Evaluation of Genotoxicity and Toxicity of Annona muricata L. Seeds and In Silico Studies
Abstract
1. Introduction
2. Results and Discussion
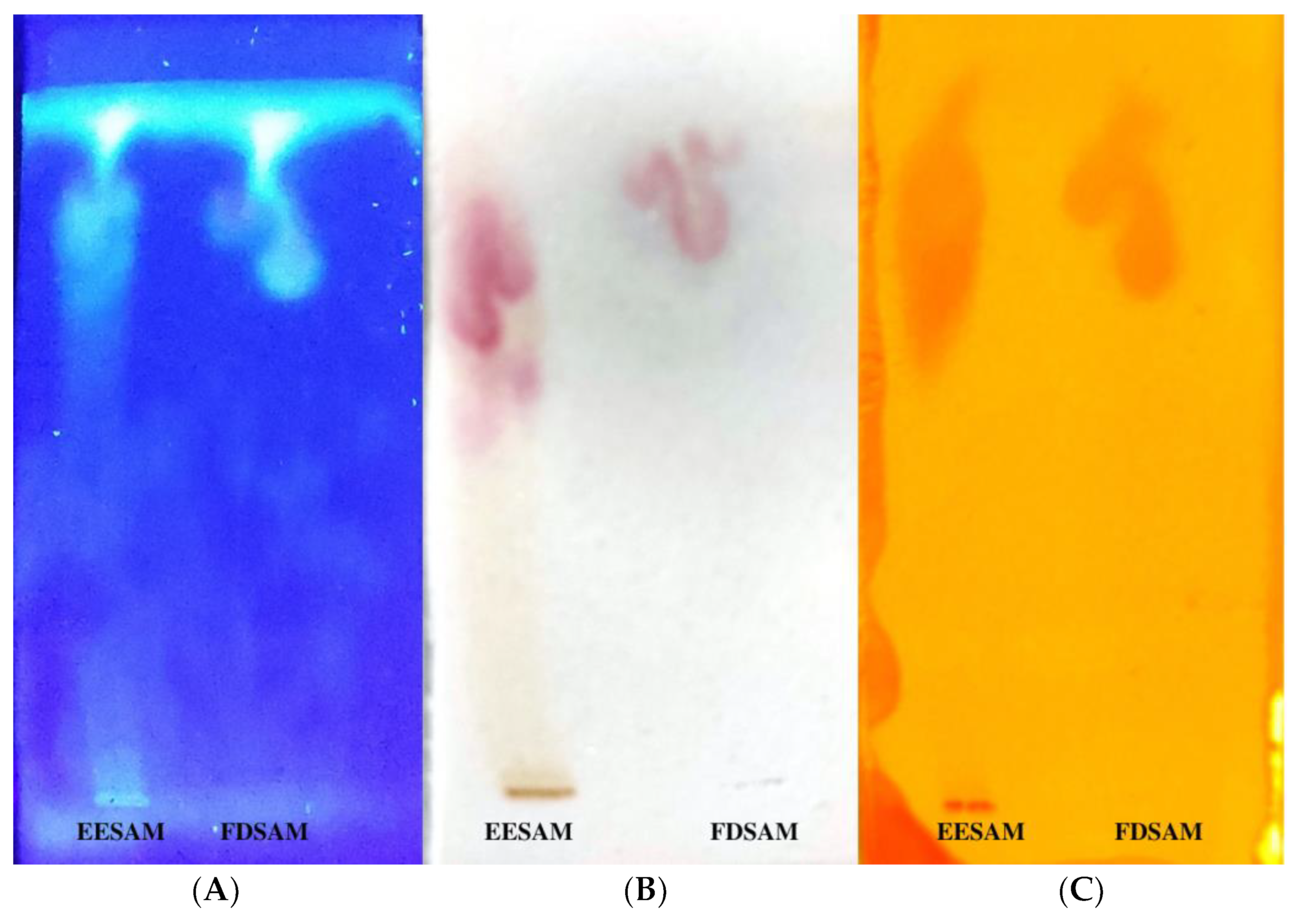
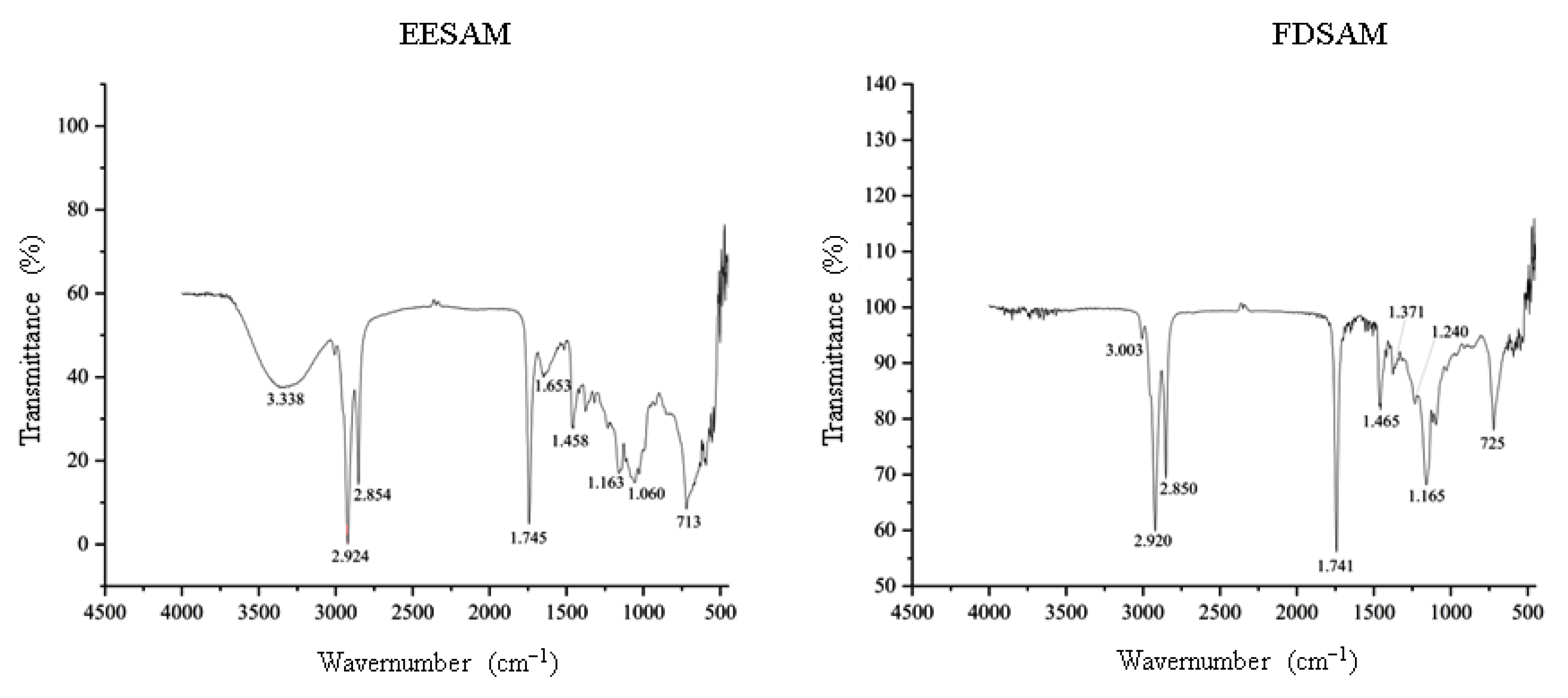
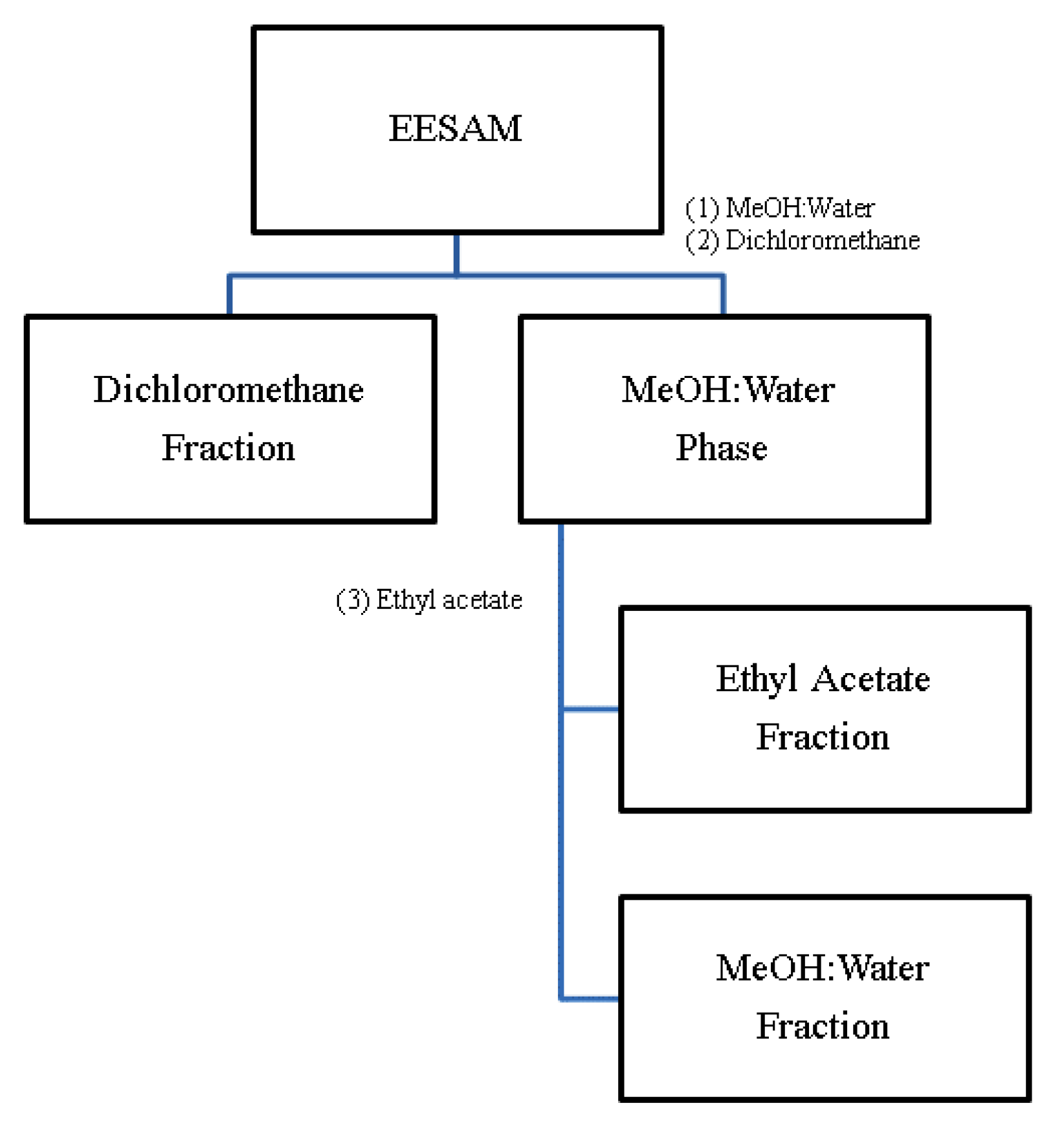
2.1. Preparation of Extract and Fraction, and Phytochemical Characterization
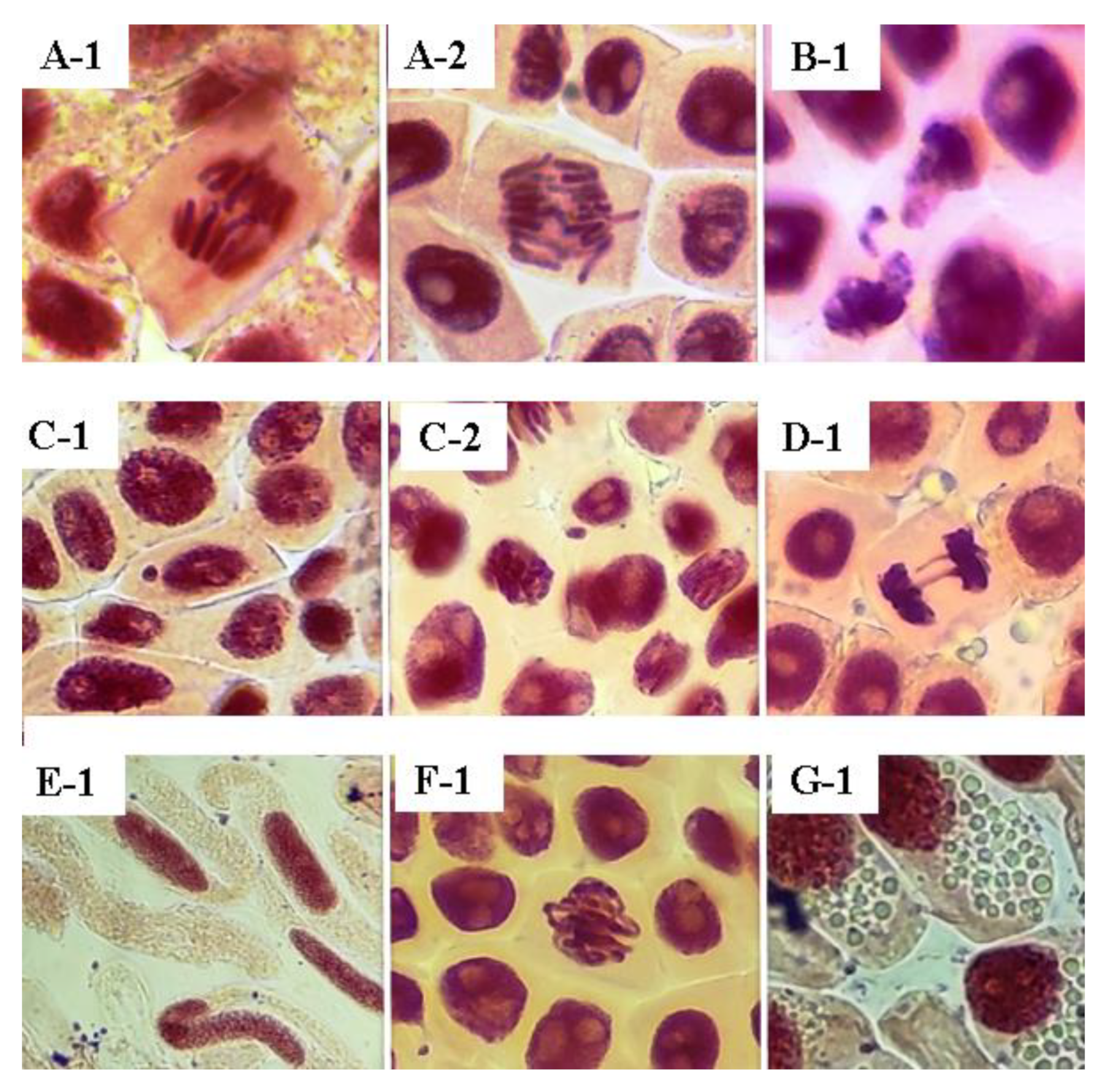
2.2. Genotoxicity in Allium cepa
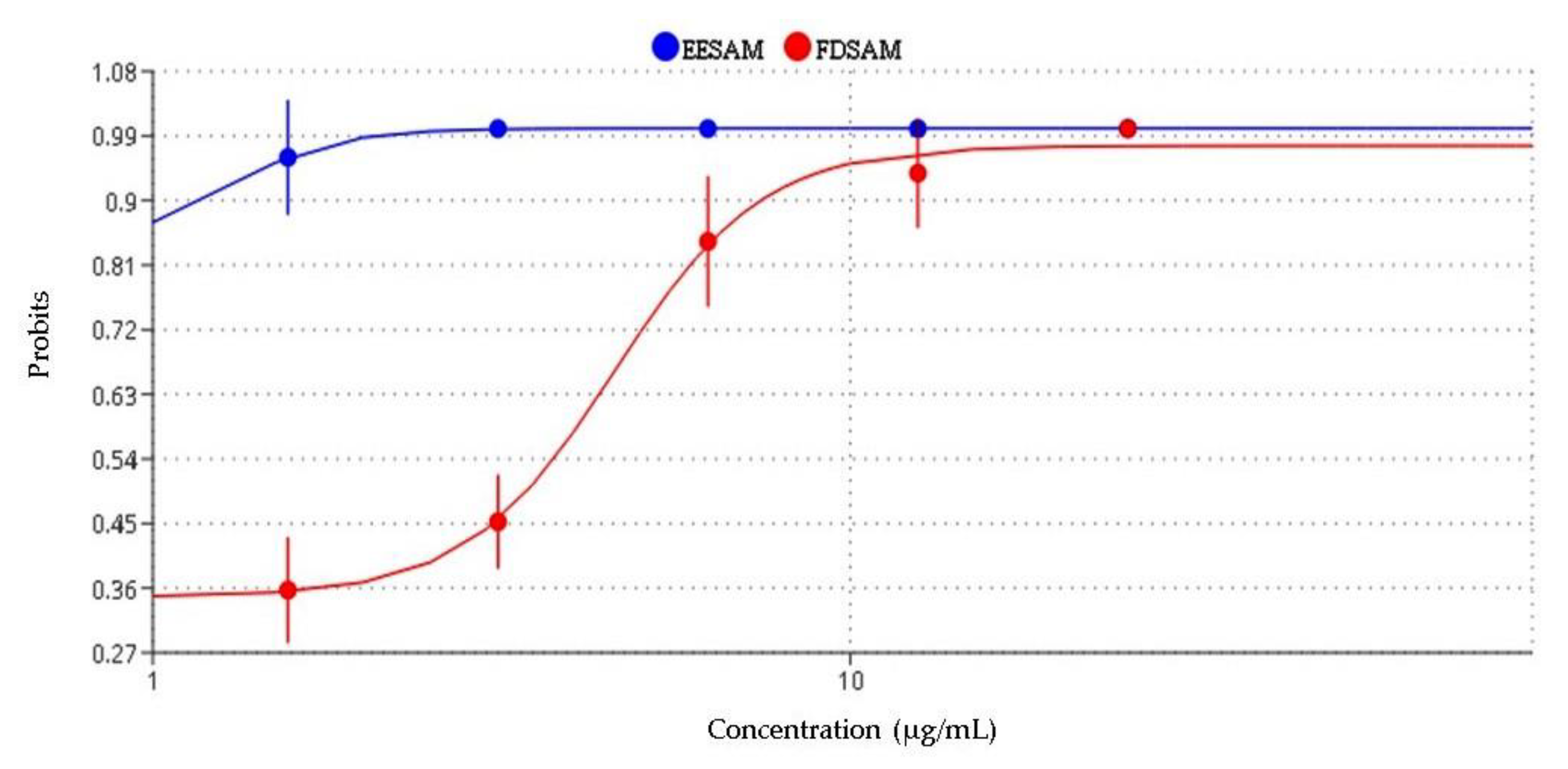
2.3. Toxicity in the Artemia salina Leach
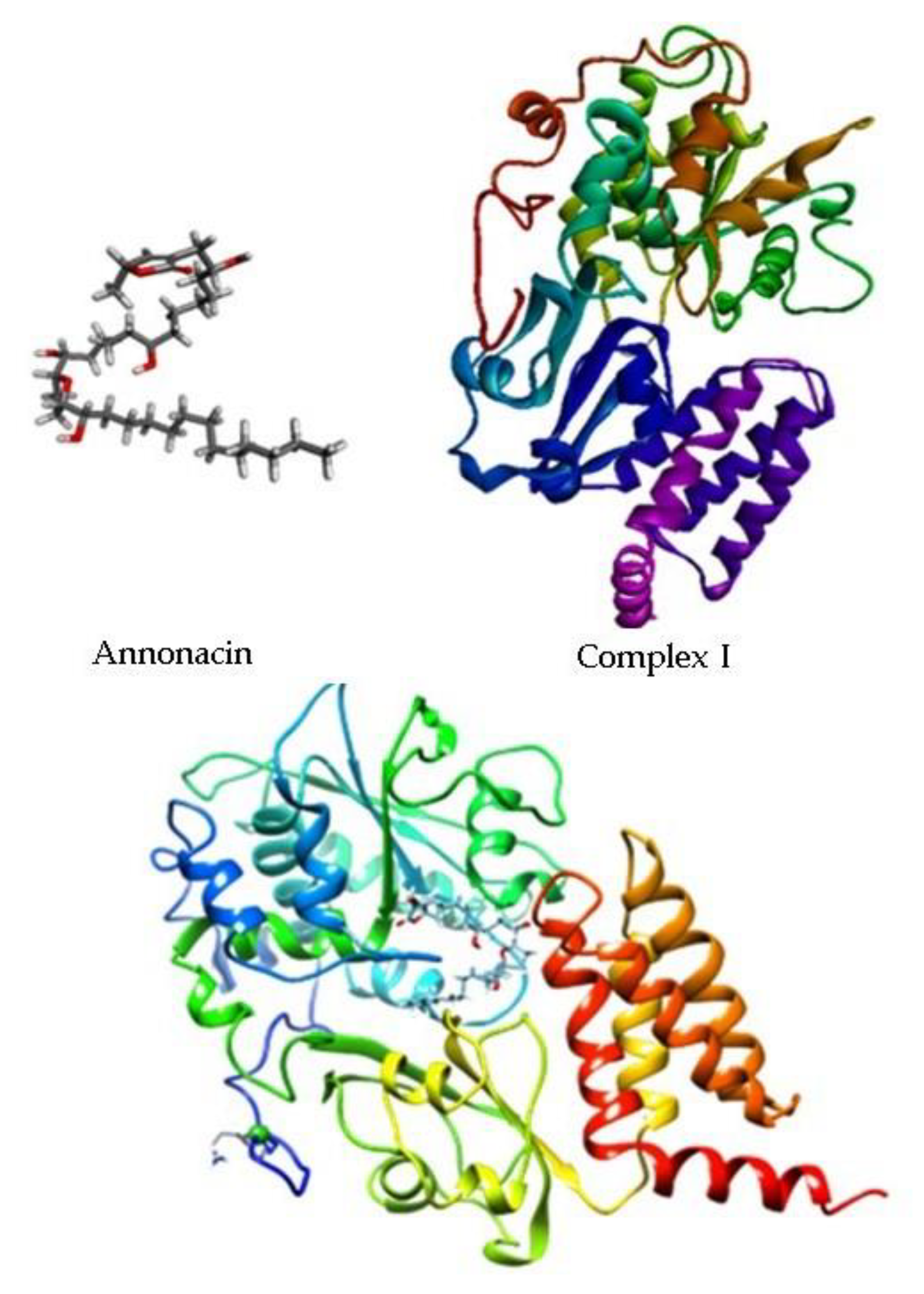
2.4. In Silico Study
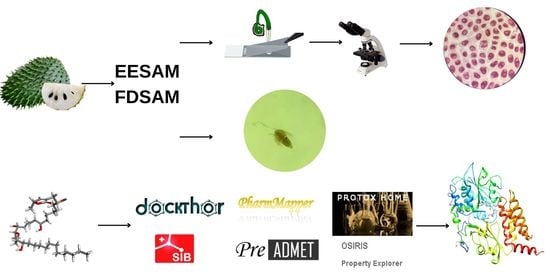
3. Materials and Methods
3.1. Plant Material, Extract and Fractions
3.2. Thin Layer Chromatography—TLC
3.3. Spectroscopy in the Infrared Region—Fourier Transform (FT-IR)
3.4. 1H Nuclear Magnetic Resonance
3.5. Allium cepa Assay
3.6. Artemia Salina Leach Assay
3.7. In Silico Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2020: Incidência de Câncer No Brasil; Instituto Nacional de Câncer José Alencar Gomes da Silva INCA: Rio de Janeiro, Brazil, 2019. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020-incidencia-de-cancer-no-brasil.pdf (accessed on 22 July 2022).
