Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer
Abstract
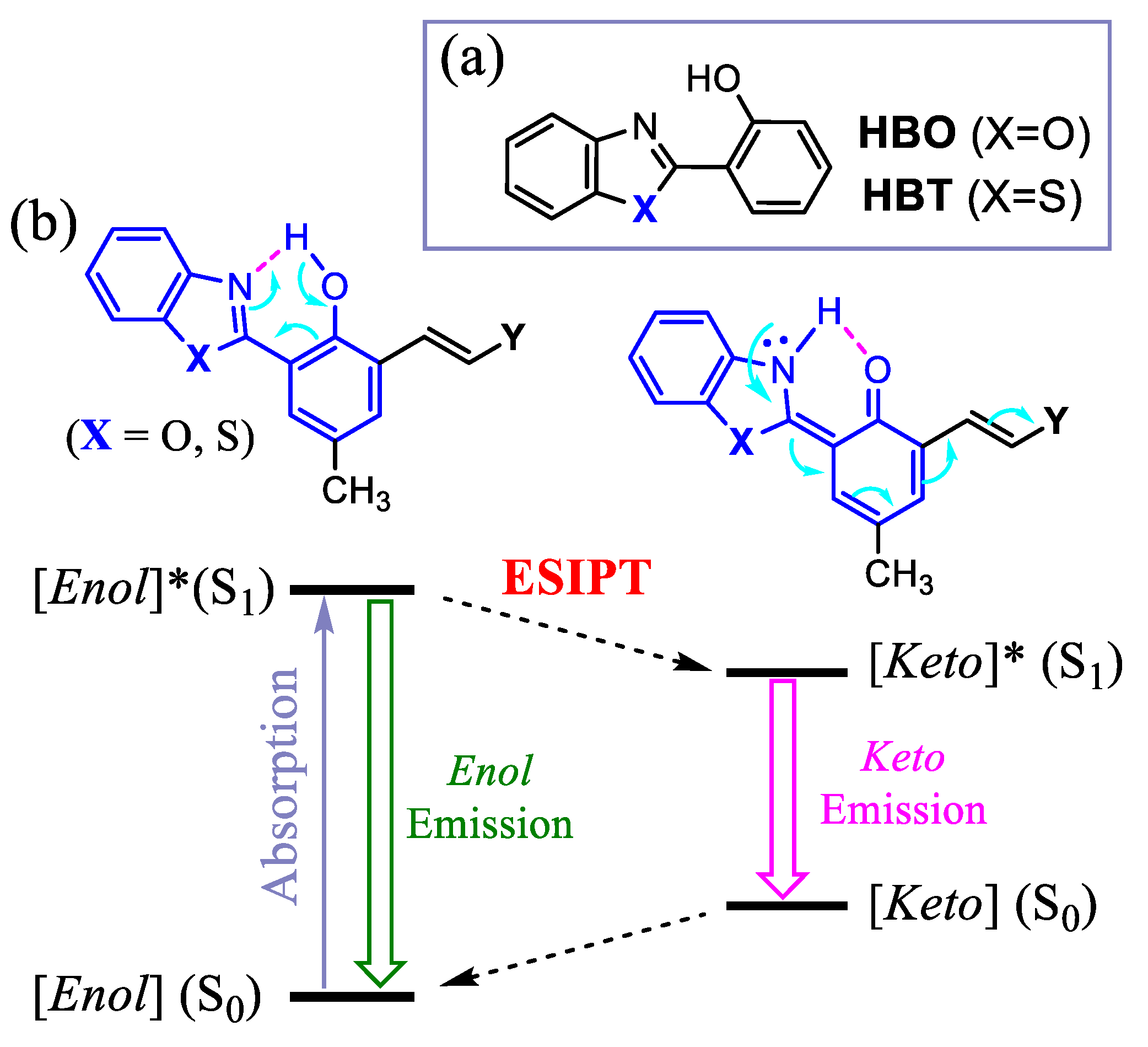
1. Introduction
2. Results and Discussion
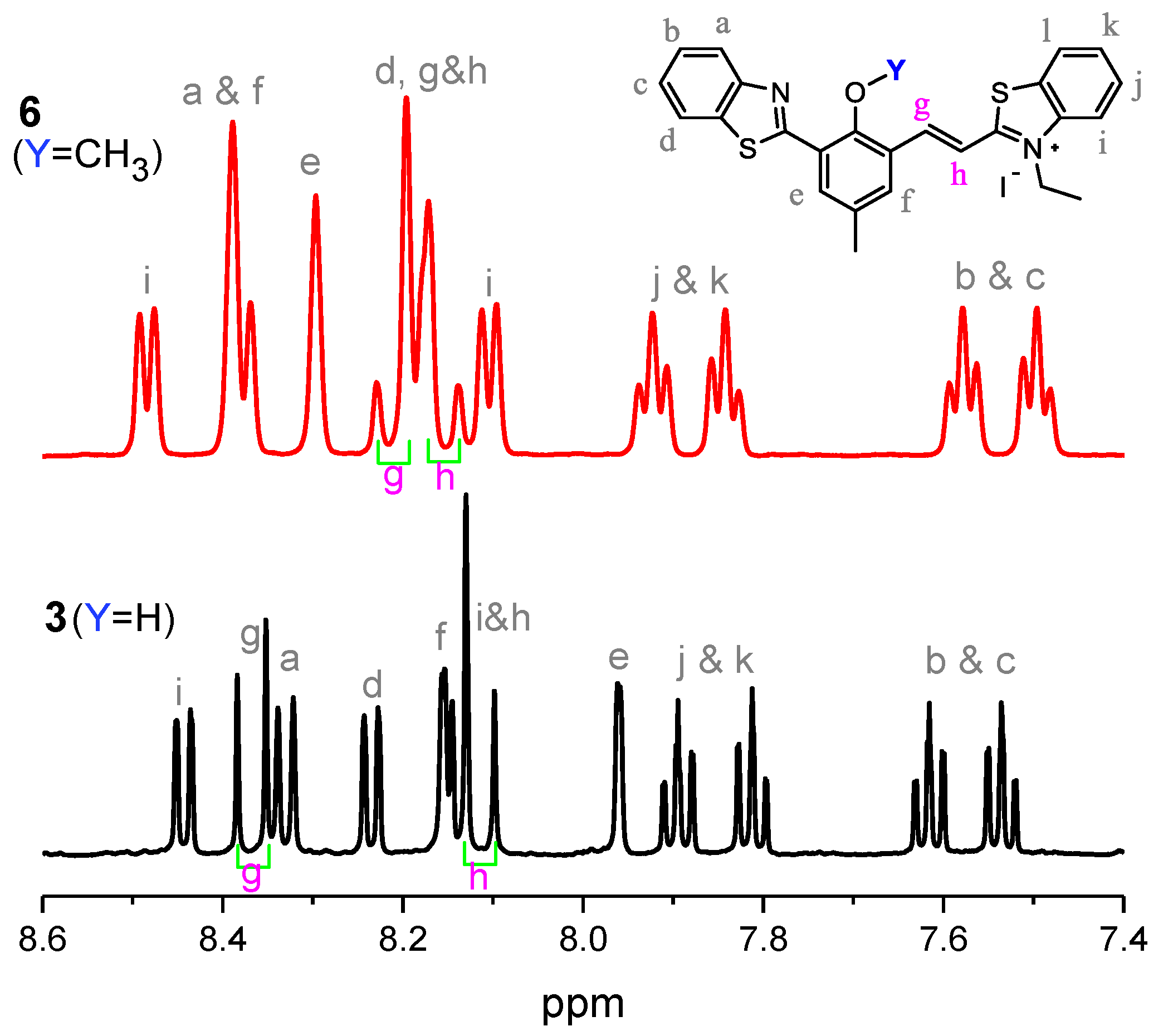
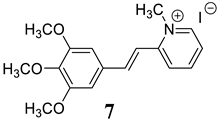
2.1. Synthesis
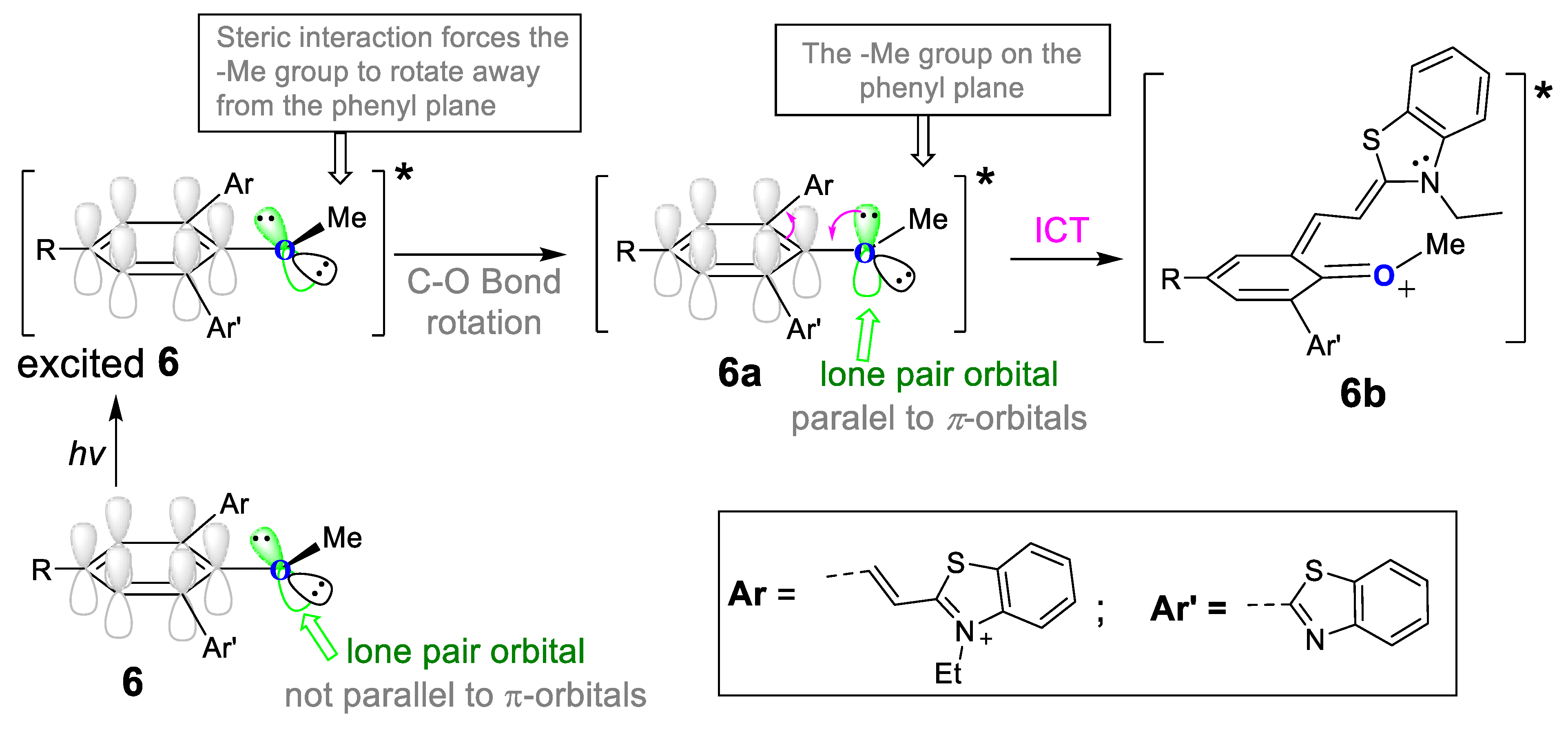
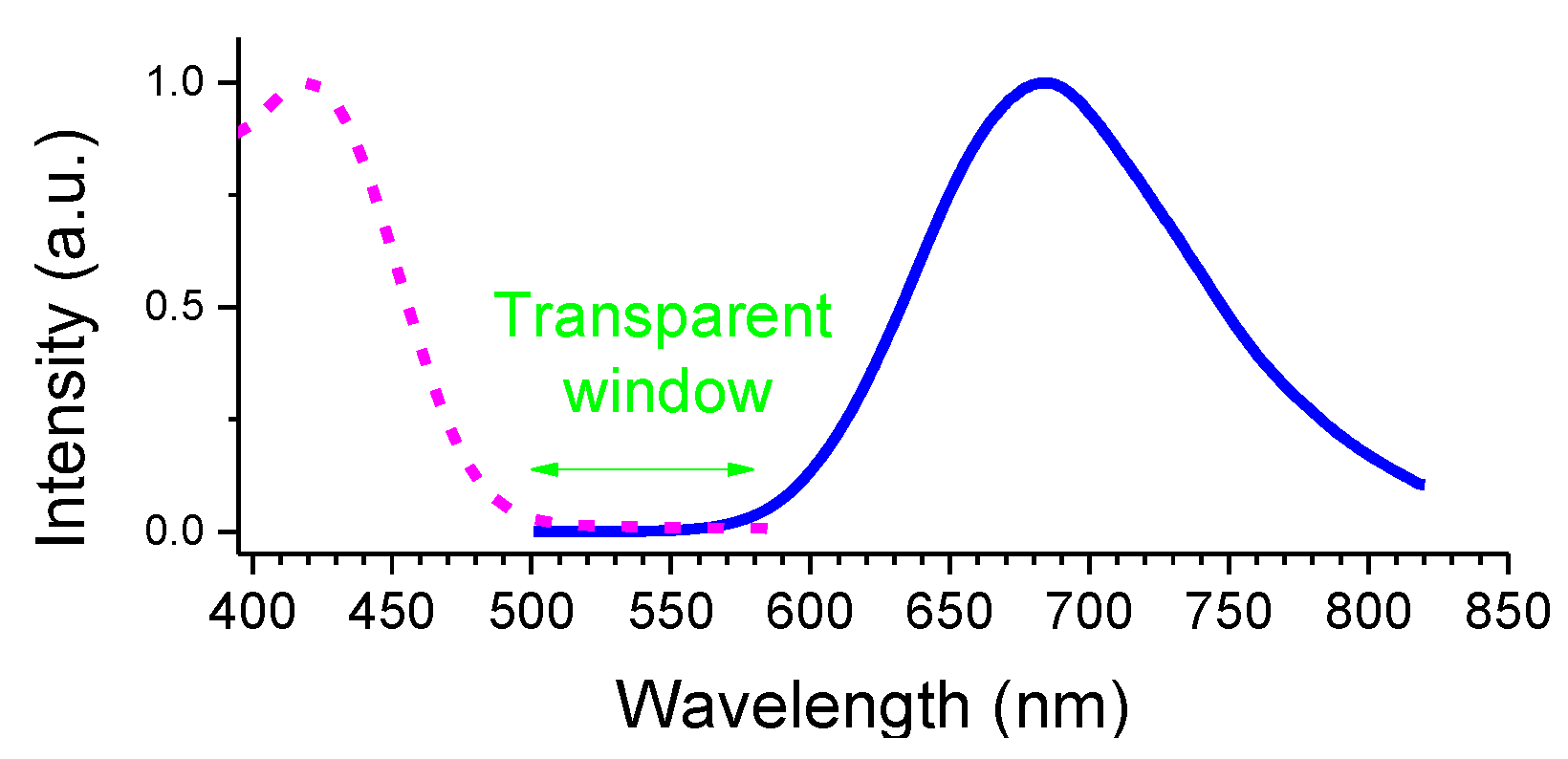
2.2. Spectroscopic Studies
Low-Temperature Fluorescence
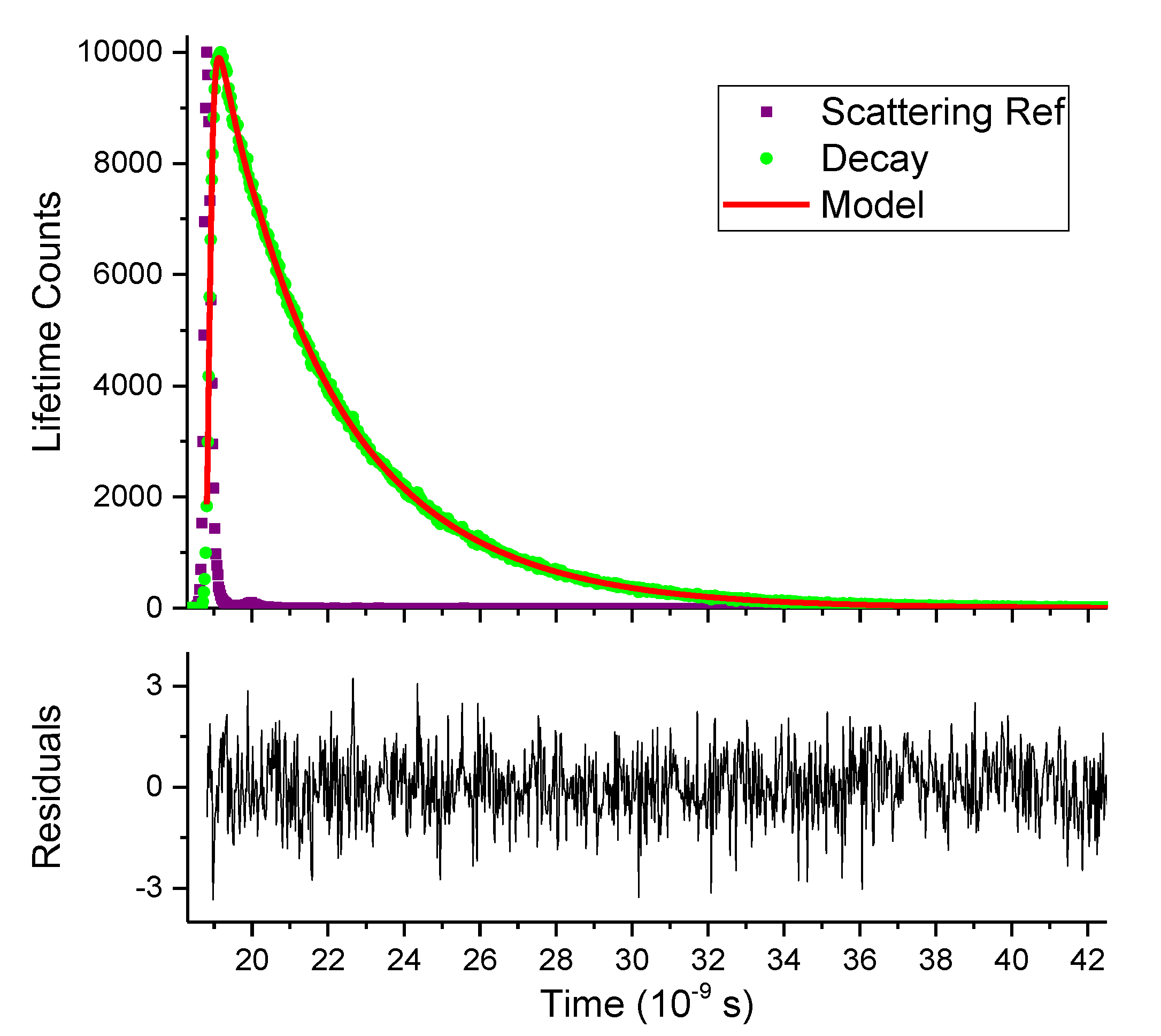
2.3. Fluorescence Lifetime
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aly, S.M.; Usman, A.; AlZayer, M.; Hamdi, G.A.; Alarousu, E.; Mohammed, O.F. Solvent-Dependent Excited-State Hydrogen Transfer and Intersystem Crossing in 2-(2′-Hydroxyphenyl)-Benzothiazole. J. Phys. Chem. B 2015, 119, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dahal, D.; Abeywickrama, C.; Pang, Y. Progress