Experiences and Perspectives of GC-MS Application for the Search of Low Molecular Weight Discriminants of Schizophrenia
Abstract
1. Introduction
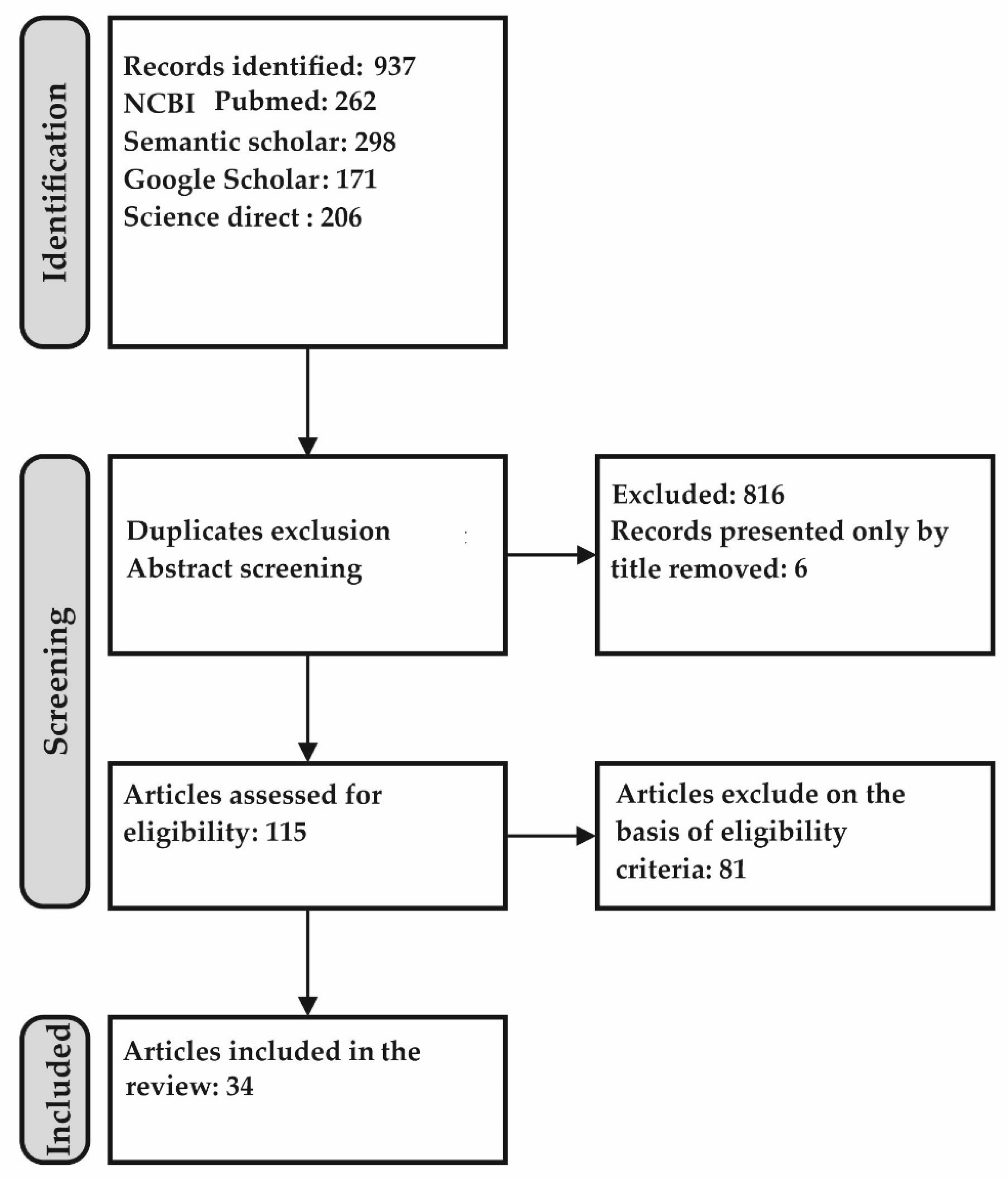
2. Methods Used for Literature Search and Publications Selection 6993452864
- The work should be an original study (not a review) involving patients with schizophrenia (or its various forms) and a control group of individuals without mental illness. Biomarker studies of other psychiatric diseases to schizophrenia as a comparison group would also be of interest to differentiate these illnesses. The diagnosis of schizophrenia should be made based on DSM-III-R or DSM-IV (V).
