Insights on Ferroptosis and Colorectal Cancer: Progress and Updates
Abstract
1. Introduction
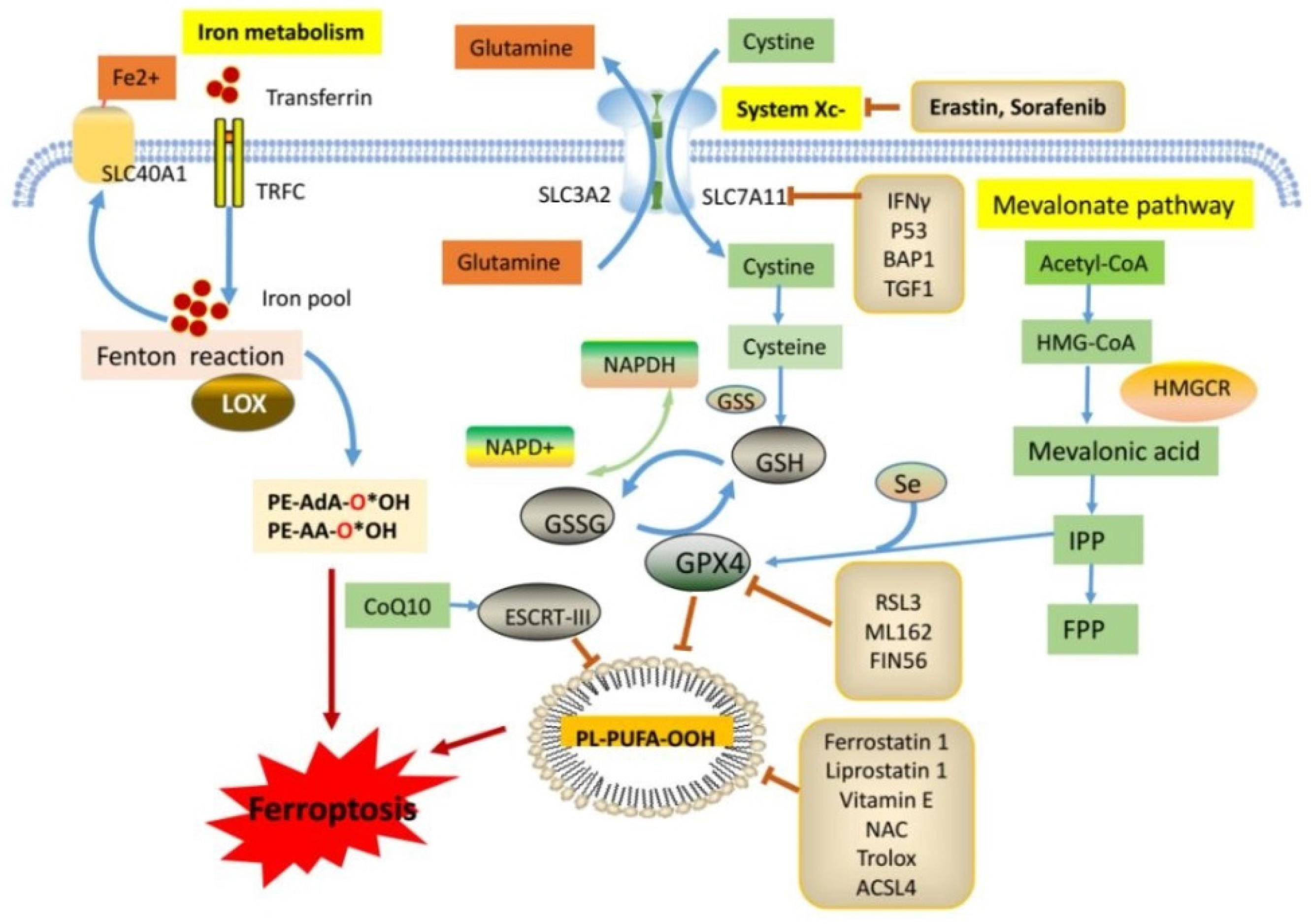
2. Summary of the Regulatory Pathways Associated with Ferroptosis
3. Molecules That Mediate Ferroptosis in CRC
3.1. Ferroptosis-Inhibiting Genes
3.2. Ferroptosis-Promoting Genes
3.3. Non-Coding RNAs Mediate Ferroptosis
3.4. KRAS Mutation and Ferroptosis in CRC
4. Agents That Induce or Inhibit Ferroptosis
4.1. Plant-Derived Small-Molecule Compounds
4.2. Other Small-Molecule Compounds
| Agents | Target | Reference |
|---|---|---|
| Plant-derived small-molecule compounds | ||
| Ginsenoside Rh4 | ROS generation | [58] |
| β-Elemonic acid (EA) | transferrin, ferroxidase, ACSL4 | [60] |
| Tetrahydrobiopterin (BH4) | NCOA4, GPX4 | [61] |
| Auriculasin | ROS generation | [62] |
| punicic acid (PunA) | MDA, lipid peroxidation | [63] |
| Tagitinin C | PERK-Nrf2-HO-1 pathway | [64] |
| Andrographis | HMOX1, GCLC, GCLM, TCF7L2 | [65] |
| Bromelain | ACSL4 | [57] |
| Betulaceae Extract | HO-1 | [66] |
| Avicequinone B | JAK-STAT, MAPK, PI3K-AKT pathway | [67] |
| Other small-molecule compounds | ||
| Propofol | GPX4 | [69] |
| Apatinib | ELOVL6/ACSL4 | [70] |
| Talaroconvolutin A | SLC7A11, ALOXE3 | [71] |
| Dichloroacetate | Iron levels | [72] |
| BSO | GSH | [73] |
| High-fat diet | CHAC1 | [74] |
4.3. Molecules That Mediate Drug Resistance in CRC by Suppressing Ferroptosis
4.4. Nanomaterials That Inhibit CRC by Inducing Ferroptosis
4.5. Gene Signatures or Clusters That Could Predict the Prognosis of CRC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ortiz, A.P.; Pinheiro, P.S.; Bandi, P.; Minihan, A.; Fuchs, H.E.; Martinez Tyson, D.; Tortolero-Luna, G.; Fedewa, S.A.; Jemal, A.M.; et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J. Clin. 2021, 71, 466–487. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Li, J.; Liu, L.J.; Zheng, N.X.; Zheng, K.; Mei, Z.; Bai, C.G.; Zhang, W. Trends, clinicopathological features, surgical treatment patterns and prognoses of early-onset versus late-onset colorectal cancer: A retrospective cohort study on 34067 patients managed from 2000 to 2021 in a Chinese tertiary center. Int. J. Surg. 2022, 104, 106780. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Li, Z.; Si, W.; Jin, W.; Yuan, Z.; Chen, Y.; Fu, L. Targeting autophagy in colorectal cancer: An update on pharmacological small-molecule compounds. Drug Discov. Today 2022, 27, 2373–2385. [Google Scholar] [CrossRef]
- Privitera, G.; Rana, N.; Scaldaferri, F.; Armuzzi, A.; Pizarro, T.T. Novel Insights into the Interactions Between the Gut Microbiome, Inflammasomes, and Gasdermins During Colorectal Cancer. Front. Cell Infect. Microbiol. 2021, 11, 806680. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L. The recent progress of the mechanism and regulation of tumor necrosis in colorectal cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 453–463. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, S.M.; Alyammahi, S.K.; Alhamad, D.W.; Abdin, S.M.; Omar, H.A. Ferroptosis: An emerging approach for targeting cancer stem cells and drug resistance. Crit. Rev. Oncol. Hematol. 2020, 155, 103095. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, E.; Bakar-Ates, F. Ferroptosis: A Trusted Ally in Combating Drug Resistance in Cancer. Curr. Med. Chem. 2022, 29, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Shin, D.; Lee, J.; Jung, A.R.; Roh, J.L. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer. Lett. 2018, 432, 180–190. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wu, X.; Xu, F.; Ma, H.; Wu, M.; Xia, Y. Targeting Ferroptosis Pathway to Combat Therapy Resistance and Metastasis of Cancer. Front. Pharmacol. 2022, 13, 909821. [Google Scholar] [CrossRef]
- Shibata, Y.; Yasui, H.; Higashikawa, K.; Miyamoto, N.; Kuge, Y. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS ONE 2019, 14, e0225931. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Yang, F.; Sun, S.Y.; Wang, S.; Guo, J.T.; Liu, X.; Ge, N.; Wang, G.X. Molecular regulatory mechanism of ferroptosis and its role in gastrointestinal oncology: Progress and updates. World J. Gastrointest. Oncol. 2022, 14, 1–18. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, W.; Wang, Y.; Leng, Y.; Xia, Z. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov. 2021, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, J.; Yuan, S.; Zhuang, X.; Qiao, T. Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Radiation-Induced Lung Injury. Oxid. Med. Cell Longev. 2022, 2022, 8973509. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Zhao, Y.; Meng, X.; Ding, W.; Wang, Q.; Liu, C.; Deng, H. Flavonoid 4,4’-dimethoxychalcone induced ferroptosis in cancer cells by synergistically activating Keap1/Nrf2/HMOX1 pathway and inhibiting FECH. Free Radic. Biol. Med. 2022, 188, 14–23. [Google Scholar] [CrossRef]
- Song, J.; Liu, T.; Yin, Y.; Zhao, W.; Lin, Z.; Yin, Y.; Lu, D.; You, F. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021, 22, e51162. [Google Scholar] [CrossRef]
- Meng, H.; Wu, J.; Shen, L.; Chen, G.; Jin, L.; Yan, M.; Wan, H.; He, Y. Microwave assisted extraction, characterization of a polysaccharide from Salvia miltiorrhiza Bunge and its antioxidant effects via ferroptosis-mediated activation of the Nrf2/HO-1 pathway. Int. J. Biol. Macromol. 2022, 215, 398–412. [Google Scholar] [CrossRef]
- Shan, Y.; Li, J.; Zhu, A.; Kong, W.; Ying, R.; Zhu, W. Ginsenoside Rg3 ameliorates acute pancreatitis by activating the NRF2/HO1mediated ferroptosis pathway. Int. J. Mol. Med. 2022, 50, 89. [Google Scholar] [CrossRef]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef]
