Phytochemical Characterization, Antioxidant and Anti-Inflammatory Effects of Cleome arabica L. Fruits Extract against Formalin Induced Chronic Inflammation in Female Wistar Rat: Biochemical, Histological, and In Silico Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic, Flavonoid and Tannins Contents
2.2. HPLC Analysis of Phenolic Compounds
2.3. Antioxidant Activity
2.4. Anti-Inflammatory Effects of Cleome on Edema Size
2.5. Hematological Findings
2.6. C-Reactive Protein (CRP) and Pro-Inflammatory Cytokines Profiling
2.7. Effect of Cleome Fruits Extract on Oxidative Stress Status
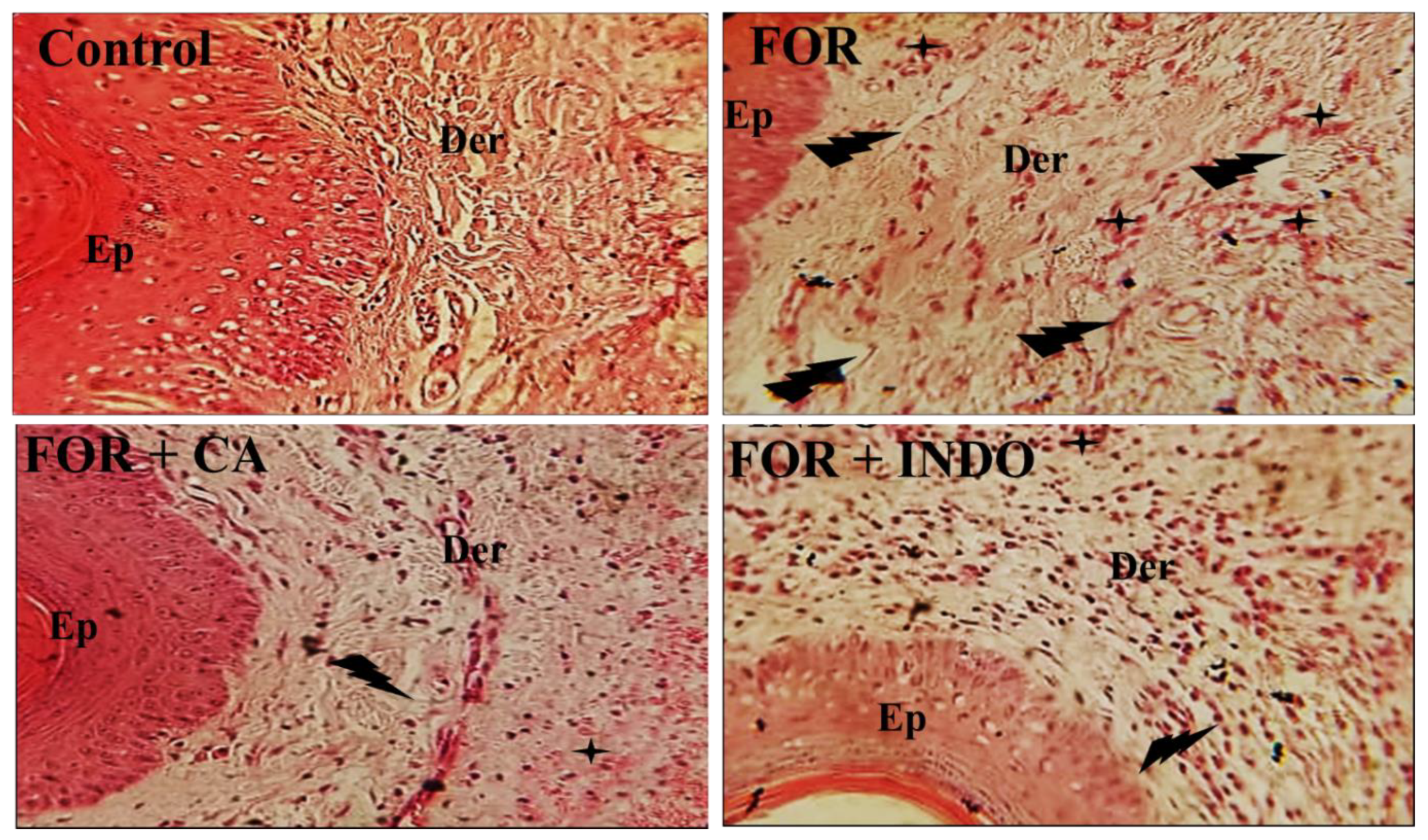
2.8. Effect of Cleome Fruits Extract on Formalin-Induced Histological Alterations
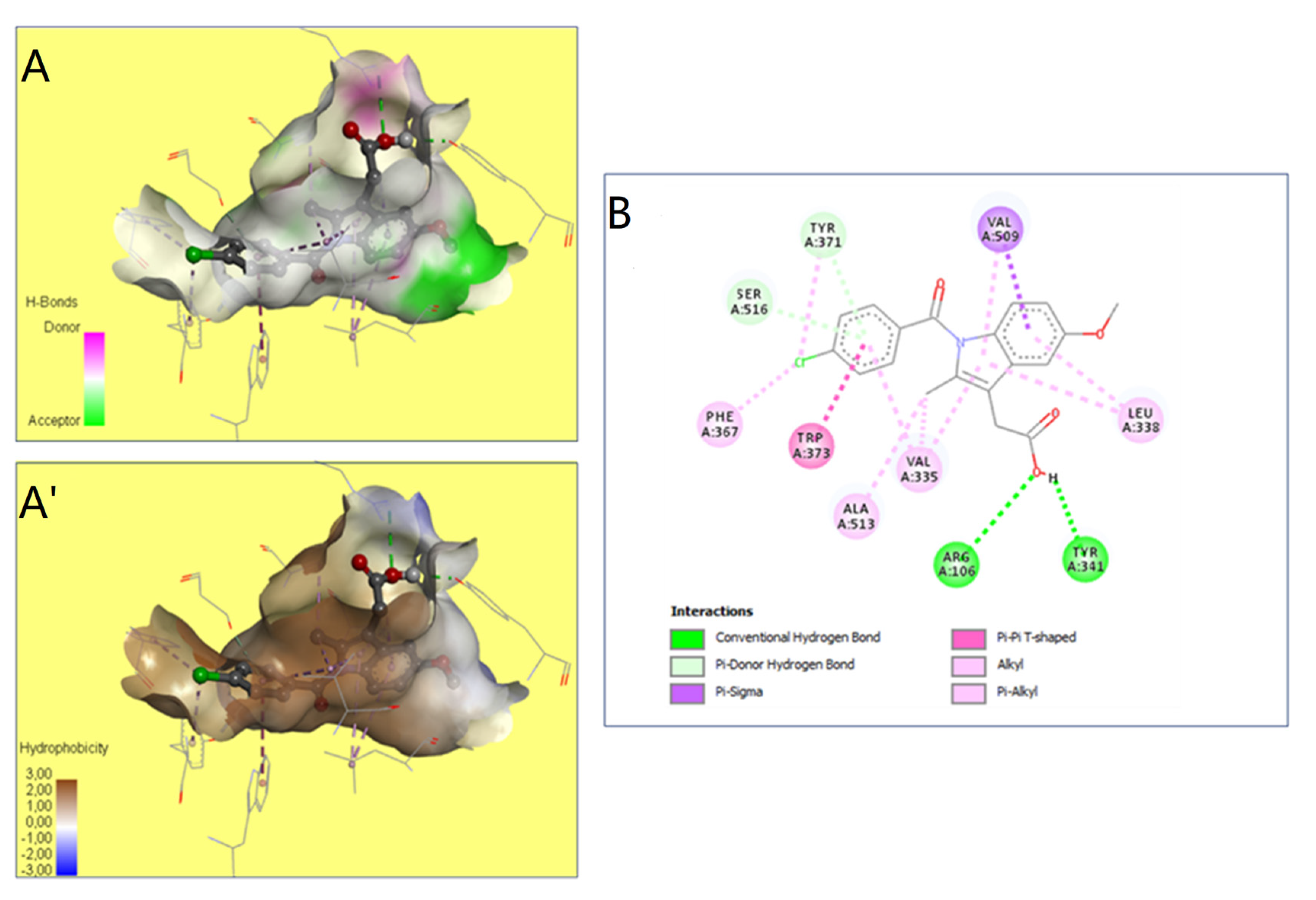
2.9. Molecular Docking Studies
3. Materials and Methods
3.1. Plant Material
3.2. Characterization of the Plant Extract
3.2.1. Preparation of the Plant Extract
3.2.2. Total Phenolic Content (TPC)
3.2.3. Total Flavonoid Content
3.2.4. Determination of Condensed Tannins Content
3.2.5. HPLC Analysis
3.2.6. DPPH• Free Radical Scavenging Activity
3.2.7. Ferric Reducing Antioxidant Power (FRAP)
3.2.8. Nitric Oxide Radical (NO·) Inhibition
3.3. In Vivo Study
3.3.1. Animals and Experiment Design
- -
- Control group: rats injected with physiological serum 0.1 mL/kg of body weight and served as a control group.
- -
- FOR group: rats injected by 0.1 mL of 2% formalin for 7 days.
- -
- FOR+CA group: rats received Cleome fruits extract (50 mg/kg of body weight), by gavage, 30 min before formalin injection.
- -
- FOR+INDO group: rats received indomethacin (10 mg/kg of body weight), by gavage, 30 min before formalin injection.