in Tuning Emission of the Excited-State Intramolecular Proton Transfer (ESIPT)-Based Fluorescent Probes. ACS Omega 2021, 6, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Barbara, P.F.; Walsh, P.K.; Brus, L.E. Picosecond Kinetic and Vibrationally Resolved Spectroscopic Studies of Intramolecular Excited-State Hydrogen Atom Transfer. J. Phys. Chem. 1989, 93, 29–34. [Google Scholar] [CrossRef]
- Abou-Zied, O.K.; Jimenez, R.; Thompson, E.H.Z.; Millar, D.P.; Romesberg, F.E. Solvent-Dependent Photoinduced Tautomerization of 2-(2′-Hydroxyphenyl)Benzoxazole. J. Phys. Chem. A 2002, 106, 3665–3672. [Google Scholar] [CrossRef]
- Mohammed, O.F.; Luber, S.; Batista, V.S.; Nibbering, E.T.J. Ultrafast Branching of Reaction Pathways in 2-(2′-Hydroxyphenyl)Benzothiazole in Polar Acetonitrile Solution. J. Phys. Chem. A 2011, 115(26), 7550–7558. [Google Scholar] [CrossRef] [PubMed]
- Dahal, D.; McDonald, L.; Bi, X.; Abeywickrama, C.; Gombedza, F.; Konopka, M.; Paruchuri, S.; Pang, Y. An NIR-Emitting Lysosome-Targeting Probe with Large Stokes Shift via Coupling Cyanine and Excited-State Intramolecular Proton Transfer. Chem. Commun. 2017, 53, 3697–3700. [Google Scholar] [CrossRef] [PubMed]
- Dahal, D.; Ojha, K.R.; Alexander, N.; Konopka, M.; Pang, Y. An NIR-Emitting ESIPT Dye with Large Stokes Shift for Plasma Membrane of Prokaryotic (E. Coli) Cells. Sens. Actuators B Chem. 2018, 259, 44–49. [Google Scholar] [CrossRef]