- Vieira, V.; Cruz, V.; Soares, N.; Silva, J.; Araújo, E. Quimioterápicos antineoplásicos derivados de plantas. Enciclopédia Biosf. 2020, 17, 444–461. [Google Scholar] [CrossRef]
- Silva, T.F. Análise Fitoquímica e Atividade Leishmanicida de Frações de Sementes de Annona muricata L. (annonaceae) de um Cultivo em Tomé-açu. Master’s Thesis, Universidade Federal do Pará, Pará, Brazil, 2017. [Google Scholar]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.K.; Lu, J.; Bai, Y. Mitochondrial Respiratory Complex I: Structure, Function and Implication in Human Diseases. Curr. Med. Chem. 2009, 16, 1266–1277. [Google Scholar] [CrossRef]
- Lima, N.N.d.C.; Faustino, D.C.; Allahdadi, K.J.; França, L.S.D.A.; Pinto, L.C. Acetogenins from Annonaceae plants: Potent antitumor and neurotoxic compounds. PharmaNutrition 2022, 20, 100295. [Google Scholar] [CrossRef]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.S. Estudo da Atividade Citotóxica de Compostos Obtidos do Extrato Acetônico das Folhas de Annona muricata L. por Fracionamento Bioguiado. Master’s Thesis, Universidade Federal do Ceára, Ceára, Brazil, 2014. [Google Scholar]