3.3.2. Sample Collection
3.3.3. Hematological Parameters Determination
3.3.4. Determination of Inflammatory Mediators
3.3.5. Assessment of Oxidative Stress Parameters in Paw Tissue
3.3.6. Histopathological Examination
3.4. Molecular Docking Procedure
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jantan, I.; Haque, M.d.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef] [PubMed]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Fruit Extract of Pithecellobium dulce (FPD) ameliorates carrageenan-induced acute inflammatory responses via regulating pro-inflammatory mediators. J. Food Biochem. 2020, 44, e13329. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2378. [Google Scholar] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mzid, M.; Ben Khedir, S.; Bardaa, S.; Sahnoun, Z.; Rebai, T. Chemical composition, phytochemical constituents, antioxidant and anti-inflammatory activities of Urtica urens L. leaves. Arch. Physiol. Biochem. 2017, 123, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Pavase, L.S.; Mane, D.V.; Baheti, K.G. Anti-inflammatory Exploration of Sulfonamide Containing Diaryl Pyrazoles with Promising COX-2 Selectivity and Enhanced Gastric Safety Profile. J. Heterocycl. Chem. 2018, 55, 913–922. [Google Scholar] [CrossRef]
- Vahedpour, T.; Kaur, J.; Hemmati, S.; Hamzeh-Mivehroud, M.; Alizadeh, A.A.; Wuest, F.; Dastmalchi, S. Synthesis and Biological Evaluation of 1,3,5-Trisubstituted 2-Pyrazolines as Novel Cyclooxygenase-2 Inhibitors with Antiproliferative Activity. Chem. Biodivers. 2021, 18, e2000832. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Labib, M.B.; Ali, W.A.M.; Kamel, G.; Azouz, A.A.; EL-Nahass, E.S. Design, synthesis, analgesic, anti-inflammatory activity of novel pyrazolones possessing aminosulfonyl pharmacophore as inhibitors of COX-2/5-LOX enzymes: Histopathological and docking studies. Bioorg. Chem. 2018, 78, 103–114. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Fadaly, W.A.A. New 1,2-diaryl-4-substituted-benzylidene-5-4H-imidazolone derivatives: Design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. Bioorg. Chem. 2017, 72, 123–129. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.A.; Chen, B.; Jin, Z.; Lin, M.L.; Li, M.; Mei, H.X.; Lu, J.C.; Gong, Y.Q.; Jin, S.W.; et al. Protectin DX promotes the inflammatory resolution via activating COX-2/L-PGDS-PGD2 and DP1 receptor in acute respiratory distress syndrome. Int. Immunopharmacol. 2022, 102, 108348. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts in vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J. Inflamm. Res. 2021, 14, 3729–3738. [Google Scholar] [CrossRef]
- Machado, G.C.; Abdel-Shaheed, C.; Underwood, M.; Day, R.O. Non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain. BMJ 2021, 104, 372. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Dargan, P.; Lanas, A.; Wiffen, P. The burden of musculoskeletal pain and the role of topical non-steroidal anti-inflammatory drugs (NSAIDs) in its treatment. Ten underpinning statements from a global pain faculty. Curr. Med. Res. Opin. 2021, 37, 287–292. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse Effects of Nonsteroidal Antiinflammatory Drugs: An Update of Gastrointestinal, Cardiovascular and Renal Complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Selvi, S.; Polat, R.; Çakılcıoğlu, U.; Celep, F.; Dirmenci, T.; Ertuğ, Z. An ethnobotanical review on medicinal plants of the Lamiaceae family in Turkey. Turk. J. Bot. 2022, 46, 283–332. [Google Scholar] [CrossRef]
