Chemistry and Biological Activities of Naturally Occurring and Structurally Modified Podophyllotoxins
Abstract
1. Introduction
2. Natural Occurring Podophyllotoxins
2.1. Podophyllotoxins
2.2. Seco-Podophyllotoxins
2.3. Podophyllotoxin Glycosides
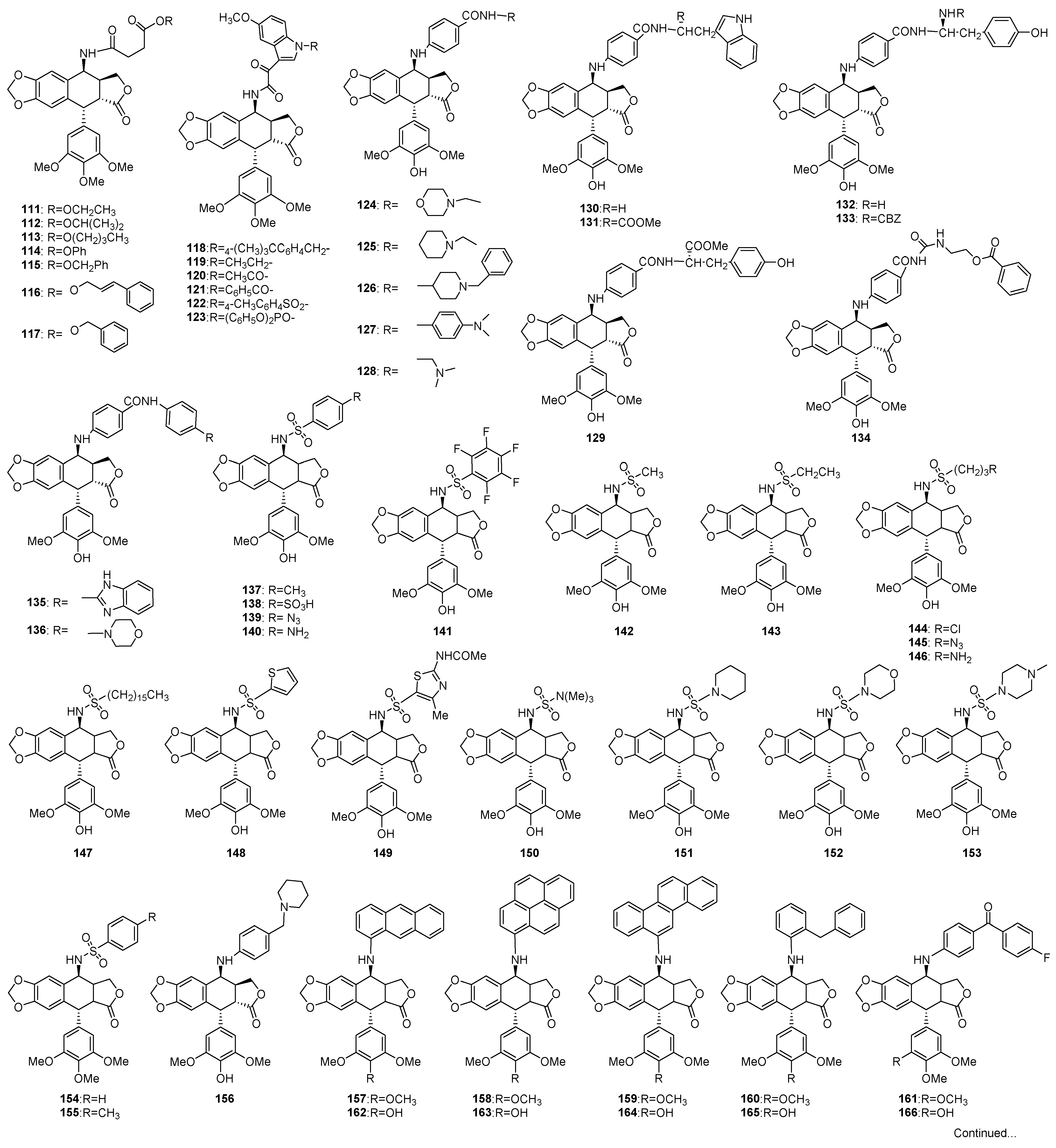
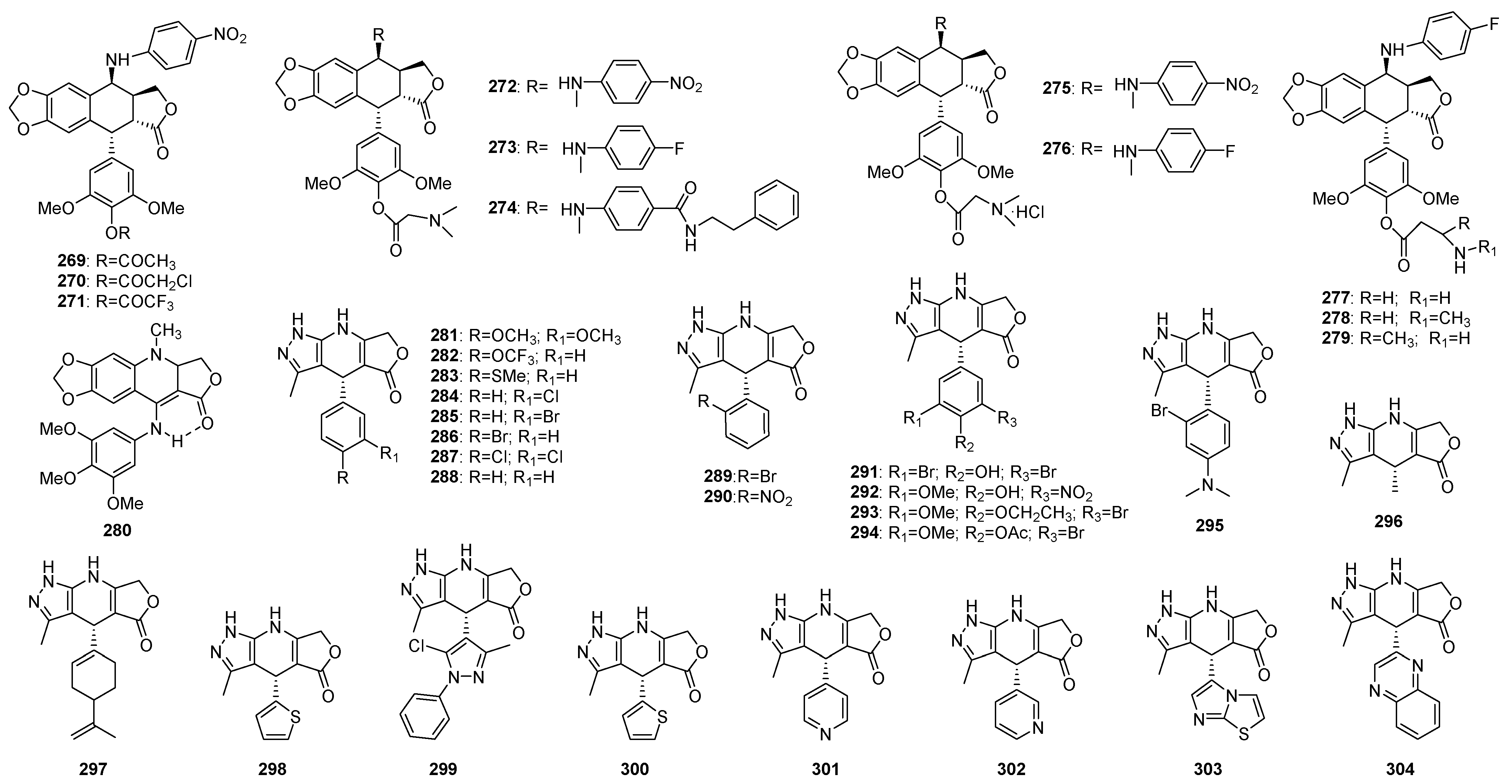
3. Structural Modification in Podophyllotoxins
3.1. Introducing Heteronuclears into Podophyllotoxins
3.1.1. Ring A
3.1.2. Ring B
3.1.3. Ring C
3.1.4. Ring D
3.1.5. Ring E
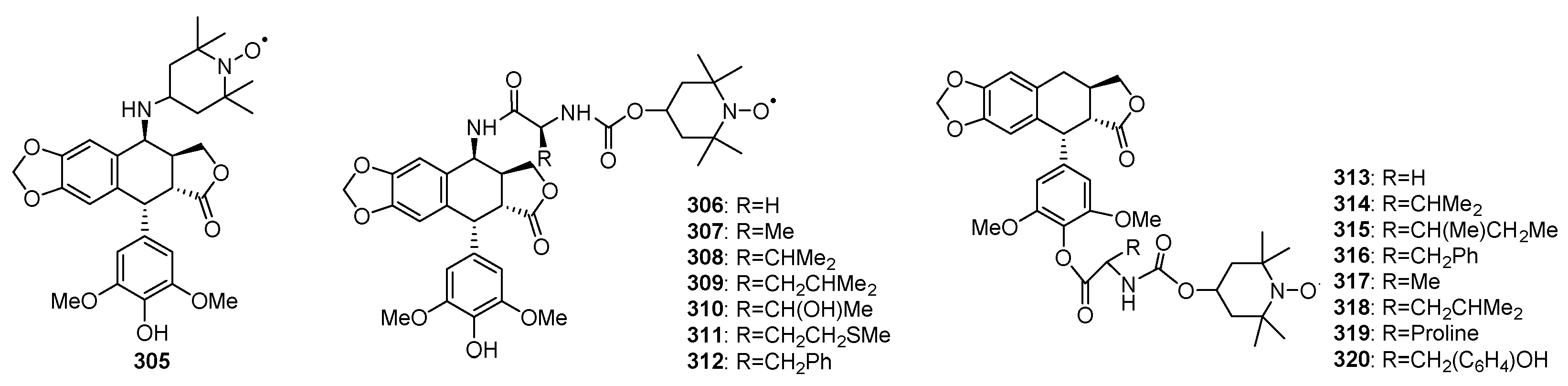
3.2. Spin Labeled Podophyllotoxins
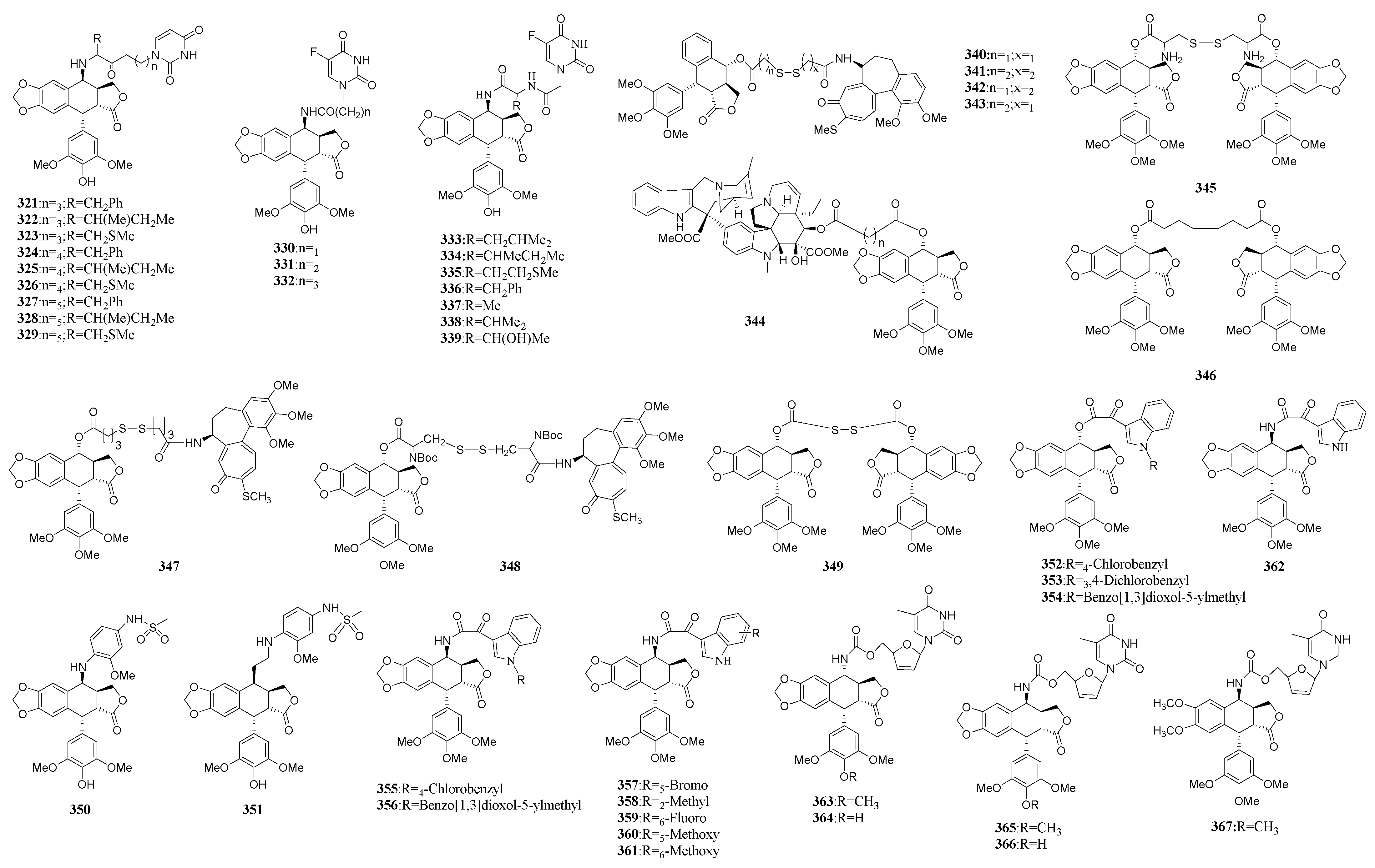
3.3. Conjugates of Podophyllotoxins
4. Biological Activities of Natural Occurring Podophyllotoxins
4.1. Antitumor Activity
4.2. Antiviral Activities
4.3. Anti-Inflammation Activities
4.4. Miscellaneous Activities
4.5. Toxicity and Protection
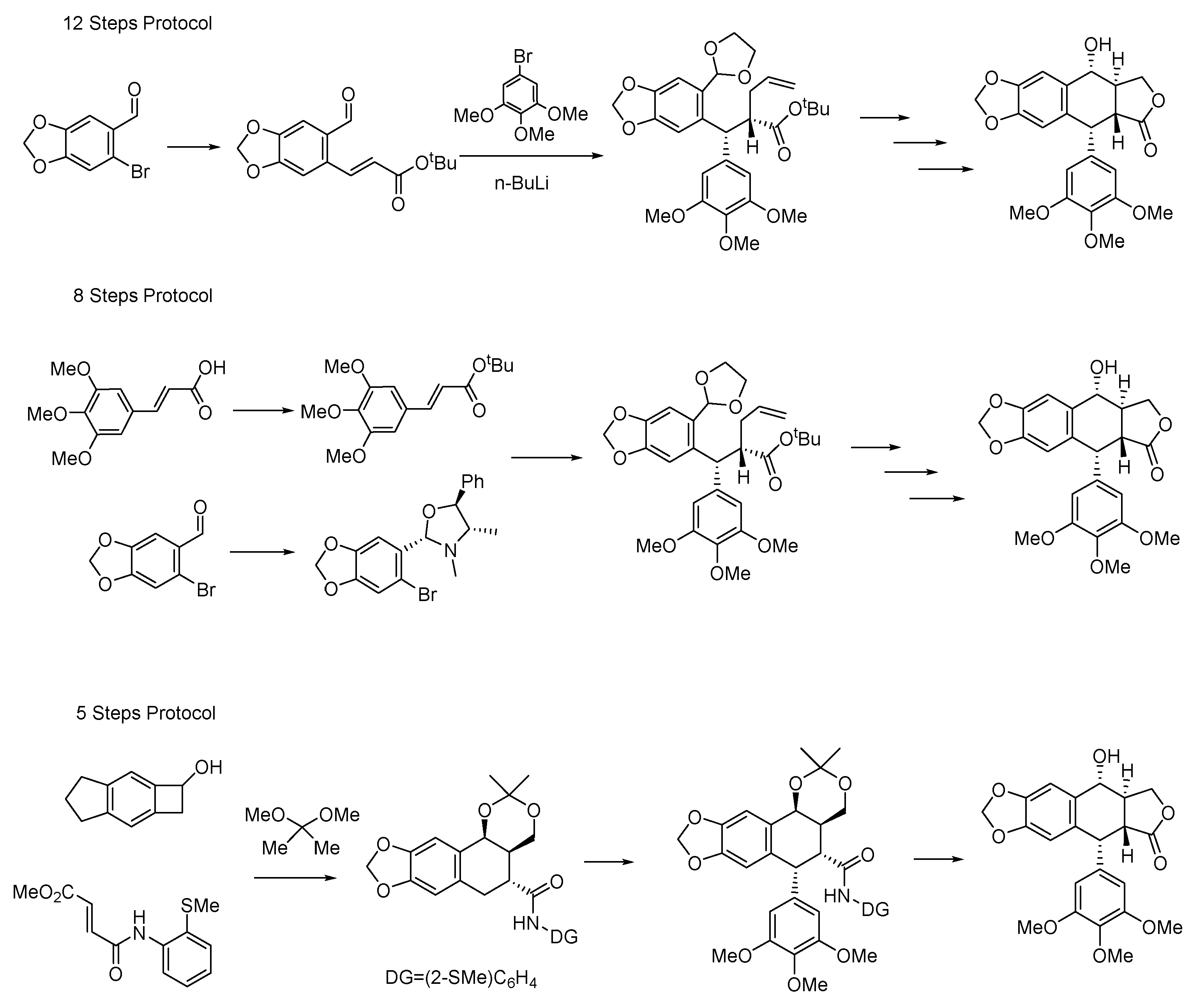
5. Total Chemical Synthesis, Biosynthesis and ADME
6. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-Ui-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin, history, recent advances and future prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Chou, S.L.; Chou, M.Y.; Kao, W.F.; Yen, D.H.T.; Huang, C.I.; Lee, C.H. Cessation of nail growth following Bajiaolian intoxication. Clin. Toxicol. 2008, 46, 159–163. [Google Scholar] [CrossRef]
- Hu, L.L.; Zhou, X.; Zhang, H.L.; Wu, L.L.; Tang, L.S.; Chen, L.L.; Duan, J.L. Exposure to podophyllotoxin inhibits oocyte meiosis by disturbing meiotic spindle formation. Sci. Rep. 2018, 8, 10145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Yang, L.; Tian, X. Podophyllotoxin, current perspectives. Curr. Bioact. Compd. 2007, 3, 37–66. [Google Scholar]
- Cheng, Y.C.; Ying, C.X.; Leung, C.H.; Li, Y. New targets and inhibitors of HBV replication to combat drug resistance. J. Clin. Virol. 2005, 34, S147–S150. [Google Scholar] [CrossRef]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a source of anticancer agents, from bench to bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Che, Z.; Xu, H. Recent advances in the chemistry and biology of podophyllotoxins. Chem. Eur. J. 2017, 23, 4467–4526. [Google Scholar] [CrossRef]
- Murray, M.L.; Meadows, J.; Doré, C.J.; Copas, A.J.; Haddow, L.J.; Lacey, C.; Jit, M.; Soldan, K.; Bennett, K.; Tetlow, M.; et al. Human papillomavirus infection, protocol for a randomised controlled trial of imiquimod cream (5%) versus podophyllotoxin cream (0.15%), in combination with quadrivalent human papillomavirus or control vaccination in the treatment and prevention of recurrence of anogenital warts (HIPvac trial). BMC Med. Res. Methodol. 2018, 18, 125. [Google Scholar]
- Sosa, S.; Balick, M.J.; Arvigo, R.; Esposito, R.G.; Pizza, C.; Altinier, G.; Tubaro, A. Screening of the topical anti-inflammatory activity of some Central American plants. J. Ethnopharmacol. 2002, 81, 211–215. [Google Scholar] [CrossRef]
- Fan, H.Y.; Zhu, Z.L.; Xian, H.C.; Wang, H.F.; Chen, B.J.; Tang, Y.J.; Tang, Y.L.; Liang, X.H. Insight into the molecular mechanism of podophyllotoxin derivatives as anticancer drugs. Front. Cell Dev. Biol. 2021, 9, 709075. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.A.; Carter, S.K. 4′-Demethyl-epipodophyllotoxin-[beta]-d-thenylidene glucoside (VM-26)-A brief review. Eur. J. Cancer 1973, 9, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Yusenko, M.; Jakobs, A.; Klempnauer, K.H. A novel cell-based screening assay for small-molecule MYB inhibitors identifies podophyllotoxins teniposide and etoposide as inhibitors of MYB activity. Sci. Rep. 2018, 8, 13159. [Google Scholar] [CrossRef]
- Tachibana, Y.; Zhu, X.K.; Krishnan, P.; Lee, K.H.; Bastow, K.F. Characterization of human lung cancer cells resistant to 4 ‘-O-demethyl-4 beta-(2″-nitro-4″-fluoroanilino)-4-desoxypodophyllotoxin, a unique compound in the epipodophyllotoxin antitumor class. Anti-Cancer Drugs 2000, 11, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.G.; Cho, J.H.; Hwang, S.G.; Lee, E.; Lee, J.; Kim, J.I.; Um, H.D.; Park, J.K. Chemosensitizing effect of podophyllotoxin acetate on topoisomerase inhibitors leads to synergistic enhancement of lung cancer cell apoptosis. Int. J. Oncol. 2016, 48, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Infante Lara, L.; Fenner, S.; Ratcliffe, S.; Isidro-Llobet, A.; Hann, M.; Bax, B.; Osheroff, N. Coupling the core of the anticancer drug etoposide to an oligonucleotide induces topoisomerase II-mediated cleavage at specific DNA sequences. Nucleic Acids Res. 2018, 46, 2218–2233. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Gupta, M.M.; Khanuja, S.P.S. Plant-based anticancer molecules, A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–5908. [Google Scholar] [CrossRef]
- Lee, N.J.; Jeong, I.C.; Cho, M.Y.; Jeon, C.W.; Yun, B.C.; Kim, Y.O.; Kim, S.H.; Chung, I. Synthesis and in vitro antitumor activity of phthalimide polymers containing podophyllotoxin. Eur. Polym. J. 2006, 42, 3352–3359. [Google Scholar] [CrossRef]
- Sun, D.; Gao, X.; Wang, Q.; Krausz, K.W.; Fang, Z.; Zhang, Y.; Xie, C.; Gonzalez, F.J. Metabolic map of the antiviral drug podophyllotoxin provides insights into hepatotoxicity. Xenobiotica 2021, 51, 1047–1059. [Google Scholar] [CrossRef]
- Gordaliza, M.; Castro, M.A.; del Corral, J.M.; López-Vázquez, M.; Feliciano, A.S.; Faircloth, G.T. In vivo immunosuppressive activity of some cyclolignans. Bioorg. Med. Chem. Lett. 1997, 7, 2781–2786. [Google Scholar] [CrossRef]
- Chou, S.L.; Chou, M.Y.; Kao, W.F.; Yen, D.H.T.; Yen, L.Y.; Huang, C.I.; Lee, C.H. Bajiaolian poisoning–a poisoning with high misdiagnostic rate. Am. J. Emerg. Med. 2010, 28, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2009, 287, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Shin, S.Y.; Lee, Y.H.; Lim, Y. Antitumor activity of deoxypodophyllotoxin isolated from Anthriscus sylvestris, Induction of G2/M cell cycle arrest and caspase-dependent apoptosis. Bioorg. Med. Chem. Lett. 2009, 19, 4367–4371. [Google Scholar] [CrossRef] [PubMed]
- Botta, B.; Delle Monache, G.; Misiti, D.; Vitali, A.; Zappia, G. Aryltetralin lignans, chemistry, pharmacology and biotransformations. Curr. Med. Chem. 2001, 8, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Lizama, C.; Ludwig, A.; Moreno, R.D. Etoposide induces apoptosis and upregulation of TACE/ADAM17 and ADAM10 in an in vitro male germ cell line model. Biochim. Biophys. Acta (BBA) 2011, 1813, 120–128. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Yang, L.; Tian, X. Design, synthesis, and biological evaluation of novel pyridine acid esters of podophyllotoxin and esters of 4′-demethylepipodophyllotoxin. Med. Chem. Res. 2008, 16, 319–330. [Google Scholar] [CrossRef]
- Girnita, A.; Girnita, L.; Del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004, 64, 236–242. [Google Scholar] [CrossRef]
- Ohshima-Hosoyama, S.; Hosoyama, T.; Nelon, L.D.; Keller, C. IGF-1 receptor inhibition by picropodophyllin in medulloblastoma. Biochem. Biophys. Res. Commun. 2010, 399, 727–732. [Google Scholar] [CrossRef]
- Xu, H.; Lv, M.; Tian, X. A Review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef]
- Gordaliza, M.; Garcia, P.A.; del Corral, J.M.M.; Castro, M.A.; Gomez-Zurita, M.A. Podophyllotoxin, distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Canel, C.; Moraes, R.M.; Dayan, F.E.; Ferreira, D. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, H.F.; von Wartburg, A. The chemical and biological route from podophyllotoxin glucoside to etoposide, ninth Cain memorial Award lecture. Cancer Res. 1991, 51, 5–15. [Google Scholar] [PubMed]
- Olaru, O.T.; Niţulescu, G.M.; Orțan, A.; Dinu-Pîrvu, C.E. Ethnomedicinal, phytochemical and pharmacological profile of Anthriscus sylvestris as an alternative source for anticancer lignans. Molecules 2015, 20, 15003–15022. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Lee, E.; Jin, M.H.; Yook, J.; Quan, Z.; Ha, K.; Moon, T.C.; Kim, M.J.; Kim, K.J.; Lee, S.H.; et al. Deoxypodophyllotoxin (DPT) inhibits eosinophil recruitment into the airway and Th2 cytokine expression in an OVA-induced lung inflammation. Planta Med. 2006, 72, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Three new cytotoxic aryltetralin lignans from Sinopodophyllum emodi. Bioorg. Med. Chem. Lett. 2011, 21, 3794–3797. [Google Scholar] [CrossRef]
- Lim, Y.H.; Leem, M.J.; Shin, D.H.; Chang, H.B.; Hong, S.W.; Moon, E.Y.; Lee, D.K.; Yoon, S.J.; Woo, W.S. Cytotoxic constituents from the roots of Anthriscus sylvestris. Arch. Pharm. Res. 1999, 22, 208–212. [Google Scholar] [CrossRef]
- Jeong, G.S.; Kwon, O.K.; Park, B.Y.; Oh, S.R.; Ahn, K.S.; Chang, M.J.; Oh, W.K.; Kim, J.C.; Min, B.S.; Kim, Y.C.; et al. Lignans and coumarins from the roots of Anthriscus sylvestris and their increase of caspase-3 activity in HL-60 cells. Biol. Pharm. Bull. 2007, 30, 1340–1343. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Litaudon, M.; Bousserouel, H.; Martin, M.T.; Thoison, O.; Leonce, S.; Dumontet, V.; Sevenet, T.; Gueritte, F. Sesquiterpenoids and cytotoxic lignans from the bark of Libocedrus chevalieri. J. Nat. Prod. 2007, 70, 1368–1370. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Arimoto, M.; Tanoguchi, M.; Ishida, T.; Inoue, M. Studies on the constituents of the seeds of Hernandia ovigera L. III. Structures of two new lignans. Chem. Pharm. Bull. 1982, 30, 3212–3218. [Google Scholar] [CrossRef]
- Hartwell, J.L.; Detty, W.E. Beta-Peltatin, a new component of podophyllin. J. Am. Chem. Soc. 1948, 70, 2833. [Google Scholar] [CrossRef]
