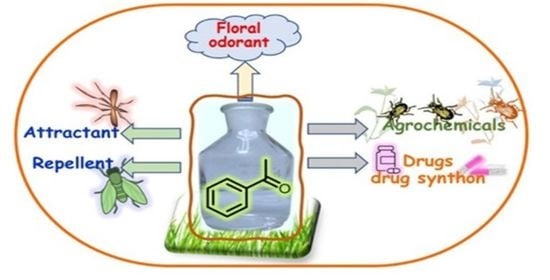
Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions
Abstract
1. Introduction
2. Historical Background
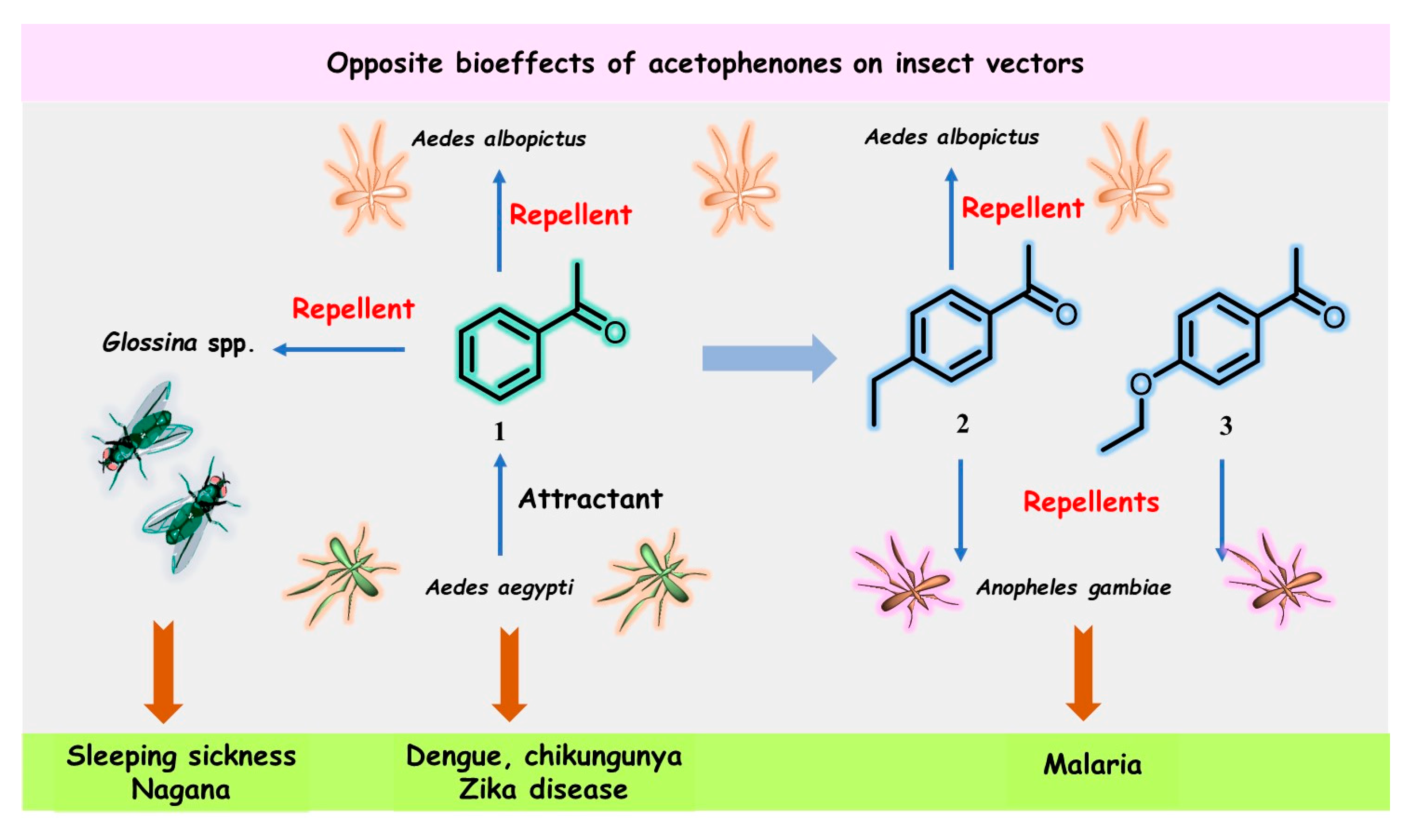
3. Acetophenone Is One of the Most Interesting Players in Skin Microbiota Manipulation by Vector-Borne Parasites Found in Animal-Feeding Insects
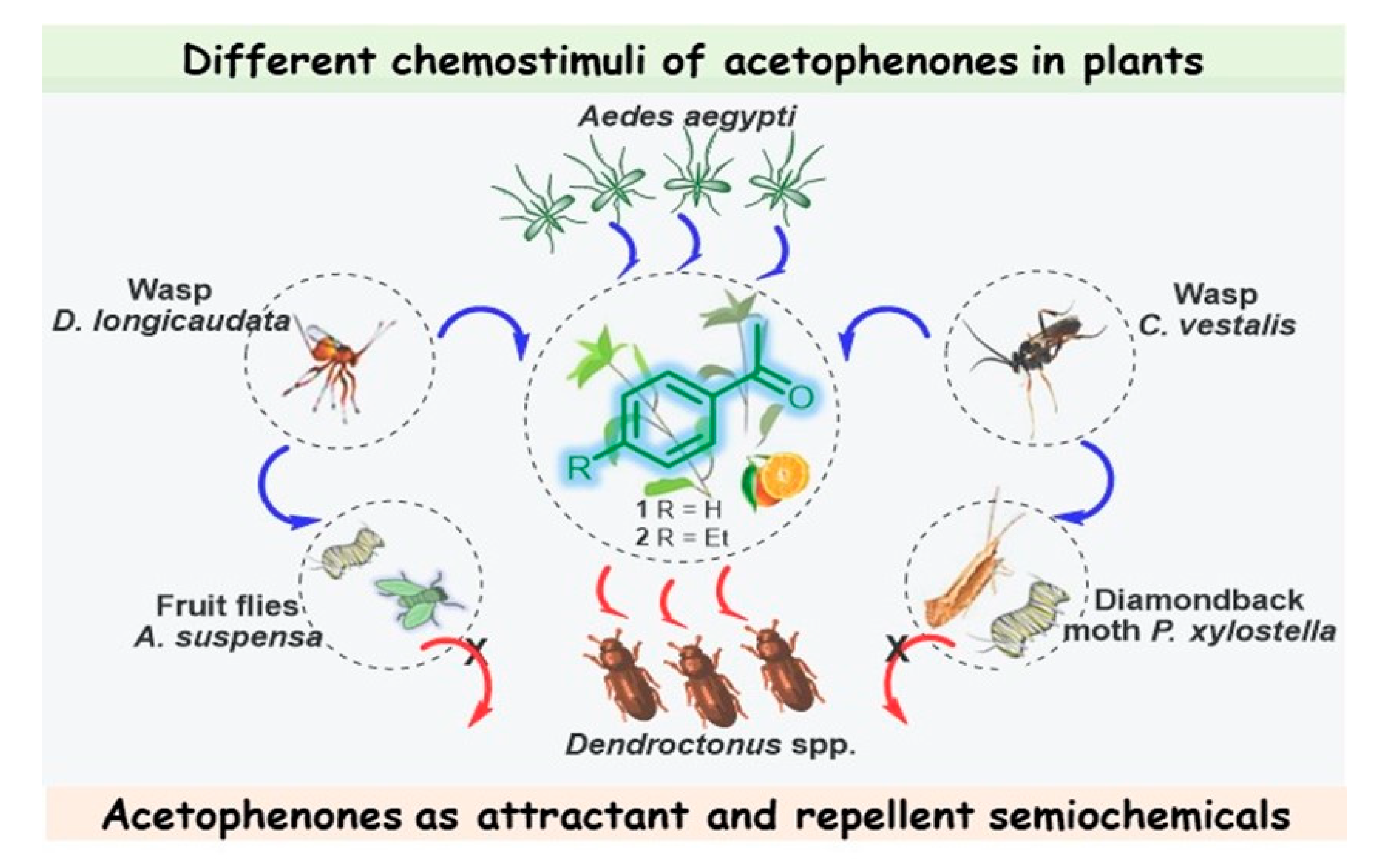
4. Acetophenone Is a Prolific Semiochemical for Plant-Feeding Insects in Complex Interspecific Communication
5. Biogenesis: How Can Acetophenone Be Formed In Vivo?
6. Industrial Production of Acetophenone: So Many Possibilities, but None Are Ideal and Sustainable
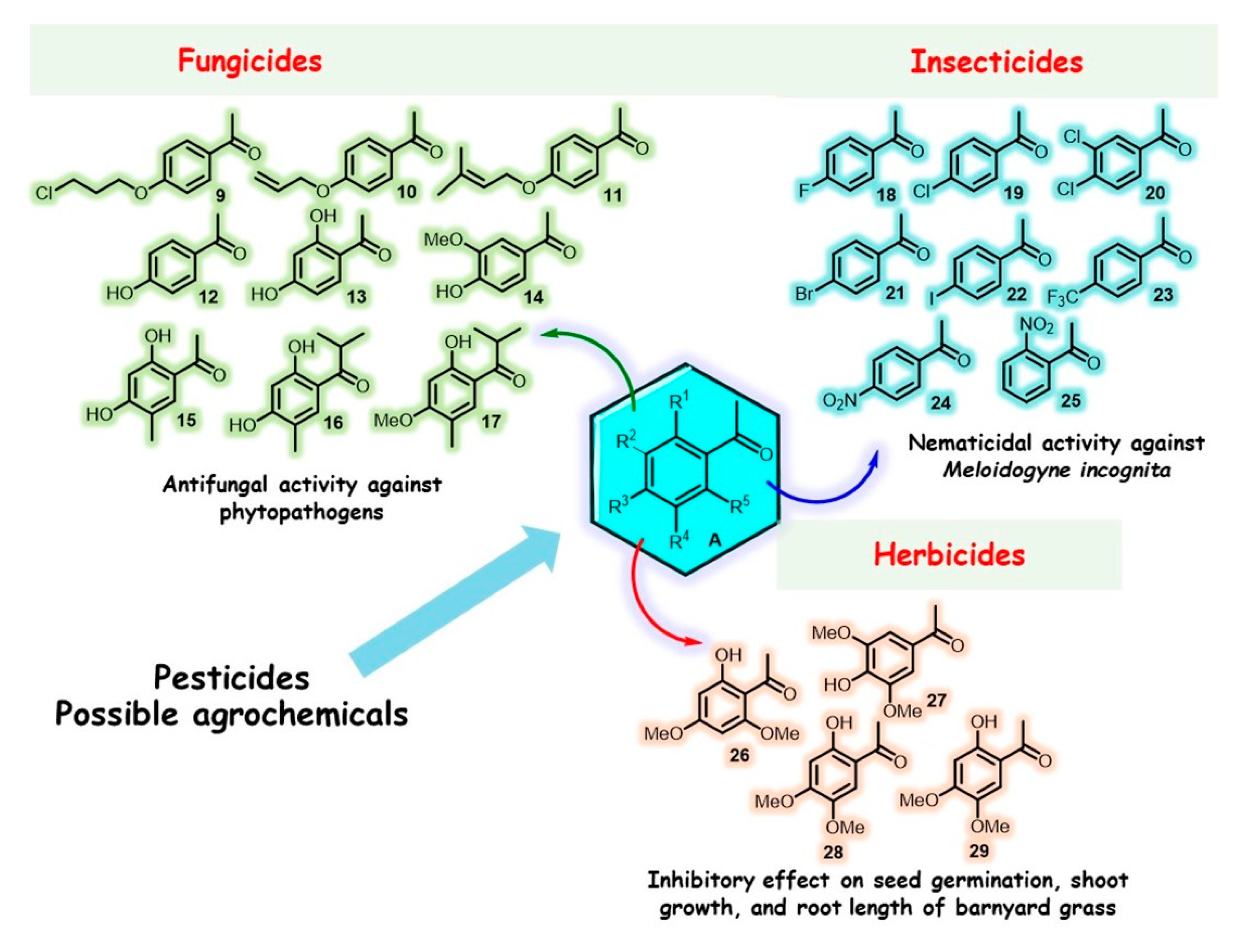
7. Natural and Synthetic Closely Related Acetophenone Cousins as Promising Agrochemicals
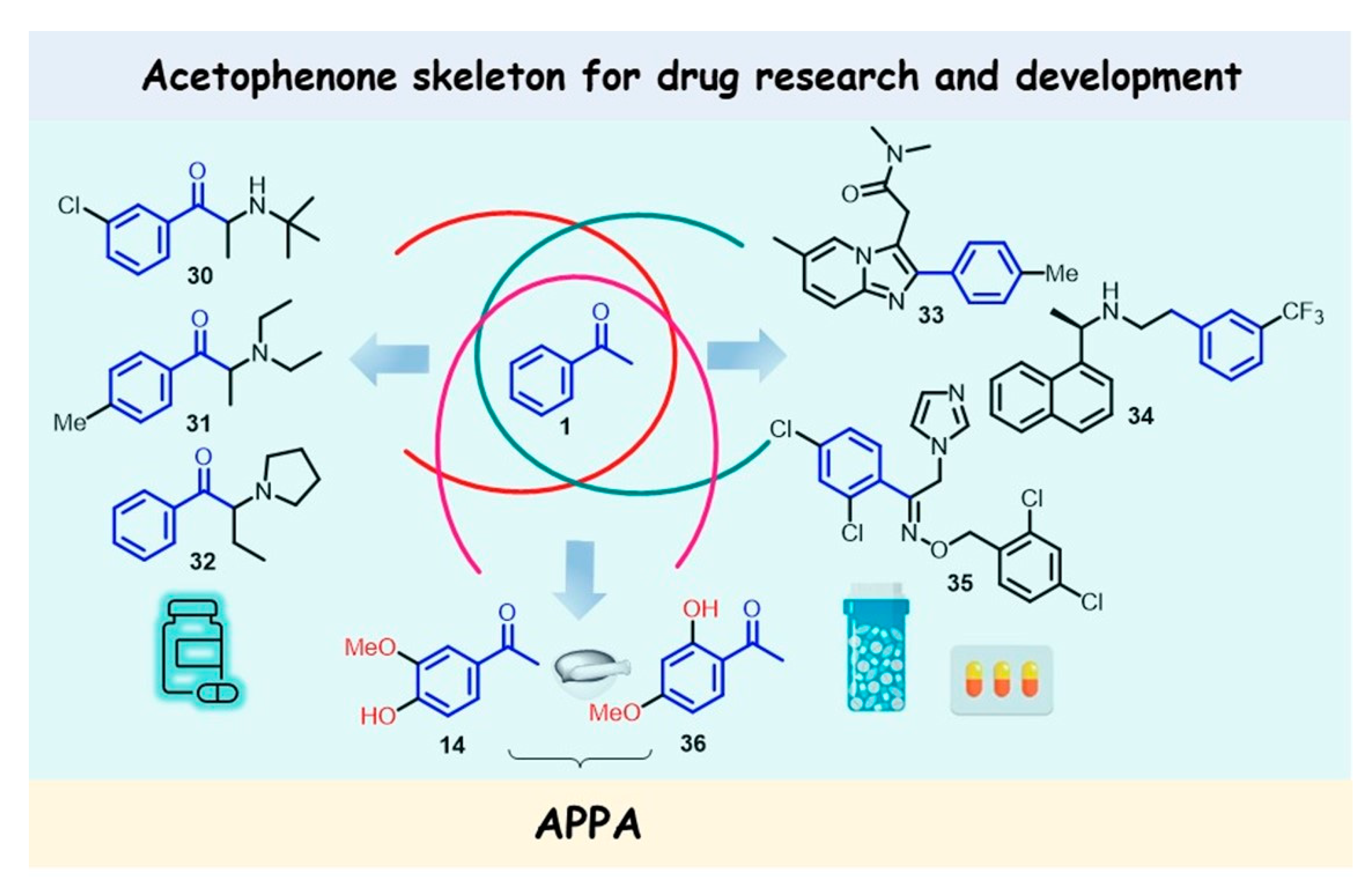
8. Acetophenone Skeleton for Developing Pharmacological Agents/Drugs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Sanders, H.J.; Keag, H.F.; McCullough, H.S. Acetophenone. Ind. Eng. Chem. 1953, 45, 2–14. [Google Scholar] [CrossRef]
- Siegel, H.; Eggersdorfer, M. Ketones. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 20, p. 202. [Google Scholar]
- Friedel, C.; Beilstein, F.K. Jahresber. Fortschritte der Chemie 1857, 270. In Handbuch der Organischen Chemie, 4th ed.; Prager, B., Jacobson, P., Schmidt, P., Stern, D., Eds.; Journal Springer: Berlin, Germany, 1925; Volume 7, p. 272. [Google Scholar]
- Granito, C.; Schultz, H.P. Decarboxylation studies. II. Preparation of alkyl phenyl ketones1, 2. J. Org. Chem. 1963, 28, 879–881. [Google Scholar] [CrossRef]
- Norman, C. Cases illustrating the Sedative Effects of Aceto-phenone (hypnone). J. Ment. Sci. 1887, 32, 519–525. [Google Scholar] [CrossRef]
- Limousin, S. Acetophenone, or hypnone, a new hypnotic agent. Am. J. Pharm. 1886, 58, 185. [Google Scholar]
- Zhang, H.; Zhu, Y.; Liu, Z.; Peng, Y.; Peng, W.; Tong, L.; Wang, J.; Liu, Q.; Wang, P.; Cheng, G. A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell 2022, 185, 2510–2522.e16. [Google Scholar] [CrossRef]
- Leslie, M. Dengue and zika viruses turn people into mosquito bait. Science 2022, 377, 137. [Google Scholar] [CrossRef]
- Du Toit, A. An attractive scent. Nat. Rev. Genet. 2022, 20, 510. [Google Scholar] [CrossRef] [PubMed]
