Abstract
Antimicrobial resistance (AMR) has arisen as a global concern in recent decades. Plant extracts used in combination with antibiotics are promising against AMR, synergistically. The purpose of this study was to evaluate the component of the bitter ginger (Zingiber zerumbet) extract in different solvents using high-performance liquid chromatography (HPLC), in addition to evaluate the antibacterial activity of these extracts, in combination with their antibiotic potential against four multi-drug resistant (MDR) bacterial strains (Lactobacillus acidophilus, Streptococcus mutans, Enterococcus faecalis and Staphylococcus aureus). Ethanol and the aqueous extracts of bitter ginger were prepared using a conventional solvent extraction method and were evaluated for their phytochemistry using HPLC, qualitatively and quantitatively. Moreover, the antibiotic susceptibility of the pathogenic isolates was determined. A disc diffusion assay was used to obtain the antimicrobial potential of the extracts alone and with antibiotics. Eight components were identified from the separation of the bitter ginger extract by HPLC. For AMR bacteria, the combination of the antibiotic solution with the bitter ginger crude extracts could improve its susceptibility of these antibiotics. This study indicates that the combination of an antibiotic solution with the bitter ginger crude extract exhibits potent antibacterial activities against MDR bacterial strains. Therefore, they can be used for the treatment of various diseases against the microbial pathogen and can be incorporated into medication for antibacterial therapy.
1. Introduction
The scientific name of bitter ginger is Zingiber zerumbet (L.) Roscoe ex Sm. [1]. It belongs to the family Zingiberaceae, the largest family of the order Zingiberales with about 57 genera of 1600 species worldwide [2,3]. The eminent members of this family, such as Curcuma longa (turmeric) [4], Zingiber officinale (ginger) [5], Elettaria cardamomum (cardamom) [6], and Zingiber zerumbet (bitter ginger), are used in folk medicine, agriculture, food condiments, and for ornamental purposes [7]. Among the member Zingiber zerumbet is commonly known as bitter ginger or shampoo ginger and is widely grown in both tropical and subtropical areas of the world [8]. Occasionally, the rhizome is the most widely explored part of the plant for its aromatic compounds. In addition, the rhizome is also reported to be rich in terpenoid (mono-terpenoids, sesquiterpenoids, and steroids) phenolic (tannins), alkaloids aromatic compounds, vanilline, zerumbone, phenolic, and flavonoid, especially kaemferol and its glucoside, along with curcumin and ginerol [9,10,11]. Zerumbone is well-known for its antimicrobial, a powerful antioxidant agent, anti-inflammatory, anticancer properties, and chondro-protective properties [12]. Other compounds of Zingiber zerumbet, such as zederone, have also reported antimicrobial activities against MDR bacterial strains, such as Staphylococcus aureus, Bacillus spices, and Pseudomonas aeruginosa. Extracts of bitter ginger (rhizome) were previously used to cure several diseases and other pathological conditions, including pain, arthritis, microbial or viral infections, gastroenteritis, anti-ageing, diabetes, gout, gastric ulcers, anti-inflammatory and anti-allergic conditions, skin ailments, and cancer [13].
Bitter ginger and its extracts have been used as a treatment for various infections for many years for their antimicrobial properties [12,14]. It shows an antimicrobial activity against many Gram-negative bacteria, such as Escherichia coli, Helicobacter pylori, and Gram-positive bacteria, such as Staphylococcus aureus, Staphylococcus epidermidis, showing a greater antibacterial effectiveness on Gram-positive organisms [12,14]. Additionally, more studies are need to determine the potential of bitter ginger as an antibacterial agent against Gram-positive microorganisms, such as Streptococcus mutans, the main agent of tooth decay, the oral infection disease most prevalent in the world that affects over 90% of school-aged children and about 100% of the world population [15].
Ethanol is a suitable solvent for the active substance in Zingiber zerumbet in the form of alkaloids, tannins, flavonoids, and terpenoids [16], and is a versatile solvent that has the ability to extract with a broad polarity, ranging from non-polar compounds to polar compounds [17]. Water (distilled water) is also considered as environmentally friendly and is generally recognized as a safe solvent by food, and nutraceutical industries. It has many advantages and plays a role as a low cost “green solvent” because it is natural, is widely available, has a high purity, and is non-toxic. The use of distilled water and an ethanol solvent for the extraction process of the Zingiber zerumbet extract will result in the profile difference of the active compound of the extract, which then will affect the biological activities of the extract.
The increasing antimicrobial resistance (AMR) among common pathogens has raised major concerns among physicians and medical professionals [18,19,20]. With the increase in AMR and the emergence of MDR organisms, such as Klebsiella pneumonia, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis, treatment options have been reduced [21,22,23]. These pathogens of the oral cavity, skin, intestinal tract, etc., are of great public health concern. The unavailability of new drugs, the misuse of antibiotics, and the emergence of drug-resistant organisms, are leading causes of death worldwide [24,25]. People are now moving towards herbal medicines and traditional remedies to overcome antibacterial resistances against available antibiotics [11].
The current study was designed to evaluate and highlight the importance of the commonly available medicinal plants, such as bitter ginger, as a treatment option. In this study, different components of bitter ginger (Z. zerumbet) were extracted by aqueous and ethanol solvents and analysed for a phytochemical analysis, and the antibacterial activity against selected MDR pathogens was evaluated.
2. Results
2.1. Phytochemical Analysis
The phytochemical analysis of the extracts (aqueous and ethanol) of bitter ginger (Z. zerumbet) revealed the presence of a significant number of secondary metabolites. Table 1 lists the tests used and the different solvent extracts of bitter ginger (Z. zerumbet), assessed qualitatively, through a phytochemical analysis, respectively.