- Hartwell, J.L. Alpha-Peltatin, a new compound isolated from podophyllum peltatum. J. Am. Chem. Soc. 1947, 69, 2918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, N.; He, W.; Li, R.; Shi, D.; Pang, L.; Dong, N.; Xu, H.; Ji, H. Simultaneous determination of three major lignans in rat plasma by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Diphylleia sinensis extract. Biomed. Chromatogr. 2014, 28, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Broomhead, A.J.; Dewick, P.M. Tumor-inhibitory aryltetralin lignans in Podophyllum versipelle, Diphylleia cymosa and Diphylleia grayi. Phytochemistry 1990, 29, 3831–3837. [Google Scholar] [CrossRef]
- Evcim, U.; Gozler, B.; Freyer, A.J.; Shamma, M. Haplomyrtin and (-)-haplomyrfolin, two lignans from Haplophyllum myrtifolium. Phytochemistry 1986, 25, 1949–1951. [Google Scholar] [CrossRef]
- Schaaf, G.M.; Feld, W.A. Optimizing the synthesis of haplomyrtin. In Abstracts of Papers of the American Chemical Society; Amer Chemical Society: Washington, DC, USA, 1999; Volume 217. [Google Scholar]
- Barrow, W.S. The Synthesis of Haplomyrtin Utilizing the Triisopropylsilyl Protecting Group. Master’s Thesis, Wright State University, Dayton, OH, USA, 2013. [Google Scholar]
- Hunter, N.E. Towards the Total Synthesis of Haplomyrtin. Master’s Thesis, Wright State University, Dayton, OH, USA, 2010. [Google Scholar]
- Sağlam, H.; Gözler, T.; Gözler, B. A new prenylated arylnaphthalene lignan from Haplophyllum myrtifolium. Fitoterapia 2003, 74, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Tanoguchi, M.; Arimoto, M.; Saika, H.; Yamaguchi, H. Studies on the constituents of the seeds of Hernandia ovigera L. VI. Isolation and structural determination of three lignans. Chem. Pharm. Bull. 1987, 35, 4162–4168. [Google Scholar] [CrossRef]
- Donoso-Fierro, C.; Tiezzi, A.; Ovidi, E.; Ceccarelli, D.; Triggiani, D.; Mastrogiovanni, F.; Taddei, A.R.; Pérez, C.; Becerra, J.; Silva, M.; et al. Antiproliferative activity of yatein isolated from Austrocedrus chilensis against murine myeloma cells, cytological studies and chemical investigations. Pharm. Biol. 2015, 53, 378–385. [Google Scholar] [CrossRef]
- Miyata, M.; Itoh, K.; Tachibana, S. Extractives of Juniperus chinensis L. I, isolation of podophyllotoxin and yatein from the leaves of J. chinensis. J. Wood Sci. 1998, 44, 397–400. [Google Scholar] [CrossRef]
- Wickramaratne, D.B.; Mar, W.; Chai, H.; Castillo, J.J.; Farnsworth, N.R.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic constituents of Bursera permollis. Planta Med. 1995, 61, 80–81. [Google Scholar] [CrossRef]
- Ito, C.; Matsui, T.; Wu, T.S.; Furukawa, H. Isolation of 6,7-demethylenedesoxy-podophyllotoxin from Hernandia ovigera. Chem. Pharm. Bull. 1992, 40, 1318–1321. [Google Scholar] [CrossRef]
- Pettit, G.R.; Meng, Y.; Gearing, R.P.; Herald, D.L.; Pettit, R.K.; Doubek, D.L.; Chapuis, J.C.; Tackett, L.P. Antineoplastic Agents. 522. Hernandia peltata (Malaysia) and Hernandia nymphaeifolia (Republic of Maldives). J. Nat. Prod. 2004, 67, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Nur-e-Alam, M.; Yousaf, M.; Qureshi, S.; Baig, I.; Nasim, S.; Attaur, R.; Choudhary, M.I. A novel dimeric podophyllotoxin-type lignan and a new withanolide from Withania coagulans. Helv. Chim. Acta 2003, 86, 607–614. [Google Scholar] [CrossRef]
- Day, S.H.; Chiu, N.Y.; Tsao, L.T.; Wang, J.P.; Lin, C.N. New lignan glycosides with potent antiinflammatory effect, isolated from Justicia ciliata. J. Nat. Prod. 2000, 63, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, Y.; Jin, C.; Lee, S.H.; Kang, M.J.; Jeong, T.C.; Jeong, S.Y.; Kim, D.H.; Yoo, H.H. Inhibitory effects of deoxypodophyllotoxin from Anthriscus sylvestris on human CYP2C9 and CYP3A4. Planta Med. 2010, 76, 701–704. [Google Scholar] [CrossRef]
- Ikeda, R.; Nagao, T.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents in Umbelliferae plants. III. Constituents in the root and the ground part of Anthriscus sylvestris Hoffm. Chem. Pharm. Bull. 1998, 46, 871–874. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Giorgetti, M.; Cervellati, R.; Innocenti, G. Deoxypodophyllotoxin content and antioxidant activity of aerial parts of Anthriscus sylvestris Hoffm. Zeitschrift für Naturforschung C 2006, 61, 658–662. [Google Scholar] [CrossRef]
- Sánchez-Monroy, M.B.; León-Rivera, I.; Llanos-Romero, R.E.; García-Bores, A.M.; Guevara-Fefer, P. Cytotoxic activity and triterpenes content of nine Mexican species of Bursera. Nat. Prod. Res. 2021, 35, 4881–4885. [Google Scholar] [CrossRef]
- Jutiviboonsuk, A.; Zhang, H.; Tan Ghee, T.; Ma, C.; Van Hung, N.; Manh Cuong, N.; Bunyapraphatsara, N.; Soejarto, D.D.; Fong Harry, H.S. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry 2005, 66, 2745–2751. [Google Scholar] [CrossRef]
- Peraza-Sanchez, S.R.; Pena-Rodriguez, L.M. Isolation of picropolygamain from the resin of Bursera simaruba. J. Nat. Prod. 1992, 55, 1768–1771. [Google Scholar] [CrossRef]
- Antúnez-Mojica, M.; Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.A.; Gonzalez-Maya, L.; Marquina, S.; Salas-Vidal, E.; Alvarez, L. Lignans from Bursera fagaroides affect in vivo cell behavior by disturbing the tubulin cytoskeleton in zebrafish embryos. Molecules 2018, 24, 8. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Aynehchi, Y. Deoxypodophyllotoxin, the cytotoxic principle of Callitris columellaris. J. Pharm. Sci. 1971, 60, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, W.; Pras, N.; Maingré, T.M. The accumulation of podophyllotoxin-β-D-glucoside by cell suspension cultures derived from the conifer Callitris drummondii. Plant Cell Rep. 1990, 9, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Frezza, C.; Serafini, M.; Bianco, A.; Mahdavi, B. The Genus Haplophyllum Juss.: Phytochemistry and Bioactivities—A Review. Molecules 2021, 26, 4664. [Google Scholar] [CrossRef]
- Gozler, B.; Arar, G.; Gozler, T.; Hesse, M. Isodaurinol, an arylnaphthalene lignan from Haplophyllum cappadocicum. Phytochemistry 1992, 31, 2473–2475. [Google Scholar]
- Gozler, B.; Gozler, T.; Saglam, H.; Hesse, M. Minor lignans from Haplophyllum cappadocicum. Phytochemistry 1996, 42, 689–693. [Google Scholar] [CrossRef]
- Dekebo, A.; Lang, M.; Polborn, K.; Dagne, E.; Steglich, W. Four lignans from Commiphora erlangeriana. J. Nat. Prod. 2002, 65, 1252–1257. [Google Scholar] [CrossRef]
- Habtemariam, S. Cytotoxic and cytostatic activity of erlangerins from Commiphora erlangeriana. Toxicon 2003, 41, 723–727. [Google Scholar] [CrossRef]
- Ma, C.; Yang, J.S.; Luo, S.R. Study on lignans from Diphylleia sinensis. Acta Pharm. Sin. 1993, 28, 690–694. [Google Scholar]
- Yuan, Y.; Wang, Y.; Huang, M.; Xu, R.; Zeng, H.; Nie, C.; Kong, J. Development and characterization of molecularly imprinted polymers for the selective enrichment of podophyllotoxin from traditional Chinese medicines. Anal. Chim. Acta 2011, 695, 63–72. [Google Scholar] [CrossRef]
- Xu, X.; Gao, X.; Jin, L.; Bhadury, P.S.; Yuan, K.; Hu, D.; Song, B.; Yang, S. Antiproliferation and cell apoptosis inducing bioactivities of constituents from Dysosma versipellis in PC3 and Bcap-37 cell lines. Cell Div. 2011, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.W.; Zhou, J.R.; Hon, P.M.; Li, S.L.; Zhou, Y.; Li, L.L.; Ye, W.C.; Xu, H.X.; Shaw, P.C.; But, P.P.H. Lignans from Dysosma versipellis with Inhibitory Effects on Prostate Cancer Cell Lines. J. Nat. Prod. 2007, 70, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, G.; Zhang, Y.; Guo, M. Potential multifunctional bioactive compounds from Dysosma versipellis explored by bioaffinity ultrafiltration-HPLC/MS with Topo I, Topo II, COX-2 and ACE2. J. Inflamm. Res. 2022, 15, 4677–4692. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiya, P.; Tsay, H.S. Therapeutic values, chemical constituents and toxicity of Taiwanese Dysosma pleiantha—A review. Toxicol. Lett. 2015, 236, 90–97. [Google Scholar] [CrossRef]
- Uden, W. The production of podophyllotoxin and related cytotoxic lignans by plant cell cultures. Plant Cell Tissue Organ. Cult. 1992, 20, 81–87. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Zhou, Q.; Wu, W.R.; Duan, Y.X.; Gao, Z.Y.; Li, Y.W.; Lu, Q. Anticancer effect of deoxypodophyllotoxin induces apoptosis of human prostate cancer cells. Oncol. Lett. 2016, 12, 2918–2923. [Google Scholar] [CrossRef]
- Santos, E.O.; Lima, L.S.; David, J.M.; Martins, L.C.; Guedes, M.L.S.; David, J.P. Podophyllotoxin and other aryltetralin lignans from Eriope latifolia and Eriope blanchetii. Nat. Prod. Res. 2011, 25, 1450–1453. [Google Scholar] [CrossRef]
- David, J.P.; Silva, E.F.; Moura, D.L.; Guedes, M.L.; Assunção, R.J.; David, J.M. Lignans and triterpenes from cytotoxic extract of Eriope blanchetii. Quim. Nova 2001, 24, 730–733. [Google Scholar] [CrossRef]
- Raffauf, R.F.; Kelley, C.J.; Ahmad, Y.; Le Quesne, P.W. Alpha- and beta-peltatin from Eriope macrostachya. J. Nat. Prod. 1987, 50, 772–773. [Google Scholar] [CrossRef]
- Lim, S.; Grassi, J.; Akhmedjanova, V.; Debiton, E.; Balansard, G.; Beliveau, R.; Barthomeuf, C. Reversal of P-glycoprotein-mediated drug efflux by eudesmin from Haplophyllum perforatum and cytotoxicity pattern versus diphyllin, podophyllotoxin and etoposide. Planta Med. 2007, 73, 1563–1567. [Google Scholar] [CrossRef]
- Bessonova, I.A. Components of Haplophyllum bucharicum. Chem. Nat. Compd. 2000, 36, 323–324. [Google Scholar] [CrossRef]