- Gul, L.; Korcsmaros, T.; Hall, N. Flaviviruses hijack the host microbiota to facilitate their transmission. Cell 2022, 185, 2395–2397. [Google Scholar] [CrossRef] [PubMed]
- Braks, M.; Anderson, R.; Knols, B. Infochemicals in Mosquito Host Selection: Human Skin Microflora and Plasmodium Parasites. Parasitol. Today 1999, 15, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.O.; Andriessen, R.; Groenhagen, U.; Kiss, G.B.; Schulz, S.; Takken, W.; Van Loon, J.J.A.; Schraa, G.; Smallegange, R.C. Differential Attraction of Malaria Mosquitoes to Volatile Blends Produced by Human Skin Bacteria. PLoS ONE 2010, 5, e15829. [Google Scholar] [CrossRef]
- Verhulst, N.O.; Takken, W.; Dicke, M.; Schraa, G.; Smallegange, R.C. Chemical ecology of interactions between human skin microbiota and mosquitoes. FEMS Microbiol. Ecol. 2010, 74, 1–9. [Google Scholar] [CrossRef]
- Verhulst, N.O.; Qiu, Y.T.; Beijleveld, H.; Maliepaard, C.; Knights, D.; Schulz, S.; Berg-Lyons, D.; Lauber, C.L.; Verduijn, W.; Haasnoot, G.W.; et al. Composition of Human Skin Microbiota Affects Attractiveness to Malaria Mosquitoes. PLoS ONE 2011, 6, e28991. [Google Scholar] [CrossRef]
- Guerenstein, P.G.; Lazzari, C.R. Host-seeking: How triatomines acquire and make use of information to find blood. Acta Trop. 2009, 110, 148–158. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Molina, J. Preliminary evidence of Rhodnius prolixus (Hemiptera: Triatominae) attraction to human skin odour extracts. Acta Trop. 2010, 113, 174–179. [Google Scholar] [CrossRef]
- Omolo, M.O.; Ndiege, I.O.; Hassanali, A. Semiochemical signatures associated with differential attraction of Anopheles gambiae to human feet. PLoS ONE 2021, 16, e0260149. [Google Scholar] [CrossRef]
- Carraretto, D.; Soresinetti, L.; Rossi, I.; Malacrida, A.R.; Gasperi, G.; Gomulski, L.M. Behavioural Responses of Male Aedes albopictus to Different Volatile Chemical Compounds. Insects 2022, 13, 290. [Google Scholar] [CrossRef]
- Fawaz, E.Y.; Allan, S.A.; Bernier, U.R.; Obenauer, P.J.; Diclaro, J.W. Swarming mechanisms in the yellow fever mosquito: Aggregation pheromones are involved in the mating behavior of Aedes aegypti. J. Vector Ecol. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Mozūraitis, R.; Hajkazemian, M.; Zawada, J.W.; Szymczak, J.; Pålsson, K.; Sekar, V.; Biryukova, I.; Friedländer, M.R.; Koekemoer, L.L.; Baird, J.K.; et al. Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nat. Ecol. Evol. 2020, 4, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Torr, S.; Mangwiro, T.; Hall, D. Responses of Glossina pallidipes (Diptera: Glossinidae) to synthetic repellents in the field. Bull. Èntomol. Res. 1996, 86, 609–616. [Google Scholar] [CrossRef]
- Olaide, O.Y.; Tchouassi, D.P.; Yusuf, A.A.; Pirk, C.W.W.; Masiga, D.K.; Saini, R.K.; Torto, B. Zebra skin odor repels the savannah tsetse fly, Glossina pallidipes (Diptera: Glossinidae). PLOS Neglected Trop. Dis. 2019, 13, e0007460. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Van Oers, M.M.; Vlak, J.M.; Vreysen, M.J.B.; Parker, A.G.; Abd-Alla, A.M.M. Virology, Epidemiology and Pathology of Glossina Hytrosavirus, and Its Control Prospects in Laboratory Colonies of the Tsetse Fly, Glossina pallidipes (Diptera; Glossinidae). Insects 2013, 4, 287–319. [Google Scholar] [CrossRef]
- Olaide, O.Y.; Tchouassi, D.P.; Yusuf, A.A.; Pirk, C.W.; Masiga, D.K.; Saini, R.K.; Torto, B. Effect of zebra skin-derived compounds on field catches of the human African trypanosomiasis vector Glossina fuscipes fuscipes. Acta Trop. 2020, 213, 105745. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Stanczyk, N.M.; Betz, H.S.; Pulido, H.; Sim, D.G.; Read, A.F.; Mescher, M.C. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 2014, 111, 11079–11084. [Google Scholar] [CrossRef]
- Yamazaki, K.; Boyse, E.A.; Bard, J.; Curran, M.; Kim, D.; Ross, S.R.; Beauchamp, G.K. Presence of mouse mammary tumor virus specifically alters the body odor of mice. Proc. Natl. Acad. Sci. USA 2002, 99, 5612–5615. [Google Scholar] [CrossRef]
- Vreysen, M.J.; Seck, M.T.; Sall, B.; Bouyer, J. Tsetse flies: Their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 2012, 112, S15–S25. [Google Scholar] [CrossRef]
- Lindh, J.M.; Torr, S.J.; Vale, G.A.; Lehane, M.J. Improving the Cost-Effectiveness of Artificial Visual Baits for Controlling the Tsetse Fly Glossina fuscipes fuscipes. PLOS Neglected Trop. Dis. 2009, 3, e474. [Google Scholar] [CrossRef]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef] [PubMed]
- Dormont, L.; Mulatier, M.; Carrasco, D.; Cohuet, A. Mosquito Attractants. J. Chem. Ecol. 2021, 47, 351–393. [Google Scholar] [CrossRef] [PubMed]