Table 1.
Phytochemical analysis of the extracts of bitter ginger (Z. zerumbet).
2.2. HPLC Analysis
The HPLC analysis of the crude extract of Z. zerumbet (aqueous extract), allowed for the identification of eight compounds, in comparison with the standard. Comparing the phenolic and flavanol retention times to the reference standards, allowed for the identification (Sigma Chemicals Co., St Louis, MO, USA). The RT for each analyte was used to pick a similar peak (in terms of the retention time), as that of the standards. The HPLC program has the ability to detect the flavonoid and phenolic compounds that showed similar peaks with similar retention time as that of the standards. This provided a base for the identification of each analyte.
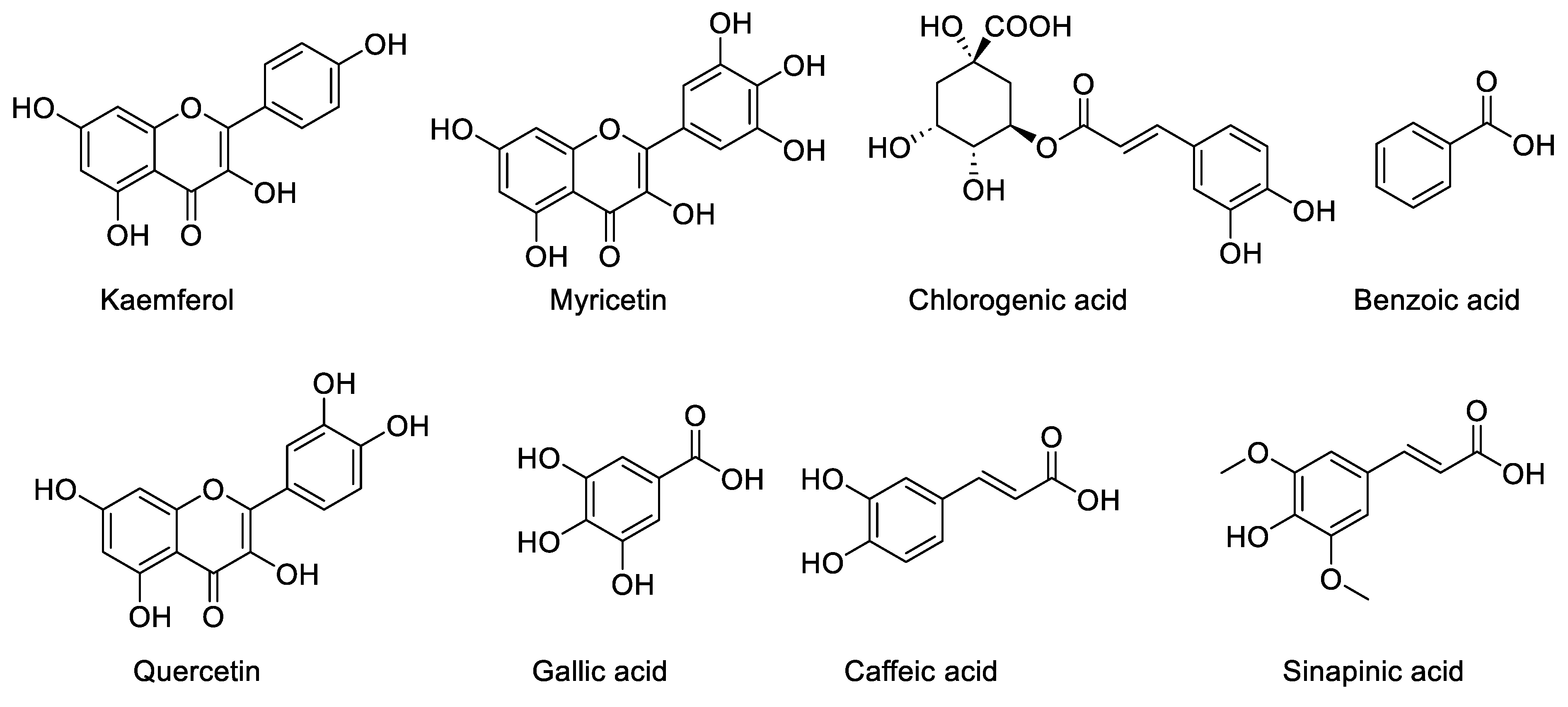
The identified components of the Z. zerumbet extract are caffeic acid, kaempferol, gallic acid, quercetin, sinapic acid, chlorogenic acid, benzoic acid, and myricetin. The identified components from the class of phenolic compounds are chlorogenic acid (16.92%, 16.67%), gallic acid (0.723%, 9.48%), sinapic acid (0.50%, 3.13%), caffeic acid (2.36%. 1.86%), myricetin (26.80%, 39.34%), benzoic acid (2.57%, 0%), kaempferol (0.06%, 0.45%), and quercetin (0.31%, 0.78%) in the aqueous and ethanolic extract, respectively. There are differences in the percentage of these components in the two crude extracts, according to the % peak area, which is illustrated in Table 2. The HPLC analysis of the bitter ginger extract is present in Figure 1 and Figure 2). Other peaks in the HPLC analysis show other biomolecules, which are present in the Z. zerumbet extract.

Table 2.
Flavonoid and phenolic contents in the Z. zerumbet rhizome extract, determined by the HPLC methods.