- Štefánik, M.; Bhosale, D.S.; Haviernik, J.; Straková, P.; Fojtíková, M.; Dufková, L.; Huvarová, I.; Salát, J.; Bartáček, J.; Svoboda, J.; et al. Diphyllin Shows a Broad-Spectrum Antiviral Activity against Multiple Medically Important Enveloped RNA and DNA Viruses. Viruses 2022, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; Abu-Zarga, M.; Sabri, S.; Atta Ur, R.; Voelter, W. A arylnaphthalene lignan from Haplophyllum buxbaumii. Phytochemistry 1998, 49, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; Sabri, S.; Zarga, M.A.; Shah, Z.; Attaur, R. Chemical constituents of the flora of Jordan, part V-B. Three new arylnaphthalene lignan glucosides from Haplophyllum buxbaumii. J. Nat. Prod. 1990, 53, 1152–1161. [Google Scholar] [CrossRef]
- Nakul, G.S.; Zarga, M.H.A.; Sabri, S.S.; Al-Eisawi, D.M. Chemical constituents of the flora of Jordan. Part III. Mono-O-acetyl diphyllin apioside, a new arylnaphthalene lignan from Haplophyllum buxbaumii. J. Nat. Prod. 1987, 50, 748–750. [Google Scholar] [CrossRef]
- Aimaiti, S.; Saito, Y.; Fukuyoshi, S.; Goto, M.; Miyake, K.; Newman, D.J.; O’Keefe, B.R.; Lee, K.H.; Nakagawa-Goto, K. Isolation, Structure Elucidation, and Antiproliferative Activity of Butanolides and Lignan Glycosides from the Fruit of Hernandia nymphaeifolia. Molecules 2019, 24, 4005. [Google Scholar] [CrossRef]
- Udino, L.; Abaul, J.; Bourgeois, P.; Gorrichon, L.; Duran, H.; Zedde, C. Lignans from the seeds of Hernandia sonora. Planta Med. 1999, 65, 279–281. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Ogata, M.; Mou, X.Y.; Tokuda, H.; Nishino, H.; Furukawa, H. Lignans as anti-tumor-promoter from the seeds of Hernandia ovigera. Planta Med. 2001, 67, 166–168. [Google Scholar] [CrossRef]
- Gu, J.Q.; Park, E.J.; Totura, S.; Riswan, S.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of the twigs of Hernandia ovigera that inhibit the transformation of JB6 murine epidermal cells. J. Nat. Prod. 2002, 65, 1065–1068. [Google Scholar] [CrossRef]
- Liu, H.P. Studies on the Chemical Constituentis of Rhamnus Hainanensis and Hernandia ovigera L. Master’s Thesis, Qingdao University of Science and Technology, Shangdou, China, 2013. [Google Scholar]
- Fragoso-Serrano, M.; Pereda-Miranda, R. Dereplication of podophyllotoxin and related cytotoxic lignans in Hyptis verticillata by ultra-high-performance liquid chromatography tandem mass spectrometry. Phytochem. Anal. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Picking, D.; Delgoda, R.; Boulogne, I.; Mitchell, S. Hyptis verticillata Jacq, a review of its traditional uses, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2013, 147, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Bridi, H.; de Carvalho Meirelles, G.; von Poser, G.L. Subtribe Hyptidinae (Lamiaceae): A promising source of bioactive metabolites. J. Ethnopharmacol. 2021, 264, 113225. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wei, B.L.; Ko, H.H.; Lin, C.N. DNA strand-scission by phloroglucinols and lignans from heartwood of Garcinia subelliptica Merr. and Justicia plants. Phytochemistry 2008, 69, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Liang, Z.Z.; Li, J.J.; Zhang, X.; Zheng, R.H.; Zhao, C.Q. Update on naturally occurring novel arylnaphthalenes from plants. Phytochem. Rev. 2020, 19, 337–403. [Google Scholar] [CrossRef]
- Al-Juaid, S.S.; Abdel-Mogib, M. A novel podophyllotoxin lignan from Justicia heterocarpa. Chem. Pharm. Bull. 2004, 52, 507–509. [Google Scholar] [CrossRef]
- Suryanarayana, A.A.; Shankar, S.; Manoharan, J.P.; Vidyalakshmi, S. In-Silico analysis and molecular docking studies of phytoconstituents of Justicia adhatoda as potential inhibitors of SARS-CoV2 target proteins. In Proceedings of the First International Conference on Combinatorial and Optimization, ICCAP 2021, Chennai, India, 7–8 December 2021. [Google Scholar]
- Weng, J.R.; Ko, H.H.; Yeh, T.L.; Lin, H.C.; Lin, C.N. Two new arylnaphthalide lignans and antiplatelet constituents from Justicia procumbens. Arch. Pharm. 2004, 337, 207–212. [Google Scholar] [CrossRef]
- Day, S.H.; Lin, Y.C.; Tsai, M.L.; Tsao, L.T.; Ko, H.H.; Chung, M.I.; Lee, J.C.; Wang, J.P.; Won, S.J.; Lin, C.N. Potent cytotoxic lignans from Justicia procumbens and their effects on nitric oxide and tumor necrosis factor-alpha production in mouse macrophages. J. Nat. Prod. 2002, 65, 379–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Ku, C.F.; Xu, X.Y.; Tsang, N.Y.; Zhu, Y.; Zhao, C.L.; Liu, K.L.; Li, C.C.; Rong, L.; Zhang, H.J. Stable axially chiral isomers of arylnaphthalene lignan glycosides with antiviral potential discovered from Justicia procumbens. J. Org. Chem. 2021, 86, 5568–5583. [Google Scholar] [CrossRef]
- Xu, X.Y.; Wang, D.Y.; Ku, C.F.; Zhao, Y.; Cheng, H.; Liu, K.L.; Rong, L.J.; Zhang, H.J. Anti-HIV lignans from Justicia procumbens. Chin. J. Nat. Med. 2019, 17, 945–952. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, T.; Xie, Z.T.; Hong, Z.C.; Lu, Y.; Long, Y.M.; Ji, C.Z.; Liu, Y.T.; Yang, Y.F.; Wu, H.Z. Effective components and mechanism analysis of anti-platelet aggregation effect of Justicia procumbens L. J. Ethnopharmacol. 2022, 294, 115392. [Google Scholar] [CrossRef]
- Hemmati, S.; Seradj, H. Justicidin B, a promising bioactive lignan. Molecules 2016, 21, 820. [Google Scholar] [CrossRef]
- Premjet, D.; Tachibana, S. Production of podophyllotoxin by immobilized cell cultures of Juniperus chinensis. Pakistan J. Biol. Sci. 2004, 7, 1130–1134. [Google Scholar]
- Xu, S.; Li, X.; Liu, S.; Tian, P.; Li, D. Juniperus sabina L. as a source of podophyllotoxins, extraction optimization and Anticholinesterase activities. Int. J. Mol. Sci. 2022, 23, 10205. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, G.; Qu, L.; Zhong, R.; Chen, P.; Lu, Z.; Zhou, J.; Guo, X.; Li, Z.; Ma, A.; et al. Podophyllotoxin Extracted from Juniperus sabina Fruit Inhibits Rat Sperm Maturation and Fertility by Promoting Epididymal Epithelial Cell Apoptosis. Evid. Based Complement Alternat. Med. 2017, 2017, 6958982. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.; Abad, A.; Montenegro, G.; Del Olmo, E.; López-Pérez, J.L.; San Feliciano, A. Analgesic and anti-inflammatory activity of podophyllotoxin derivatives. Pharm. Biol. 2013, 51, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Kašparová, M.; Jand, M.; Tůmová, L.; Spilková, J. Production of podophyllotoxin by plant tissue cultures of Juniperus virginiana. Nat. Prod. Commun. 2017, 12, 101–103. [Google Scholar] [CrossRef]
- Maqbool, M.; Cushman, K.E.; Gerard, P.D.; Bedir, E.; Lata, H.; Moraes, R.M. Podophyllotoxin content in leaves of Eastern red cedar (Juniperus virginiana). Acta Hortic. 2004, 629, 87–92. [Google Scholar] [CrossRef]
- Belma, K. Arytetralin lignans from Linum catharticum L. Biochem. Syst. Ecol. 1998, 26, 795–796. [Google Scholar] [CrossRef]
- Klaes, M.; Ellendorff, T.; Schmidt, T.J. 6-Methoxypodophyllotoxin-7-O-n-Hexanoate, a new aryltetralin lignan ester from seeds of Linum flavum. Planta Med. 2010, 76, 719. [Google Scholar] [CrossRef]
- Mikame, K.; Sakakibara, N.; Umezawa, T.; Shimada, M. Lignans of Linum flavum var. compactum. J. Wood Sci. 2002, 48, 440–445. [Google Scholar] [CrossRef]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) Hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghzadeh, A.; Gholami, A.; Soltani, M.; Hemmati, S.; Alfermann, A.W. Linum mucronatum, Organ to organ lignan variations. Z. Nat. C 2005, 60, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghzadeh, A.; Hemmati, S.; Mehregan, I.; Alfermann, A.W. Linum persicum, lignans and placement in Linaceae. Phytochem. Rev. 2004, 2, 363–369. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R.; Rezai, H.; Jafari, A.; Azarmehr, A.; Amirghofran, Z. Biological Activity and Aryltetraline Lignans of Linum persicum. Pharm. Biol. 2005, 43, 547–550. [Google Scholar] [CrossRef]
- Vasilev, N.P.; Ionkova, I.I. Isolation and structure elucidation of aryltetralin lignans from Linum tauricum ssp. bulgaricum. Pharmacogn. Mag. 2006, 2, 169–174. [Google Scholar]
- Anand, U.; Biswas, P.; Kumar, V.; Ray, D.; Ray, P.; Loake, V.I.P.; Kandimalla, R.; Chaudhary, A.; Singh, B.; Routhu, N.K.; et al. Podophyllum hexandrum and its active constituents, Novel radioprotectants. Biomed. Pharmacother. 2022, 146, 112555. [Google Scholar] [CrossRef]
- Zilla, M.K.; Nayak, D.; Amin, H.; Nalli, Y.; Rah, B.; Chakraborty, S.; Kitchlu, S.; Coswami, A.; Ali, A. 4′-Demethyl-deoxypodophyllotoxin glucoside isolated from Podophyllum hexandrum exhibits potential anticancer activities by altering Chk-2 signaling pathway in MCF-7 breast cancer cells. Chem.Biol. Interact. 2014, 224, 100–107. [Google Scholar] [CrossRef]
- Jackson, D.E.; Dewick, P.M. Aryltetralin lignans from Podophyllum hexandrum and Podophyllum peltatum. Phytochemistry 1984, 23, 1147–1152. [Google Scholar] [CrossRef]
- Hoffmann, J.J.; Wiedhopf, R.M.; Cole, J.R. Cytotoxic and tumor inhibitory agent from Polygala macradenia gray (Polygalaceae): 4′-demethyldeoxypodophyllotoxin. J. Pharm. Sci. 1977, 66, 586–587. [Google Scholar] [CrossRef]
- Zhao, C.; Nagatsu, A.; Hatano, K.; Shirai, N.; Kato, S.; Ogihara, Y. New lignan glycosides from Chinese medicinal plant, Sinopodophillum emodi. Chem. Pharm. Bull. 2003, 51, 255–261. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, W.; Nagatsu, A.; Ogihara, Y. Three new glycosides from Sinopodophyllum emodi (WALL.) YING. Chem. Pharm. Bull. 2001, 49, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Yamada, C.; Saito, M.; Yokogawa, S.; Mimaki, Y. Chemical constituents of the leaves of Thujopsis dolabrata and their cytotoxicity. Chem. Pharm. Bull. 2022, 70, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Hande, K.R. Etoposide, four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.P.; Jablonksy, M.J.; Shadid, M.; Romaine, I.; Dunlap, N.; Anklin, C.; Graves, D.E.; Osheroff, N. Substituents on etoposide that interact with human topoisomerase II alpha in the binary enzyme-drug complex, Contributions to etoposide binding and activity. Biochemistry 2008, 47, 4501–4509. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Jin, F.; Zhao, L.; Guo, Y.J.; Zhao, W.J.; Zhang, H.; Wang, H.T.; Shao, T.; Zhang, S.L.; Wei, Y.J.; Feng, J.; et al. Influence of Etoposide on anti-apoptotic and multidrug resistance-associated protein genes in CD133 positive U251 glioblastoma stem-like cells. Brain Res. 2010, 1336, 103–111. [Google Scholar] [CrossRef]