- Güler, O.; Polat, R.; Karakose, M.; Cakılcıoğlu, U.; Akbulut, S. An ethnoveterinary study on plants used for the treatment of livestock diseases in the province of Giresun (Turkey). S. Afr. J. Bot. 2021, 142, 53–62. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Płotka-Wasylka, J.; Andruch, V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind. Crops Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Çakılcıoğlu, U. An ethnobotanical field study; Traditional foods production and medicinal utilization of Gundelia L. species in Tunceli (Turkey). Indian J. Tradit. Knowl. 2020, 19, 714–718. [Google Scholar]
- Chand, J.; Panda, S.R.; Jain, S.; Murty, U.; Das, A.M.; Kumar, G.J.; Naidu, V. Phytochemistry and polypharmacology of cleome species: A comprehensive Ethnopharmacological review of the medicinal plants. J. Ethnopharmacol. 2022, 282, 114600. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.-F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, A.; Omezzine, F.; DellaGreca, M.; Zarrelli, A.; Zuppolini, S.; Haouala, R. Phytotoxic activity of Cleome arabica L. and its principal discovered active compounds. S. Afr. J. Bot. 2013, 88, 341–351. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, A.; Mishra, A.K. The chemistry and pharmacology of Cleome genus: A review. Biomed. Pharmacother. 2018, 101, 37–48. [Google Scholar] [CrossRef]
- Bouriche, H.; Selloum, L.; Tigrine, C.; Boudoukha, C. Effect of Cleome arabica Leaf Extract on Rat Paw Edema and Human Neutrophil Migration. Pharm. Biol. 2003, 41, 10–15. [Google Scholar] [CrossRef]
- Tigrine, C.; Bulzomi, P.; Leone, S.; Bouriche, H.; Kameli, A.; Marino, M. Cleome arabica leaf extract has anticancer properties in human cancer cells. Pharm. Biol. 2013, 51, 1508–1514. [Google Scholar] [CrossRef]
- Cercato, L.M.; Araújo, J.M.D.; Oliveira, A.S.; Melo, A.J.O.; Lima, B.S.; dos Santos, E.W.P.; Neto, A.G.D.S.; de Albuquerque-Júnior, R.L.C.; Duarte, M.C.; Araujo, A.A.S.; et al. Reduced cutaneous inflammation associated with antioxidant action after topical application of the aqueous extract of Annona muricata leaves. Inflammopharmacology 2021, 29, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]
- Moridi Farimani, M.; Nazarianpoor, E.; Rusaie, A.; Akhbari, M. Phytochemical constituents and biological activities of Cleome iberica DC. Nat. Prod. Res. 2017, 31, 1329–1332. [Google Scholar] [CrossRef]
- Costa, G.; Francisco, V.C.; Lopes, M.T.; Cruz, M.T.; Batista, M. Intracellular Signaling Pathways Modulated by Phenolic Compounds: Application for New Anti-Inflammatory Drugs Discovery. Curr. Med. Chem. 2012, 19, 2876–2900. [Google Scholar] [CrossRef]
- Fawole, O.A.; Ndhlala, A.R.; Amoo, S.O.; Finnie, J.F.; Van Staden, J. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J. Ethnopharmacol 2009, 123, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants-Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhou, P.; Peng, Z.; Wang, C. Antioxidant and Antibacterial Activities of Dodecyl Tannin Derivative Linked with 1,2,3-Triazole. Chem. Biodivers. 2022, 19, e202100558. [Google Scholar] [CrossRef] [PubMed]
- Samout, N.; Bouzenna, H.; Ettaya, A.; Elfeki, A.; Hfaiedh, N. Antihypercholesterolemic effect of Cleome arabica L. on high cholesterol diet induced damage in rats. Excli J. 2015, 14, 791–800. [Google Scholar]