- Nunes, C.R. Estudo Químico e Avaliação da Atividade Antineoplásica de Annona muricata L. Doctoral´s Thesis, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Lima, M.D. Perfil Cromatográfico dos Extratos Brutos das Sementes de Annona muricata L. e Annona squamosa L. Através da Cromatografia Líquida de alta Eficiência. Master’s Thesis, Universidade Federal de Alagoas, Maceió, Brazil, 2007. [Google Scholar]
- Simões, C.F.D.S. Perfil Fitoquímico e Estudo das Atividades Antimicrobiana, Citotóxica e Anti-Inflamatória de Annona muricata L. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2015. [Google Scholar]
- Castro, A.L.G.; Cruz, J.N.; Sodré, D.F.; Correa-Barbosa, J.; Azonsivo, R.; de Oliveira, M.S.; Siqueira, J.E.d.S.; Galucio, N.C.D.R.; Bahia, M.D.O.; Burbano, R.M.R.; et al. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated from Eleutherine plicata herb. using bioassays and in silico approaches. Arab. J. Chem. 2021, 14, 103084. [Google Scholar] [CrossRef]
- Farias, M.S. Dano ao DNA, Citotoxicidade, Efeito Antiproliferativo e Antitumoral de 1, 4-Naftoquinonas Substituídas. Doctoral’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2014. [Google Scholar]
- Araujo Soria, J.M. Efecto Protector del Extracto Etanolico de Annona Muricata Sobre la Citotoxicidad y Genotoxicidad del Sorbato de Potasio en Meristemos Radiculares de Allium cepa. Monograph’s Thesis, Universidad Nacional de Trujillo, Trujillo, Peru, 2012. [Google Scholar]
- Chávez Aldave, M.I. Efecto del Extracto Etanólico de Annona Muricata a Diferentes Tiempos y Concentraciones Sobre el Ciclo Celular en Allium Cepa. Doctoral’s Thesis, Universidad Nacional de Trujillo, Trujillo, Peru, 2015. [Google Scholar]
- Bibi, R. Cytotoxic and Genotoxic Efects of Morinda Citrifolia and Annona Muricata on Allium Cepa Root Tip Cells. Master’s Thesis, University of the South Pacific, Suva, Fiji, 2017. [Google Scholar]
- Fajardo, V.G.F. Cytological Study on Allium cepa (onion) root tip treated with Annona muricata (guyabano) bark and leaves extract. Ascend. Asia J. Multidiscip. Res. Abstr. 2018, 2, 3. Available online: https://ojs.aaresearchindex.com/index.php/AAJMRA/article/view/4045 (accessed on 15 July 2022).