- Biological samples should be represented by biomaterial available for minimally invasive acquisition and suitable for early diagnosis (plasma/serum, urine, saliva, sweat, heart, exhaled air). Some works using cerebrospinal fluid were of interest as demonstrating the potential of GC-MS but, strictly speaking, did not meet the criteria of this review due to the invasiveness of the intervention and were referred to as background information. Post-mortem studies, animal brain tissue, and human cadaver studies were excluded due to possible post-mortem changes.
- The original articles were selected so that individuals preferably represented the group of patients with schizophrenia with a first episode or with suspected schizophrenia before receiving therapy (drug-naïve schizophrenia, hereafter referred to as T0). However, the treated cases were also of interest (Tn, where n is the duration of therapy in months or relapse of schizophrenia without indication of the period of illness) as a comparison group. A control group of persons without psychiatric disorders should be present regardless of whether thT0 or Tn represents the patient group.
- The substances of endogenous origin/products of human metabolism should be the subject of the work; research on pharmaceuticals and narcotic substances is beyond the scope of this review.
- The main analytical method used in studies should be gas chromatography with mass spectrometric detection (GC-MS), irrespective of ionization types of mass spectrometers. Studies involving other methods (biochemical, immunochemical, etc.) were considered as reference or complementary approaches.
- The age of the articles was not limited, but the study did not include papers whose data are presented only by title and in which at least an abstract is not available.
- Thus, processes of the studies identification were performed (Figure 1). In total, 34 papers were considered to meet best these systematic review criteria.
3. Results
3.1. The Peculiar “Smell of Schizophrenia” and Search for Volatile Organic Biomarkers of Schizophrenia
3.2. GC-MS Applications for the Search of Non-Volatile Biomarkers of Schizophrenia
3.2.1. Analysis of Amino Acids and Their Derivatives
3.2.2. Glucose Metabolites and Energy Metabolism
3.2.3. Lipid Metabolism
3.2.4. Endogenous Cannabinoids (Endocannabinoids)
3.2.5. Neurotransmitters and Their Metabolites
3.2.6. Markers of Oxidative Stress
3.2.7. Steroid Hormones and Their Metabolites
3.2.8. Identification of Schizophrenia by Metabolites Set/Fingerprint/Pattern
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| AIC | Akaike Information Criterion |
| AUC | Area Under the Curve |
| BSTFA | bis–trimethylsilyltrifluoroacetamide |
| CSF | Cerebrospinal fluid |
| DSM-III-R | Diagnostic and Statistical Manual of Mental Disorders–3rd Edition Revised |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders, 4th Edition |
| IDMS | Isotope Dilution Mass Spectrometry |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| PAA | phenylacetic acid |
| PEA | phenylethylamine |
| PUFA | polyunsaturated fatty acids |
| RBC | Red Blood Cells |
| TMHA | trans-3-methyl-2-hexenoic acid |
| TCA | tricarboxylic acid cycle |
| TFO | Time Of Flight |
| VOC | Volatile Organic Compounds |
| VOM | Volatile organic metabolites |
References
- Li, R.; Ma, X.; Wang, G.; Yang, J.; Wang, C. Why sex differences in schizophrenia? J. Transl. Neurosci. 2016, 1, 37–42. [Google Scholar] [PubMed]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Winkleby, M.A.; Sundquist, K.; Sundquist, J. Comorbidities and mortality in persons with schizophrenia: A Swedish national cohort study. Am. J. Psychiatry 2013, 170, 324–333. [Google Scholar] [CrossRef] [PubMed]
- WHO. Schizophrenia Key Facts. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia (accessed on 18 January 2022).