- Allagui, I.; Hcini, K.; Msalbi, D.; Saoudi, M.; El Feki, A.; Jordan, M.J.; Alwasel, S.; Harrath, A.H.; Allagui, M.S. Phytochemical screening, antioxidant properties, anti-apoptotic effects and molecular docking study of Tunisian cleome (Cleome arabica L.) fruits extract under optimized extraction conditions. Int. J. Food Prop. 2022, 25, 2107–2120. [Google Scholar] [CrossRef]
- Chen, X.; Ru, Y.; Chen, F.; Wang, X.; Zhao, X.; Ao, Q. FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll. 2013, 31, 435–437. [Google Scholar] [CrossRef]
- Erdogan Orhan, I.; Küpeli Akkol, E.; Suntar, I.; Yesilada, E. Assessment of anticholinesterase and antioxidant properties of the extracts and (+)-catechin obtained from Arceuthobium oxycedri (D.C.) M. Bieb (dwarf mistletoe). S. Afr. J. Bot. 2019, 120, 309–312. [Google Scholar] [CrossRef]
- Greenwald, R.A. Animal models for evaluation of arthritis drugs. Methods Find. Exp. Clin. Pharmacol. 1991, 13, 75–83. [Google Scholar]
- Norma, A.; Claudia, S.E.G.; Tatiana, C.D.C.; Carlos, R.M.G.; Marsen, G.P.C.; Roberto, S.D.M.; Elisabeth, M. Anti-inflammatory and antinociceptive activity of field-growth plants and tissue culture of Cleome spinosa (Jacq.) in mice. J. Med. Plants Res. 2013, 7, 1043–1049. [Google Scholar]
- Bouriche, H.; Arnhold, J. Effect of Cleome arabica Leaf Extract Treated by Naringinase on Human Neutrophil Chemotaxis. Nat. Prod. Commun. 2010, 5, 1934578X1000500315. [Google Scholar] [CrossRef]
- de Visser, K.E.; Korets, L.V. Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, V.; Gupta, P.K.; Singh, S. Anti-arthritic activity of Barleria prionitis Linn. leaves in acute and chronic models in Sprague Dawley rats. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 199–209. [Google Scholar] [CrossRef]
- Rajaram, C.; Reddy, K.R.; Sekhar, K.B.C. Evaluation of anti-arthritic activity of Caesalpinia pulcherrima in freund’s complete adjuvant induced arthritic rat model. J. Young-Pharm. 2015, 7, 128–132. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, H.; Shang, C. Farrerol ameliorate adjuvant-induced ankle injury via alteration of PPAR-γ signal pathway. J. Food Biochem. 2021, 45, e13585. [Google Scholar] [CrossRef] [PubMed]
- John, N.A.A.; Shobana, G. Anti-inflammatory activity of Talinum fruticosum L. on formalin induced paw edema in albino rats. J. Appl. Pharm. Sci. 2012, 2, 123–127. [Google Scholar]
- Sato, A.; Nakashima, H.; Nakashima, M.; Ikarashi, M.; Nishiyama, K.; Kinoshita, M.; Seki, S. Involvement of the TNF and FasL Produced by CD11b Kupffer Cells/Macrophages in CCl4-Induced Acute Hepatic Injury. PLoS ONE 2014, 9, e92515. [Google Scholar] [CrossRef]
- Mohammadi, S.; Karimzadeh Bardei, L.; Hojati, V.; Ghorbani, A.; Nabiuni, M. Anti-Inflammatory Effects of Curcumin on Insulin Resistance Index, Levels of Interleukin-6, C-Reactive Protein, and Liver Histology in Polycystic Ovary Syndrome-Induced Rats. Cell J. 2017, 19, 425–433. [Google Scholar]
- Zhou, P.; Li, Q.; Su, S.; Dong, W.; Zong, S.; Ma, Q.; Yang, X.; Zuo, D.; Zheng, S.; Meng, X.; et al. Interleukin 37 Suppresses M1 Macrophage Polarization Through Inhibition of the Notch1 and Nuclear Factor Kappa B Pathways. Front. Cell Dev. Biol. 2020, 8, 56. [Google Scholar] [CrossRef]
- Ceciliani, F.; Giordano, A.; Spagnolo, V. The Systemic Reaction During Inflammation: The Acute-Phase Proteins. Protein Pept. Lett. 2002, 9, 211–223. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Fenkci, V.; Fenkci, S.; Yilmazer, M.; Serteser, M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Steril. 2003, 80, 123–127. [Google Scholar] [CrossRef]