- Vasilcanu, D.; Weng, W.H.; Girnita, A.; Lui, W.O.; Vasilcanu, R.; Axelson, M.; Larsson, O.; Larsson, C.; Girnita, L. The insulin-like growth factor-1 receptor inhibitor PPP produces only very limited resistance in tumor cells exposed to long-term selection. Oncogene 2006, 25, 3186–3195. [Google Scholar] [CrossRef]
- Lima, L.M.; Barreiro, E.J. Bioisosterism, A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef]
- Bertounesque, E.; Meresse, P.; Monneret, C.; Florent, J.C. Synthetic approach to condensed heterocyclic analogues from etoposide revisited. Synthesis of A-ring pyridazine etoposide. Tetrahedron Lett. 2007, 48, 5781–5784. [Google Scholar] [CrossRef]
- Zhu, X.K.; Guan, J.; Xiao, Z.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents. Part 61, Anti-HIV activity of new podophyllotoxin derivatives. Bioorg. Med. Chem. 2004, 12, 4267–4273. [Google Scholar] [CrossRef]
- Magedov, I.V.; Manpadi, M.; Van Slambrouck, S.; Steelant, W.F.A.; Rozhkova, E.; Przheval’skii, N.M.; Rogelj, S.; Kornienko, A. Discovery and investigation of antiproliferative and apoptosis-inducing properties of new heterocyclic podophyllotoxin analogues accessible by a one-step multicomponent synthesis. J. Med. Chem. 2007, 50, 5183–5192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Feng, M.; Bai, S.F.; Zhang, Y.; Chao Bi, W.; Chen, H. Synthesis and antitumor activity of novel podophyllotoxin derivatives. Chin. Chem. Lett. 2009, 20, 901–904. [Google Scholar] [CrossRef]
- Kamal, A.; Ashwini Kumar, B.; Arifuddin, M.; Dastidar, S.G. Synthesis of 4[beta]-amido and 4[beta]-sulphonamido analogues of podophyllotoxin as potential antitumour agents. Bioorg. Med. Chem. 2003, 11, 5135–5142. [Google Scholar] [CrossRef]
- Xiao, Z.; Bastow, K.F.; Vance, J.R.; Lee, K.H. Antitumor agents. Part 227, Studies on novel 4′-O-demethyl-epipodophyllotoxins as antitumor agents targeting topoisomerase II. Bioorg. Med. Chem. 2004, 12, 3339–3344. [Google Scholar]
- Xi, W.L.; Cai, Q.; Tang, Y.B.; Sun, H.; Xiao, Z.Y. Design and synthesis of novel cytotoxic podophyllotoxin derivatives. Chin. Chem. Lett. 2010, 21, 1153–1156. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Yang, Z.; Cao, B.; Zheng, Y.; Chen, H. Synthesis of podophyllotoxin derivatives and their antitumor activity in vitro. Chin. J. Med. Chem. 2009, 19, 85–88. [Google Scholar]
- Lu, Y.; Zuo, S.; Shi, S.; Li, X.; Zhang, Y.; Lu, J.; Chen, H. Synthesis and antitumor activities of podophyllotoxin derivatives. Chin. J. Med. Chem. 2010, 20, 90–95. [Google Scholar]
- Guianvarc’h, D.; Duca, M.; Boukarim, C.; Kraus-Berthier, L.; Léonce, S.; Pierré, A.; Pfeiffer, B.; Renard, P.; Arimondo, P.B.; Monneret, C.; et al. Synthesis and biological activity of sulfonamide derivatives of epipodophyllotoxin. J. Med. Chem. 2004, 47, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Xu, D.Q.; Du, W.T.; Qian, S.J.; Wang, L.; Lou, J.S.; He, Q.J.; Yang, B.; Hu, Y.Z. Novel 4 beta-anilino-podophyllotoxin derivatives, design synthesis and biological evaluation as potent DNA-topoisomerase II poisons and anti-MDR agents. Mol. Biosyst. 2010, 6, 410–420. [Google Scholar] [CrossRef]
- Kamal, A.; Kumar, B.A.; Suresh, P.; Agrawal, S.K.; Chashoo, G.; Singh, S.K.; Saxena, A.K. Synthesis of 4[beta]-N-polyaromatic substituted podophyllotoxins, DNA topoisomerase inhibition, anticancer and apoptosis-inducing activities. Bioorg. Med. Chem. 2010, 18, 8493–8500. [Google Scholar] [CrossRef]
- Bhat Bilal, A.; Reddy, P.B.; Agrawal Satyam, K.; Saxena, A.K.; Kumar, H.M.S.; Qazi, G.N. Studies on novel 4beta-[(4-substituted)-1,2,3-triazol-1-yl] podophyllotoxins as potential anticancer agents. Eur. J. Med. Chem. 2008, 43, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Han, Y.; An, L.; Yang, J.; Wang, Q. Seleno-podophyllotoxin derivatives induce hepatoma SMMC-7721 cell apoptosis through Bax pathway. Cell Bio. Int. 2008, 32, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Zhang, Q.; Yuan, W.B.; Wang, L.F.; Song, Y.M.; Liu, H.H. Studies on the mechanism of the interaction between hydrazide-podophyllic Ni(II), Co(II), Zn(II) metal complexes and DNA. Spectrosc. Spectr. Anal. 2006, 26, 1298–1302. [Google Scholar]
- Usami, Y.; Arimoto, M.; Kobayashi, K.; Honjou, M.; Yamanaka, M.; Miyao, M.; Ichikawa, H.; Bastow, K.F.; Lee, K.H. Synthesis of aryltetralin type 2-azalignans using schollkopf’s bislactim-ether methodology. Heterocycles 2009, 78, 2041–2052. [Google Scholar] [CrossRef]
- Iida, A.; Kano, M.; Kubota, Y.; Koga, K.; Tomioka, K. Podophyllotoxin aza-analogue, a novel DNA topoisomerase II inhibitor. Chem. Pharm. Bull. 2000, 48, 486–489. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Kobayashi, M.; Morita, H.; Itokawa, H.; Takeya, K. Synthesis of (-)-4-aza-4-deoxypodophyllotoxin from (-)-podophyllotoxin. Tetrahedron Lett. 1999, 40, 9107–9110. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Fukuyo, M.; Tsuda, K.; Kobayashi, M.; Ozeki, A.; Itokawa, H.; Takeya, K. 4-aza-2,3-dehydro-4-deoxypodophyllotoxins, Simple aza-podophyllotoxin analogues possessing potent cytotoxicity. Bioorg. Med. Chem. Lett. 2000, 10, 315–317. [Google Scholar] [CrossRef]
- Shi, C.L.; Wang, J.X.; Chen, H.; Shi, D.Q. Regioselective synthesis and in vitro anticancer activity of 4-aza-podophyllotoxin derivatives catalyzed by l-proline. J. Comb. Chem. 2010, 12, 430–434. [Google Scholar] [CrossRef]
- Labruere, R.; Gautier, B.; Testud, M.; Seguin, J.; Lenoir, C.; Desbene-Finck, S.; Helissey, P.; Garbay, C.; Chabot, G.G.; Vidal, M.; et al. Design, synthesis, and biological evaluation of the first podophyllotoxin analogues as potential vascular-disrupting agents. Chem. Med. Chem. 2010, 5, 2016–2025. [Google Scholar] [CrossRef]
- Singh, P.; Faridi, U.; Srivastava, S.; Kumar, J.K.; Darokar, M.P.; Luqman, S.; Shanker, K.; Chanotiya, C.S.; Gupta, A.; Gupta, M.M.; et al. Design and synthesis of C-ring lactone- and lactam-based podophyllotoxin analogs as anticancer agents. Chem. Pharm. Bull. 2010, 58, 242–246. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, J.H.; Ding, H.X.; Xiong, Y.; Cheng, C.H.K.; Hao, X.J.; Zhang, Y.M.; Pan, Y.J.; Gueritte, F.; Wu, X.M.; et al. Synthesis and cytotoxicity of racemic isodeoxypodophyllotoxin analogues with isoprene-derived side chains. J. Nat. Prod. 2006, 69, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Garcia, P.A.; Gomez-Zurita, M.A.; San Feliciano, A. Synthesis and cytotoxic evaluation of C-9 oxidized podophyllotoxin derivatives. Bioorg. Med. Chem. 2007, 15, 1670–1678. [Google Scholar] [CrossRef]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; García, P.A.; Gómez-Zurita, M.A.; García-Grávalos, M.D.; de la Iglesia-Vicente, J.; Gajate, C.; An, F.; Mollinedo, F.; et al. Synthesis and biological evaluation of new selective cytotoxic cyclolignans derived from podophyllotoxin. J. Med. Chem. 2004, 47, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Miguel del Corral, J.M.; Garcia, P.A.; Rojo, M.V.; de la Iglesia-Vicente, J.; Mollinedo, F.; Cuevas, C.; San Feliciano, A. Synthesis and Biological Evaluation of New Podophyllic Aldehyde Derivatives with Cytotoxic and Apoptosis-Inducing Activities. J. Med. Chem. 2010, 53, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Imperio, D.; Pirali, T.; Galli, U.; Pagliai, F.; Cafici, L.; Canonico, P.L.; Sorba, G.; Genazzani, A.A.; Tron, G.C. Replacement of the lactone moiety on podophyllotoxin and steganacin analogues with a 1,5-disubstituted 1,2,3-triazole via ruthenium-catalyzed click chemistry. Bioorg. Med. Chem. 2007, 15, 6748–6757. [Google Scholar] [CrossRef]
- Xiao, Z.; Han, S.; Bastow, K.F.; Lee, K.H. Antitumor agents. Part 232, Synthesis of cyclosulfite podophyllotoxin analogues as novel prototype antitumor agents. Bioorg. Med. Chem. Lett. 2004, 14, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Vance, J.R.; Bastow, K.F.; Brossi, A.; Wang, H.K.; Lee, K.H. Antitumor agents. Part 235, Novel 4′-ester etoposide analogues as potent DNA topoisomerase II inhibitors with improved therapeutic potential. Bioorg. Med. Chem. 2004, 12, 3363–3369. [Google Scholar] [CrossRef]
- Wu, C.C.; Li, T.K.; Farh, L.; Lin, L.Y.; Lin, T.S.; Yu, Y.J.; Yen, T.J.; Chiang, C.W.; Chan, N.L. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science 2011, 333, 459–462. [Google Scholar] [CrossRef]
- Labruere, R.; Helissey, P.; Desbene-Finck, S.; Giorgi-Renault, S. Design and effective synthesis of the first 4-aza-2,3-didehydropodophyllotoxin rigid aminologue, a N-methyl-4-[(3,4,5- trimethoxyphenyl) amino]]-1,2- dihydroquinoline-lactone. J. Org. Chem. 2008, 73, 3642–3645. [Google Scholar] [CrossRef]
- Wright, J.L.; Gregory, T.F.; Kesten, S.R.; Boxer, P.A.; Serpa, K.A.; Meltzer, L.T.; Wise, L.D.; Espitia, S.A.; Konkoy, C.S.; Whittemore, E.R.; et al. Subtype-Selective N-Methyl-D-Aspartate Receptor Antagonists, Synthesis and Biological Evaluation of 1-(Heteroarylalkynyl)-4-benzylpiperidines. J. Med. Chem. 2000, 43, 3408–3419. [Google Scholar] [CrossRef]