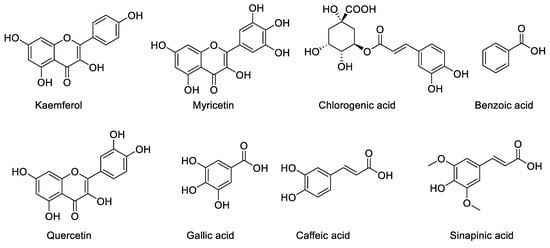
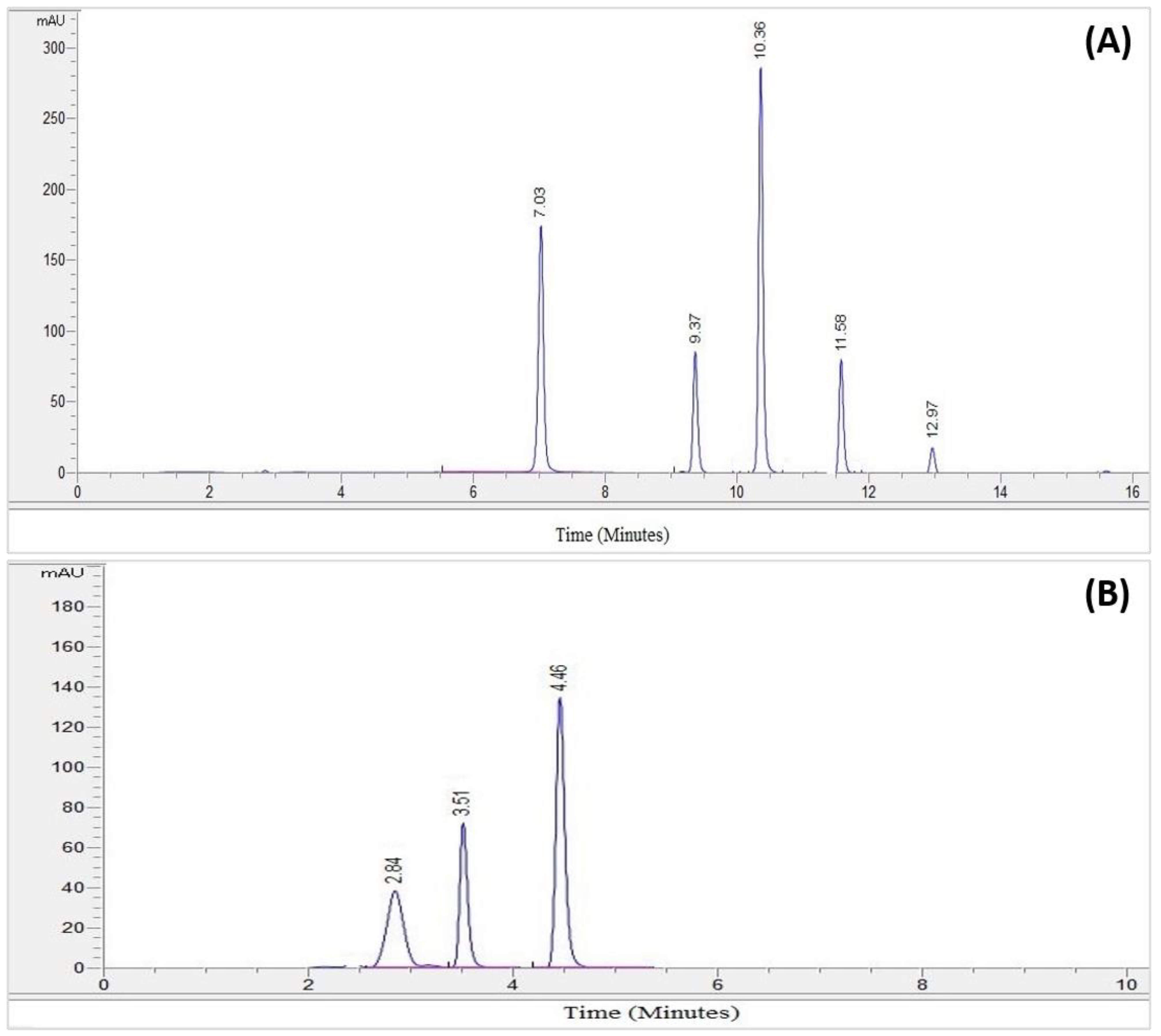
Figure 1.
(A) The chromatogram of the standard phenolic compounds. (B) The chromatogram of the standard flavonoid compounds.
Figure 2.
The chemical structure of the phenolic compounds.
Benzoic acid was the compound and it is one of the phenolic compounds present in the aqueous extract of Z. zerumbet, but it is not present in the ethanolic extracts.
The HPLC analysis of the standard phenolics and flavonoids is given in Figure 1 and Table 3. HPLC analysis of the various extracts (organic/aqueous) of Z. zerumbet, the peak assignment of the component includes chlorogenic acid, gallic acid, sinapic acid, caffeic acid, myricetin, benzoic acid, kaempferol, and quercetin.

Table 3.
Standard flavonoid and phenolic compounds determined by the HPLC methods.
2.3. Antibacterial Activity of the Plant Extract Combined with the Antibiotic Solution
When the antibiotics were tested without the prepared extracts, the tested bacterial strains showed resistance against the tested drugs. Lactobacillus acidophilus showed a resistance against ciprofloxacin, chloramphenicol, and tetracycline. Streptococcus mutans, Enterococcus faecalis, and Staphylococcus aureus showed resistance to all of the tested drugs, to varying degrees, as shown in Table 4.

Table 4.
Antibiotic susceptibility testing of the bacterial isolates with and without the bitter ginger aqueous extracts.
The synergistic mixture used in the study of the antibiotics and bitter ginger (aqueous and ethanolic) extracts is a combination of antibiotics at a concentration of 30 µg /mL (except CIP 5 µg/ mL) and the bitter ginger (aqueous and ethanolic) extracts at a concentration 10 mg/mL. When the extracts combined with antibiotics were used, the different resistance patterns of the tested organisms were observed, as shown in Table 4 and Table 5. The larger ZOI was observed and noted for the antibiotics treated with the bitter ginger aqueous and ethanolic extracts.