- Kapsetaki, S.-E.; Tzelepis, I.; Avgousti, K.; Livadaras, I.; Garantonakis, N.; Varikou, K.; Apidianakis, Y. The bacterial metabolite 2-aminoacetophenone promotes association of pathogenic bacteria with flies. Nat. Commun. 2014, 5, 4401. [Google Scholar] [CrossRef]
- Fikrig, K.; Johnson, B.J.; Fish, D.; Ritchie, S.A. Assessment of synthetic floral-based attractants and sugar baits to capture male and female Aedes aegypti (Diptera: Culicidae). Parasites Vectors 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Lahondère, C.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffell, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA 2019, 117, 708–716. [Google Scholar] [CrossRef]
- von Oppen, S.; Masuh, H.; Licastro, S.; Zerba, E.; Gonzalez-Audino, P. A floral-derived attractant for Aedes aegypti mosquitoes. Èntomol. Exp. Appl. 2015, 155, 184–192. [Google Scholar] [CrossRef]
- Ovruski, S.; Aluja, M.; Sivinski, J.; Wharton, R. Hymenopteran Parasitoids on Fruit-infesting Tephritidae (Diptera) in Latin America and the Southern United States: Diversity, Distribution, Taxonomic Status and their use in Fruit Fly Biological Control. Integr. Pest Manag. Rev. 2000, 5, 81–107. [Google Scholar] [CrossRef]
- Rohrig, E.; Sivinski, J.; Teal, P.; Stuhl, C.; Aluja, M. A Floral-Derived Compound Attractive to the Tephritid Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). J. Chem. Ecol. 2008, 34, 549–557. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Reynolds, O.L.; Chen, W.; He, W.; You, M.; Gurr, G.M. Alyssum (Lobularia maritima) selectively attracts and enhances the performance of Cotesia vestalis, a parasitoid of Plutella xylostella. Sci. Rep. 2020, 10, 6447. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Borden, J.H. New repellent semiochemicals for three species of Dendroctonus (Coleoptera: Scolytidae). Chemoecology 2004, 14, 67–75. [Google Scholar] [CrossRef]
- Sullivan, B.T. Electrophysiological and Behavioral Responses of Dendroctonus frontalis (Coleoptera: Curculionidae) to Volatiles Isolated from Conspecifics. J. Econ. Èntomol. 