101.3 | 12.11 | 0.12 | 96.9 | 12.47 | 0.20 | 99.8 |
| W-2_2 | 12.75 | 0.43 | 102.0 | 12.51 | 0.17 | 100.1 | 12.94 | 0.10 | 103.5 |
| W-2_3 | 12.39 | 0.33 | 99.1 | 13.19 | 0.12 | 105.5 | 12.50 | 0.14 | 100.0 |
| JB-2a_1 | 29.54 | 0.46 | 98.5 | 29.29 | 0.16 | 97.7 | 28.66 | 0.17 | 95.6 |
| JB-2a_2 | 28.85 | 0.44 | 96.2 | 29.54 | 0.20 | 98.5 | 28.92 | 1.54 | 96.5 |
| JB-2a_3 | 28.02 | 0.45 | 93.5 | 29.41 | 0.19 | 98.1 | 28.99 | 0.29 | 96.7 |
| JR-2_1 | 145.8 | 2.5 | 100.5 | 149.8 | 1.0 | 103.3 | 141.6 | 0.8 | 97.6 |
| JR-2_2 | 145.5 | 1.1 | 100.3 | 150.9 | 5.7 | 104.0 | 143.2 | 0.7 | 98.7 |
| JR-2_3 | 151.0 | 1.0 | 104.1 | 151.4 | 0.7 | 104.4 | 142.6 | 1.7 | 98.4 |
| Method | High-pressure closed digestion method | ||||||||
| Sample | Freshly prepared | Within 20 days | Within 30 days | ||||||
| Content μg/g | 2σ | Recovery % | Content μg/g | 2σ | Recovery % | Content μg/g | 2σ | Recovery % | |
| W-2_1 | 8.32 | 0.05 | 66.6 | 9.84 | 0.08 | 78.8 | 6.63 | 0.12 | 53.1 |
| W-2_2 | 8.41 | 0.13 | 67.2 | 11.06 | 0.12 | 88.5 | 7.17 | 0.09 | 57.3 |
| W-2_3 | 8.33 | 0.12 | 66.7 | 11.19 | 0.18 | 89.5 | 7.00 | 0.13 | 56.0 |
| JB-2a_1 | 14.84 | 0.11 | 49.5 | 20.49 | 0.16 | 68.4 | 13.08 | 0.14 | 43.6 |
| JB-2a_2 | 15.96 | 0.12 | 53.2 | 23.13 | 0.24 | 77.1 | 14.89 | 0.15 | 49.7 |
| JB-2a_3 | 14.54 | 0.17 | 48.5 | 22.19 | 0.19 | 74.0 | 14.61 | 0.16 | 48.7 |
| JR-2_1 | 139.0 | 0.6 | 95.9 | 125.6 | 1.1 | 86.6 | 85.35 | 0.56 | 58.9 |
| JR-2_2 | 129.5 | 0.6 | 89.3 | 122.6 | 1.4 | 84.6 | 83.58 | 0.40 | 57.6 |
| JR-2_3 | 142.1 | 1.2 | 98.0 | 134.1 | 1.1 | 92.5 | 93.19 | 0.71 | 64.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Zhou, R.; Feng, Y.; Liang, T. In-Depth Method Investigation for Determination of Boron in Silicate Samples Using an Improved Boron–Mannitol Complex Digestion Method by Inductively Coupled Plasma Mass Spectrometry. Molecules 2023, 28, 441. https://doi.org/10.3390/molecules28010441
Tan X, Zhou R, Feng Y, Liang T. In-Depth Method Investigation for Determination of Boron in Silicate Samples Using an Improved Boron–Mannitol Complex Digestion Method by Inductively Coupled Plasma Mass Spectrometry. Molecules. 2023; 28(1):441. https://doi.org/10.3390/molecules28010441
Chicago/Turabian StyleTan, Xijuan, Ruili Zhou, Yonggang Feng, and Ting Liang. 2023. "In-Depth Method Investigation for Determination of Boron in Silicate Samples Using an Improved Boron–Mannitol Complex Digestion Method by Inductively Coupled Plasma Mass Spectrometry" Molecules 28, no. 1: 441. https://doi.org/10.3390/molecules28010441
APA StyleTan, X., Zhou, R., Feng, Y., & Liang, T. (2023). In-Depth Method Investigation for Determination of Boron in Silicate Samples Using an Improved Boron–Mannitol Complex Digestion Method by Inductively Coupled Plasma Mass Spectrometry. Molecules, 28(1), 441. https://doi.org/10.3390/molecules28010441