- Christensen, M.K.; Lim, C.C.W.; Saha, S.; Plana-Ripoll, O.; Cannon, D.; Presley, F.; Weye, N.; Momen, N.C.; Whiteford, H.A.; Iburg, K.M.; et al. The cost of mental disorders: A systematic review. Epidemiol. Psychiatr. Sci. 2020, 29, e161. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Teoh, S.L.; Wu, D.B.; Kotirum, S.; Chiou, C.F.; Chaiyakunapruk, N. Global economic burden of schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 357–373. [Google Scholar] [CrossRef]
- Horvitz-Lennon, M.; Predmore, Z.; Orr, P.; Hanson, M.; Hillestad, R.; Durkin, M.; El Khoury, A.C.; Mattke, S. The Predicted Long-Term Benefits of Ensuring Timely Treatment and Medication Adherence in Early Schizophrenia. Adm. Policy Ment. Health 2020, 47, 357–365. [Google Scholar] [CrossRef]
- Davison, J.; O’Gorman, A.; Brennan, L.; Cotter, D.R. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 2018, 195, 32–50. [Google Scholar] [CrossRef]
- Schmuckler, A. Biomarkers of schizophrenia. In Mental Health: Social, Clinical and Organisational and Scientific Aspects; KDU Publishing House: Moscow, Russia, 2017; pp. 167–174. [Google Scholar]
- Zaikin, V.; Halket, J.M. A Handbook of Derivatives for Mass Spectrometry; IM Publications: Charlton, UK, 2009. [Google Scholar]
- Koureas, M.; Kalompatsios, D.; Amoutzias, G.D.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Comparison of Targeted and Untargeted Approaches in Breath Analysis for the Discrimination of Lung Cancer from Benign Pulmonary Diseases and Healthy Persons. Molecules 2021, 26, 2609. [Google Scholar] [CrossRef]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef]
- Smith, K.; Sines, J.O. Demonstration of a peculiar odor in the sweat of schizophrenic patients. AMA Arch. Gen. Psychiatry 1960, 2, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Thompson, G.F.; Koster, H.D. Sweat in schizophrenic patients: Identification of the odorous substance. Science 1969, 166, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.; Wise, C.D. Possible etiology of schizophrenia: Progressive damage to the noradrenergic reward system by 6-hydroxydopamine. Science 1971, 171, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.L.; Melancon, S.B.; Lesk, D.; Hansen, S. Failure to detect trans-3-methyl-2-hexenoic acid in the sweat of schizophrenic patients. Clin. Chim. Acta 1970, 30, 721–725. [Google Scholar] [CrossRef]
- Gordon, S.G.; Smith, K.; Rabinowitz, J.L.; Vagelos, P.R. Studies of trans-3-methyl-2-hexenoic acid in normal and schizophrenic humans. J. Lipid Res. 1973, 14, 495–503. [Google Scholar] [CrossRef]
- Barzantny, H.; Brune, I.; Tauch, A. Molecular basis of human body odour formation: Insights deduced from corynebacterial genome sequences. Int. J. Cosmet. Sci. 2012, 34, 2–11. [Google Scholar] [CrossRef]
- Brewer, W.J.; Edwards, J.; Anderson, V.; Robinson, T.; Pantelis, C. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol. Psychiatry 1996, 40, 1021–1031. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid Volatolomics and Disease Detection. Angew. Chem.-Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Phillips, M.; Sabas, M.; Greenberg, J. Increased pentane and carbon disulfide in the breath of patients with schizophrenia. J. Clin. Pathol. 1993, 46, 861–864. [Google Scholar] [CrossRef]
- Yuan, X.; Kang, Y.; Zhuo, C.; Huang, X.F.; Song, X. The gut microbiota promotes the pathogenesis of schizophrenia via multiple pathways. Biochem. Biophys. Res. Commun. 2019, 512, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Piatoikina, A.S.; Lyakhova, A.A.; Semennov, I.V.; Zhilyaeva, T.V.; Kostina, O.V.; Zhukova, E.S.; Shcherbatyuk, T.G.; Kasyanov, E.D.; Blagonravova, A.S.; Mazo, G.E. Association of antioxidant deficiency and the level of products of protein and lipid peroxidation in patients with the first episode of schizophrenia. J. Mol. Neurosci. 2022, 72, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Erickson, G.A.; Sabas, M.; Smith, J.P.; Greenberg, J. Volatile organic compounds in the breath of patients with schizophrenia. J. Clin. Pathol. 1995, 48, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Human Metabolome Database. University of Alberta and The Metabolomics Innovation Centre. 2017. Available online: https://hmdb.ca/ (accessed on 18 January 2022).