- Song, X.; Zhu, S.; Chen, P.; Hou, W.; Wen, Q.; Liu, J.; Xie, Y.; Liu, J.; Klionsky, D.J.; Kroemer, G.; et al. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc(-) Activity. Curr. Biol. 2018, 28, 2388–2399.e5. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ban, F.; Peng, S.; Xu, D.; Li, H.; Mo, H.; Hu, L.; Zhou, X. Exogenous Iron Induces NADPH Oxidases-Dependent Ferroptosis in the Conidia of Aspergillus flavus. J. Agric. Food Chem. 2021, 69, 13608–13617. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fan, Y.Q.; Liu, B.H.; Zhou, H.; Wang, J.M.; Chen, Q.X. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol. Rep. 2020, 43, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Meng, L.; Kang, R.; Wang, X.; Tang, D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Y.; Li, Q.; Ling, Y.; Zhou, Y.; Chu, K.; Xue, L.; Tao, S. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death Dis. 2022, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.; Ichu, T.A.; Milosevich, N.; Melillo, B.; Schafroth, M.A.; Otsuka, Y.; Scampavia, L.; Spicer, T.P.; Cravatt, B.F. LPCAT3 Inhibitors Remodel the Polyunsaturated Phospholipid Content of Human Cells and Protect from Ferroptosis. ACS Chem. Biol. 2022, 17, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Tyurin, V.A.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Anthonymuthu, T.S.; Zhai, Y.J.; Pan, M.H.; Gong, H.B.; Lu, D.H.; Sun, J.; et al. Phospholipase iPLA2beta averts ferroptosis by eliminating a redox lipid death signal. Nat. Chem. Biol. 2021, 17, 465–476. [Google Scholar] [CrossRef]
- Wang, R.; Su, Q.; Yin, H.; Wu, D.; Lv, C.; Yan, Z. Inhibition of SRSF9 enhances the sensitivity of colorectal cancer to erastin-induced ferroptosis by reducing glutathione peroxidase 4 expression. Int. J. Biochem. Cell Biol. 2021, 134, 105948. [Google Scholar] [CrossRef]
- Wang, R.; Hua, L.; Ma, P.; Song, Y.; Min, J.; Guo, Y.; Yang, C.; Li, J.; Su, H. HSPA5 repressed ferroptosis to promote colorectal cancer development by maintaining GPX4 stability. Neoplasma 2022, 69, 1054–1069. [Google Scholar] [CrossRef]
- Liu, M.Y.; Li, H.M.; Wang, X.Y.; Xia, R.; Li, X.; Ma, Y.J.; Wang, M.; Zhang, H.S. TIGAR drives colorectal cancer ferroptosis resistance through ROS/AMPK/SCD1 pathway. Free Radic. Biol. Med. 2022, 182, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Wei, C.; Zheng, D.; Lu, X.; Yang, Y.; Luo, A.; Zhang, K.; Duan, X.; Wang, Y. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur. J. Pharm. Sci. 2020, 152, 105450. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, W.; Liu, F.; Wang, Q.; Song, M.; Yu, Q.; Tang, K.; Teng, T.; Wu, D.; Wang, X.; et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid. Med. Cell Longev. 2020, 2020, 1675613. [Google Scholar] [CrossRef]
- Lu, D.; Yang, Z.; Xia, Q.; Gao, S.; Sun, S.; Luo, X.; Li, Z.; Zhang, X.; Li, X. ACADSB regulates ferroptosis and affects the migration, invasion, and proliferation of colorectal cancer cells. Cell Biol. Int. 2020, 44, 2334–2343. [Google Scholar] [CrossRef]
- Singhal, R.; Mitta, S.R.; Das, N.K.; Kerk, S.A.; Sajjakulnukit, P.; Solanki, S.; Andren, A.; Kumar, R.; Olive, K.P.; Banerjee, R.; et al. HIF-2alpha activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J. Clin. Investig. 2021, 131, e143691. [Google Scholar] [CrossRef]