Table 5.
Antibiotic susceptibility testing of the bacterial isolates with and without the bitter ginger ethanolic extracts.
3. Materials and Methods
3.1. Extraction
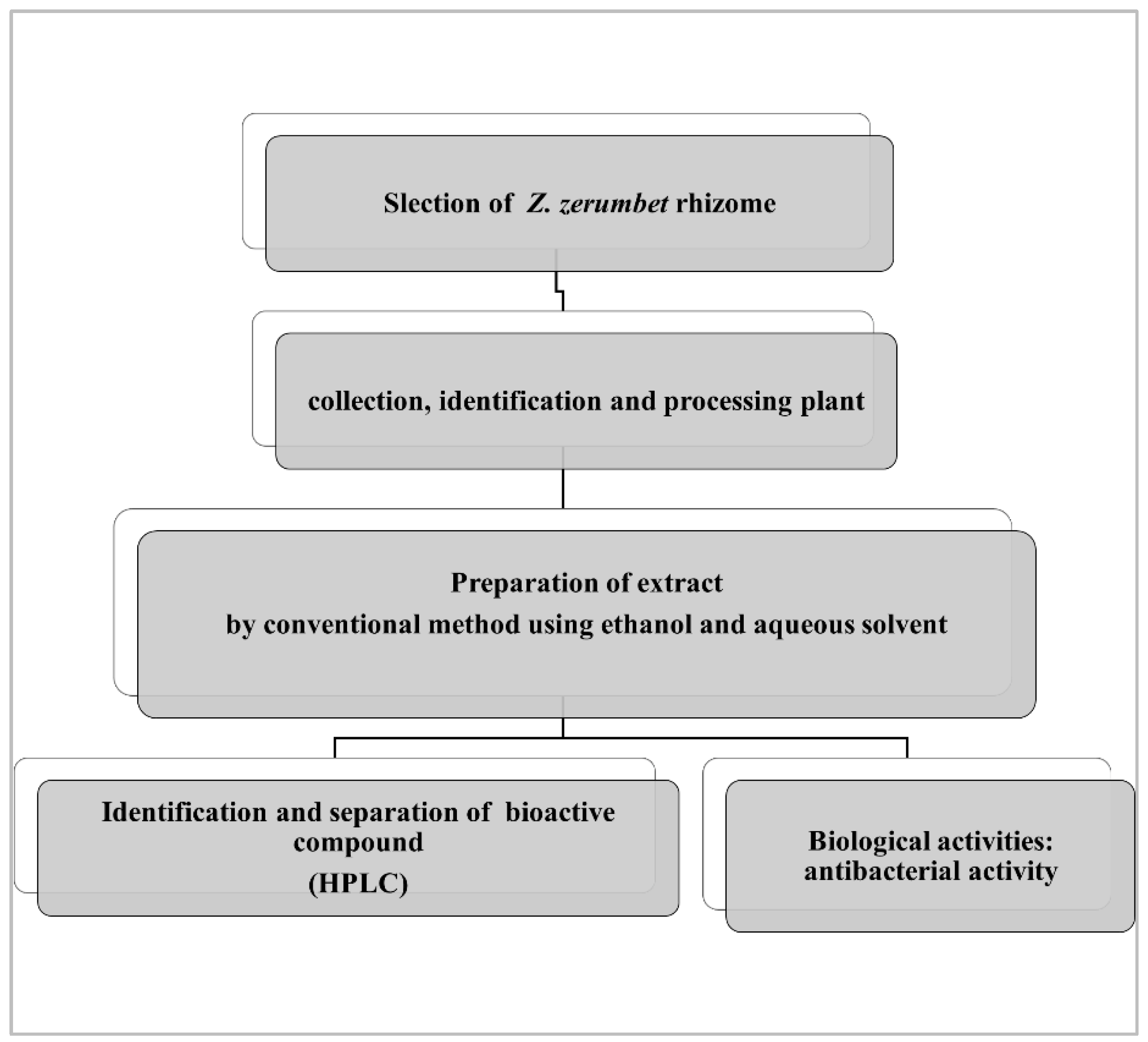
A fresh rhizome of a Z. zerumbet sample was obtained from the local market of Old Lahore (the walled city of Lahore). Following the collection, the rhizomes were washed thoroughly with distilled water, shade dried (one month), and then identified using their vernacular name by the Department of Botany, University of the Punjab, Lahore (voucher Number: LAH#280921). Following the shade drying step, the grinding of the plant was carried out to convert it into a fine powder, approximately 500 g, and the powder was processed for aqueous extraction. The aqueous (distilled water) and 95% ethanol extracts of the bitter ginger rhizome were prepared. Samples of 50 g were extracted for 5 days with 250 mL of the respective solvent, at 40 °C, in a shaking incubator at 200 rpm. Then, the extracts (mixtures) were initially filtered twice with nylon cloth, to remove the solid residue and then were further filtered using a Whatman filter paper. The fresh filtrate was left to evaporate using a rotary evaporator [26,27]. The final weighted extract was transferred to a clean airtight glass bottle and stored at 2 to 8 °C, and processed for the phytochemical analysis, and antibacterial activities (Figure 3).