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; Van Der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J. Breath Res. 2019, 13, 032001. [Google Scholar] [CrossRef] [PubMed]
- Mednova, I.A.; Serebrov, V.Y.; Baikov, A.N.; Bohan, N.A.; Ivanova, S.A. Amino acids and acylcarnitines as potential metabolomic markers of schizophrenia: New approaches to diagnostics and therapy. Bull. Sib. Med. 2019, 18, 197–208. [Google Scholar] [CrossRef]
- Saleem, S.; Shaukat, F.; Gul, A.; Arooj, M.; Malik, A. Potential role of amino acids in pathogenesis of schizophrenia. Int. J. Health Sci. 2017, 11, 63–68. [Google Scholar]
- Baruah, S.; Waziri, R.; Hegwood, T.S.; Mallis, L.M. Plasma serine in schizophrenics and controls measured by gas chromatography-mass spectrometry. Psychiatry Res. 1991, 37, 261–270. [Google Scholar] [CrossRef]
- Xuan, J.; Pan, G.; Qiu, Y.; Yang, L.; Su, M.; Liu, Y.; Chen, J.; Feng, G.; Fang, Y.; Jia, W.; et al. Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J. Proteome Res. 2011, 10, 5433–5443. [Google Scholar] [CrossRef]
- Yang, J.; Chen, T.; Sun, L.; Zhao, Z.; Qi, X.; Zhou, K.; Cao, Y.; Wang, X.; Qiu, Y.; Su, M.; et al. Potential metabolite markers of schizophrenia. Mol. Psychiatry 2013, 18, 67–78. [Google Scholar] [CrossRef]
- Al Awam, K.; Haussleiter, I.S.; Dudley, E.; Donev, R.; Brune, M.; Juckel, G.; Thome, J. Multiplatform metabolome and proteome profiling identifies serum metabolite and protein signatures as prospective biomarkers for schizophrenia. J. Neural Transm. 2015, 122 (Suppl. S1), S111–S122. [Google Scholar] [CrossRef]
- Lee, Y.R.; Hong, J.; Chung, B.C. Mass Spectrometry-based Hair Metabolomics for Biomarker Discovery. Mass Spectrom. Lett. 2022, 13, 2–10. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, H.; Zhu, J.; Hara, K.; Liu, J. Determination of Tyramine in Hair Samples by GC–MS. Chromatographia 2016, 79, 103–108. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zheng, P.; Liu, Z.; Xu, Y.; Mu, J.; Guo, J.; Huang, T.; Meng, H.-Q.; Xie, P. GC-MS based metabolomics identification of possible novel biomarkers for schizophrenia in peripheral blood mononuclear cells. Mol. BioSyst. 2014, 10, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Zhang, X.T.; Du, X.Y.; Fang, Z.; Liu, Z.; Xu, Y.; Zheng, P.; Xu, X.J.; Cheng, P.F.; Huang, T.; et al. Severe disturbance of glucose metabolism in peripheral blood mononuclear cells of schizophrenia patients: A targeted metabolomic study. J. Transl. Med. 2015, 13, 226. [Google Scholar] [CrossRef]
- Walker, N.P.; Fox, H.C.; Whalley, L.J. Lipids and schizophrenia. Br. J. Psychiatry 1999, 174, 101–104. [Google Scholar] [CrossRef]
- Glen, A.I.; Glen, E.M.; Horrobin, D.F.; Vaddadi, K.S.; Spellman, M.; Morse-Fisher, N.; Ellis, K.; Skinner, F.S. A red cell membrane abnormality in a subgroup of schizophrenic patients: Evidence for two diseases. Schizophr. Res. 1994, 12, 53–61. [Google Scholar] [CrossRef]
- Sethom, M.M.; Fares, S.; Bouaziz, N.; Melki, W.; Jemaa, R.; Feki, M.; Hechmi, Z.; Kaabachi, N. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 131–136. [Google Scholar] [CrossRef]
- Bentsen, H.; Solberg, D.K.; Refsum, H.; Gran, J.M.; Bøhmer, T.; Torjesen, P.A.; Halvorsen, O.; Lingjærde, O. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol. Psychiatry 2011, 70, 97–105. [Google Scholar] [CrossRef]
- Bentsen, H.; Solberg, D.K.; Refsum, H.; Bøhmer, T. Clinical and biochemical validation of two endophenotypes of schizophrenia defined by levels of polyunsaturated fatty acids in red blood cells. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 35–41. [Google Scholar] [CrossRef]