- Fan, H.; Ai, R.; Mu, S.; Niu, X.; Guo, Z.; Liu, L. MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2. Bioengineered 2022, 13, 12021–12029. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, L.; Song, Q.; Shan, Y.; Yin, G.; Zhu, H.; Kong, W.; Zhou, C. miR-545 promotes colorectal cancer by inhibiting transferring in the non-normal ferroptosis signaling. Aging 2021, 13, 26137–26147. [Google Scholar] [CrossRef]
- Bak, R.O.; Mikkelsen, J.G. miRNA sponges: Soaking up miRNAs for regulation of gene expression. Wiley Interdiscip. Rev. RNA 2014, 5, 317–333. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, S.; Wei, J.; Zhou, H.; Wang, W.; Yang, J.; Deng, Q.; Wang, H.; Fu, Z. Long noncoding RNA LINC01606 protects colon cancer cells from ferroptotic cell death and promotes stemness by SCD1-Wnt/beta-catenin-TFE3 feedback loop signalling. Clin. Transl. Med. 2022, 12, e752. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wei, X. Circ_0007142 downregulates miR-874-3p-mediated GDPD5 on colorectal cancer cells. Eur. J. Clin. Investig. 2021, 51, e13541. [Google Scholar] [CrossRef]
- Xian, Z.Y.; Hu, B.; Wang, T.; Cai, J.L.; Zeng, J.Y.; Zou, Q.; Zhu, P.X. CircABCB10 silencing inhibits the cell ferroptosis and apoptosis by regulating the miR-326/CCL5 axis in rectal cancer. Neoplasma 2020, 67, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Bartolacci, C.; Andreani, C.; Vale, G.; Berto, S.; Melegari, M.; Crouch, A.C.; Baluya, D.L.; Kemble, G.; Hodges, K.; Starrett, J.; et al. Author Correction: Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat. Commun. 2022, 13, 4640. [Google Scholar] [CrossRef] [PubMed]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Yang, J.; Mo, J.; Dai, J.; Ye, C.; Cen, W.; Zheng, X.; Jiang, L.; Ye, L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, J.; Kim, M.; Jin, E.J. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim. Cells. Syst. 2018, 22, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.I.; Kim, D.S.; Lee, Y.H.; Park, J.D.; Lee, C.B.; Kim, S.I. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta. Med. 1996, 62, 86–87. [Google Scholar] [CrossRef]
- Wu, Y.; Pi, D.; Chen, Y.; Zuo, Q.; Zhou, S.; Ouyang, M. Ginsenoside Rh4 Inhibits Colorectal Cancer Cell Proliferation by Inducing Ferroptosis via Autophagy Activation. Evid. Based Complement. Alternat. Med. 2022, 2022, 6177553. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, J.; Li, C.; Hao, X.; Fan, H.; Zhao, Y.; Tang, J.; Wan, X.; Lian, S.; Yang, J. TMT-Based Quantitative Proteomics Analysis Reveals the Panoramic Pharmacological Molecular Mechanism of beta-Elemonic Acid Inhibition of Colorectal Cancer. Front. Pharmacol. 2022, 13, 830328. [Google Scholar] [CrossRef]