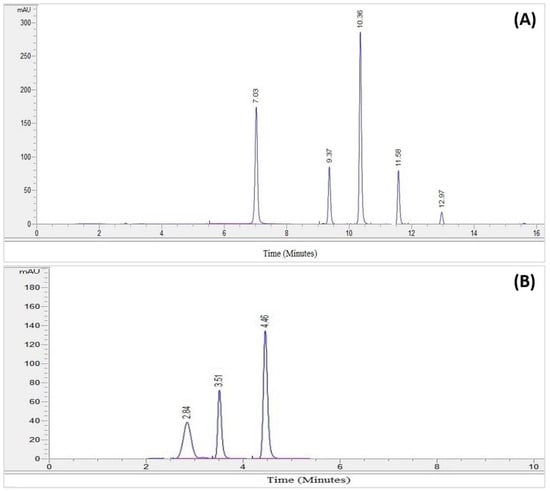
Figure 3.
General flow chart of the plant extraction and biological activity.
3.2. Preliminary Phytochemical Screening
A preliminary phytochemical examination was performed using the standard protocols, to test for the existence of glycosides, tannins, alkaloids, flavonoids, terpenoids, and quinones, qualitatively [28]. To determine the presence of alkaloids, Wagner’s test was used. To check for saponins, in six test tubes, 5 mg of the powdered extract was added, followed by 20 mL of 95% ethanol. The mixture was subjected to stirring for 12–15 min. The soapy appearance of the sample indicated the presence of saponin. To test for the presence of tannins, six tubes were loaded with 5 mg of the powdered extract and 5 mL of 95% ethanol, followed by the addition of 5% ferric chloride. The appearance of blue or a dark colour was indicative of the presence of tannins. Then, 3 mL of sodium hydroxide solution was added to each tube containing flavonoids extracted with six different solvents. A gentle stirring produced the colour, and its absence showed flavonoids. For the terpenoids test, 5 mg of the extracts were dissolved in 2 mL of pure chloroform and 5M H2SO4 was added to it. A red-brown precipitate suggested terpenoids [29]. To test for glycosides, 5 mg of powdered extracts were placed in six tubes (separately) with 5 mL of six different solvents, 2 mL of glacial acetic acid, 2% ferric chloride, and 1 mL of H2SO4. At the interface of the two liquids, glycosides produce a brown or violet ring [30]. Quinone was extracted six times with concentrated H2SO4. Red precipitates indicated the presence of quinones [31].
3.3. High-Performance Liquid Chromatography Analysis
The Z. zerumbet extracts were analysed for the phytochemical analysis using high-performance liquid chromatography (HPLC) techniques. Eight standards were tested, i.e., chlorogenic acid, quercetin, sinapic acid, gallic acid, benzoic acid, kaempferol, caffeic acid, and myricetin using HPLC. For this, 5 mg of the powdered materials of the standards and plant extract were dissolved in 2 mL of 95% ethanol, followed by filtration (0.45 µm syringe filter). An HPLC instrument (model 1260 Agilent, CA, USA) equipped with a quaternary pump 1260 and a DAD detector was used. For the data analysis, ChemStation software was used. A 20 µL volume of the filtered sample was injected into Zorbax Eclips Plus (Agilent, CA, USA), using a reverse phase (C18) column (4.6 × 250 mm; 5 µm particle size). The mobile phase used was a mixture of distilled methanol (solvent A) and 1% acetic acid (solvent B). The flow rate was of 1 mL/min in a linear gradient, following the scheme (t in min; %A): (0; 60%), (5; 35%), (10; 10%), (15, 60%), and (20; 60%). The column temperature was maintained at 25 °C. The chromatograms were recorded at 280 nm. In the case of the flavonoids, two solvent systems were utilized for the mobile phase: A, which included 3% trifluoroacetic acid, and B, which contained acetonitrile and methanol (80:20 v/v), respectively. The mobile phase, a mixture of solvents A and B (50:50 v/v), which was first filtered under vacuum through a 0.45 µm membrane, was eluted by isocratic elution at a flow rate of 1 mL/min at 30 °C. A wavelength of 360 nm was used for the detection process.
3.4. Collection and Preparation of the Bacterial Strains
For testing the antibacterial activity, the Lactobacillus acidophilus, Streptococcus mutans, Enterococcus faecalis, and Staphylococcus aureus strains were obtained from the laboratory of Microbiology, the University of Veterinary and Animal Sciences (UVAS), Lahore, Punjab, Pakistan. In the UVAS, these bacterial strains were isolated and identified from different animal and human clinical samples, and then saved for further research purposes in the bacterial strains store bank. First, these bacterial strains were sub-cultured on blood agar, nutrient agar, and nutrient broth, to obtain the fresh bacterial colonies. The plates were incubated at 37 °C for 24 h. Following the incubation period, the bacterial colonies were identified using different biochemical tests, including catalase, coagulase, DNA, and the bile esculin test [24,32,33].
3.4.1. Antibiotic Susceptibility of the Four Pathogenic Strains
The disk diffusion method was used, as per the standard guidelines from the Clinical Laboratory Standard Institute (CLSI) [32]. First, the antibacterial patterns of the bacterial isolates were obtained without any bitter ginger extract, using the disk diffusion technique. For this, 0.5% McFarland standard was prepared using Turbidimeter (Oxoid, Basingstoke, UK) for each bacterial isolate. This suspension was spread onto a Muller Hinton (MH) agar to make a bacterial lawn. Antibiotics (discs) of tetracycline (30 µg), chloramphenicol (30 µg), and ciprofloxacin (5 µg) were placed onto the agar plates followed by an incubation period of 24 h at 37 °C. Following the incubation period, the zone of inhibition (ZOI) was measured and noted.