- Solberg, D.K.; Refsum, H.; Andreassen, O.A.; Bentsen, H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. 2019, 31, 202–212. [Google Scholar] [CrossRef]
- Berdyshev, E.; Bronova, I.; Leung, D.Y.M.; Goleva, E. Methodological Considerations for Lipid and Polar Component Analyses in Human Skin Stratum Corneum. Cell Biochem. Biophys. 2021, 79, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Smesny, S.; Schmelzer, C.E.H.; Hinder, A.; Köhler, A.; Schneider, C.; Rudzok, M.; Schmidt, U.; Milleit, B.; Milleit, C.; Nenadic, I.; et al. Skin ceramide alterations in first-episode schizophrenia indicate abnormal sphingolipid metabolism. Schizophr. Bull. 2013, 39, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Rog, J.; Błażewicz, A.; Juchnowicz, D.; Ludwiczuk, A.; Stelmach, E.; Kozioł, M.; Karakula, M.; Niziński, P.; Karakula-Juchnowicz, H. The Role of GPR120 Receptor in Essential Fatty Acids Metabolism in Schizophrenia. Biomedicines 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, X.; Yuan, X.; Pang, L.; Hu, S.; Wang, Y.; Huang, X.; Song, X. The Role of Butyric Acid in Treatment Response in Drug-Naive First Episode Schizophrenia. Front. Psychiatry 2021, 12, 724664. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, X.; Pang, L.; Zhang, S.; Li, Y.; Huang, X.; Fan, X.; Song, X. nThe effect of serum lipids and short-chain fatty acids on cognitive functioning in drug-naïve, first episode schizophrenia patients. Psychiatry Res. 2022, 313, 114582. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Giuffrida, A.; Wurster, U.; Emrich, H.M.; Piomelli, D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999, 10, 1665–1669. [Google Scholar] [CrossRef]
- Mele, T.; Carman-Krzan, M.; Juric, D.M. Regulatory role of monoamine neurotransmitters in astrocytic NT-3 synthesis. Int. J. Dev. Neurosci. 2010, 28, 13–19. [Google Scholar] [CrossRef]
- Parnetti, L.; Gottfries, J.; Karlsson, I.; Långström, G.; Gottfries, C.G.; Svennerholm, L. Monoamines and their metabolites in cerebrospinal fluid of patients with senile dementia of Alzheimer type using high performance liquid chromatography and gas chromatography-mass spectrometry. Acta Psychiatr. Scand. 1987, 75, 542–548. [Google Scholar] [CrossRef]
- Davis, B.A. Biogenic amines and their metabolites in body fluids of normal, psychiatric and neurological subjects. J. Chromatogr. A 1989, 466, 89–218. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Kaku, H.; Shimogawa, S.; Watanabe, A.; Nakagawara, M.; Takahashi, R. Urinary trace amine excretion and platelet monoamine oxidase activity in schizophrenia. Psychiatry Res. 1987, 21, 229–236. [Google Scholar] [CrossRef]
- O’Reilly, R.; Davis, B.A.; Durden, D.A.; Thorpe, L.; Machnee, H.; Boulton, A.A. Plasma phenylethylamine in schizophrenic patients. Biol. Psychiatry 1991, 30, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Narasimhachari, N.; Himwich, H.E. GC-MS identification of bufotenin in urine samples from patients with schizophrenia or infantile autism. Life Sci. 1973, 12, 475–478. [Google Scholar] [CrossRef]
- Kärkkäinen, J.; Räisänen, M.; Huttunen, M.O.; Kallio, E.; Naukkarinen, H.; Virkkunen, M. Urinary excretion of bufotenin (N,N-dimethyl-5-hydroxytryptamine) is increased in suspicious violent offenders: A confirmatory study. Psychiatry Res. 1995, 58, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Colombo, R.; Martinelli, V.; Brondino, N.; Marini, M.; Boso, M.; Barale, F.; Politi, P. Elevated urine levels of bufotenine in patients with autistic spectrum disorders and schizophrenia. Neuro Endocrinol. Lett. 2010, 31, 117–121. [Google Scholar]