2005, 98, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Erbilgin, N.; Gillette, N.E.; Mori, S.R.; Stein, J.D.; Owen, D.R.; Wood, D.L. Acetophenone as an Anti-attractant for the Western Pine Beetle, Dendroctonus Brevicomis LeConte (Coleoptera: Scolytidae). J. Chem. Ecol. 2007, 33, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Erbilgin, N.; Gillette, N.E.; Owen, D.R.; Mori, S.R.; Nelson, A.S.; Uzoh, F.; Wood, D.L. Acetophenone superior to verbenone for reducing attraction of western pine beetle Dendroctonus brevicomis to its aggregation pheromone. Agric. For. Entomol. 2008, 10, 433–441. [Google Scholar] [CrossRef]
- Fettig, C.J.; McKelvey, S.R.; Dabney, C.P.; Huber, D.P.W. Responses of Dendroctonus brevicomis (Coleoptera: Curculionidae) in behavioral assays: Implications to development of a semiochemical-based tool for tree protection. J. Econ. Èntomol. 2012, 105, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jonfia-Ess, W.; Alderson, P.; Tucker, G.; Linforth, R.; West, G. The Growth of Tribolium castaneum (Herbst) and Lasioderma serricorne (Fabricius) on Feed Media Dosed with Flavour Volatiles Found in Dry Cocoa Beans. Pak. J. Biol. Sci. 2007, 10, 1301–1304. [Google Scholar] [CrossRef][Green Version]
- Nones, S.; Sousa, E.; Holighaus, G. Symbiotic fungi of an ambrosia beetle alter the volatile bouquet of cork oak seedlings. Phytopathology 2022, 112, 1965–1978. [Google Scholar] [CrossRef]
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav. Ecol. Sociobiol. 2011, 65, 1015–1027. [Google Scholar] [CrossRef]
- Hernández, M.M.; Sanz, I.; Adelantado, M.; Ballach, S.; Primo, E. Electroantennogram activity from antennae ofCeratitis capitata (Wied.) to fresh orange airborne volatiles. J. Chem. Ecol. 1996, 22, 1607–1619. [Google Scholar] [CrossRef]
- Murugan, R.; Mallavarapu, G.R.; Padmashree, K.V.; Rao, R.R.; Livingstone, C. Volatile Oil Composition of Pogostemon heyneanus and Comparison of its Composition with Patchouli Oil. Nat. Prod. Commun. 2010, 5, 1961–1964. [Google Scholar] [CrossRef]
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Murugan, R.; Pandian, S.K. Essential oils from commercial and wild Patchouli modulate Group A Streptococcal biofilms. Ind. Crop. Prod. 2015, 69, 180–186. [Google Scholar] [CrossRef]
- Bodor, N.; Gabanyi, Z.; Wong, C.K. A new method for the estimation of partition coefficient. J. Am. Chem. Soc. 1989, 111, 3783–3786. [Google Scholar] [CrossRef]
- Acetophenone. Available online: http://www.stenutz.eu/chem/solv6.php?name=acetophenone (accessed on 29 July 2022).