- Hu, Q.; Wei, W.; Wu, D.; Huang, F.; Li, M.; Li, W.; Yin, J.; Peng, Y.; Lu, Y.; Zhao, Q.; et al. Blockade of GCH1/BH4 Axis Activates Ferritinophagy to Mitigate the Resistance of Colorectal Cancer to Erastin-Induced Ferroptosis. Front. Cell Dev. Biol. 2022, 10, 810327. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Chen, L.H.; Zhuang, H.B.; Shi, Z.S.; Chen, Z.C.; Pan, J.P.; Hong, Z.S. Auriculasin enhances ROS generation to regulate colorectal cancer cell apoptosis, ferroptosis, oxeiptosis, invasion and colony formation. Biochem. Biophys. Res. Commun. 2022, 587, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, P.; Vancoppenolle, M.; Dierge, E.; Mignolet, E.; Cuvelier, G.; Knoops, B.; Page, M.; Debier, C.; Feron, O.; Larondelle, Y. Punicic Acid Triggers Ferroptotic Cell Death in Carcinoma Cells. Nutrients 2021, 13, 2751. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Zhao, Y.; Wang, J.; Yang, X.; Li, S.; Wang, Y.; Yang, X.; Fei, J.; Hao, X.; Zhao, Y.; et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021, 17, 2703–2717. [Google Scholar] [CrossRef]
- Shimura, T.; Sharma, P.; Sharma, G.G.; Banwait, J.K.; Goel, A. Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci. Rep. 2021, 11, 7548. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef]
- Ocampo, Y.; Caro, D.; Rivera, D.; Piermattey, J.; Gaitan, R.; Franco, L.A. Transcriptome Changes in Colorectal Cancer Cells upon Treatment with Avicequinone B. Adv. Pharm. Bull. 2020, 10, 638–647. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S.; Jiang, W.; Xue, F.; Zhu, X. Effects of propofol on the development of cancer in humans. Cell Prolif. 2020, 53, e12867. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F. Propofol induces the ferroptosis of colorectal cancer cells by downregulating STAT3 expression. Oncol. Lett. 2021, 22, 767. [Google Scholar] [CrossRef]
- Tian, X.; Li, S.; Ge, G. Apatinib Promotes Ferroptosis in Colorectal Cancer Cells by Targeting ELOVL6/ACSL4 Signaling. Cancer Manag. Res. 2021, 13, 1333–1342. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, S.; Li, C.; Ai, Z.; Shen, W.; Ren, W.; Yang, X. Discovery of a novel ferroptosis inducer-talaroconvolutin A-killing colorectal cancer cells in vitro and in vivo. Cell Death Dis. 2020, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cheng, X.; Pan, S.; Wang, L.; Dou, W.; Liu, J.; Shi, X. Dichloroacetate attenuates the stemness of colorectal cancer cells via trigerring ferroptosis through sequestering iron in lysosomes. Environ. Toxicol. 2021, 36, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Araki, H.; Ishikawa, Y.; Kitazawa, S.; Hata, A.; Soga, T.; Hara, T. Low tumor glutathione level as a sensitivity marker for glutamate-cysteine ligase inhibitors. Oncol. Lett. 2018, 15, 8735–8743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Ma, Y.; Zhao, X.; He, L.; Sun, P.; Wang, H. High-fat diet aggravates colitis-associated carcinogenesis by evading ferroptosis in the ER stress-mediated pathway. Free Radic. Biol. Med. 2021, 177, 156–166. [Google Scholar] [CrossRef]