- Angrist, B.; Gershon, S.; Sathananthan, G.; Walker, R.W.; Lopez-Ramos, B.; Mandel, L.R.; Vandenheuvel, W.J.A. Dimethyltryptamine levels in blood of schizophrenic patients and control subjects. Psychopharmacology 1976, 47, 29–32. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef]
- Halliwell, B.; Lee, C.Y. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid. Redox Signal. 2010, 13, 145–156. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Scheffer, R.; Correnti, E.E.; Mahadik, J.S. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol. Psychiatry 1998, 43, 674–679. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Olas, B. Isoprostenes as indicators of oxidative stress in schizophrenia. World J. Biol. Psychiatry 2009, 10, 27–33. [Google Scholar] [CrossRef]
- Jordan, W.; Dobrowolny, H.; Bahn, S.; Bernstein, H.-G.; Brigadski, T.; Frodl, T.; Isermann, B.; Lessmann, V.; Pilz, J.; Rodenbeck, A.; et al. Oxidative stress in drug-naïve first episode patients with schizophrenia and major depression: Effects of disease acuity and potential confounders. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 129–143. [Google Scholar] [CrossRef]
- Cai, H.; Cao, T.; Zhou, X.; Yao, J.K. Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Front. Psychiatry 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H.L. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Di Michele, F.; Caltagirone, C.; Bonaviri, G.; Romeo, E.; Spalletta, G. Plasma dehydroepiandrosterone levels are strongly increased in schizophrenia. J. Psychiatr. Res. 2005, 39, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, G.; Romeo, E.; Bonaviri, G.; Bernardi, G.; Caltagirone, C.; di Michele, F. Preliminary evidence for an association between aggressive and hostile behaviour and 3alpha,5alpha-tetrahydroprogesterone plasma levels in schizophrenia. J. Psychiatry Neurosci. JPN 2005, 30, 49–52. [Google Scholar]
- Bicikova, M.; Hill, M.; Ripova, D.; Mohr, P. Altered levels of circulating GABAergic 5α/β-reduced pregnane and androstane steroids in schizophrenic men. Horm. Mol. Biol. Clin. Investig. 2011, 6, 227–230. [Google Scholar] [CrossRef]
- Bicikova, M.; Hill, M.; Ripova, D.; Mohr, P.; Hampl, R. Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J. Steroid Biochem. Mol. Biol. 2013, 133, 77–83. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Tang, J.; Dai, X.; Huang, H.; Cao, R.; Hu, J. Dysregulation of amino acids and lipids metabolism in schizophrenia with violence. BMC Psychiatry 2020, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Haworth, J.J.; Pitcher, C.K.; Ferrandino, G.; Hobson, A.R.; Pappan, K.L.; Lawson, J.L.D. Breathing new life into clinical testing and diagnostics: Perspectives on volatile biomarkers from breath. Crit. Rev. Clin. Lab. Sci. 2022, 59, 353–372. [Google Scholar] [CrossRef]
| Reference | Population Sample Schizophrenic/Other Mental Disease/Healthy | Biosample | VOCs | Claimed Outcomes |
|---|---|---|---|---|
| [15] | 14/0/0 | Sweat | TMHA | Detected and identified in schizophrenics |
| [17] | 11/0/0 | Sweat | TMHA | Failure to detect in schizophrenics |
| [18] | 7/0/12 | Sweat | TMHA | Detected in schizophrenics and controls. Cannot be considered as a marker of disease |
| [23] | 25/26/37 | Exhaled air | Pentane, Carbon disulfide | CS2 concentrations discriminate schizophrenics from other mental patients and healthy controls, pentane only from healthy controls |