- Rehana, B.; Ketan, C.; Marta, F.; Nwangburuka, C. Analysis of the mitotic effect of Annona muricata leaf extracts on Allium cepa root tip cells. Afr. J. Biotechnol. 2019, 18, 155–162. [Google Scholar] [CrossRef]
- Ruiz Baca, J. Efecto de Diferentes Concentraciones del Extracto Clorofórmico de Hojas de Annona muricata L. “Guanábana” y Tiempos de Exposición Sobre el Índice Mitótico de Allium Cepa L. “Cebolla” var. Arequipeña. Monograph´s Thesis, Universidad Nacional de Trujillo, Trujillo, Peru, 2019. [Google Scholar]
- Jacobo-Herrera, N.; Perez-Plasencia, C.; Castro-Torres, V.A.; Martínez-Vázquez, M.; González-Esquinca, A.R.; Zentella-Dehesa, A. Selective Acetogenins and Their Potential as Anticancer Agents. Front. Pharmacol. 2019, 10, 783. [Google Scholar] [CrossRef]
- Paes, M.M.; Vega, M.R.G.; Cortes, D.; Kanashiro, M.M. Potencial citotóxico das acetogeninas do gênero Annona. Rev. Virtual De Química 2016, 8, 945–980. [Google Scholar] [CrossRef]
- Khah, M.A.; Alshehri, M.A.; Filimban, F.Z.; Alam, Q.; Aloufi, S. Influence of Colchicine in Causing Severe Chromosomal Damage in Microsporocytes of Hard Wheat (Triticum durum Desf.): Possible Mechanisms and Genotoxic Relevance. Cytologia 2022, 87, 137–143. [Google Scholar] [CrossRef]
- Zafra-Polo, M.C.; Figadère, B.; Gallardo, T.; Tormo, J.; Cortes, D. Natural acetogenins from annonaceae, synthesis and mechanisms of action. Phytochemistry 1998, 48, 1087–1117. [Google Scholar] [CrossRef]
- Grba, D.N.; Blaza, J.N.; Bridges, H.R.; Agip, A.-N.A.; Yin, Z.; Murai, M.; Miyoshi, H.; Hirst, J. Cryo-electron microscopy reveals how acetogenins inhibit mitochondrial respiratory complex I. J. Biol. Chem. 2022, 298, 1–13. [Google Scholar] [CrossRef]
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. In Membrane Protein Complexes: Structure and Function. Subcellular Biochemistry; Harris, J., Boekema, E., Eds.; Springer: Singapore, 2018; Volume 87. [Google Scholar] [CrossRef]
- Luna, J.D.S.; De Carvalho, J.M.; De Lima, M.R.F.; Bieber, L.W.; Bento, E.D.S.; Franck, X.; Sant’Ana, A.E.G. Acetogenins in Annona muricata L. (annonaceae) leaves are potent molluscicides. Nat. Prod. Res. 2006, 20, 253–257. [Google Scholar] [CrossRef]
- Silva, E.M.F.; Nascimento, R.B.D.C.; Barreto, F.S.; Moraes Filho, M.O.D.; Griz, S.D.A.S.; Santos, A.F.D.; Mousinho, K.C. Estudo in vitro do potencial citotóxico da Annona muricata L. Rev. De Ciências Farm. Básica Apl. 2015, 36, 277–283. [Google Scholar]
- Hoe, P.K.; Yiu, P.H.; Ee, G.C.L.; Wong, S.C.; Rajan, A.; Bong, C.F. Biological Activity of Annona muricata Seed Extracts. Malays. J. Sci. 2010, 29, 153–159. [Google Scholar] [CrossRef]
- Barros, M.E.S.B. Estudos de Docking Molecular, Síntese e Atividade Biológica de Análogos da (-)-Massoialactona e da Combretastatina A-4. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2015. [Google Scholar]
- Sugita, S.; Enokida, H.; Yoshino, H.; Miyamoto, K.; Yonemori, M.; Sakaguchi, T.; Osako, Y.; Nakagawa, M. HRAS as a potential therapeutic target of salirasib RAS inhibitor in bladder cancer. Int. J. Oncol. 2018, 53, 725–736. [Google Scholar] [CrossRef]