- Deligeoroglou, E.; Vrachnis, N.; Athanasopoulos, N.; Iliodromiti, Z.; Sifakis, S.; Siristatidis, C.; Creatsas, G.; Iliodromiti, S. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol. Endocrinol. 2012, 28, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Q.; Amin, S.; Kawa, I.A.; Jeelani, H.; Manzoor, S.; Rizvi, S.M.; Rashid, F. Evaluation of antioxidant defense markers in relation to hormonal and insulin parameters in women with polycystic ovary syndrome (PCOS): A case-control study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1957–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, H.; Bai, H.; Zhang, Y.; Liu, Q.; Guan, L.; Fan, P. Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin. Endocrinol. 2017, 86, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Drishya, S.; Dhanisha, S.S.; Guruvayoorappan, C. Anti-Inflammatory Potential Exhibited by Amomum subulatum Fruits Mitigates Experimentally Induced Acute and Chronic Inflammation in Mice: Evaluation of Antioxidant Parameters, Pro-Inflammatory Mediators and HO-1 Pathway. J. Am. Coll. Nutr. 2021, 40, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kang, J.S.; Park, J.H.Y.; Lee, Y.J.; Choi, J.S.; Kang, Y.H. Polyphenolic Flavonoids Differ in Their Antiapoptotic Efficacy in Hydrogen Peroxide–Treated Human Vascular Endothelial Cells. J. Nutr. 2003, 133, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free. Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Cin, A.N. Identifying Characteristic Properties and Antioxidant Effect of Quercetin Loaded Titanium Dioxide Nanoparticles. Ph.D. Thesis, Sabancı University, Tuzla, Turkey, 2021. [Google Scholar]
- Salganik, R.I. The Benefits and Hazards of Antioxidants: Controlling Apoptosis and Other Protective Mechanisms in Cancer Patients and the Human Population. J. Am. Coll. Nutr. 2013, 20, 464S–472S. [Google Scholar] [CrossRef]
- Yasmen, N.; Aziz, M.A.; Tajmim, A.; Akter, M.I.; Hazra, A.K.; Rahman, S.M.M. Analgesic and Anti-Inflammatory Activities of Diethyl Ether and n-Hexane Extract of Polyalthia suberosa Leaves. Evid.-Based Complement. Altern. Med. 2018, 2018, e5617234. [Google Scholar] [CrossRef]
- Eltom, S.; Abdellatif, A.; Maswadeh, H.; Al-Omar, M.; Abdel-Hafez, A.; Mohammed, H.; Agabein, E.; Alqasoomi, I.; Alrashidi, S.; Sajid, M.; et al. The Anti-Inflammatory Effect of a γ-Lactone Isolated from Ostrich Oil of Struthio camelus (Ratite) and Its Formulated Nano-Emulsion in Formalin-Induced Paw Edema. Molecules 2021, 26, 3701. [Google Scholar] [CrossRef]
- Kim, H.-D.; Cho, K.-H.; Lee, B.-W.; Kwon, Y.-S.; Lee, H.-S.; Choi, S.-H.; Ku, S.-K. Effects of Magnetic Infrared Laser Irradiation on Formalin-Induced Chronic Paw Inflammation of Mice. J. Phys. Ther. Sci. 2010, 22, 395–404. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.S.; Waheed, A.; Farman, M.; Qayyum, A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J. Ethnopharmacol. 2010, 129, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Malik, A.; Arooj, M.; Butt, T.T.; Zahid, S.; Zahid, F.; Jafar, T.H.; Waquar, S.; Gan, S.H.; Ahmad, S.; Mirza, M.U. In silico and in vivo characterization of cabralealactone, solasodin and salvadorin in a rat model: Potential anti-inflammatory agents. Drug Des. Devel. Ther. 2018, 12, 1431–1443. [Google Scholar] [CrossRef]
- Meena, A.; Yadav, D.K.; Srivastava, A.; Khan, F.; Chanda, D.; Chattopadhyay, S.K. In Silico Exploration of Anti-Inflammatory Activity of Natural Coumarinolignoids. Chem. Biol. Drug Des. 2011, 78, 567–579. [Google Scholar] [CrossRef]