| [26] | 25/26/37 | Exhaled air | 2-Methylbutane Trichlorofluoromethane 2-Pentanol Pentane Dichloromethane Trichloroethene Benzene 1-Chloro-2-methylbutane 2,3,3-Trimethylpentane 2,2-Dimethylbutane Tetrachloroethene | Patients with schizophrenia can be distinguished from two other groups with a sensitivity of 80.0% and a specificity of 61.9%. |
| Reduced Compared to Control (Multiplicity, FC *)/Matrix | Reference | Increased Compared to Control (Multiplicity, FC)/Matrix | Reference |
|---|---|---|---|
| 2-Aminoadipic acid (−1.27), urine | [33] | 2-Aminobutyrate (1.28), serum | [33] |
| Aspartate (−2.20), serum | [32] | 2-Aminobutyric acid (1,45), urine | [33] |
| Catechol (−1.83), urine | [33] | 2-Hydroxybutyrate (2.45), serum | [33] |
| Cystine (−1,36), serum | [33] | 5-Oxoproline (1.35), serum | [33] |
| Glycine (−1,8), serum | [32] | Aspartate (1.38) serum | [33] |
| Glycocyamine (−1.89), urine | [33] | Cystine (1.54), urine | [33] |
| N-Acetylaspartate (−1.96), serum | [32] | Glutamate (1.35), urine | [33] |
| Tryptophan (−2.07), serum | [32] | Glutamate (1.63), serum | [33] |
| Isoleucine (1.3), urine | [33] | ||
| Phenylalanine (1.14), serum | [33] | ||
| Pipecolinic acid (1.65), urine | [33] | ||
| Pyroglutamic acid (1.25), urine | [33] | ||
| Serine (1.13) | [33] | ||
| Tyramine, (>2) hair samples | [36] | ||
| Valine (1.07), urine, plasma | [33,37] |
| Reduced Compared to Control (Multiplicity, FC)/Matrix | Reference | Increased Compared to Control (Multiplicity, FC)/Matrix | Reference |
|---|---|---|---|
| Citrate (−2.66), serum | [32,38] | Citrate (1.45), serum | [33] |
| Citrate (−0.71), PBMCs | [38] | cis-Aconitic acid (1.28), urine | [33] |
| α-Ketoglutarate (−1.52), serum | [32] | 2-Oxoglutarate (1.59), serum | [33] |
| Lactic acid, PBMCs | [38] | Lactate (1.24–2.32), serum | [32,33] |
| Octanoic acid (−0.85), plasma | [37] | Malate (1.57), serum | [33] |
| Dihydroxyacetone phosphate (−1.28), PBMCs | [38] | Creatinine (0.35), plasma | [37] |
| Glycerol 3-phosphate (−0,49), PBMCs | [38] | Pyruvate (0.82), PBMCs | [38] |
| Glyceraldehyde-3-phosphate (−1.05), PBMCs | [38] | Pyruvate (1.88), serum | [33] |
| 1,3-Bisphosphoglycerate (−1.61), serum | [32] | fructose (0.48), PBMCs | [38] |
| fructose 6-phosphate (1.06), PBMCs | [38] | ||
| Fumaric acid (0.24), plasma | [37] | ||
| Maltose (0.51), plasma | [37] | ||
| Glucose (0.67–1.50), serum, PBMCs | [32,38] | ||
| Glucose 6-phosphate (1.48), PBMCs | [38] | ||
| Sorbitol (0.31), plasma | [37] | ||
| Succinic acid (0.76), PBMCs | [38] |
| Reduced Compared to Control (Multiplicity, FC)/Matrix | Reference | Increased Compared to Control (Multiplicity, FC)/Matrix | Reference |
|---|---|---|---|
| 2,3-Dihydroxybutanoic acid (−1.3), urine | [33] | 3-Hydroxybutyric acid (1.37), urine | [33] |
| Arachidonic acid, RBC | [40,41,43] | 2-Hydroxybutyric acid (1.41), urine | [33] |
| Cholest-3,5-diene, serum | [34] | Tetradecanoic acid (1.45), serum | [33] |
| Cholest-5-en-3-ol, serum | [34] | 4-Pentenoic acid (1.54), urine | [33] |
| Docosahexaenoic acid, RBC | [40,41,43] | 3-Hydroxysebacic (5.55) acid, urine | [33] |
| Ethoxy- cholest-5-ene, serum | [34] | Glycerate (2.57), serum | [33] |
| Heptadecanoic acid, serum | [34] | Suberic acid (1.59), urine | [33] |
| Hydroxyacetic acid (−1.36), urine | [33] | β-Hydroxybutyrate (2.61), serum | [33] |
| Palmitic acid (−1.77), serum | [32] | Palmitic acid, RBC | [41] |
| Pentadecanoic acid, serum | [34] | Threonic acid (1.21), urine | [33] |
| Stearic acid (−1.81), serum | [32] | 2-Ethyl-3-hydroxypropionic acid (1.29), urine | [33] |
| Eicosanoic acid, serum | [34] | Eicosenoic acid (1.96), serum | [33] |
| Linoleic acid (−2.69), serum | [32] | Linoleate (1.18), serum | [33] |