3.4.2. Preparation of the Synergistic Mixture for the Antibacterial Activity
The synergistic mixture was prepared in different types of combination of antibiotics and plant extracts. It was prepared with a combination of plant extracts (aqueous and ethanolic) at a concentration of 10 mg/mL and different antibiotics at a concentration of 30 µg/mL, respectively, in 1:1 ratio, except for ciprofloxacin (concentration 5 µg/mL)
3.4.3. Antibacterial Effect of the Z. zerumbet Extracts Combined with Antibiotics
The synergistic effect of the bitter ginger extracts (aqueous and ethanolic) and three common antibiotics was investigated. The antibiotics discs were tetracycline (TE, 30 µg), chloramphenicol (C, 30 µg), and ciprofloxacin (CIP, 5 µg). As a control, the antibiotics were tested without the bitter ginger extract (2.5.1). For studying the synergistic effect of the bitter ginger extracts with the antibiotic solution. 20 µL of the desired mixture was added to the paper disc. A disc of each set was displaced on an agar plate inoculated with the tested bacterium. The zones of inhibition produced by the plant extract with the antibiotic’s solution after the incubation period at 37 °C for 24 h, were estimated. One percent di-methyl sulfoxide (DMSO) was used as a negative control for the test. If the zone of the combination treatment was greater than the zone of the plant extract plus the zone of the corresponding antibiotics, this was interpreted as synergism; if it was equal to the zone of the plant extract plus the zone of the corresponding antibiotics, this was interpreted as additive; and if it was less than the zone of the plant extract plus the zone of the corresponding antibiotics, this was interpreted as antagonism. The activity was described as sensitive and resistant by a zone of inhibition (ZOI). A zone diameter of ≥21 mm was deemed sensitive for CIP, C. The sensitivity zone for TE was around 23 mm. The Clinical and Laboratory Standard Institute’s guidelines (2017) were used to see the zone of inhibition for the antibacterial test [34].
3.5. Statistical Analysis
The data were analysed using the Statistical Package for Social Sciences (SPSS version 20). The standard deviation and mean values were used to define the data. The ZOI was also calculated in terms of the standard deviation.
4. Discussion
The present study was designed to obtain the preliminary information on the in vitro antimicrobial activities of the bitter ginger extract combined with an antibiotic solution on four MDR bacterial strains. A disc diffusion method was preferred to be used in this study, two crude extracts were analysed using HPLC. The results showed that the extraction using distilled water and ethanol has antimicrobial activity against the bacterial strains. This may be because of the presence of phenolic, flavonoid, and other bioactive compounds in the bitter ginger extract [35]. Moreover, our results showed that there is a noticeable difference in the antimicrobial activity of the extract of bitter ginger when distilled water is the solvent, than the extract when ethanol is the solvent, as seen in Table 4 and Table 5. There are two points which can help explain this. The first point is that, as a result of the variation in the chemical composition between the two bitter ginger extracts, i.e., the variations related to the presence of benzoic acid in the aqueous extract but not in the ethanolic extract, as determined in Table 2. The second point is that because of the difference in the concentrations of materials, i.e., benzoic acid in the two extracts, this may lead to a different antimicrobial potency, this difference in concentration could be due to the nature of the solvent used for the extraction. Furthermore, the results for both extracts, combined with the antibiotic solution, were effective against all strains. This is due to the negative charge of the surface of the Gram-positive wall, which may reduce their resistance to an antibacterial component.
The phenolic acid of the extract of bitter ginger was detected using HPLC. The seven standards available are: chlorogenic acid, quercetin, sinapic acid, gallic acid, benzioc acid, kaempferol, caffeic acid, and myricetin [7]. In the current study we found various phenolic and flavonoid compounds in bitter ginger, including chlorogenic acid, quercetin, sinapic acid, gallic acid, benzioc acid, kaempferol, caffeic acid and myricetin. The current study demonstrated that the amount of phenolic and flavonoid compounds differed greatly from one extract to the next. These phenolic acids are found naturally in medicinal plants, reported to be prominent bioactive compounds in most medicinal plants. The findings in this study agreed with a previous study [2]. Elguindy et al., 2016 found that the main phenolic compound identified in the cardamom extract (Zingiberaceae), was caffeic acid. The aqueous extracts showed the presence of the highest concentrations of chlorogenic acid. Similar findings were reported in previous studies, in which they reported that compounds in different extracts differed in concentrations [36].
In the current study, generally, Z. zerumbet extracts (aqueous and ethanol) did not show any antimicrobial potential against all strains. Therefore, there were no synergistic effects between the Z. zerumbet extracts (aqueous and ethanol) and the three different antibiotics studied against the resistant bacterial isolates. However, there was considerable improvement in the ciprofloxacin, chloramphenicol, and tetracycline action when the Z. zerumbet extracts were added to the ciprofloxacin, chloramphenicol, and tetracycline solutions (Table 4 and Table 5). This interesting finding was in harmony with those demonstrated by [37]. They stated that, the antibacterial activities of some antibiotics, including ampicillin, kanamycin, erythromycin, and chloramphenicol, have been enhanced in the presence silver nanoparticles against Gram positive and Gram-negative bacteria. Generally, the obtained result demonstrated that the combination of antibiotics with the Z. zerumbet extracts have a better antimicrobial potential. Bitter ginger’s antimicrobial properties were the primary rationale for its use, implying that ginger itself or its extracts could be used as a treatment for infections of a bacterial origin [6]. Combining two drugs may have additive, antagonistic, or synergistic effects. Many in vitro studies have shown that combining plant extracts with antibiotics has a synergistic impact that lowers the level of MICs for the antibiotics significantly. The combined action of an antibiotic drug and a plant extract against certain MDR bacterial strains was one of the primary goals of the current investigation. The results of this research may aid in understanding the synergistic impact of combination therapy, which, in more recent times may have the potential to provide a fresh approach to the management of bacterial infections. Antibiotic plant extracts showed a synergistic efficacy between the plant extracts and antibiotics against the MDR bacterial isolates shown to be effective previously. At current, tetracycline, ciprofloxacin, and chloramphenicol were used. According to the findings of this study, Lactobacillus acidophilus. S. mutans, and E. faecalis Staphylococcus aureus were found to be the most susceptible to ethanol extracts containing antibiotics, as compared to the aqueous extract containing antibiotics.