- Zaki, A.A.; Al-Karmalawy, A.A.; El-Amier, Y.A.; Ashour, A. Molecular docking reveals the potential of Cleome amblyocarpa isolated compounds to inhibit COVID-19 virus main protease. New J. Chem. 2020, 44, 16752–16758. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Han, Y. Cyclooxygenase-2 and β-Catenin as Potential Diagnostic and Prognostic Markers in Endometrial Cancer. Front. Oncol. 2020, 10, 56. [Google Scholar] [CrossRef]
- Dowlati Beirami, A.; Hajimahdi, Z.; Zarghi, A. Docking-based 3D-QSAR (CoMFA, CoMFA-RG, CoMSIA) study on hydroquinoline and thiazinan-4-one derivatives as selective COX-2 inhibitors. J. Biomol. Struct. Dyn. 2019, 37, 2999–3006. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- John, B.C.T.S.; George, S.; Reddy, V.R.K. Total phenolics and flavonoids in selected medicinal plants from Kerala. International J. Pharm. Pharm. Sci. 2014, 6, 406–408. [Google Scholar]
- Saad, H.; Charrier-El Bouhtoury, F.; Pizzi, A.; Rode, K.; Charrier, B.; Ayed, N. Characterization of pomegranate peels tannin extractives. Ind. Crops Prod. 2012, 40, 239–246. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Seddighfar, M.; Mirghazanfari, S.M.; Dadpay, M. Analgesic and anti-inflammatory properties of hydroalcoholic extracts of Malva sylvestris, Carum carvi or Medicago sativa, and their combination in a rat model. J. Integr. Med. 2020, 18, 181–188. [Google Scholar] [CrossRef]
- Al-Hejjaj, W.K.G.; Numan, I.T.; Al-Sa’ad, R.Z.; Hussain, S.A. Anti-inflammatory activity of telmisartan in rat models of experimentally-induced chronic inflammation: Comparative study with dexamethasone. Saudi Pharm. J. 2011, 19, 29–34. [Google Scholar] [CrossRef]
- Morse, L.R.; Stolzmann, K.; Nguyen, H.P.; Jain, N.B.; Zayac, C.; Gagnon, D.R.; Tun, C.G.; Garshick, E. Association Between Mobility Mode and C-Reactive Protein Levels in Men With Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2008, 89, 726–731. [Google Scholar] [CrossRef]
- Oh, L. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free. Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 114–120. [Google Scholar]
- Jollow, D.J.; Thorgeirsson, S.S.; Potter, W.Z.; Hashimoto, M.; Mitchell, J.R. Acetaminophen-Induced Hepatic Necrosis. Pharmacology 1974, 12, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J.; et al. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorg. Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef]




| Extract | Total Phenolic Content (mg GAE/g DW) | Total Flavonoids Content (mg QE/g DW) | Total Tannins Content (mg CatE/g DW) |
|---|---|---|---|
| Aqueous | 230.22 ± 7.08 | 55.08 ± 1.28 | 15.17 ± 0.47 |
| Phenolic Compounds | RT (min) | Quantification (mg/g DW) | Chemical Structure |
|---|---|---|---|
| Quercetin | 5.98 | 3.45 | 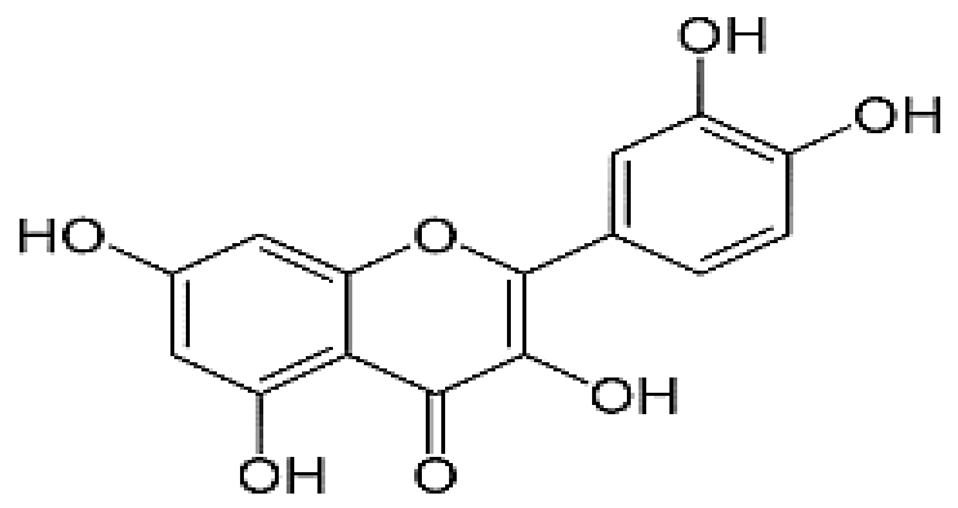 |
| Cathechin | 13.78 | 5.42 | 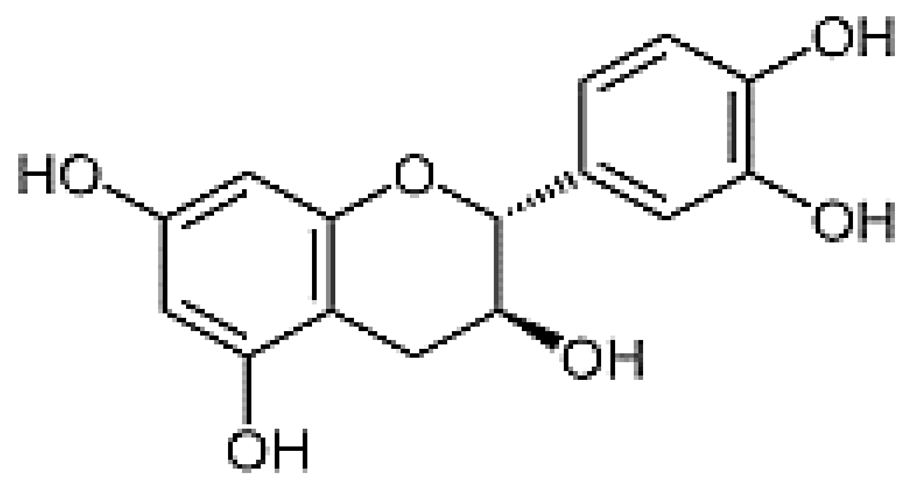 |
| Kaempferol | 15.21 | 3.23 | 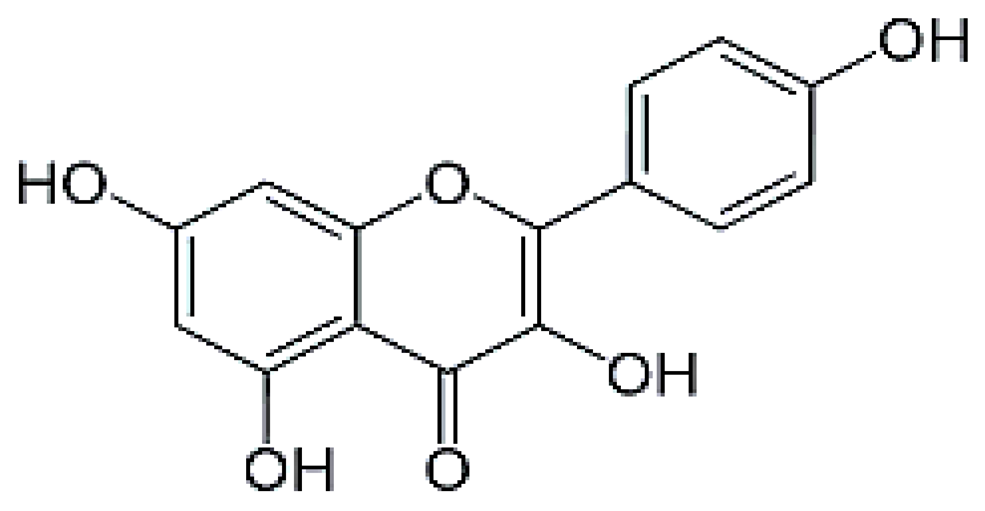 |
| Rosmarinic acid | 21.78 | 0.12 |  |
| Naringenin | 24.45 | 2.84 |  |
| Extract | DPPH IC50 (mg/mL) | FRAP IC50 (mg/mL) | NO· IC50 (mg/mL) |
|---|---|---|---|
| Aqueous | 3.346 ± 0.12 | 2.306 ± 0.14 | 0.023 ± 0.001 |
| Vit C | 0.032 ± 0.0005 | 0.154 ± 0.002 | 0.006 ± 0.001 |
| Groups | Mean Paw Thickness (mm) at Zero Time | Mean Paw Thickness (mm) after 7 Days | Mean Increase in Paw Thickness (mm) after 7 Days | Inflammation (%) | Inhibition of Inflammation (%) |
|---|---|---|---|---|---|
| Control | 3.43 ± 0.07 | 3.5 ± 0.03 | 0.17 ± 0.01 | 2.04 NS | - |