- Neugut, A.I.; Lin, A.; Raab, G.T.; Hillyer, G.C.; Keller, D.; O’Neil, D.S.; Accordino, M.K.; Kiran, R.P.; Wright, J.; Hershman, D.L. FOLFOX and FOLFIRI Use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin. Colorectal Cancer 2019, 18, 133–140. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef]
- Sharma, P.; Shimura, T.; Banwait, J.K.; Goel, A. Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of beta-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis 2020, 41, 1385–1394. [Google Scholar] [CrossRef]
- Lorenzato, A.; Magri, A.; Matafora, V.; Audrito, V.; Arcella, P.; Lazzari, L.; Montone, M.; Lamba, S.; Deaglio, S.; Siena, S.; et al. Vitamin C Restricts the Emergence of Acquired Resistance to EGFR-Targeted Therapies in Colorectal Cancer. Cancers 2020, 12, 685. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Ma, J.; Yang, L.; Song, Q.; Wang, H.; Lv, G. Glutathione Peroxidase 4 as a Therapeutic Target for Anti-Colorectal Cancer Drug-Tolerant Persister Cells. Front. Oncol. 2022, 12, 913669. [Google Scholar] [CrossRef]
- He, Z.; Yang, J.; Sui, C.; Zhang, P.; Wang, T.; Mou, T.; Sun, K.; Wang, Y.; Xu, Z.; Li, G.; et al. FAM98A promotes resistance to 5-fluorouracil in colorectal cancer by suppressing ferroptosis. Arch. Biochem. Biophys. 2022, 722, 109216. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Hu, P.S.; Wang, Y.Y.; Tan, Y.T.; Yu, K.; Liao, K.; Wu, Q.N.; Li, T.; Meng, Q.; Lin, J.Z.; et al. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal. Transduct. Target. Ther. 2022, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Choudhary, B.S.; Shah, S.G.; Khapare, N.; Dwivedi, N.; Gaikwad, A.; Joshi, N.; Raichanna, J.; Basu, S.; Gurjar, M.; et al. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int. J. Cancer 2021, 149, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Y.; Lin, S.; Liu, Y.; Li, W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging 2021, 13, 13515–13534. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Y.; Luo, J.; Chen, S.; Xie, J.; Liu, H.; Wang, Y.; Li, Z. Integrated Analysis of a Ferroptosis-Related LncRNA Signature for Evaluating the Prognosis of Patients with Colorectal Cancer. Genes 2022, 13, 1094. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Beretta, G.L. Nanoparticles for Ferroptosis Therapy in Cancer. Pharmaceutics 2021, 13, 1785. [Google Scholar] [CrossRef]
- Li, Q.; Su, R.; Bao, X.; Cao, K.; Du, Y.; Wang, N.; Wang, J.; Xing, F.; Yan, F.; Huang, K.; et al. Glycyrrhetinic acid nanoparticles combined with ferrotherapy for improved cancer immunotherapy. Acta Biomater. 2022, 144, 109–120. [Google Scholar] [CrossRef]
- Pan, X.; Qi, Y.; Du, Z.; He, J.; Yao, S.; Lu, W.; Ding, K.; Zhou, M. Zinc oxide nanosphere for hydrogen sulfide scavenging and ferroptosis of colorectal cancer. J. Nanobiotechnol. 2021, 19, 392. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Qi, Y.; Wang, S.; Li, L.; Li, W.; Xie, T.; Zhu, H.; Tang, Z.; Zhou, M. H2 S-Scavenged and Activated Iron Oxide-Hydroxide Nanospindles for MRI-Guided Photothermal Therapy and Ferroptosis in Colon Cancer. Small 2020, 16, e2001356. [Google Scholar] [CrossRef]