Staphylococcus spp. is resistant to the anti-biofilm effects of quercetin, which impairs the quorum sensing. Additionally, quercetin is a well-known cancer-fighting and anti-oxidant agent [38,39]. In the current study, quercetin was abundantly detected in the Z. zerumbet extract, but due to their high polarity, their concentrations were highest in the ethanol and water extracts of Z. zerumbet. Saponins, tannins, glycosides, terpenoids, quinones, and flavonoids were found in the present study. Saponins have the ability to deal with bacteria and fungi [40]. Flavonoids are the main substance having significant anti-oxidation and antimicrobial potentials [41].
5. Conclusions
The results of the present study have shown that the Z. zerumbet extracts have sufficient antibacterial activities for their various solvent-based extracts. The combination of the plant extract with antibiotics will open new approaches in the pharmaceutical sector for the manufacture of pharmaceutical products, which may help in utilizing these extracts in the treatment of various infections.
Author Contributions
Conceptualization, B.Z.; methodology, M.R.; software, M.R.; validation; formal analysis, M.R.; investigation, M.R.; resources, B.Z.; data curation, M.R.; writing—original draft preparation, M.R.; writing—review and editing, B.Z.; visualization, M.R.; supervision, B.Z.; project administration, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support is provided by the University of Central Punjab and Universiti Malaysia Sabah, Malaysia.
Institutional Review Board Statement
The study was conducted according to the Declaration of Helsinki, and ethical approval was obtained from the Human Research Ethics Committee, Faculty of Life Sciences, University of Central Punjab, Lahore, Pakistan, with code HREC UCP Code: CP/FOLS/210419-6.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to thank the Faculty of Science and Technology, the University of Central Punjab for providing the research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Prakash, R.O.; Rabinarayan, A.; Kumar, M.S. Zingiber zerumbet (L.) Sm., a reservoir plant for therapeutic uses: A review. Int. J. Res. Ayurveda Pharm. 2011, 2, 543216. [Google Scholar]
- Saensouk, P.; Saensouk, S. Diversity, traditional uses and conservation status of Zingiberaceae in Udorn Thani Province, Thailand. Biodiversitas J. Biol. Divers. 2021, 22, 3083–3097. [Google Scholar] [CrossRef]
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Ahmed, N.; Karobari, M.I.; Yousaf, A.; Mohamed, R.N.; Arshad, S.; Basheer, S.N.; Peeran, S.W.; Noorani, T.Y.; Assiry, A.A.; Alharbi, A.S. The Antimicrobial Efficacy Against Selective Oral Microbes, Antioxidant Activity and Preliminary Phytochemical Screening of Zingiber officinale. Infect. Drug Resist. 2022, 15, 2773. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. The diversity of biologically active compounds in the rhizomes of recently discovered Zingiberaceae plants native to North Eastern Thailand. Pharmacogn. J. 2019, 11, 1014–1022. [Google Scholar] [CrossRef]
- Saensouk, S.; Saensouk, P.; Pasorn, P.; Chantaranothai, P. Diversity and uses of Zingiberaceae in Nam Nao National Park, Chaiyaphum and Phetchabun provinces, Thailand, with a new record for Thailand. Agric. Nat. Resour. 2016, 50, 445–453. [Google Scholar] [CrossRef]
- Chang, C.J.; Tzeng, T.-F.; Liou, S.-S.; Chang, Y.-S.; Liu, I.-M. Acute and 28-day subchronic oral toxicity of an ethanol extract of Zingiber zerumbet (L.) Smith in rodents. Evid.-Based Complementary Altern. Med. 2012, 2012, 608284. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef]
- Koga, A.Y.; Beltrame, F.L.; Pereira, A.V. Several aspects of Zingiber zerumbet: A review. Rev. Bras. Farmacogn. 2016, 26, 385–391. [Google Scholar] [CrossRef]
- Liu, W.Y.; Tzeng, T.-F.; Liu, I.-M. Healing potential of zerumbone ointment on experimental full-thickness excision cutaneous wounds in rat. J. Tissue Viability 2017, 26, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jantan, I. Recent Updates on the Phytochemistry, Pharmacological, and Toxicological Activities of Zingiber zerumbet (L.) Roscoe ex Sm. Curr. Pharm. Biotechnol. 2017, 18, 696–720. [Google Scholar] [CrossRef] [PubMed]
- Sidahmed, H.M.A.; Hashim, N.M.; Abdulla, M.A.; Ali, H.M.; Mohan, S.; Abdelwahab, S.I.; Taha, M.M.E.; Fai, L.M.; Vadivelu, J. Antisecretory, gastroprotective, antioxidant and anti-Helicobcter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith. PLoS ONE 2015, 10, e0121060. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. Strengthening of oral health systems: Oral health through primary health care. Med. Princ. Pract. 2014, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pasril, Y.; Yuliasanti, A. Anti-Bacterial Power of Red Batel Leaves (Piper Crocatum) to Enterococcus Faecalis Bacteria as Medi. Insisiva Dent. J. 2014, 3, 88–95. [Google Scholar]
- Sam, M.F.R.; Hamid, A.; Ghazali, A.R.; Louis, S.R.; Budin, S.B. Protective effects of zingiber zerumbet ethyl acetate extract on hydrogen peroxide-induced damage of red blood cells. Sains Malays. 2019, 48, 781–790. [Google Scholar]