| FOR | 3.79 ± 0.28 | 9.77 ± 0.36 *** | 5.98 ± 0.26 *** | 157.78 *** | - |
| FOR+CA | 3.67 ± 0.23 | 6.36 ± 0.43 *** | 2.69 ± 0.27 ** | 73.29 *** | 55.01 |
| FOR+INDO | 3.32 ± 0.11 | 6.6 ± 0.12 *** | 3.28 ± 0.09 ** | 98.79 *** | 45.15 |
| Groups | Control | FOR | FOR+CA | FOR+INDO |
|---|---|---|---|---|
| MDA (nmol MDA/g of protein) | 11.5 ± 0.63 | 14.61 ± 0.22 ** | 9.76 ± 0.18 @@@ | 11.56 ± 0.07 |
| AOPP (µmol/mg of protein) | 1.2 ± 0.03 | 1.44 ± 0.02 *** | 1.01 ± 0.007 **@@@ | 0.97 ± 0.007 *** |
| SOD (U/mg of protein) | 1.9 ± 0.09 | 1.16 ± 0.006 ** | 1.74 ± 1.74 @@ | 1.49 ± 0.003 * |
| GPx (µmol of GSH/mg of protein/min) | 3.29 ± 0.13 | 0.75 ± 0.04 *** | 3.11 ± 0.19 @@@ | 2.95 ± 0.2 |
| GSH (µmol GSH/mg of protein) | 1.19 ± 0.03 | 0.52 ± 0.002 *** | 1.02 ± 0.002 ***@@@ | 1.05 ± 0.008 *** |
| Ligand | Binding Affinity (kcal/mol) | Intermolecular Interactions | |
|---|---|---|---|
| Conventional Hydrogen Bonds | Interacting Amino Acid Residues | ||
| Quercetin (C) | −6.5 | 3 | Val102, Arg106 *, Val335, Leu338, Tyr341 *, Leu345, Tyr371 *, Ala513, Leu517 |
| Catechin (C) | −9.8 | 6 | His75 *, Gln178 *, Val335 *, Leu338 *, Ser339 *, Trp373, Val509, Ser516 * |
| Kaempferol (C) | −9.4 | 2 | Val335, Leu338, Tyr341 *, Tyr371 *, Val509, Ala513, |
| Rosmarinicacid (C) | −8.0 | 5 | Arg106 *, Leu338 *, Tyr341 *, Phe504, Met508 *, Val509, Gly512, Ser516 * |
| Naringenin (C) | −7.5 | 2 | Val335, Leu338, Tyr341, Tyr371 *, Val509, Gly512, Ala513, Ser516 * |
| Indomethacin (R) | −5.4 | 2 | Arg106 *, Val335, Leu338, Tyr341 *, Phe367, Tyr371, Trp373, Val509, Ala513, Ser516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allagui, I.; Horchani, M.; Zammel, N.; Jalouli, M.; Elfeki, A.; Kallel, C.; Mansour, L.; Alwasel, S.; Harrath, A.H.; Jannet, H.B.; et al. Phytochemical Characterization, Antioxidant and Anti-Inflammatory Effects of Cleome arabica L. Fruits Extract against Formalin Induced Chronic Inflammation in Female Wistar Rat: Biochemical, Histological, and In Silico Studies. Molecules 2023, 28, 26. https://doi.org/10.3390/molecules28010026
Allagui I, Horchani M, Zammel N, Jalouli M, Elfeki A, Kallel C, Mansour L, Alwasel S, Harrath AH, Jannet HB, et al. Phytochemical Characterization, Antioxidant and Anti-Inflammatory Effects of Cleome arabica L. Fruits Extract against Formalin Induced Chronic Inflammation in Female Wistar Rat: Biochemical, Histological, and In Silico Studies. Molecules. 2023; 28(1):26. https://doi.org/10.3390/molecules28010026
Chicago/Turabian StyleAllagui, Ikram, Mabrouk Horchani, Nourhene Zammel, Maroua Jalouli, Abdelfatteh Elfeki, Choumous Kallel, Lamjed Mansour, Salah Alwasel, Abdel Halim Harrath, Hichem Ben Jannet, and et al. 2023. "Phytochemical Characterization, Antioxidant and Anti-Inflammatory Effects of Cleome arabica L. Fruits Extract against Formalin Induced Chronic Inflammation in Female Wistar Rat: Biochemical, Histological, and In Silico Studies" Molecules 28, no. 1: 26. https://doi.org/10.3390/molecules28010026
APA StyleAllagui, I., Horchani, M., Zammel, N., Jalouli, M., Elfeki, A., Kallel, C., Mansour, L., Alwasel, S., Harrath, A. H., Jannet, H. B., Salah Allagui, M., & Hcini, K. (2023). Phytochemical Characterization, Antioxidant and Anti-Inflammatory Effects of Cleome arabica L. Fruits Extract against Formalin Induced Chronic Inflammation in Female Wistar Rat: Biochemical, Histological, and In Silico Studies. Molecules, 28(1), 26. https://doi.org/10.3390/molecules28010026