- Hodge, R.G.; Schaefer, A.; Howard, S.V.; Der, C.J. RAS and RHO family GTPase mutations in cancer: Twin sons of different mothers? Crit. Rev. Biochem. Mol. Biol. 2020, 55, 386–407. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Sengupta, S.; McComb, M.E.; Théberge, R.; Wilson, W.G.; Costello, C.E.; Jacobsen, D.W. In Vitro and in Vivo Interactions of Homocysteine with Human Plasma Transthyretin. J. Biol. Chem. 2003, 278, 49707–49713. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Ding, X.; Zhao, T.; Wu, L.; Perkins, S.; Du, H.; Yan, C. Transthyretin Stimulates Tumor Growth through Regulation of Tumor, Immune, and Endothelial Cells. J. Immunol. 2018, 202, 991–1002. [Google Scholar] [CrossRef]
- Haagenson, K.K.; Wu, G.S. Mitogen activated protein kinase phosphatases and cancer. Cancer Biol. Ther. 2010, 9, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Ahmadi, A.; Mortezaee, K. Extracellular-signal-regulated kinase/mitogen-activated protein kinase signaling as a target for cancer therapy: An updated review. Cell Biol. Int. 2019, 43, 1206–1222. [Google Scholar] [CrossRef]
- Selvakumar, P.; Lakshmikuttyamma, A.; Dimmock, J.R.; Sharma, R.K. Methionine aminopeptidase 2 and cancer. Biochim. Et Biophys. Acta 2006, 1765, 148–154. [Google Scholar] [CrossRef]
- Grocin, A.G.; Kallemeijn, W.W.; Tate, E.W. Targeting methionine aminopeptidase 2 in cancer, obesity, and autoimmunity. Trends Pharmacol. Sci. 2021, 42, 870–882. [Google Scholar] [CrossRef]
- Carvalho, L.S. Efeito Depressor e Toxicidade do Extrato Etanólico da Casca de Aspidosperma Subincanum (Apocynaceae) em Camundongos. Master’s Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2013. [Google Scholar]
- Djouad, S.-E.; Berredjem, M.; Aoul, F.Z.H.; Bouchareb, F.; Guerfi, M.; Ben Hadda, T.; Aissaoui, M.; Belhani, B. In silico drug design and molecular docking of novel amidophosphonates and sulfamidophosphonates as inhibitors of urokinase-type plasminogen activator. J. Indian Chem. Soc. 2022, 99, 100650. [Google Scholar] [CrossRef]
- Vilar, J.B.; Ferreira, F.L.; Ferri, P.H.; Guillo, L.A.; Chen, L.C. Assessment of the mutagenic, antimutagenic and cytotoxic activities of ethanolic extract of araticum (Annona crassiflora Mart. 1841) by micronucleus test in mice. Braz. J. Biol. 2008, 68, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Colom, O.; Salvatore, A.; Willink, E.; Ordóñez, R.; Isla, M.I.; Neske, A.; Bardón, A. Insecticidal, Mutagenic and Genotoxic Evaluation of Annonaceous Acetogenins. Nat. Prod. Commun. 2010, 5, 391–394. [Google Scholar] [CrossRef]
- Champy, P.; Höglinger, G.; Féger, J.; Gleye, C.; Hocquemiller, R.; Laurens, A.; Guérineau, V.; Laprévote, O.; Medja, F.; Lombès, A.; et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: Possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 2003, 88, 63–69. [Google Scholar] [CrossRef]