- Rizvi, A.; Saeed, M.U.; Nadeem, A.; Yaqoob, A.; Rabaan, A.A.; Bakhrebah, M.A.; Al Mutair, A.; Alhumaid, S.; Aljeldah, M.; Al Shammari, B.R.; et al. Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients in Lahore, Pakistan. Medicina 2022, 58, 904. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alhumaid, S.; Mutair, A.A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Bshabshe, A.A.; Sulaiman, T.; AlFonaisan, M.K. Application of Artificial Intelligence in Combating High Antimicrobial Resistance Rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef]
- Zeb, S.; Mushtaq, M.; Ahmad, M.; Saleem, W.; Rabaan, A.A.; Naqvi, B.S.Z.; Garout, M.; Aljeldah, M.; Al Shammari, B.R.; Al Faraj, N.J. Self-Medication as an Important Risk Factor for Antibiotic Resistance: A Multi-Institutional Survey among Students. Antibiotics 2022, 11, 842. [Google Scholar] [CrossRef]
- Ahmed, N.; Zeshan, B.; Naveed, M.; Afzal, M.; Mohamed, M. Antibiotic resistance profile in relation to virulence genes fimH, hlyA and usp of uropathogenic E. coli isolates in Lahore, Pakistan. Trop. Biomed. 2019, 36, 559–568. [Google Scholar] [PubMed]
- Hussain, S.; Zeshan, B.; Arshad, R.; Kabir, S.; Ahmed, N. MRSA Clinical Isolates Harboring mecC Gene Imply Zoonotic Transmission to Humans and Colonization by Biofilm Formation. Pakistan J. Zool. 2022. [Google Scholar] [CrossRef]
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z. The Molecular Characterization of Virulence Determinants and Antibiotic Resistance Patterns in Human Bacterial Uropathogens. Antibiotics 2022, 11, 516. [Google Scholar] [CrossRef]
- Parveen, S.; Saqib, S.; Ahmed, A.; Shahzad, A.; Ahmed, N. Prevalence of MRSA colonization among healthcare-workers and effectiveness of decolonization regimen in ICU of a Tertiary care Hospital, Lahore, Pakistan. Adv. Life Sci. 2020, 8, 38–41. [Google Scholar]
- Ahmed, N.; Ali, Z.; Riaz, M.; Zeshan, B.; Wattoo, J.I.; Aslam, M.N. Evaluation of antibiotic resistance and virulence genes among clinical isolates of Pseudomonas aeruginosa from cancer patients. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1333. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, A.; Verma, A.K.; Singh, S.; Roy, M.K.; Daimari, M. Antioxidant and antiproliferative activity of selected medicinal plants of lower Assam, India: An in vitro and in silico study. Anti-Cancer Agents Med. Chem. 2021, 21, 267–277. [Google Scholar] [CrossRef]
- Ling, J.W.A.; Chang, L.S.; Mohd Khalid, R.; Wan Mustapha, W.A.; Sofian-Seng, N.S.; Mohd Razali, N.S.; Rahman, H.A.; Mohd Zaini, N.A.; Lim, S.J.; Preservation. Sequential extraction of red button ginger (Costus woodsonii): Phytochemical screening and antioxidative activities. J. Food Processing 2020, 44, e14776. [Google Scholar] [CrossRef]
- Yahaya, T.; Mungadi, A.; Obadiah, C. Phytoconstituent Screening of roselle (Hibiscus sabdariffa), moringa (Moringa oleifera), ginger (Zingiber officinale) and fluted pumpkin (Telfairia occidentalis) leaves. J. Appl. Sci. Environ. Manag. 2017, 21, 253–256. [Google Scholar] [CrossRef][Green Version]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- De Zoysa, M.H.N.; Rathnayake, H.; Hewawasam, R.P.; Wijayaratne, W.M.D.G.B. Determination of in vitro antimicrobial activity of five Sri Lankan medicinal plants against selected human pathogenic bacteria. Int. J. Microbiol. 2019, 2019, 7431439. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Karobari, M.I.; Heboyan, A.; Mohamed, R.N.; Mustafa, M.; Basheer, S.N.; Desai, V.; Batool, S.; Ahmed, N.; Zeshan, B. Synthesis of silver nanoparticles from extracts of wild ginger (Zingiber zerumbet) with antibacterial activity against selective multidrug resistant oral bacteria. Molecules 2022, 27, 2007. [Google Scholar] [CrossRef]
- Clinical Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef]
- Zakaria, Z.; Mohamad, A.; Chear, C.; Wong, Y.; Israf, D.; Sulaiman, M. Antiinflammatory and antinociceptive activities of Zingiber zerumbet methanol extract in experimental model systems. Med. Princ. Pract. 2010, 19, 287–294. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Zulkifli, I.; Hair Bejo, M.; Farida, A.; Somchit, M. Acute toxicity study and phytochemical screening of selected herbal aqueous extract in broiler chickens. Int. J. Pharm. 2008, 4, 352–360. [Google Scholar] [CrossRef]
- Kader, M.G.; Habib, M.R.; Nikkon, F.; Yeasmin, T.; Rashid, M.A.; Rahman, M.M.; Gibbons, S. Zederone from the rhizomes of Zingiber zerumbet and its anti-staphylococcal activity. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2010, 9, 63–68. [Google Scholar]
- Reginato, F.Z.; da Silva, A.R.H.; de Freitas Bauermann, L. Evaluation of the flavonoides use in the treatment of the inflammation. Rev. Cuba. Farm. 2015, 49, 569–582. [Google Scholar]
- Liu, X.; Jia, J.; Jing, X.; Li, G. Antioxidant activities of extracts from sarcocarp of Cotoneaster multiflorus. J. Chem. 2018, 2018, 4619768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).