- Wilkening, R.R.; Ratcliffe, R.W.; Fried, A.K.; Meng, D.; Sun, W.; Colwell, L.; Lambert, S.; Greenlee, M.; Nilsson, S.; Thorsell, A.; et al. Estrogen receptor beta-subtype selective tetrahydrofluorenones, use of a fused pyrazole as a phenol bioisostere. Bioorg. Med. Chem. Lett. 2006, 16, 3896–3901. [Google Scholar] [CrossRef] [PubMed]
- Magedov, I.V.; Manpadi, M.; Rozhkova, E.; Przheval’skii, N.M.; Rogelj, S.; Shors, S.T.; Steelant, W.F.A.; slambrouck, S.V.; Kornienko, A. Structural simplification of bioactive natural products with multicomponent synthesis: Dihydropyridopyrazole analogues of podophyllotoxin. Bioorg. Med. Chem. Lett. 2007, 17, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Herr, K.; Fleckenstein, M.; Brodrecht, M.; Höfler, M.V.; Heise, H.; Aussenac, F.; Gutmann, T.; Reggelin, M.; Buntkowsky, G. A novel strategy for site selective spin-labeling to investigate bioactive entities by DNP and EPR spectroscopy. Sci. Rep. 2021, 11, 13714. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Nan, X.; Li, W.Q.; Wang, M.J.; Zhao, X.B.; Liu, Y.Q.; Zhang, Z.J.; Lee, K.H. Synthesis of novel spin-labeled podophyllotoxin derivatives as potential antineoplastic agents, Part XXV. Med. Chem. Res. 2014, 23, 4926–4931. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, S.W.; Tian, X. Synthesis and biological evaluation of new spin-labeled derivatives of podophyllotoxin. Bioorg. Med. Chem. 2006, 14, 3062–3068. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhang, Z.W.; Hui, L.; Chen, S.W.; Tian, X. Novel semisynthetic spin-labeled derivatives of podophyllotoxin with cytotoxic and antioxidative activity. Bioorg. Med. Chem. Lett. 2009, 20, 983–986. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhang, Z.W.; Hui, L.; Tian, X. Design, synthesis and biological evaluation of novel spin-labeled derivatives of podophyllotoxin. Nat. Prod. Commun. 2010, 5, 241–244. [Google Scholar] [CrossRef]
- Kou, L.; Wang, M.J.; Wang, L.T.; Zhao, X.B.; Nan, X.; Yang, L.; Liu, Y.Q.; Morris-Natschke, S.L.; Lee, K.H. Toward synthesis of third-generation spin-labeled podophyllotoxin derivatives using isocyanide multicomponent reactions. Eur. J. Med. Chem. 2014, 75, 282–288. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.Y.; Li, W.G.; Wu, Y.J.; Tian, X. Antioxidative and antitumor activity of derivatives of 4-beta -amino-4′-demethylepipodophyllotoxin and their structure-activity relationship. Pharmazie 2007, 62, 432–438. [Google Scholar]
- Zhang, Z.W.; Zhang, J.Q.; Hui, L.; Chen, S.W.; Tian, X. First synthesis and biological evaluation of novel spin-labeled derivatives of deoxypodophyllotoxin. Eur. J. Med. Chem. 2010, 45, 1673–1677. [Google Scholar] [CrossRef]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 2020, 119, 199–245. [Google Scholar] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer, crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Baniyash, M. Chronic inflammation, immunosuppression and cancer, new insights and outlook. Semin. Cancer Biol. 2006, 16, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging, A role for prostate cancer? Biochim. Biophys. Acta (BBA) 2009, 1795, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.J.; van der Wilt, C.L.; van Moorsel, C.J.; Kroep, J.R.; Bergman, A.M.; Ackland, S.P. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol. Ther. 2000, 87, 227–253. [Google Scholar] [CrossRef]
- Chen, S.W.; Tian, X.; Tu, Y.Q. Synthesis and cytotoxic activity of novel derivatives of 4′-demethylepipodophyllotoxin. Bioorg. Med. Chem. Lett. 2004, 14, 5063–5066. [Google Scholar] [CrossRef]
- Zhang, F.M.; Yao, X.J.; Tian, X.; Tu, Y.Q. Synthesis and biological evaluation of new 4beta-5-Fu-substituted 4′-demethylepipodophyllotoxin derivatives. Molecules 2006, 11, 849–857. [Google Scholar] [CrossRef]
- Danieli, B.; Giardini, A.; Lesma, G.; Passarella, D.; Peretto, B.; Sacchetti, A.; Silvani, A.; Pratesi, G.; Zunino, F. Thiocolchicine-podophyllotoxin conjugates, Dynamic libraries based on disulfide exchange reaction. J. Org. Chem. 2006, 71, 2848–2853. [Google Scholar] [CrossRef]
- Passarella, D.; Giardini, A.; Peretto, B.; Fontana, G.; Sacchetti, A.; Silvani, A.; Ronchi, C.; Cappelletti, G.; Cartelli, D.; Borlak, J.; et al. Inhibitors of tubulin polymerization, Synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III. Bioorgan. Med. Chem. 2008, 16, 6269–6285. [Google Scholar] [CrossRef]
- Passarella, D.; Peretto, B.; Yepes, R.B.Y.; Cappelletti, G.; Cartelli, D.; Ronchi, C.; Snaith, J.; Fontana, G.; Danieli, B.; Borlak, J. Synthesis and biological evaluation of novel thiocolchicine-podophyllotoxin conjugates. Eur. J. Med. Chem. 2010, 45, 219–226. [Google Scholar] [CrossRef]
- Arimondo, P.; Boukarim, C.; Bailly, C.; Dauzonne, D.; Monneret, C. Design of two etoposide-amsacrine conjugates, topoisomerase II and tubuline polymerization inhibition and relation to cytotoxicity. Anti-Cancer Drug Des. 2000, 15, 413–421. [Google Scholar]
- Yu, P.F.; Chen, H.; Wang, J.; He, C.X.; Cao, B.; Li, M.; Yang, N.; Lei, Z.Y.; Cheng, M.S. Design, synthesis and cytotoxicity of novel podophyllotoxin derivatives. Chem. Pharm. Bull. 2008, 56, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bi, W.C.; Cao, B.; Yang, Z.X.; Chen, S.W.; Shang, H.; Yu, P.F.; Yang, J. A novel podophyllotoxin derivative (YB-1EPN) induces apoptosis and down-regulates express of P-glycoprotein in multidrug resistance cell line KBV200. Eur. J. Pharmacol. 2010, 627, 69–74. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Zhang, J.Z.; Wang, Y.Z.; Cao, B.; Bai, S.F.; Yu, P.F.; Bi, W.C.; Xie, W.L. L1EPO, a Novel Podophyllotoxin Derivative Overcomes P-Glycoprotein-Mediated Multidrug Resistance in K562/A02 Cell Line. Biol. Pharm. Bull. 2009, 32, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Wang, Y.H.; Jin, Y.; Tian, X.; Zheng, Y.T.; Luo, D.Q.; Tu, Y.Q. Synthesis and anti-HIV-1 activities of novel podophyllotoxin derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 2091–2095. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, Y.; Wang, J.S.; Kong, W.; Jin, Y.H. Etoposide Induces Mitochondria-Associated Apoptotic Cell Death in Human Gastric Carcinoma Cells. Chem. Res. Chin. Univ. 2008, 24, 597–602. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, C.; Liu, S.; Chen, H.; Yang, C. Effect of podophyllotoxin on human gastric cancer cell line SGC 7901. Zhongnan Daxue Xuebao Yixueban 2008, 33, 718–722. [Google Scholar]
- Chirumbolo, S.; Conforti, A.; Lussignoli, S.; Metelmann, H.; Bellavite, P. Effects of Podophyllum peltatum compounds in various preparations and dilutions on human neutrophil functions in vitro. Br. Homeopath. J. 1997, 86, 16–26. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wu, L.Q.; Liu, H.L.; Ye, L.Y.; Xin, Y.L.; Grau, G.E.; Lou, J.N. Abnormal blood vessels formed by human liver cavernous hemangioma endothelial cells in nude mice are suitable for drug evaluation. Microvasc. Res. 2009, 78, 379–385. [Google Scholar] [CrossRef]
- Lata, M.; Prasad, J.; Singh, S.; Kumar, R.; Singh, L.; Chaudhary, P.; Arora, R.; Chawla, R.; Tyagi, S.; Soni, N.L.; et al. Whole body protection against lethal ionizing radiation in mice by REC-2001, A semi-purified fraction of Podophyllum hexandrum. Phytomedicine 2009, 16, 47–55. [Google Scholar] [CrossRef]
- Bala, M.; Goel, H.C. Radioprotective effect of podophyllotoxin in Saccharomyces cerevisiae. J. Environ. Pathol. Toxicol. Oncol. 2004, 23, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Chawla, R.; Dhaker, A.S.; Adhikari, M.; Sharma, J.; Singh, S.; Gupta, D.; Kumar, R.; Sharma, A.; Sharma, R.K.; et al. Podophyllum hexandrum as a potential botanical supplement for the medical management of nuclear and radiological emergencies (NREs) and free radical-mediated ailments, leads from in vitro/in vivo radioprotective efficacy evaluation. J. Diet. Suppl. 2010, 7, 31–50. [Google Scholar] [CrossRef]
- Chawla, R.; Arora, R.; Kumar, R.; Sharma, A.; Prasad, J.; Singh, S.; Sagar, R.; Chaudhary, P.; Shukla, S.; Kaur, G.; et al. Antioxidant activity of fractionated extracts of rhizomes of high-altitude Podophyllum hexandrum, Role in radiation protection. Mol. Cell. Biochem. 2005, 273, 193–208. [Google Scholar] [CrossRef]
- Singh Pankaj, K.; Kumar, R.; Sharma, A.; Arora, R.; Chawla, R.; Jain Swatantra, K.; Sharma Rakesh, K. Podophyllum hexandrum fraction (REC-2006) shows higher radioprotective efficacy in the p53-carrying hepatoma cell line, a role of cell cycle regulatory proteins. Integr. Cancer Ther. 2009, 8, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Razuvaev, A.; Henderson, B.; Girnita, L.; Larsson, O.; Axelson, M.; Hedin, U.; Roy, J. The cyclolignan picropodophyllin attenuates intimal hyperplasia after rat carotid balloon injury by blocking insulin-like growth factor-1 receptor signaling. J. Vasc. Surg. 2007, 46, 108–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, C.X.; Son, M.J.; Ju, H.K.; Moon, T.C.; Lee, E.; Kim, S.H.; Kim, M.J.; Son, J.K.; Lee, S.H.; Chang, H.W. Deoxypodophyllotoxin, a naturally occurring lignan, inhibits the passive cutaneous anaphylaxis reaction. Planta Med. 2004, 70, 474–476. [Google Scholar]
- Xu, P.; Sun, Q.; Wang, X.J.; Zhang, S.G.; An, S.S.; Cheng, J.; Gao, R.; Xiao, H. Pharmacological effect of deoxypodophyllotoxin, A medicinal agent of plant origin, on mammalian neurons. Neurotoxicology 2010, 31, 680–686. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, M.; Xu, H. Insecticidal activity of twin compounds from podophyllotoxin and cytisine. Bioorg. Med. Chem. Lett. 2021, 43, 128104. [Google Scholar] [CrossRef]