- Escobar-Khondiker, M.; Hollerhage, M.; Muriel, M.-P.; Champy, P.; Bach, A.; Depienne, C.; Respondek, G.; Yamada, E.S.; Lannuzel, A.; Yagi, T.; et al. Annonacin, a Natural Mitochondrial Complex I Inhibitor, Causes Tau Pathology in Cultured Neurons. J. Neurosci. 2007, 27, 7827–7837. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Ryan, S.; McDonald, M.; Thomas, A.L.; Maia, J.G.S.; Smith, R.E. Annonacin and Squamocin Contents of Pawpaw (Asimina triloba) and Marolo (Annona crassiflora) Fruits and Atemoya (A. squamosa × A. cherimola) Seeds. Biol. Trace Elem. Res. 2020, 199, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, N.; Schmitz-Afonso, I.; Brunelle, A.; Touboul, D.; Champy, P. Method development for quantification of the environmental neurotoxin annonacin in Rat plasma by UPLC–MS/MS and application to a pharmacokinetic study. J. Chromatogr. B 2015, 1004, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Amarante, C.B.D.; Müller, A.H.; Póvoa, M.M.; Dolabela, M.F. Estudo fitoquímico biomonitorado pelos ensaios de toxicidade frente à Artemia salina e de atividade antiplasmódica do caule de aninga (Montrichardia linifera). Acta Amaz. 2011, 41, 431–434. [Google Scholar] [CrossRef]
- Bioquest, A.A.T. Inc. Quest Graph™ IC50 Calculator; Bioquest, A.A.T. Inc.: Pleasanton, CA, USA, 2020. [Google Scholar]
- Almeida, D.M. Dockthor: Implementação, Aprimoramento e Validação de um Programa de Atracamento Receptor-Ligante. Master’s Thesis, Laboratório Nacional de Computação Científica, Petrópolis, Brazil, 2011. [Google Scholar]
- Bitencourt-Ferreira, G.; Azevedo, W.F.D. Docking with SwissDock. In Docking Screens for Drug Discovery; Humana: New York, NY, USA, 2019; pp. 189–202. [Google Scholar] [CrossRef]
- Ferreira, G.G.; Brandão, D.L.D.N.; Dolabela, M.F. Predição do comportamento farmacocinético, toxicidade e de atividades biológicas de alcaloides isolados de Geissospermum laeve (Vell.) Miers. Res. Soc. Dev. 2020, 9, e27991211056. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 2149–2159. [Google Scholar] [CrossRef]
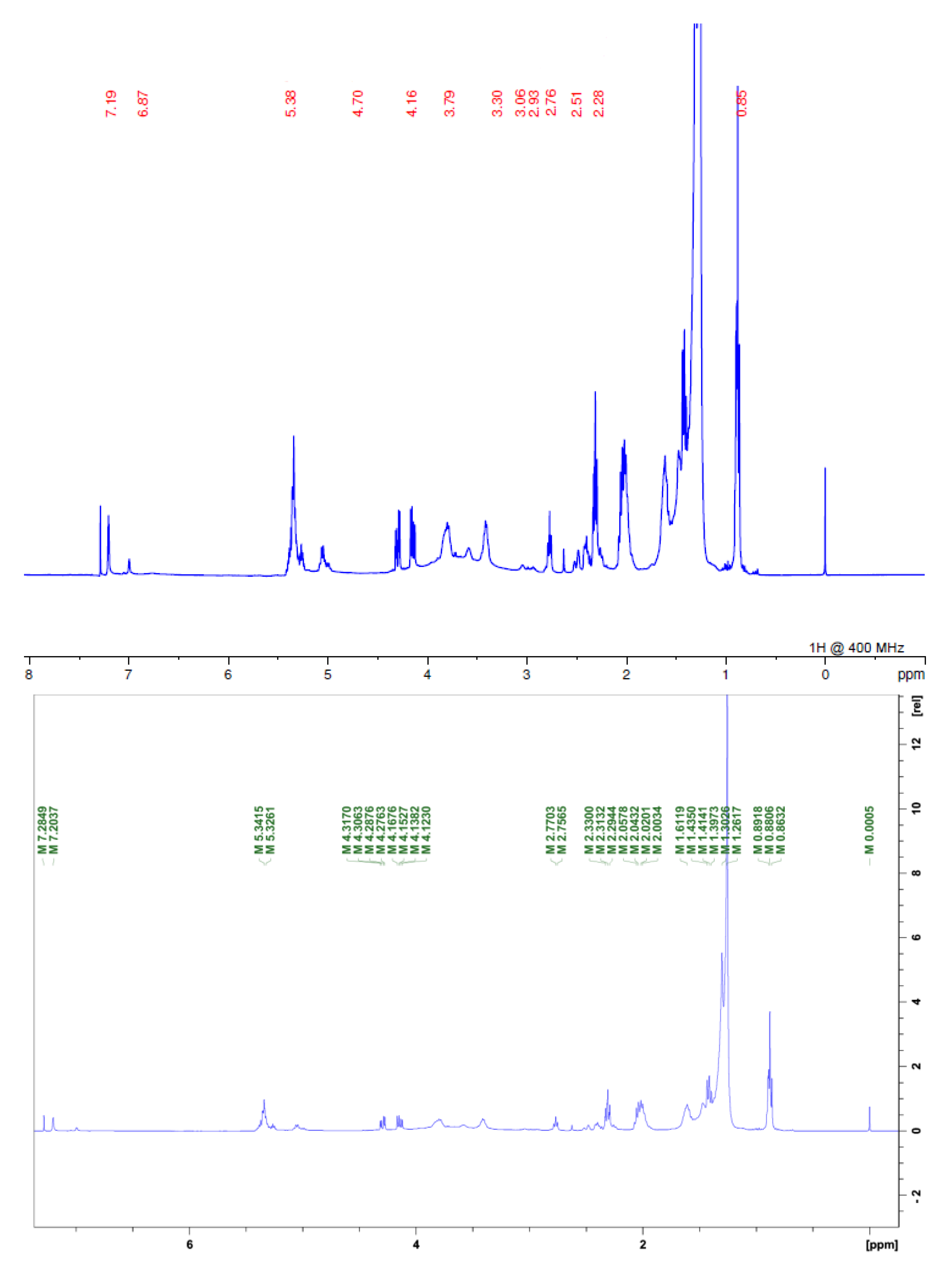
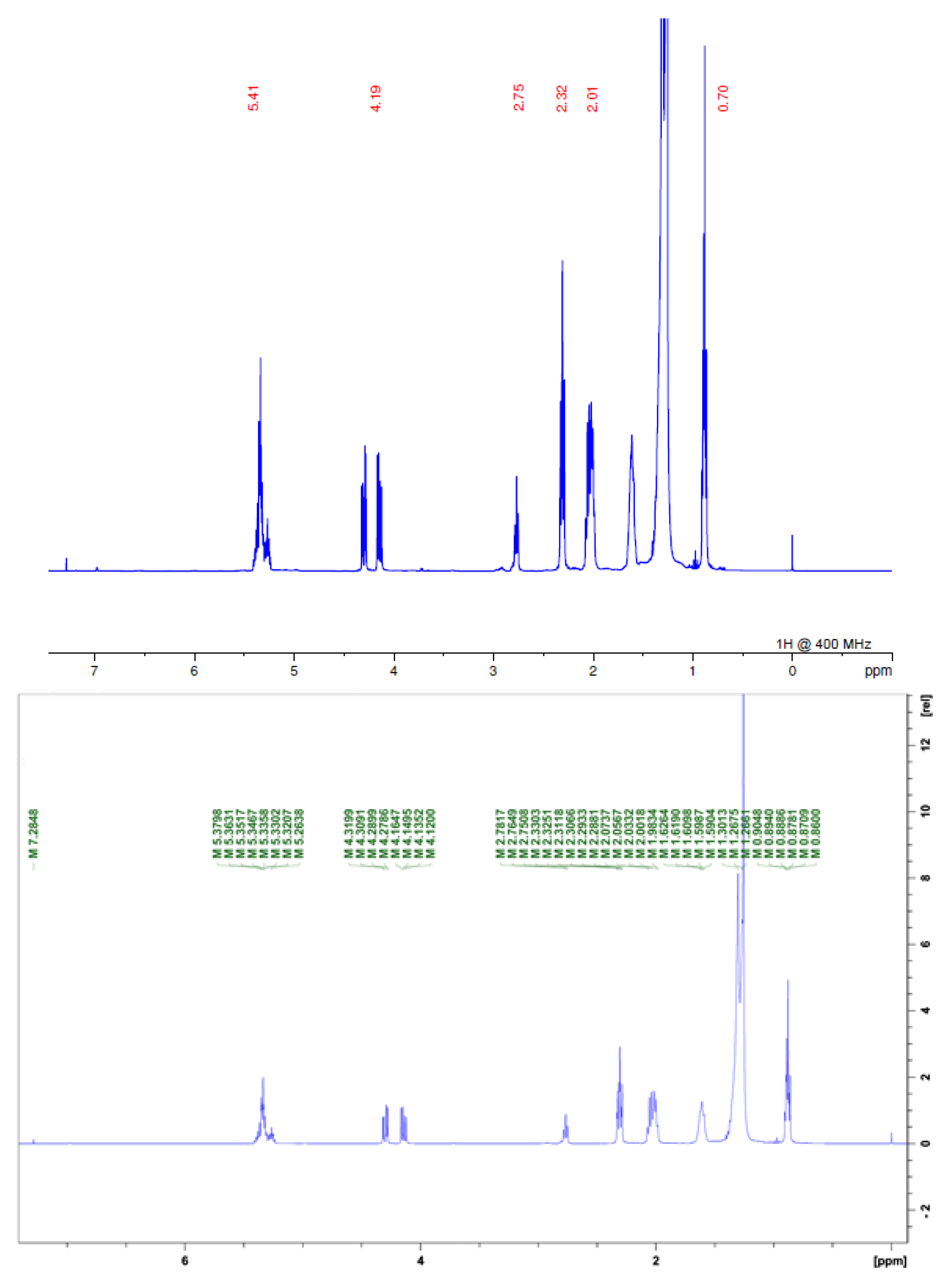

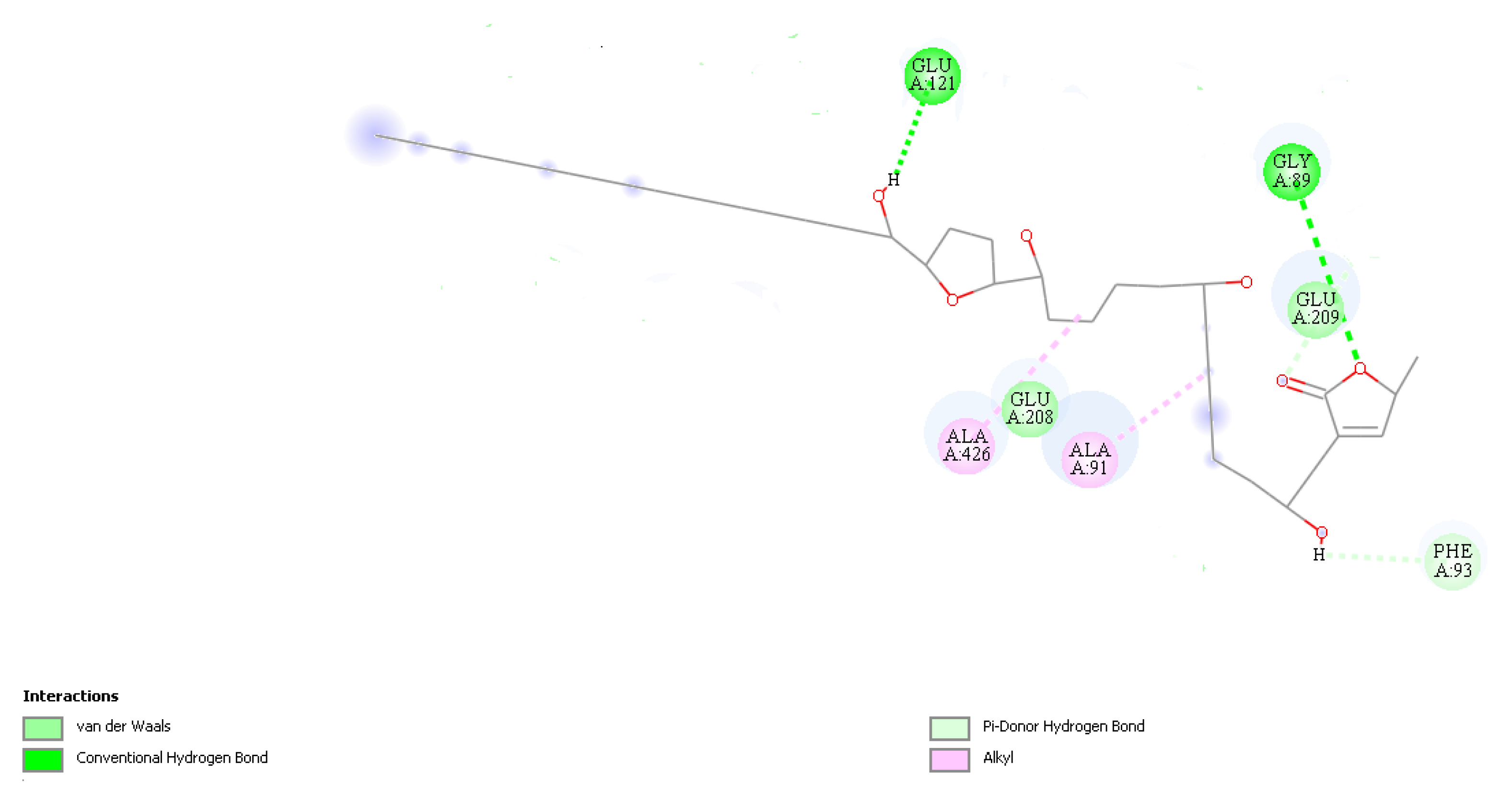
| α,β-unsaturated y-lactone | H-2 | H-3 | H-4 | H-35 (33) | H-36 (34) | H-37 (35) |
| - | 2.26 | 1.52 | 6.95 | 4.99 | 1.42 | |
| THF ring | δ | |||||
| 3.0–4.2 | ||||||
| Olefinic C-H | δ | |||||
| 5.0–5.8 |
| Mitotic Index (MI) % | Chromosomal Aberrations (CA) % | |||||
|---|---|---|---|---|---|---|
| Sample | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
| Distilled water (NC) | 16.5 | 16.9 | 13.2 | * | 0.001 | 0.001 |
| Colchicine (PC) | ||||||
| 1.5625 µg/mL | 20.5 | 18.7 | 15.7 | 0.23 | 0.25 | 0.34 |
| 3.125 µg/mL | 19.1 | 16.4 | 13.8 | 0.36 | 0.4 | 0.45 |
| 6.25 µg/mL | 15.9 | 13.7 | 10.3 | 0.68 | 0.52 | 0.75 |
| 12.5 µg/mL | 11.3 | 8.7 | 6.1 | 0.81 | 0.89 | 1.23 |
| 25 µg/mL | 5.9 | 4.2 | 1.2 | 1.75 | 1.96 | 2.08 |
| EESAM | ||||||
| 1.5625 µg/mL | 28.6 | 28.2 | 25.4 | 0.26 | 0.2 | 0.31 |
| 3.125 µg/mL | 25.1 | 22.6 | 20.7 | 0.38 | 0.42 | 0.4 |
| 6.25 µg/mL | 20.5 | 19.8 | 18.1 | 0.38 | 0.45 | 0.58 |