- Zhou, N.; Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database 2020, 2020, baaa021. [Google Scholar] [CrossRef]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H. Prognostic and Predictive Models for Left- and Right- Colorectal Cancer Patients: A Bioinformatics Analysis Based on Ferroptosis-Related Genes. Front. Oncol. 2022, 12, 833834. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, Y.; Liu, L.; Wu, Y.; Fu, P.; Cao, Y.; Xiong, J.; Tu, Y.; Li, Z.; Liu, Y.; et al. Comprehensive Analysis of Immune Infiltrates of Ferroptosis-Related Long Noncoding RNA and Prediction of Colon Cancer Patient Prognoses. J. Immunol. Res. 2022, 2022, 9480628. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shen, J.; Qiao, X.; Gao, Y.; Su, H.; Zhang, S. Long Non-Coding RNA Signatures Associated with Ferroptosis Predict Prognosis in Colorectal Cancer. Int. J. Gen. Med. 2022, 15, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, X.; Fang, X.; Xin, Z. Construction on of a Ferroptosis-Related lncRNA-Based Model to Improve the Prognostic Evaluation of Gastric Cancer Patients Based on Bioinformatics. Front. Genet. 2021, 12, 739470. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, Z.; Li, L.; Ma, M.; Long, F.; Wu, R.; Huang, L.; Chou, J.; Yang, K.; Zhang, Y.; et al. Identification and Validation of Ferroptosis-Related LncRNA Signatures as a Novel Prognostic Model for Colon Cancer. Front. Immunol. 2021, 12, 783362. [Google Scholar] [CrossRef]
- Cai, H.J.; Zhuang, Z.C.; Wu, Y.; Zhang, Y.; Liu, X.; Zhuang, J.; Yang, Y.; Gao, Y.; Chen, B.; Guan, G. Development and validation of a ferroptosis-related lncRNAs prognosis signature in colon cancer. Bosn. J. Basic Med. Sci. 2021, 5, 569–576. [Google Scholar] [CrossRef]
- Du, S.; Zeng, F.; Sun, H.; Liu, Y.; Han, P.; Zhang, B.; Xue, W.; Deng, G.; Yin, M.; Cui, B. Prognostic and therapeutic significance of a novel ferroptosis related signature in colorectal cancer patients. Bioengineered 2022, 2, 2498–2512. [Google Scholar] [CrossRef]
- Yang, Y.B.; Zhou, J.X.; Qiu, S.H.; He, J.; Pan, J.; Pan, Y. Identification of a Novel Ferroptosis-Related Gene Prediction Model for Clinical Prognosis and Immunotherapy of Colorectal Cancer. Dis. Markers 2021, 2021, 4846683. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, H.B.; Chen, Z.M.; Meng, L.; Xu, A. Identification of a ferroptosis-related gene signature predictive model in colon cancer. World J. Surg. Oncol. 2021, 1, 135. [Google Scholar] [CrossRef]