- Linder, S.; Shoshan, M.C.; Gupta, R.S. Picropodophyllotoxin or podophyllotoxin does not induce cell death via insulin-like growth factor-I receptor. Cancer Res. 2007, 67, 2899. [Google Scholar] [CrossRef][Green Version]
- Gao, R.; Gao, C.; Tian, X.; Yu, X.; Di, X.; Xiao, H.; Zhang, X. Insecticidal activity of deoxypodophyllotoxin, isolated from Juniperus sabina, and related lignans against larvae of Pieris rapae. Pest Manag. Sci. 2004, 60, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Xie, X. Podophyllotoxin induces CREB phosphorylation and CRE-driven gene expression via PKA but not MAPKs. Mol. Cells 2010, 29, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.J.; Wang, Y.J.; Liang, Y.C.; Jeng, J.H.; Lee, W.S.; Lin, J.K.; Chen, C.H.; Liu, I.C.; Ho, Y.S. Microtubule damaging agents induce apoptosis in HL 60 cells and G2/M cell cycle arrest in HT 29 cells. Toxicology 2002, 175, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, L.; Zhen, H.; Zhou, L.; Shi, P.; Huang, Z. Proteomic changes induced by podophyllotoxin in human cervical carcinoma HeLa cells. Am. J. Chin. Med. 2013, 41, 163–175. [Google Scholar] [CrossRef]
- Naik, P.K.; Dubey, A.; Soni, K.; Kumar, R.; Singh, H. The binding modes and binding affinities of epipodophyllotoxin derivatives with human topoisomerase II[alpha]. J. Mol. Graph. Model 2010, 29, 546–564. [Google Scholar] [CrossRef]
- Asami, Y.; Jia, D.W.; Tatebayashi, K.; Yamagata, K.; Tanokura, M.; Ikeda, H. Effect of the DNA topoisomerase II inhibitor VP-16 on illegitimate recombination in yeast chromosomes. Gene 2002, 291, 251–257. [Google Scholar] [CrossRef]
- Karasic, T.B.; Hei, T.K.; Ivanov, V.N. Disruption of IGF-1R signaling increases TRAIL-induced apoptosis, A new potential therapy for the treatment of melanoma. Exp. Cell Res. 2010, 316, 1994–2007. [Google Scholar] [CrossRef]
- Colon, E.; Zaman, F.; Axelson, M.; Larsson, O.; Carlsson-Skwirut, C.; Svechnikov Konstantin, V.; Soder, O. Insulin-like growth factor-I is an important antiapoptotic factor for rat leydig cells during postnatal development. Endocrinology 2007, 148, 128–139. [Google Scholar] [CrossRef]
- Duan, Z.; Choy, E.; Harmon, D.; Yang, C.; Ryu, K.; Schwab, J.; Mankin, H.; Hornicek, F.J. Insulin-like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Mol. Cancer Ther. 2009, 8, 2122–2130. [Google Scholar] [CrossRef]
- Tanaka, T.; Halicka, H.D.; Traganos, F.; Seiter, K.; Darzynkiewicz, Z. Induction of ATM activation, histone H2AX phosphorylation and apoptosis by etoposide-Relation to cell cycle phase. Cell Cycle 2007, 6, 371–376. [Google Scholar] [CrossRef]
- Xu, X.Y.; Wang, D.Y.; Li, Y.P.; Deyrup, S.T.; Zhang, H.J. Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef] [PubMed]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Kuramochi, K.; Imai, T.; Takata, K.I.; Takehara, M.; Kobayashi, S.; Sakaguchi, K.; Sugawara, F. Podophyllotoxin directly binds a hinge domain in E2 of HPV and inhibits an E2/E7 interaction in vitro. Bioorg. Med. Chem. 2008, 16, 5815–5825. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Akhtar, M.F.; Haris, M.; Abdel-Daim, M.M. Recent updates on immunological, pharmacological, and alternative approaches to combat COVID-19. Inflammopharm 2021, 29, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, S.; Branch, S.; Harrelson, S.; Hussain, M.K.; Saquib, M.; Khan, S. Primed for global coronavirus pandemic: Emerging research and clinical outcome. Eur. J. Med. Chem. 2021, 209, 112862. [Google Scholar] [CrossRef]
- Patel, M.; Dominguez, E.; Sacher, D.; Desai, P.; Chandar, A.; Bromberg, M.; Caricchio, R.; Criner, G.J.; Temple University COVID-19 Research Group. Etoposide as salvage therapy for cytokine storm due to coronavirus disease 2019. Chest 2021, 159, e7–e11. [Google Scholar] [CrossRef]
- Rashid, H.U.; Ahmad, N.; Abdalla, M.; Khan, K.; Martines, M.A.U.; Shabana, S. Molecular docking and dynamic simulations of Cefixime, Etoposide and Nebrodenside A against the pathogenic proteins of SARS-CoV-2. J. Mol. Struct. 2022, 1247, 131296. [Google Scholar] [CrossRef]
- Jin, M.H.; Moon, T.C.; Quan, Z.J.; Lee, E.; Kim, Y.K.; Yang, J.H.; Suh, S.J.; Jeong, T.C.; Lee, S.H.; Kim, C.H.; et al. The naturally occurring flavolignan, deoxypodophyllotoxin, inhibits lipopolysaccharide-induced iNOS expression through the NF-kappa B activation in RAW264.7 macrophage cells. Biol. Pharm. Bull. 2008, 31, 1312–1315. [Google Scholar] [CrossRef]
- Jin, M.; Lee, E.; Yang, J.H.; Lu, Y.; Kang, S.; Chang, Y.C.; Lee, S.H.; Suh, S.J.; Kim, C.H.; Chang, H.W. Deoxypodophyllotoxin Inhibits the Expression of Intercellular Adhesion Molecule-1 Induced by Tumor Necrosis Factor-alpha in Murine Lung Epithelial Cells. Biol. Pharm. Bull. 2010, 33, 1–5. [Google Scholar] [CrossRef]
- Suh, S.J.; Kim, J.R.; Jin, U.H.; Choi, H.S.; Chang, Y.C.; Lee, Y.C.; Kim, S.H.; Lee, I.S.; Moon, T.C.; Chang, H.W.; et al. Deoxypodophyllotoxin, flavolignan, from Anthriscus sylvestris Hoffm. inhibits migration and MMP-9 via MAPK pathways in TNF-alpha-induced HASMC. Vasc. Pharmacol. 2009, 51, 13–20. [Google Scholar] [CrossRef]
- Petersen, C.S.; Agner, T.; Ottevanger, V.; Larsen, J.; Ravnborg, L. A single-blind study of podophyllotoxin cream 0.5% and podophyllotoxin solution 0.5% in male patients with genital warts. Genitourin Med. 1995, 71, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, F.; Puebla-Pérez, A.M.; González-Pozos, S.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; Alvarez, L.P.; Tapia-Pastrana, G.; Castillo-Romero, A. Antigiardial Activity of Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides. Molecules 2017, 22, 799. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Yu, X.; Zhi, X.; Fan, L.; Yao, X.; Xu, H. Synthesis of novel 4α-(acyloxy)-2′(2′,6′)-(di)halogenopodophyllotoxin derivatives as insecticidal agents. J. Agric. Food Chem. 2013, 61, 8148–8155. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhi, X.; Li, J.; Xu, H. Synthesis of Novel Oxime Sulfonate Derivatives of 2′(2′,6′)-(Di)chloropicropodophyllotoxins as Insecticidal Agents. J. Agric. Food Chem. 2015, 63, 6668–6674. [Google Scholar] [CrossRef]
- Filley, C.M.; Graff-Richard, N.R.; Lacy, J.R.; Heitner, M.A.; Earnest, M.P. Neurologic manifestations of podophyllin toxicity. Neurology 1982, 32, 308–311. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.; Keohane, C.; Jacobs, J.; O’Riordain, D.; Whelton, M. Neuropathy due to podophyllin intoxication. J. Neurol. 1990, 237, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.F.; Hung, D.Z.; Tsai, W.J.; Lin, K.P.; Deng, J.F. Podophyllotoxin intoxication: Toxic effect of Bajiaolian in herbal therapeutics. Hum. Exp. Toxicol. 1992, 11, 480–487. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Xia, D.; Xu, X. The Immunosuppression of Etoposide Deserves Attention in Chemoimmunotherapy. Am. J. Clin. Oncol. 2021, 44, 224–225. [Google Scholar] [CrossRef]
- Li, J.; Dai, C.X.; Sun, H.; Jin, L.; Guo, C.Y.; Cao, W.; Wu, J.; Tian, H.Y.; Luo, C.; Ye, W.C.; et al. Protective effects and mechanisms of curcumin on podophyllotoxin toxicity in vitro and in vivo. Toxicol. Appl. Pharmacol. 2012, 265, 190–199. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Jin, L.; Cao, W.; Zhang, J.; Guo, C.Y.; Ding, K.; Luo, C.; Ye, W.C.; Jiang, R.W. Alleviation of podophyllotoxin toxicity using coexisting flavonoids from Dysosma versipellis. PLoS ONE 2013, 8, e72099. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Renata, H. Asymmetric Chemoenzymatic Synthesis of (-)-Podophyllotoxin and Related Aryltetralin Lignans. Angew. Chem. Int. Ed. Engl. 2019, 58, 11657–11660. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, H.; Zhao, Y.; Zhao, J.; Chen, J.; Li, L. A New and Efficient Strategy for the Synthesis of Podophyllotoxin and Its Analogues. Org. Lett. 2007, 9, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, J.; Chen, J.; Pan, C.; Li, L.; Zhang, H. Enantioselective sequential conjugate addition-allylation reactions, a concise total synthesis of (+)-podophyllotoxin. Org. Lett. 2009, 11, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.P.; Maimone, T.J. C-H Bond Arylation in the Synthesis of Aryltetralin Lignans: A Short Total Synthesis of Podophyllotoxin. Angew. Chem. Int. Ed. Engl. 2014, 53, 3115–3119. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cong, X.W.; Yang, G.Z.; Wang, Y.W.; Peng, Y. Divergent Asymmetric Syntheses of Podophyllotoxin and Related Family Members via Stereoselective Reductive Ni-Catalysis. Org. Lett. 2018, 20, 1651–1654. [Google Scholar] [CrossRef]
- Hajra, S.; Garai, S.; Hazra, S. Catalytic Enantioselective Synthesis of (-)-Podophyllotoxin. Org. Lett. 2017, 19, 6530–6533. [Google Scholar] [CrossRef]
- Anrini, M.; Jha, S. Characterization of Podophyllotoxin Yielding Cell Lines of Podophyllum hexandrum. Caryologia 2009, 62, 220–235. [Google Scholar] [CrossRef]
- Shen, S.; Tong, Y.; Luo, Y.; Huang, L.; Gao, W. Biosynthesis, total synthesis, and pharmacological activities of aryltetralin-type lignan podophyllotoxin and its derivatives. Nat. Prod. Rep. 2022, 39, 1856–1875. [Google Scholar] [CrossRef]
- Sedaghat, S.; Ezatzadeh, E.; Alfermann, A.W. Podophyllotoxin from suspension culture of Linum album. Nat. Prod. Res. 2008, 22, 984–989. [Google Scholar] [CrossRef]
- Baldi, A.; Srivastava, A.K.; Bisaria, V.S. Effect of Aeration on Production of Anticancer Lignans by Cell Suspension Cultures of Linum album. Appl. Biochem. Biotech. 2008, 151, 547–555. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshoft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yao, T.; Zhao, W.; Li, H.; Tang, Y.J. Research progress in biosynthesis of podophyllotoxin and its derivatives. Chin. J. Biotechnol. 2021, 37, 2026–2038. [Google Scholar]