- Marchiosi, R.; Dos Santos, W.D.; Constantin, R.P.; De Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.D.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Lattanzio, V. Relationship of Phenolic Metabolism to Growth in Plant and Cell Cultures Under Stress. In Plant Cell and Tissue Differentiation and Secondary Metabolites. Reference Series in Phytochemistry; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 837–868. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A Familiar Ring to It: Biosynthesis of Plant Benzoic Acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388. [Google Scholar] [CrossRef]
- Lapadatescu, C.; Giniès, C.; Le Quéré, J.-L.; Bonnarme, P. Novel Scheme for Biosynthesis of Aryl Metabolites from l -Phenylalanine in the Fungus Bjerkandera adusta. Appl. Environ. Microbiol. 2000, 66, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, Z.; Baldermann, S.; Kajitani, Y.; Ota, S.; Kasuga, H.; Imazeki, Y.; Ohnishi, T.; Watanabe, N. Characterization of l-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of Camellia sinensis using stable isotope labeling. J. Plant Physiol. 2011, 169, 217–225. [Google Scholar] [CrossRef]
- Kroschwitz, J.I.; Howe-Gremt, M. (Eds.) Encyclopedia of Chemical Technology, 4th ed.; Wiley Interscience: New York, NY, USA, 1993; Volume 11, p. 1055. [Google Scholar]
- Zakoshansky, V.M. The cumene process for phenol-acetone production. Pet. Chem. 2007, 47, 273–284. [Google Scholar] [CrossRef]
- Schmidt, R.J. Industrial catalytic processes—Phenol production. Appl. Catal. A Gen. 2005, 280, 89–103. [Google Scholar] [CrossRef]
- Drönner, J.; Hausoul, P.; Palkovits, R.; Eisenacher, M. Solid Acid Catalysts for the Hock Cleavage of Hydroperoxides. Catalysts 2022, 12, 91. [Google Scholar] [CrossRef]
- Unnarkat, A.P.; Sonani, J.; Baldha, J.; Agarwal, S.; Manvar, K.; Faraji, A.R.; Arshadi, M. Catalytic oxidation of ethylbenzene: Kinetic modeling, mechanism, and implications. Chem. Pap. 2022, 76, 995–1008. [Google Scholar] [CrossRef]
- Becker, M. Preparation of Hydroperoxides. U.S. Patent 4,262,143 A, 1981. [Google Scholar]
- Schmidt, J.P. Preparation of Ethylbenzene Hydroperoxide. U.S. Patent 4,066,706 A, 1978. [Google Scholar]
- Roohi, H.; Rajabi, M. Noncatalytic Liquid Phase Air Oxidation of Ethylbenzene to 1-Phenyl Ethyl Hydroperoxide in Low Oxygen Volume Fraction. Org. Process Res. Dev. 2018, 22, 136–146. [Google Scholar] [CrossRef]
- Acetophenone Market. Available online: https://www.futuremarketinsights.com/reports/acetophenone-market#:~:text=%5B250%20Pages%20Report%5D%20The%20global,valuation%20of%20US%24%20335%20Million (accessed on 29 July 2022).
- Dataintelo. Available online: https://dataintelo.com/report/global-acetophenone-market/ (accessed on 29 July 2022).
- Liu, S.-H.; Yu, C.-F.; Das, M. Thermal hazardous evaluation of autocatalytic reaction of cumene hydroperoxide alone and mixed with products under isothermal and non-isothermal conditions. J. Therm. Anal. Calorim. 2019, 140, 2325–2336. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Rathod, S.; Bansal, V.; Bokade, V.V. A Review on Selective Production of Acetophenone from Oxidation of Ethylbenzene over Heterogeneous Catalysts in a Decade. Catal. Lett. 2021, 151, 2116–2131. [Google Scholar] [CrossRef]
- Rahman, M.; Ara, M.G.; Rahman, S.; Uddin, S.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Recent Development of Catalytic Materials for Ethylbenzene Oxidation. J. Nanomater. 2020, 2020, 7532767. [Google Scholar] [CrossRef]
- Gutmann, B.; Elsner, P.; Roberge, D.; Kappe, C.O. Homogeneous Liquid-Phase Oxidation of Ethylbenzene to Acetophenone in Continuous Flow Mode. ACS Catal. 2013, 3, 2669–2676. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Kheilkordi, Z.; Mohajer, F. Recent advances in the application of acetophenone in heterocyclic compounds synthesis. J. Iran. Chem. Soc. 2019, 17, 247–282. [Google Scholar] [CrossRef]
- Rajai-Daryasarei, S.; Gohari, M.H.; Mohammadi, N. Reactions involving aryl methyl ketone and molecular iodine: A powerful tool in the one-pot synthesis of heterocycles. New J. Chem. 2021, 45, 20486–20518. [Google Scholar] [CrossRef]
- Mohammed, S.; Mitton-Fry, M.J.; West, J.S. Aryl Methyl Ketones: Versatile Synthons in the Synthesis of Heterocyclic Compounds. SynOpen 2022, 06, 110–131. [Google Scholar] [CrossRef]
- Deka, B.; Rastogi, G.K.; Deb, M.L.; Baruah, P.K. Ten Years of Glory in the α-Functionalizations of Acetophenones: Progress Through Kornblum Oxidation and C–H Functionalization. Top. Curr. Chem. 2021, 380, 1–38. [Google Scholar] [CrossRef]
- Moonen, M.J.H.; Kamerbeek, N.M.; Westphal, A.H.; Boeren, S.A.; Janssen, D.B.; Fraaije, M.W.; van Berkel, W.J.H. Elucidation of the 4-Hydroxyacetophenone Catabolic Pathway in Pseudomonas fluorescens ACB. J. Bacteriol. 2008, 190, 5190–5198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Negrel, J.; Javelle, F. The biosynthesis of acetovanillone in tobacco cell-suspension cultures. Phytochemistry 2010, 71, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Parent, G.J.; Giguère, I.; Mageroy, M.; Bohlmann, J.; MacKay, J.J. Evolution of the biosynthesis of two hydroxyacetophenones in plants. Plant, Cell Environ. 2018, 41, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Soucy, N.V. Acetophenone in “Encyclopedia of Toxicology”, 3rd ed.; Wexler, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 43–45. [Google Scholar]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop. Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2012, 33, 243–255. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2019, 140, 104095. [Google Scholar] [CrossRef]
- Ridomil Glod®SL; Product No. A13947A; Syngenta Crop Protection, LLC: Greensboro, NC, USA, 2015.