| Oleic acid (−2.52), serum | [32,34] | Oleic acid (2.09), serum | [33] |
| Glycerol (−0.35), plasma | [37] | Glycerol (1.42), serum | [32] |
| Cholesterol | [34] | Cholesterol (1.43), serum | [32] |
| Hexadecanoic acid (1.4), serum | [33] | ||
| 3-Hydroxyadipic acid (2.06), urine | [33] | ||
| Octadecanoic acid (1.14), serum | [33] |
| Reduced Compared to Control (Multiplicity, FC)/Matrix | Reference | Increased Compared to Control (Multiplicity, FC)/Matrix | Reference |
|---|---|---|---|
| 2-Piperidinec carboxylic acid, serum | [34] | 5-HO-DMT | [56,57,58] |
| 6-Deoxy-mannofuranose > (−60), serum | [34] | Dopamine (0.31), plasma | [37] |
| Aspartic acid (−1.05), plasma | [37] | N,N-dimethyltryptamine | [59] |
| Homoserine (−0.50), plasma | [37] | ||
| Oxoproline, serum | [34] |
| Reduced Compared to Control (Multiplicity, FC)/Matrix | Reference | Increased Compared to Control (Multiplicity, FC)/Matrix | Reference |
|---|---|---|---|
| Hydroxylamine (−0.29), plasma | [37] | Pyroglutamic acid (0.13), plasma | [37] |
| γ-Tocopherol (−1.53), serum | [32] | γ-Tocopherol (0.34), plasma | [37] |
| α-Tocopherol (0.19), plasma | [37] | ||
| Serum SOD | [64] | ||
| Urine 8-iso-PGF2α/creatinine (T0) | [64] |
| Study | [32] | [33] | [37] | [34] |
|---|---|---|---|---|
| GC-MS platform | GC-Q-MS | GC-TOF-MS | GC-Q-MS | GC-Q-MS |
| Matrix | Serum | Serum | PBMCs | Serum |
| Discriminating metabolites | Glucose 1,3-Bisphosphoglycerate Lactate Citrate α-Ketoglutarate Allantoin Uric acid γ-Tocopherol N-Acetylaspartate Aspartate Glycine Tryptophan Myo-inositol Glucuronic acid Linoleic acid Oleic acid Stearic acid Palmitic acid Glycerol Cholesterol Lactobionic acid Erythrose | Glycerate Eicosenoic acid β-Hydroxybutyrate Pyruvate Cystine Malate Elaidic acid 2-Hydroxybutyrate Tetradecanoic acid Hexadecanoic acid Aspartate α-Oxo-pentanedioic acid Pyroglutamic acid Glutamate Citrate Phenylalanine Lactate Octadecanoic acid 2-Aminobutyrate Cholesterol Linoleic acid myo-lnositol | Octanoic acid Fumaric acid Valine Creatinine Inositol Sorbitol Maltose Hydroxylamine Pyroglutamic acid Tocopherol-g Tocopherol-α Aspartic acid Homoserine Dopamine Benzoic acid 2-Hydroxyethyl palmitate Glycerol Methyl Phosphate | 1-Oxo-proline 2-Piperidinec carboxylic acid 6-Deoxy-mannofuranose Galactose oxime Oleic acid Pentadecanoic acid Heptadecanoic acid Eicosanoic acid Cholesterol |
| AUC, training set | - | 0.945 | 0.82 | - |
| AUC, test set | 0.958 | 0.895 | 0.71 | 0.76–0.93 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porozova, N.; Danilova, E.; Senshinov, I.; Tsakalof, A.; Nosyrev, A. Experiences and Perspectives of GC-MS Application for the Search of Low Molecular Weight Discriminants of Schizophrenia. Molecules 2023, 28, 324. https://doi.org/10.3390/molecules28010324
Porozova N, Danilova E, Senshinov I, Tsakalof A, Nosyrev A. Experiences and Perspectives of GC-MS Application for the Search of Low Molecular Weight Discriminants of Schizophrenia. Molecules. 2023; 28(1):324. https://doi.org/10.3390/molecules28010324
Chicago/Turabian StylePorozova, Natalia, Elena Danilova, Igor Senshinov, Andreas Tsakalof, and Alexander Nosyrev. 2023. "Experiences and Perspectives of GC-MS Application for the Search of Low Molecular Weight Discriminants of Schizophrenia" Molecules 28, no. 1: 324. https://doi.org/10.3390/molecules28010324
APA StylePorozova, N., Danilova, E., Senshinov, I., Tsakalof, A., & Nosyrev, A. (2023). Experiences and Perspectives of GC-MS Application for the Search of Low Molecular Weight Discriminants of Schizophrenia. Molecules, 28(1), 324. https://doi.org/10.3390/molecules28010324