- Jia, K.Z.; Zhan, X.; Li, H.M.; Li, S.; Shen, Y.; Qingsheng, Q.; Zhang, Y.; Li, Y.Z.; Tang, Y.J. A novel podophyllotoxin derivative with higher anti-tumor activity produced via 4′-demethylepipodophyllotoxin biotransformation by Penicillium purpurogenum. Process Biochem. 2020, 96, 220–227. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.Y.; Mi, Z.Y.; Li, D.S.; Tang, Y.J. Novel biotransformation process of podophyllotoxin to produce podophyllic acid and picropodophyllotoxin by Pseudomonas aeruginosa CCTCC AB93066, part II: Process optimization. Bioresour. Technol. 2009, 100, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Messiano, G.B.; Wijeratne, E.M.; Lopes, L.M.; Gunatilaka, A.A. Microbial transformations of aryltetralone and aryltetralin lignans by Cunninghamella echinulata and Beauveria bassiana. J. Nat. Prod. 2010, 73, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Xu, X.L.; Zhong, J.J. A novel biotransformation process of 4′-demethylepipodophyllotoxin to 4′-demethylepipodophyllic acid by Bacillus fusiformis CICC 20463, Part II: Process optimization. Bioprocess. Biosyst. Eng. 2010, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.P.; Julsing, M.K.; Koulman, A.; Clarkson, C.; Woerdenbag, H.J.; Ionkova, I.; Bos, R.; Jaroszewski, J.W.; Kayser, O.; Quax, W.J. Bioconversion of deoxypodophyllotoxin into epipodophyllotoxin in E. coli using human cytochrome P450 3A4. J. Biotechnol. 2006, 126, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Julsing, M.K.; Vasilev, N.P.; Schneidman-Duhovny, D.; Muntendam, R.; Woerdenbag, H.J.; Quax, W.J.; Wolfson, H.J.; Ionkova, I.; Kayser, O. Metabolic stereoselectivity of cytochrome P450 3A4 towards deoxypodophyllotoxin, In silico predictions and experimental validation. Eur. J. Med. Chem. 2008, 43, 1171–1179. [Google Scholar] [CrossRef]
- Kitamura, H.; Okudela, K.; Yazawa, T.; Sato, H.; Shimoyamada, H. Cancer stem cell, Implications in cancer biology and therapy with special reference to lung cancer. Lung Cancer 2009, 66, 275–281. [Google Scholar] [CrossRef]
- Cheng, J.X.; Liu, B.L.; Zhang, X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat. Rev. 2009, 35, 403–408. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers, Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Bjerkvig, R.; Johansson, M.; Miletic, H.; Niclou, S.P. Cancer stem cells and angiogenesis. Semin. Cancer Biol. 2009, 19, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Nassar, D.; Blanpain, C. Cancer Stem Cells, Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Salnikov, A.V.; Kusumawidjaja, G.; Rausch, V.; Bruns, H.; Gross, W.; Khamidjanov, A.; Ryschich, E.; Gebhard, M.M.; Moldenhauer, G.; Büchler, M.W.; et al. Cancer stem cell marker expression in hepatocellular carcinoma and liver metastases is not sufficient as single prognostic parameter. Cancer Lett. 2009, 275, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, W.; Qian, H.; Zhu, W.; Yan, Y.; Zhou, H.; Zhang, X.; Xu, X.; Li, J.; Chen, Z.; et al. Mesenchymal stem cell-like cells derived from human gastric cancer tissues. Cancer Lett. 2009, 274, 61–71. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression. The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef]
- Apte, R.N.; Krelin, Y.; Song, X.; Dotan, S.; Recih, E.; Elkabets, M.; Carmi, Y.; Dvorkin, T.; White, R.M.; Gayvoronsky, L.; et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur. J. Cancer 2006, 42, 751–759. [Google Scholar] [CrossRef]
- Liu, J.; Gao, M.; Yang, Z.; Zhao, Y.; Guo, K.; Sun, B.; Gao, Z.; Wang, L. Macrophages and Metabolic Reprograming in the Tumor Microenvironment. Front. Oncol. 2022, 12, 795159. [Google Scholar] [CrossRef]

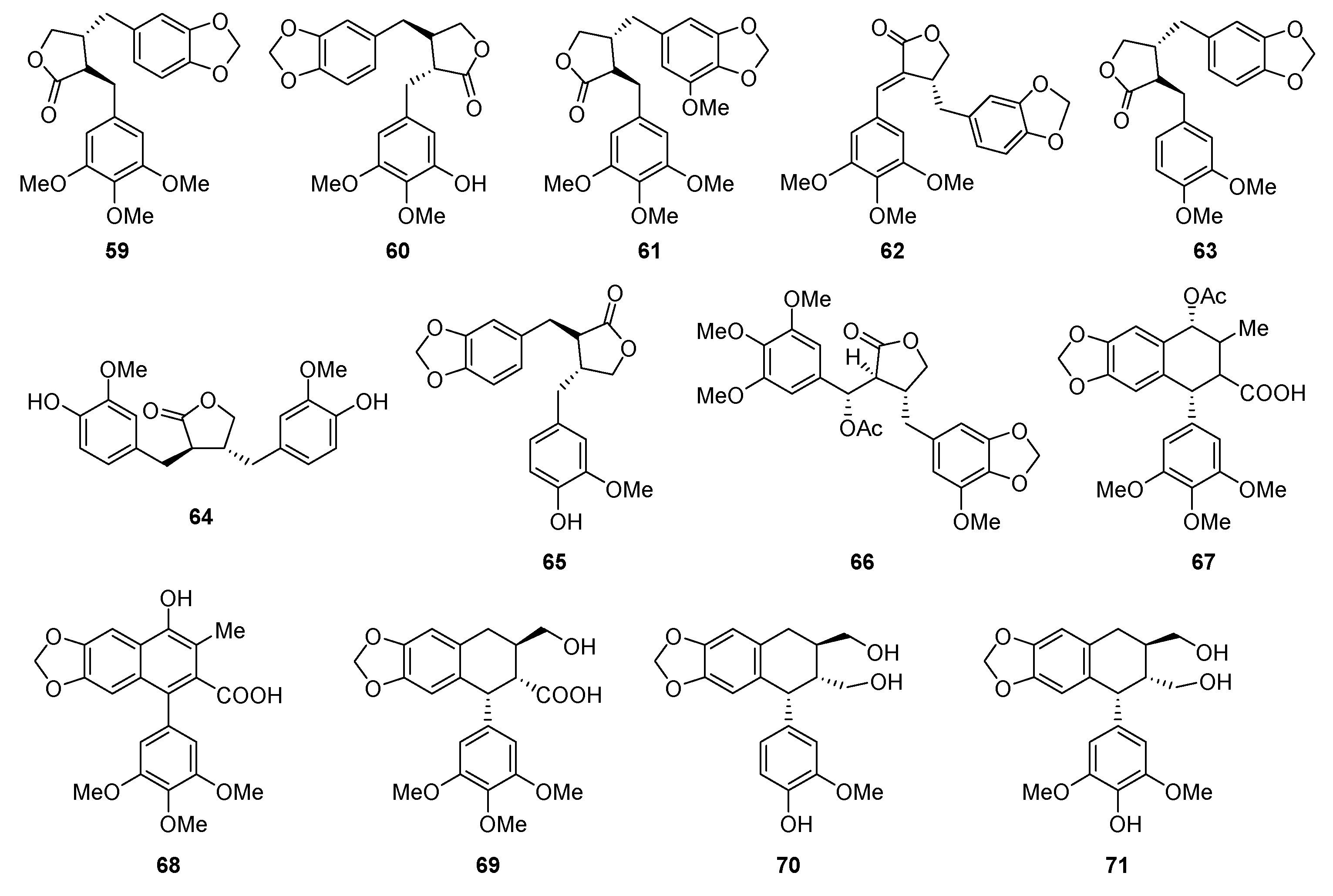
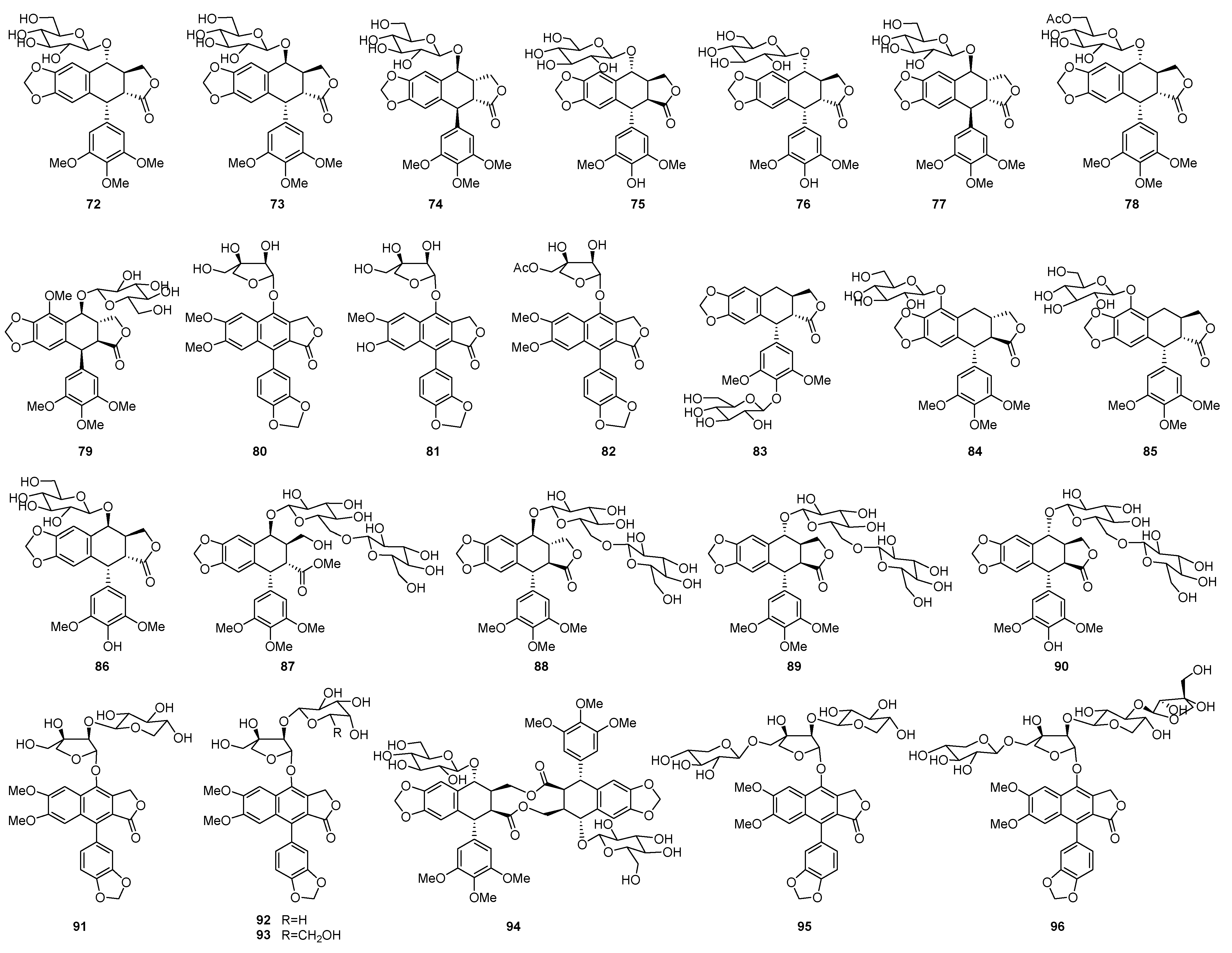
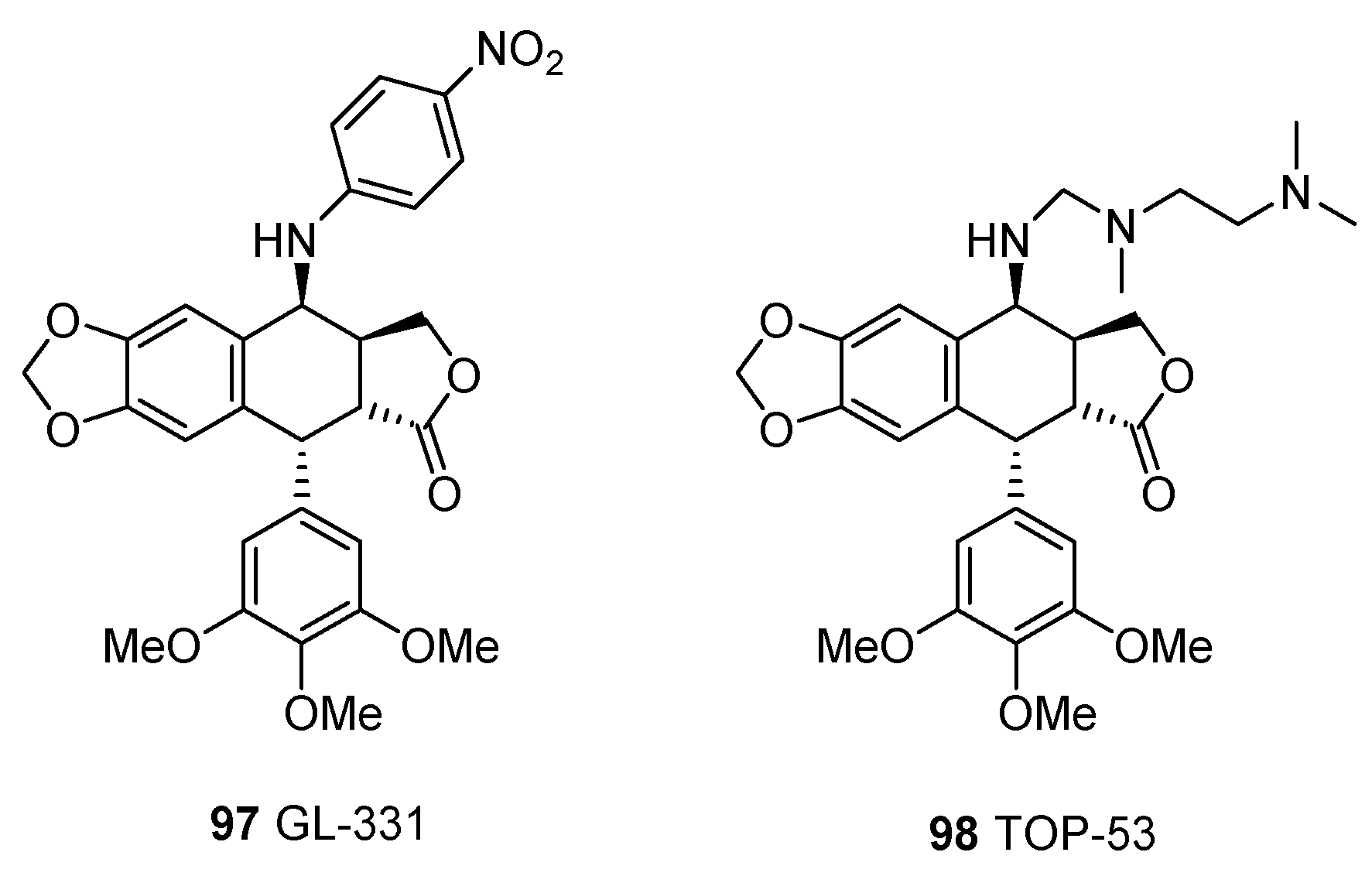
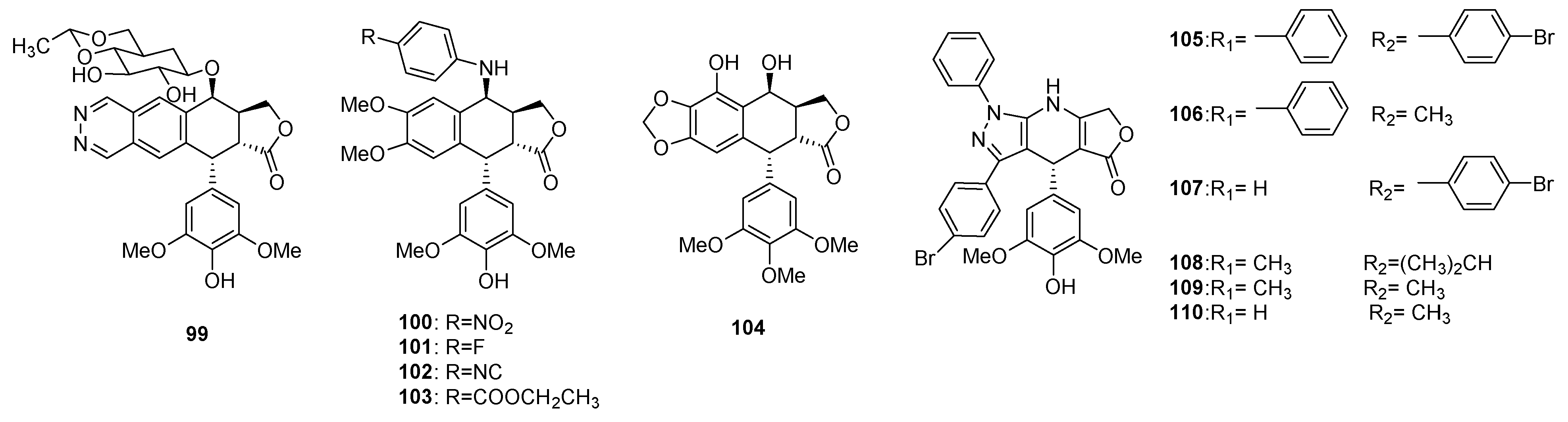
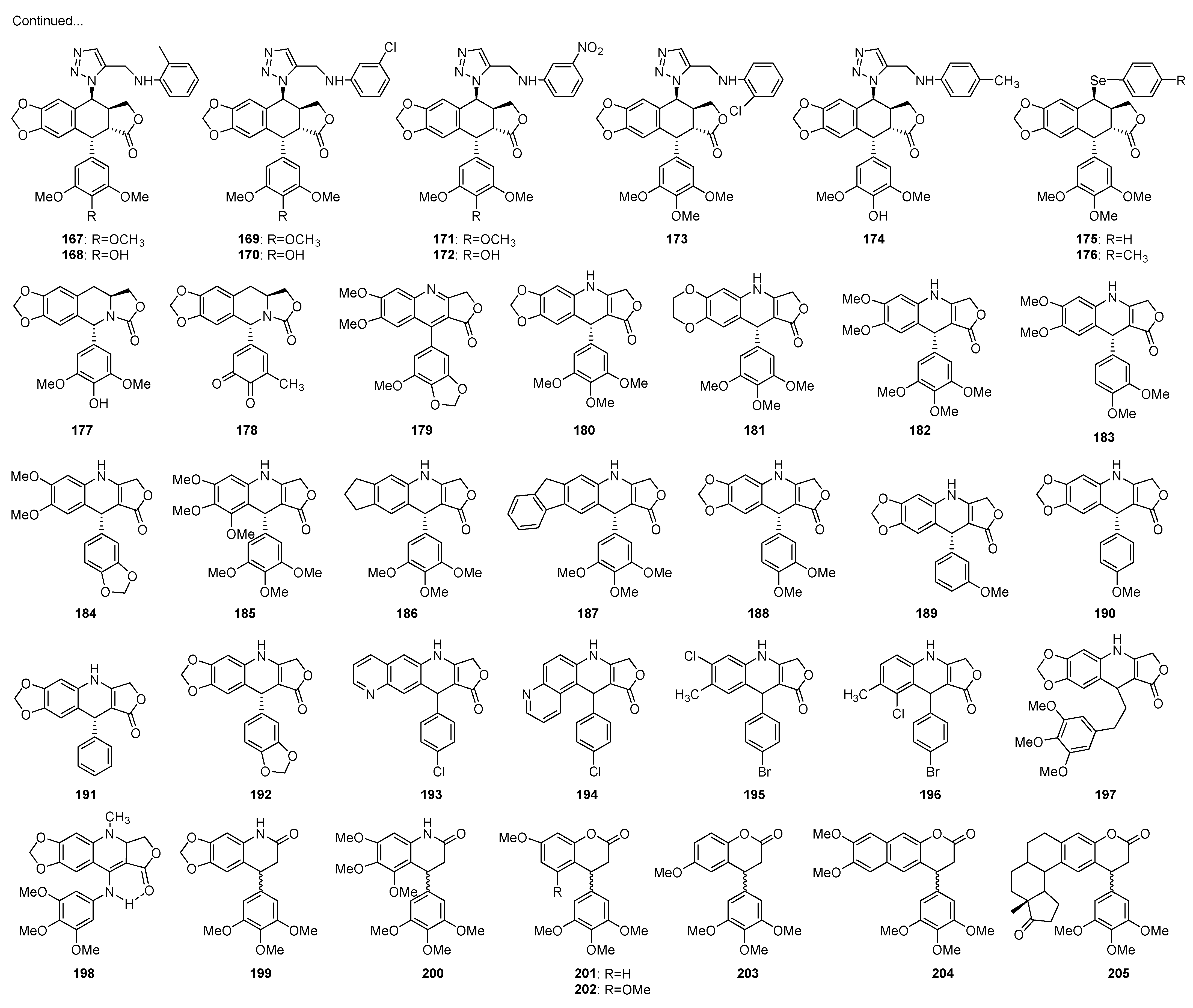
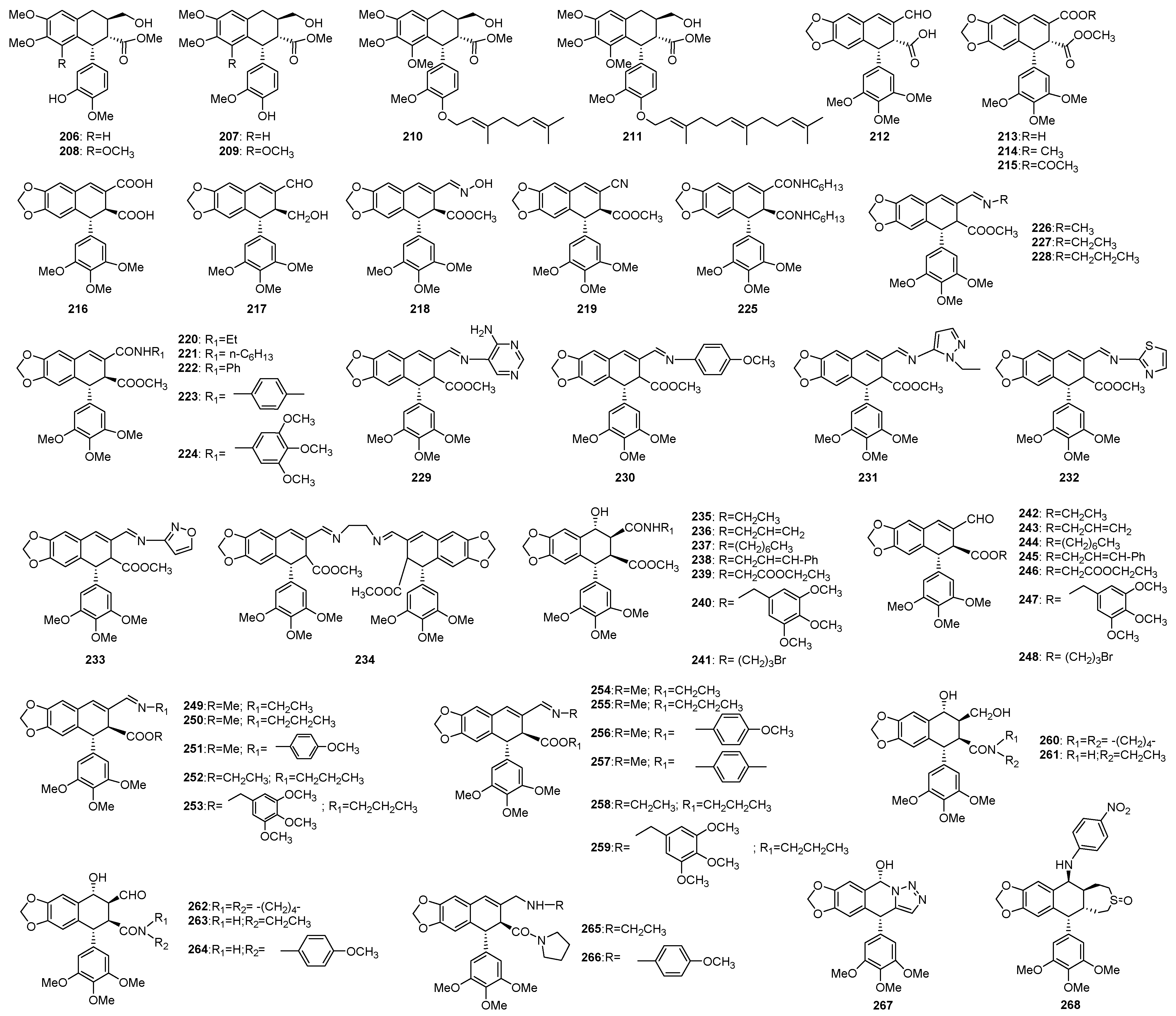
| Plant | Compounds | Biological Activities | References |
|---|---|---|---|
| Anthriscus neglecta | Deoxypodophyllotoxin 14 | Antitumor (induction of apoptosis) and inhbiton of CYP2C9 and CYP3A4 enzymes | [57] |
| Anthriscus sylvestris | Picropodophyllin 3 | Antitumor (induction of HL-60 apoptosis) | [33,37,58,59] |
| Deoxypodophyllotoxin 14 | Antitumor (inhibtions on MK-1, HeLa and B16F10 cells; induction of HL-60 apoptosis) | ||
| Deoxypicropodophyllin 15 | Antitumor (induction of HL-60 apoptosis) | ||
| Angeloyl podophyllotoxin 25 | Antitumor (induction of HL-60 apoptosis) | ||
| Nemerosin 62 | Antitumor (inhibition against MK-1, HeLa, and B16F10 cells) | ||
| Bursera morelensis | Deoxypodophyllotoxin 14 | Antitumor (inhibition agains HCT-15 and SK-LU1 cells) | [60] |
| 5′-demethoxydeoxypodophyllotoxin 40 | Antitumor (inhibition agains HCT-15 and SK-LU1 cells) | ||
| Bursera tonkinensis | 4′-demethyldesoxypodophyllotoxin 17 | Antitumor (inhibition agains KB, Col2 and LNCaP cells) | [61] |
| Bursehernin 63 | - | ||
| Isolariciresinol 70 | - | ||
| 5-methoxy-Isolariciresinol 71 | - | ||
| 4-demethyldesoxypodophyllotoxin-4-O-β-D-glucoside 83 | - | ||
| Bursera simaruba | Picropolygamain 52 | - | [62] |
| Bursera fagaroides | Acetyl podophyllotoxin 20 | Antitumor (disturbing tubulin) | [63] |
| Peltatin-A-methyl ether 32 | Antitumor (disturbing tubulin) | ||
| 5′-desmethoxy-β-Peltatin-A-methyl ether 38 | Antitumor (disturbing tubulin) | ||
| Callitris columellaris | Deoxypodophyllotoxin 14 | Antitumor (induction of apoptosis) | [64,65] |
| Callitris drummondi | Podophyllotoxin 1 | - | [66] |
| Haplophyllum cappadocicum | 4-deoxyisodiphyllin 37 | - | [67,68,69] |
| Tuberculatin 80 | - | ||
| Matairesinol 64 | - | ||
| Justicidin A 47 | - | ||
| Justicidin B 48 | - | ||
| Diphyllin 46 | - | ||
| Haplomyrtoside 81 | - | ||
| Haplomyrtin 50 | - | ||
| 1β-Polygamain 51 | - | ||
| Majidine 91 | - | ||
| Commiphora erlangeriana | Erlangerin A 53 | - | [70,71] |
| Erlangerin B 54 | - | ||
| Erlangerin C 55 | Cytotoxicity in RAW 264.7 cells; antitumor (inhibtion of HeLa, EAhy926 and L929 cells) | ||
| Erlangerin D 56 | Cytotoxicity in RAW 264.7 cells; antitumor (inhibtion of HeLa, EAhy926 and L929 cells) | ||
| Podophyllotoxin 1 | Cytotoxicity in RAW 264.7 cells; antitumor (inhibtion of HeLa, EAhy926 and L929 cells) | ||
| Diphylleia sinensis | Deoxypodophyllotoxin 14 | - | [42,72,73] |
| Isopicropodophyllone 10 | - | ||
| Diphyllin 46 | - | ||
| Picropodophyllin 3 | - | ||
| Podophyllotoxone 8 | - | ||
| Justicidin A 47 | - | ||
| 4′-demethylpodophyllotoxin 6 | - | ||
| Picropodophyllin glucoside 74 | - | ||
| 4′-demethylpodophyllotoxin 6 | - | ||
| Dysosma versipellis | Podophyllotoxin 1 | Antitumor (inhibition of LNCaP, PC-3, A549 and HT-29 cells) | [74,75,76] |
| 4′-demethyldeoxypodophyllotoxin 17 | Antitumor (inhibition against PC3 and LNcap-37 cells) | ||
| Dehydropodophyllotoxin 12 | - | ||
| Diphyllin 46 | - | ||
| Podophyllotoxone 8 | - | ||
| 4′-demethyldehydropodophyllotoxin 13 | - | ||
| Isopicropodophyllone 10 | - | ||
| Isodiphyllin 36 | - | ||
| Picropodophyllotoxin-4-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside 90 | - | ||
| L-picropodophyllotoxin-4-O-β-D-glucopyranoside 77 | - | ||
| 4′-demethyl podophyllotoxone 11 | - | ||
| Podophyllotoxone 8 | Antitumor (inhibition against PC3 and LNcap-37 cells) | ||
| α-peltatin 34 | - | ||
| β-peltatin 35 | - | ||
| Deoxypodophyllotoxin 14 | Antitumor (inhibition of LNCaP and PC-3 cells) | ||
| Podophyllotoxin-4-O-β-D-glucoside 72 | - | ||
| 4-demethylpodophyllotoxin-7¢-O-β-D-glucopyranoside 76 | - | ||
| α-peltatin-5-O-β-D-glucopyranoside 84 | - | ||
| β-peltatin-5-O-β-D-glucopyranoside 85 | - | ||
| 4′-demethylpodophyllotoxin 6 | Antitumor (inhibition of LNCaP and PC-3 cells) | ||
| Dysosma pleiantha | Deoxypodophyllotoxin 14 | Antiviral, anticancer | [77] |
| Podophyllotoxone 8 | |||
| 4′-demethylpodophyllotoxin 6 | |||
| 4′-demethyldesoxypodophyllotoxin 17 | |||
| 4′-demethyl podophyllotoxone 3 | |||
| Podophyllotoxin 1 | |||
| 4′-demethylpodophyllotoxin 6 | |||
| Diphylleia cymosa | 4′-demethyldesoxypodophyllotoxin 17 | - | [43] |
| Diphyllin 46 | - | ||
| 4′-demethylpodophyllotoxin 6 | - | ||
| Podophyllotoxin 1 | - | ||
| β-peltatin 35 | - | ||
| Diphylleia grayi | Picropodophyllin 3 | - | [43,78,79] |
| Deoxypodophyllotoxin 14 | Antitumor (inhibition of the prostate cancer cells through Akt/p53/Bax/PTEN pathway) | ||
| Diphyllin 46 | - | ||
| Podophyllotoxin 1 | Antitumor targeting the mitosis | ||
| β-apopicropodophyllin 18 | - | ||
| Eriope blanchetii | β-peltatin 35 | - | [80,81] |
| α-peltatin 34 | - | ||
| Yatein 59 | - | ||
| Podophyllotoxin 1 | - | ||
| Eriope macrostachya | β-peltatin 35 | - | [82] |
| α-peltatin 34 | - | ||
| Haplophyllum perforatum | Diphyllin 46 | Antitumor (inhibition against PC3, DLD1, A549, MDCK, MDCK-MDR1 cells) | [83] |
| Haplophyllum myrtifolium | 7-O-(3-methyl-2-butenyl)isodaurinol 58 | - | [44,48] |
| Haplomyrtin 50 | - | ||
| (-)-haplomyrfolin 65 | - | ||
| 1β-Polygamain 51 | - | ||
| Haplophyllum bucharicum | Justicidin B 48 | - | [84,85] |
| Diphyllin 46 | - | ||
| Haplophyllum buxbaumii | Justicidin B 48 | - | [86,87,88] |
| Diphyllin 46 | - | ||
| (-)-Tuberculatin 80 | - | ||
| Mono-O-acetyldiphyllin apioside 82 | - | ||
| Ciliatoside A 95 | - | ||
| Ciliatoside B 96 | - | ||
| Majidine 91 | - | ||
| Hernandia peltata | Deoxypodophyllotoxin 14 | - | [54] |
| Deoxypicropodophyllin 15 | - | ||
| 5′-methoxyyatein 61 | - | ||