| 12.5 µg/mL | 15.4 | 11.3 | 12.6 | 0.78 | 0.69 | 0.89 |
| 25 µg/mL | 10.2 | 9.6 | 8.9 | 1.35 | 2.39 | 3.06 |
| FDSAM | ||||||
| 1.5625 µg/mL | 27.9 | 24.3 | 26.8 | 0.38 | 0.29 | 0.31 |
| 3.125 µg/mL | 24.9 | 22.8 | 21.2 | 0.4 | 0.42 | 0.53 |
| 6.25 µg/mL | 19.6 | 20.2 | 16 | 0.38 | 0.49 | 0.48 |
| 12.5 µg/mL | 9.9 | 8.9 | 8.1 | 0.88 | 0.79 | 1.02 |
| 25 µg/mL | 7.1 | 4.9 | 6.9 | 0.98 | 1.51 | 2.35 |
| Order | Code PDB | Target Name | Fit Score |
|---|---|---|---|
| 1 | 5P21 | GTPase HRas | 5.24 |
| 2 | 1RLB | Transtirretina | 4.77 |
| 3 | 1CBS | Cellular retinoic acid binding protein (CRABP2) | 4.72 |
| 4 | 1R5L | Alpha-tocopherol transfer protein | 4.49 |
| 5 | 1O1V | Ileal lipid binding protein (ILBP) | 4.39 |
| 6 | 1SR7 | Progesterone receptor | 4.37 |
| 7 | 1M7Q | Mitogen-activated protein kinase (MAPK14) | 4.37 |
| 8 | 1BOA | Methionine Aminopeptidase 2 | 4.32 |
| 9 | 1GNI | Seroalbumin | 4.30 |
| 10 | 1V4S | Glucokinase | 4.30 |
| ADMETLab | |
| hERG | Negative |
| hepatotoxicity | Negative |
| skin sensitization | Negative |
| Reduction of liver damage | Negative |
| AMES | Negative |
| PreADME | |
| Algae | Toxic |
| Medaka sp. | Very toxic |
| Daphnia | Toxic |
| Minnow | Toxic |
| Ames | Negative |
| Carcinogenic (rats) | Negative |
| Carcinogen (mice) | Positive |
| hERG | Negative |
| Osiris | |
| Mutagen | Negative |
| Tumorigenic | Negative |
| Irritating | Negative |
| Effect on reproduction | Negative |
| ProTox | |
| Hepatotoxicity | Negative |
| Carcinogenicity | Negative |
| Immunogenicity | Positive |
| Mutagenicity | Negative |
| Cytotoxicity | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, G.G.; Quaresma, A.C.S.; Brandão, D.L.d.N.; Marinho, A.M.d.R.; Siqueira, J.E.d.S.; Correa, K.L.; Silva-Júnior, J.O.C.; Percario, S.; Dolabela, M.F. Evaluation of Genotoxicity and Toxicity of Annona muricata L. Seeds and In Silico Studies. Molecules 2023, 28, 231. https://doi.org/10.3390/molecules28010231
Ferreira GG, Quaresma ACS, Brandão DLdN, Marinho AMdR, Siqueira JEdS, Correa KL, Silva-Júnior JOC, Percario S, Dolabela MF. Evaluation of Genotoxicity and Toxicity of Annona muricata L. Seeds and In Silico Studies. Molecules. 2023; 28(1):231. https://doi.org/10.3390/molecules28010231
Chicago/Turabian StyleFerreira, Gleison Gonçalves, Ana Carolina Sousa Quaresma, Dayse Lúcia do Nascimento Brandão, Andrey Moacir do Rosario Marinho, José Edson de Sousa Siqueira, Kamila Leal Correa, José Otávio Carréra Silva-Júnior, Sandro Percario, and Maria Fâni Dolabela. 2023. "Evaluation of Genotoxicity and Toxicity of Annona muricata L. Seeds and In Silico Studies" Molecules 28, no. 1: 231. https://doi.org/10.3390/molecules28010231
APA StyleFerreira, G. G., Quaresma, A. C. S., Brandão, D. L. d. N., Marinho, A. M. d. R., Siqueira, J. E. d. S., Correa, K. L., Silva-Júnior, J. O. C., Percario, S., & Dolabela, M. F. (2023). Evaluation of Genotoxicity and Toxicity of Annona muricata L. Seeds and In Silico Studies. Molecules, 28(1), 231. https://doi.org/10.3390/molecules28010231