- Ma, Y.-T.; Fan, H.-F.; Gao, Y.-Q.; Zhang, A.-L.; Gao, J.-M.; Li, H. Natural Products as Sources of New Fungicides (I): Synthesis and Antifungal Activity of Acetophenone Derivatives Against Phytopathogenic Fungi. Chem. Biol. Drug Des. 2013, 81, 545–552. [Google Scholar] [CrossRef]
- Nandinsuren, T.; Shi, W.; Zhang, A.-L.; Bai, Y.; Gao, J.-M. Natural products as sources of new fungicides (II): Antiphytopathogenic activity of 2,4-dihydroxyphenyl ethanone derivatives. Nat. Prod. Res. 2015, 30, 1166–1169. [Google Scholar] [CrossRef]
- Shi, W.; Dan, W.-J.; Tang, J.-J.; Zhang, Y.; Nandinsuren, T.; Zhang, A.-L.; Gao, J.-M. Natural products as sources of new fungicides (III): Antifungal activity of 2,4-dihydroxy-5-methylacetophenone derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luong, T.T.M.; Dan, W.-J.; Ren, Y.; Nien, H.X.; Zhang, A.-L.; Gao, J.-M. Natural products as sources of new fungicides (IV): Synthesis and biological evaluation of isobutyrophenone analogs as potential inhibitors of class-II fructose-1,6-bisphosphate aldolase. Bioorganic Med. Chem. 2018, 26, 386–393. [Google Scholar] [CrossRef]
- Luong, T.T.M.; Wang, W.-W.; Zhang, F.; Dan, W.-J.; Nien, H.X.; Zhang, A.-L.; Li, D.; Gao, J.-M. Structure-antifungal relationships and preventive effects of 1-(2,4-dihydroxyphenyl)-2-methylpropan-1-one derivatives as potential inhibitors of class-II fructose-1,6-bisphosphate aldolase. Pestic. Biochem. Physiol. 2019, 159, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.X.; Song, B. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef] [PubMed]
- Tocco, G.; Eloh, K.; Onnis, V.; Sasanelli, N.; Caboni, P. Haloacetophenones as newly potent nematicides against Meloidogyne incognita. Ind. Crop. Prod. 2017, 110, 94–102. [Google Scholar] [CrossRef]
- Caboni, P.; Aissani, N.; Demurtas, M.; Ntalli, N.; Onnis, V. Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita and structure-activity considerations. Pest Manag. Sci. 2015, 72, 125–130. [Google Scholar] [CrossRef]
- Oliveira, D.F.; Costa, V.A.; Terra, W.C.; Campos, V.P.; Paula, P.M.; Martins, S.J. Impact of phenolic compounds on Meloidogyne incognita in vitro and in tomato plants. Exp. Parasitol. 2019, 199, 17–23. [Google Scholar] [CrossRef]
- Charoenying, P.; Teerarak, M.; Laosinwattana, C. An allelopathic substance isolated from Zanthoxylum limonella Alston fruit. Sci. Hortic. 2010, 125, 411–416. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. [Google Scholar] [CrossRef]
- Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and identification of two allelopathic substances in Eleocharis atropurpurea. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 155, 510–516. [Google Scholar] [CrossRef]
- Chotpatiwetchkul, W.; Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Structure–Activity Relationship Study of Xanthoxyline and Related Small Methyl Ketone Herbicides. ACS Omega 2022, 7, 29002–29012. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.F.; DeSanty, K.P.; Kast, R.E. Bupropion: Pharmacology and therapeutic applications. Expert Rev. Neurother. 2006, 6, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Ioannides-Demos, L.L.; Piccenna, L.; McNeil, J.J. Pharmacotherapies for Obesity: Past, Current, and Future Therapies. J. Obes. 2010, 2011, 179674. [Google Scholar] [CrossRef]
- Meltzer, P.C.; Butler, D.; Deschamps, J.R.; Madras, B.K. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) Analogues: A Promising Class of Monoamine Uptake Inhibitors. J. Med. Chem. 2006, 49, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Sumalatha, Y.; Reddy, T.R.; Reddy, P.P.; Satyanarayana, B. A simple and efficient synthesis of hypnotic agent, zolpidem and its related substances. Arkivoc 2009, 2009, 315–320. [Google Scholar] [CrossRef]
- Catozzi, N.; Foletto, J.; Forcato, M.; Giovanetti, R.; Soriato, G.; Verzini, M. Process for Preparing Cinacalcet. U.S. Patent No. 8,614,353, 24 December 2013. [Google Scholar]
- Polak, A. Oxiconazole, a new imidazole derivative. Evaluation of antifungal activity in vitro and in vivo. Arzneimittelforschung 1982, 32, 17–24. [Google Scholar]
- Fukuhara, Y.; Yoshida, D. Paeonol: A Bio-antimutagen Isolated from a Crude Drug, Moutan Cortex. Agric. Biol. Chem. 1987, 51, 1441–1442. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shimamura, H.; Nakamura, S.-I.; Kameoka, H. Antimutagenic Activity of (+)-β-Eudesmol and Paeonol from Dioscorea japonica. J. Agric. Food Chem. 1996, 44, 1647–1650. [Google Scholar] [CrossRef]
- Papandreou, V.; Magiatis, P.; Chinou, I.; Kalpoutzakis, E.; Skaltsounis, A.-L.; Tsarbopoulos, A. Volatiles with antimicrobial activity from the roots of Greek Paeonia taxa. J. Ethnopharmacol. 2002, 81, 101–104. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.-C.; Liu, L.-F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Chu, H.; Wu, Z.; Sun, J. Paeonol Derivatives and Pharmacological Activities: A Review of Recent Progress. Mini-Reviews Med. Chem. 2020, 20, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Adki, K.M.; Kulkarni, Y.A. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 2020, 250, 117544. [Google Scholar] [CrossRef] [PubMed]