| Bursehernin 63 | - | ||
| Hernandia nymphaeifolia | Deoxypodophyllotoxin 14 | - | [54,89] |
| Deoxypicropodophyllin 15 | - | ||
| Bursehernin 63 | - | ||
| Yatein 59 | - | ||
| 5′-methoxyYatein 61 | - | ||
| Hernandia sonora | Podophyllotoxin 1 | - | [90] |
| Picropodophyllin 3 | - | ||
| Deoxypodophyllotoxin 14 | - | ||
| Hernandin 33 | - | ||
| Podophyllotoxin acetate 51 | - | ||
| 5-Methoxypodophyllotoxin 26 | - | ||
| 5-methoxypodophyllotoxin acetate 30 | - | ||
| (3S,4R)-3-[(S)-(acetyloxy)(3,4,5-trimethoxyphenyl) | - | ||
| methyl]dihydro-4-[(7-methoxy-1,3-benzodioxol-5-yl) | - | ||
| methyl]-2(3H)-Furanone 66 | - | ||
| Hernandia ovigera | 6,7-demethylenedesoxypodophyllotoxin 45 | Antivirus (inhibition against EBV early antigen activation) | [39,49,53,91,92,93] |
| Deoxypicropodophyllin 15 | - | ||
| Podophyllotoxin 1 | - | ||
| Bursehernin 63 | Antivirus (inhibition against EBV early antigen activation) | ||
| Hernandin 33 | - | ||
| Dehydrodeoxypodophyllotoxin 19 | Antivirus (inhibition against EBV early antigen activation) | ||
| Yatein 59 | - | ||
| Dehydropodophyllotoxin 12 | Antivirus (inhibition against EBV early antigen activation) | ||
| Deoxypodophyllotoxin 14 | Antivirus (inhibition against EBV early antigen activation) | ||
| 5-methoxy-desoxypodophyllotoxin 29 | - | ||
| Hyptis verticillata | 4′-Demethylpicropodophyllotoxin 7 | - | [94,95,96] |
| 4′-demethyldeoxypodophyllotoxin 17 | Mitosis disturbance and antifungus | ||
| β-peltatin 35 | Mitosis disturbance and antivirus | ||
| Dehydropodophyllotoxin 12 | Mitosis disturbance and antibacteria | ||
| Dehydrodeoxypodophyllotoxin 19 | - | ||
| Yatein 59 | Mitosis disturbance and antifungus | ||
| Isodeoxypodophyllotoxin 16 | Mitosis disturbance | ||
| Deoxypicropodophyllin 15 | Mitosis disturbance | ||
| β-apopicropodophyllin 18 | Mitosis disturbance and antifungus | ||
| Justicia ciliata | Justicidin A 47 | [56,97,98] | |
| Justicidin B 49 | - | ||
| Cilinaphthalide A 41 | - | ||
| Cilinaphthalide B 42 | Antiplatelet | ||
| Ciliatoside A 95 | DNA damage and anti-inflammation (inhibited the accumulation of NO in RAW 264.7) | ||
| Ciliatoside B 96 | Anti-inflammation (inhibition of NO in RAW 264.7) | ||
| Diphyllin 46 | - | ||
| Justicia heterocarpa | Furo[3′,4′:6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 5,8,8a,9-tetrahydro-5a-hydroxy-5-(6-methoxy-1,3-benzodioxol-5-yl)-, (5S,5aS,8aS)- 57 | - | [99] |
| Justicia adhatoda | Diphyllin 46 | Antitumor, antivirus (SARS-CoV2) | [100] |
| Justicidin B 49 | Antiinflammatory, antiplatelet aggregation, cytotoxicity, antiviral (SARS-CoV2), fungicidal | ||
| Justicidin A 47 | Antivirus (SARS-CoV2) | ||
| Podophyllotoxin 1 | Antitumor, antivirus (SARS-CoV2) | ||
| Justicia procumbens | Tuberculation 80 | Antitumor (breakage of plasmid), enhancement of TNF-alpha generation and antiplatelet | [97,101,102,103,104,105,106] |
| Justicidin A 47 | Antiplatelet; cytotoxicity and enhancement of TNF-alpha generation | ||
| procumbenoside A 92 | Antitumor (breakage of plasmid) and antivirus (HIV-1) | ||
| procumbenoside B 93 | - | ||
| Ciliatoside A 95 | Antitumor (breakage of plasmid) | ||
| Ciliatoside B 96 | - | ||
| Justicidin C 49 | - | ||
| Justicidin B 48 | Antitumor, antiplatelet, anti-inflammation, antifungus, antivirus and antibacteria | ||
| Diphyllin 46 | Cytotoxic and antivirus (HIV-1) | ||
| Mono-O-acetyldiphyllin apioside 82 | - | ||
| Isodiphyllin 36 | - | ||
| Juniperus chinensis | Podophyllotoxin 1 | - | [51,107] |
| Yatein 59 | - | ||
| Juniperus sabina | epipicropodophyllotoxin 4 | - | [108,109] |
| 4-acetyl epipodophyllotoxin 22 | - | ||
| 4-acetyl epipicropodophyllotoxin 23 | - | ||
| 4-acetyl junaphtoic acid 67 | |||
| Junaphtoic acid 68 | - | ||
| Podophyllotoxin 1 | Anticholinesterase, antifertility effect (inducing epididymal epithelial cell apoptosis) | ||
| Deoxypodophyllotoxin 14 | Anticholinesterase | ||
| 3-O-demethylYatein 60 | - | ||
| Juniperus thurifera | Podophyllotoxone 8 | Analgesic and anti-inflammation | [110] |
| Deoxypodophyllotoxin 14 | Analgesic and anti-inflammation | ||
| Juniperus virginiana | Podophyllotoxin 1 | - | [111,112] |
| Libocedrus chevalieri | 5-methoxy-4-epipodophyllotoxin 27 | Antitumor (inhibition against leukemia L1210 cells) | [38] |
| 5-Methoxypodophyllotoxin 26 | Antitumor (inhibition against cancer cells) | ||
| 5-methoxypodophyllotoxin-4-O-β-D-glucoside 79 | - | ||
| Podophyllotoxin-4-O-β-D-glucoside 72 | - | ||
| Linum catharticum | Podophyllotoxin 1 | - | [113] |
| β-peltatin 35 | - | ||
| Podophyllotoxin-4-O-β-D-glucoside 72 | - | ||
| 5-Methoxypodophyllotoxin 26 | - | ||
| 5-methoxy podophyllotoxin acetate 30 | - | ||
| 5-methoxypodophyllotoxin-4-O-β-D-glucoside 79 | - | ||
| Linum flavum | 5-Methoxypodophyllotoxin 26 | - | [114,115,116] |
| 5-methoxypodophyllotoxin-7-O-n-hexanoate 31 | - | ||
| β-peltatin 35 | - | ||
| α-peltatin 34 | - | ||
| Podophyllotoxin 1 | - | ||
| 5-methoxypodophyllotoxin-4-O-β-D-glucoside 79 | - | ||
| α-peltatin-5-O-β-D-glucopyranoside 84 | - | ||
| β-peltatin-5-O-β-D-glucopyranoside 85 | - | ||
| Linum mucronatum | 5-Methoxypodophyllotoxin 26 | - | [117] |
| β-peltatin 35 | - | ||
| 5′-demethoxy-methoxypodophyllotoxin 39 | - | ||
| Podophyllotoxin 1 | - | ||
| Yatein 59 | - | ||
| Linum persicum | 5-Methoxypodophyllotoxin 26 | - | [118,119] |
| 5-methoxy podophyllotoxin acetate 30 | - | ||
| Podophyllotoxin 1 | - | ||
| β-peltatin 35 | - | ||
| α-peltatin 34 | - | ||
| Linum tauricum | 4′-demethyl-6-methoxypodophyllotoxin 28 | - | [120] |
| Podophyllotoxin 1 | - | ||
| 4′-Demethylpodophyllotoxin 6 | - | ||
| Podophyllum hexandrum | Podophyllotoxin 1 | Antitumor (inhibition against MCF-7 cells) | [121,122] |
| Isopicropodophyllotoxin 5 | - | ||
| Sinolignan A 78 | - | ||
| Sinolignan B 90 | - | ||
| Deoxypodophyllotoxin 14 | |||
| Isopicropodophyllone 10 | - | ||
| Picropodophyllone 9 | - | ||
| Podophyllotoxone 8 | - | ||
| Picropodophyllin 3 | - | ||
| Deoxypicropodophyllin 15 | - | ||
| Dehydropodophyllotoxin 12 | - | ||
| Isopicropodophyllone 10 | - | ||
| 4′-demethyl-picropodophyllotoxin 7 | - | ||
| 3′,4′-demethylene-podophyllotoxin 43 | - | ||
| 3′,4′-demethylene-4-demethyl-podophyllotoxin 44 | - | ||
| 4′-demethyl-deoxypodophyllotoxin 17 | Antitumor (inhibition against MCF-7 cells) | ||
| 4′-demethyl-podophyllotoxin 6 | Antitumor (inhibition against MCF-7 cells) | ||
| 4′-demethyl-dehydropodophyllotoxin 13 | - | ||
| 4-demethylpodophyllotoxin-7¢-O-β-D-glucopyranoside 76 | - | ||
| Podophyllotoxin-4-O-β-D-glucopyranoside 72 | - | ||
| 4-Demethyl-deoxypodophyllotoxin-4-O-β-D-glucopyranoside 83 | Antitumor (inhibition against MCF-7 cells) | ||
| Picropodophyllotoxin-7′-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside 90 | - | ||
| Isopodophyllotoxin-7′-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside 88 | - | ||
| Me epipodophyllate 7′-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside 87 | - | ||
| Diphyllin 46 | - | ||
| Podophyllum peltatum | Epipodophyllotoxin 2 | - | [40,41,123] |
| Isopicropodophyllone 10 | - | ||
| β-peltatin 35 | - | ||
| α-peltatin 34 | - | ||
| Podophyllotoxone 8 | - | ||
| 4′-demethyl podophyllotoxone 11 | - | ||
| 4′-demethyldeoxypodophyllotoxin 17 | - | ||
| 4′-demethylpodophyllotoxin 6 | - | ||
| Podophyllotoxin 1 | Antioxidant | ||
| Deoxypodophyllotoxin 14 | Antioxidant | ||
| 4-O-β-D-glucopyranoside epipodophyllotoxin 73 | - | ||
| Polygala macradenia | 4′-demethyldeoxypodophyllotoxin 17 | Antitumor (inhibition against P-388 lymphocytic leukemia and human epidermoid carcinoma) | [124] |
| Sinopodophyllum emodi | Podophyllotoxin 1 | - | [35,125,126] |
| isopicropodophyllone 10 | |||
| Dehydropodophyllotoxin 12 | - | ||
| Deoxypodophyllotoxin 14 | - | ||
| Picropodophyllin acetate 21 | Antitumor (inhibition against HeLa and KB cells) | ||
| 4′-acetyl-4′-demethyl-podophyllotoxin 24 | Antitumor (inhibition against HeLa and KB cells) | ||
| 4-O-β-D-glucopyranoside 4′-demethylpicropodophyllotoxin 75 | Antitumor (inhibition against HeLa and KB cells) | ||
| 4-O-β-D-glucopyranosyl-(1 →6)-β-D-glucopyranoside of picropodophyllotoxin 89 | - | ||
| 4-O-β-D-glucopyranoside 4′-demethylepipodophyllotoxin 86 | - | ||
| Thujopsis dolabrata | Deoxypodophyllotoxin 14 | Cytotoxic (inhibition against HL-60 and Caki-1 cells) | [127] |
| Desoxypodophillic acid 69 | - | ||
| Desoxypicopodophyllin 15 | - | ||
| β-peltatin 35 | Cytotoxic (inhibition against HL-60 and Caki-1 cells) | ||
| β-peltatin-A-methylether 32 | - | ||
| Withania coagulans | bispicropodophyllin glucoside 94 | - | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Song, Z.; Cai, F.; Ruan, L.; Jiang, R. Chemistry and Biological Activities of Naturally Occurring and Structurally Modified Podophyllotoxins. Molecules 2023, 28, 302. https://doi.org/10.3390/molecules28010302
Jin L, Song Z, Cai F, Ruan L, Jiang R. Chemistry and Biological Activities of Naturally Occurring and Structurally Modified Podophyllotoxins. Molecules. 2023; 28(1):302. https://doi.org/10.3390/molecules28010302
Chicago/Turabian StyleJin, Lu, Zhijun Song, Fang Cai, Lijun Ruan, and Renwang Jiang. 2023. "Chemistry and Biological Activities of Naturally Occurring and Structurally Modified Podophyllotoxins" Molecules 28, no. 1: 302. https://doi.org/10.3390/molecules28010302
APA StyleJin, L., Song, Z., Cai, F., Ruan, L., & Jiang, R. (2023). Chemistry and Biological Activities of Naturally Occurring and Structurally Modified Podophyllotoxins. Molecules, 28(1), 302. https://doi.org/10.3390/molecules28010302