- Zhang, H.C.; Deng, S.H.; Pi, Y.N.; Guo, J.; Xi, H.; Shi, X.; Yang, X.; Zhang, B.; Xue, W.; Cui, B.; et al. Identification and Validation in a Novel Quantification System of Ferroptosis Patterns for the Prediction of Prognosis and Immunotherapy Response in Left- and Right-Sided Colon Cancer. Front. Immunol. 2022, 13, 855849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kong, W.; Xie, Z. Expression and Prognostic Characteristics of Ferroptosis-Related Genes in Colon Cancer. Int. J. Mol. Sci. 2021, 11, 5652. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Dai, W.; Li, Q.; Mo, S.; Han, L.; Xiao, X.; Gu, R.; Xiang, W.; Ye, L.; Wang, R.; et al. Ferroptosis-associated molecular classification characterized by distinct tumor microenvironment profiles in colorectal cancer. Int. J. Biol. Sci. 2022, 5, 1773–1794. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Zhang, Y.; Wang, F.; Cao, F.; Duan, X.; Bai, J. Classification of colorectal carcinoma subtypes based on ferroptosis-associated molecular markers. World J. Surg. Oncol. 2022, 1, 117. [Google Scholar] [CrossRef]

| Type of RNA | Component of Gene Signature or Clusters | Reference |
|---|---|---|
| lncRNA | AL161729.4, AC010973.2, CCDC144NL-AS1, AC009549.1, LINC01857, AP003555.1, AC099850.3, AC008494.3 | [85] |
| lncRNA | ZEB1-AS1, LINC01011, AC005261.3, LINC01063, LINC02381, ELFN1-AS1, AC009283.1, LINC02361, AC105219.1, AC002310.1, AL590483.1, MIR4435-2HG, NKILA, AC021054.1, AL450326.1 | [93] |
| LncRNA | AP003555.1, AC099850.3, AL031985.3, LINC01857, STPG3-AS1, AL137782.1, AC124067.4, AC012313.5, AC083900.1, AC010973.2, ALMS1-IT1, AC013652.1, AC133540.1, AP006621.2, AC018653.3 | [94] |
| LncRNA | VCAN-AS1, OVAAL, AC105383.1, AC063952.1, AC129507.1, ITGB1-DT, C15orf54, AC018781.1, NDST1-AS1, AC090204.1, AC011352.1, FAM239A, LINC01210, AC130324.2, LINC01775, AC093458.1, AL022316.1 | [95] |
| LncRNA | AC104819.3, AP003555.1, AC005841.1, LINC02381 | [96] |
| LncRNA | LINC01503, AC004687.1, AC010973.2, AP001189.3, ARRDC1-AS1, OIP5-AS1, NCK1-DT | [97] |
| Gene | ACACA, GSS, NFS1 | [98] |
| Gene | AKR1C1, ALOX12, ATP5MC3, CARS1, HMGCR, CRYAB, FDFT1, and PHKG2 | [99] |
| Gene | NOX4, SCP2, CARS1, ULK1, WIPI1, CDKN2A, BRD4, DRD4, SLC2A3, TFAP2C | [100] |
| Gene | NOS2 and IFNG for LCRC; NOS2 and ALOXE for RCRC | [92] |
| Gene | Two ferroptosis-gene clusters | [101] |
| Gene | Two ferroptosis-gene clusters | [102] |
| Gene | Three ferroptosis clusters (FAC1, FAC2 and FAC3) | [103] |
| Gene | Four subtypes of CRC (C1, C2, C3, C4) | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Yin, Y.; Li, S.; Guo, X. Insights on Ferroptosis and Colorectal Cancer: Progress and Updates. Molecules 2023, 28, 243. https://doi.org/10.3390/molecules28010243
Hu B, Yin Y, Li S, Guo X. Insights on Ferroptosis and Colorectal Cancer: Progress and Updates. Molecules. 2023; 28(1):243. https://doi.org/10.3390/molecules28010243
Chicago/Turabian StyleHu, Bangli, Yixin Yin, Siqi Li, and Xianwen Guo. 2023. "Insights on Ferroptosis and Colorectal Cancer: Progress and Updates" Molecules 28, no. 1: 243. https://doi.org/10.3390/molecules28010243
APA StyleHu, B., Yin, Y., Li, S., & Guo, X. (2023). Insights on Ferroptosis and Colorectal Cancer: Progress and Updates. Molecules, 28(1), 243. https://doi.org/10.3390/molecules28010243