- Schmiedeberg, O. Über die wirksamen bestandtheile der wurzel von Apocynum canabinum L. Arch. Exp. Pathol. Pharmakol. 1883, 16, 161–164. [Google Scholar]
- Finnemore, H. The constituents of Canadian hemp. Part I. Apocynin. J. Chem. Soc. Trans. 1908, 93, 1513–1519. [Google Scholar] [CrossRef]
- Basu, K.; Dasgupta, B.; Bhattacharya, S.K.; Debnath, P.K. Chemistry and pharmacology of apocynin, isolated from Picrorhiza kurroa Royle ex Benth. Curr. Sci. 1971, 40, 603–604. [Google Scholar]
- Simons, J.; Hart, L.; Van Dijk, H.; Fischer, F.; De Silva, K.; Labadie, R. Imunodulatory compounds from Picrorhiza kurroa: Isolation and characterization of two anti-complementary polymeric fractions from an aqueous root extract. J. Ethnopharmacol. 1989, 26, 169–182. [Google Scholar] [CrossRef]
- Boshtam, M.; Kouhpayeh, S.; Amini, F.; Azizi, Y.; Najaflu, M.; Shariati, L.; Khanahmad, H. Anti-inflammatory effects of apocynin: A narrative review of the evidence. All Life 2021, 14, 997–1010. [Google Scholar] [CrossRef]
- Hart, B.A.T.; Copray, S.; Philippens, I. Apocynin, a Low Molecular Oral Treatment for Neurodegenerative Disease. BioMed. Res. Int. 2014, 2014, 298020. [Google Scholar] [CrossRef]
- Sandrini, L.; Ieraci, A.; Amadio, P.; Popoli, M.; Tremoli, E.; Barbieri, S.S. Apocynin Prevents Abnormal Megakaryopoiesis and Platelet Activation Induced by Chronic Stress. Oxidative Med. Cell. Longev. 2017, 2017, 9258937. [Google Scholar] [CrossRef]
- Pandey, A.; Kour, K.; Bani, S.; Suri, K.A.; Satti, N.K.; Sharma, P.; Qazi, G.N. Amelioration of adjuvant induced arthritis by apocynin. Phytotherapy Res. 2009, 23, 1462–1468. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.-A.; Park, M.-K.; Park, Y.-D.; Na, H.-J.; Kim, H.-M.; Shin, M.-K.; Ahn, K.-S. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α. Int. Immunopharmacol. 2004, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.-F.; Duan, H.; Luo, L.; Cao, W.-G.; Han, F.-K.; Shan, L.-L.; Fang, X.-M. Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology 2017, 25, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, W.; Song, Q.; Zheng, X.; He, R.; Liu, J. Paeonol Ameliorates Ovalbumin-Induced Asthma through the Inhibition of TLR4/NF-κB and MAPK Signaling. Evidence-Based Complement. Altern. Med. 2018, 2018, 3063145. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.L.; Hawkes, J.; Wright, H.L.; Moots, R.J.; Edwards, S.W. APPA (apocynin and paeonol) modulates pathological aspects of human neutrophil function, without supressing antimicrobial ability, and inhibits TNFα expression and signalling. Inflammopharmacology 2020, 28, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef]
- Fernandez-Moreno, M.; Larkins, N.; Reynolds, A.; Hermida-Gomez, T.; Blanco, F. Biological effect of APPA -apocynin and paeonol- in human articular chondrocytes. Osteoarthr. Cartil. 2021, 29, S358. [Google Scholar] [CrossRef]
- Larkins, N. Efficacy and safety of the combination of apocynin and paeonol (APPA) in patients with osteoarthritis: An uncontrolled patient case series. Ann. Rheum. Dis. 2020, 79 (Suppl. S1), 1738. [Google Scholar] [CrossRef]
- Greener, M. How close are disease-modifying drugs for osteoarthritis? Prescriber 2021, 32, 9–12. [Google Scholar] [CrossRef]
- Karim, M.S.A.; Nasouddin, S.S.; Othman, M.; Adzahan, M.N.; Hussin, S.R. Consumers’ Knowledge and Perception towards Melicope Ptelefolia (Daun Tenggek Burung): A Preliminary Qualitative Study. Int. Food Res. J. 2011, 18, 1481–1488. Available online: https://www.proquest.com/scholarly-journals/consumers-knowledge-perception-towards-melicope/docview/927983422/se-2 (accessed on 29 July 2022).
- Ng, C.H.; Rullah, K.; Aluwi, M.F.F.M.; Abas, F.; Lam, K.W.; Ismail, I.S.; Narayanaswamy, R.; Jamaludin, F.; Shaari, K. Synthesis and Docking Studies of 2,4,6-Trihydroxy-3-Geranylacetophenone Analogs as Potential Lipoxygenase Inhibitor. Molecules 2014, 19, 11645–11659. [Google Scholar] [CrossRef]
- Chan, Y.H.; Liew, K.Y.; Tan, J.W.; Shaari, K.; Israf, D.A.; Tham, C.L. Pharmacological Properties of 2,4,6-Trihydroxy-3-Geranyl Acetophenone and the Underlying Signaling Pathways: Progress and Prospects. Front. Pharmacol. 2021, 12, 2227. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubkov, F.I.; Kouznetsov, V.V. Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions. Molecules 2023, 28, 370. https://doi.org/10.3390/molecules28010370
Zubkov FI, Kouznetsov VV. Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions. Molecules. 2023; 28(1):370. https://doi.org/10.3390/molecules28010370
Chicago/Turabian StyleZubkov, Fedor I., and Vladimir V. Kouznetsov. 2023. "Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions" Molecules 28, no. 1: 370. https://doi.org/10.3390/molecules28010370
APA StyleZubkov, F. I., & Kouznetsov, V. V. (2023). Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions. Molecules, 28(1), 370. https://doi.org/10.3390/molecules28010370