- Dahal, D.; Pokhrel, S.; McDonald, L.; Bertman, K.; Paruchuri, S.; Konopka, M.; Pang, Y. NIR-Emitting Hemicyanines with Large Stokes’ Shifts for Live Cell Imaging: From Lysosome to Mitochondria Selectivity by Substituent Effect. ACS Appl. Bio. Mater. 2019, 2, 4037–4043. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.; Dahal, D.; Konopka, M.; Liu, Q.; Pang, Y. An NIR Emitting Styryl Dye with Large Stokes Shift to Enable Co-Staining Study on Zebrafish Neuromast Hair Cells. Bioorg. Chem. 2019, 89, 103040. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dahal, D.; Kuang, Z.; Wang, X.; Song, H.; Guo, Q.; Pang, Y.; Xia, A. Ultrafast Excited State Intramolecular Proton/Charge Transfers in Novel NIR-Emitting Molecules. AIP Adv. 2019, 9, 015229. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Hu, J.; Wang, X.; Jie, J.; Guo, Q.; Chen, C.; Xia, A. Ultrafast Investigation of Intramolecular Charge Transfer and Solvation Dynamics of Tetrahydro [5]-Helicene-Based Imide Derivatives. Sci. Rep. 2016, 6, 24313. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Pang, Y. Molecular Aggregation of Poly[(1,3-Phenyleneethynylene)-Alt-Oligo(2,5-Dialkoxy-1,4-Phenyleneethynylene)]: Effects of Solvent, Temperature, and Polymer Conformation. Macromolecules 2003, 36, 4614–4618. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin, Germany, 2006; Time-Domain Lifetime Measurements; pp. 98–157. [Google Scholar]
- Shynkar, V.V.; Mély, Y.; Duportail, G.; Piémont, E.; Klymchenko, A.S.; Demchenko, A.P. Picosecond Time-Resolved Fluorescence Studies Are Consistent with Reversible Excited-State Intramolecular Proton Transfer in 4‘-(Dialkylamino)-3-Hydroxyflavones. J. Phys. Chem. A 2003, 107, 9522–9529. [Google Scholar] [CrossRef]
- Ramadass, R.; Bereiter-Hahn, J. Photophysical Properties of DASPMI as Revealed by Spectrally Resolved Fluorescence Decays. J. Phys. Chem. B 2007, 111, 7681–7690. [Google Scholar] [CrossRef] [PubMed]
- Glasbeek, M.; Zhang, H. Femtosecond Studies and Intramolecular Configurational Dynamics of Fluorophores in Liquid Solution. Chem. Rev. 2004, 104, 1929. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, B.; Consiglio, G.; Elisei, F.; Fortuna, C.G.; Mazzucato, U.; Spalletti, A. Intramolecular Charge Transfer of Push–Pull Pyridinium Salts in the Singlet Manifold. J. Phys. Chem. A 2014, 118, 3580–3592. [Google Scholar] [CrossRef] [PubMed]
- Pines, D.; Pines, E.; Rettig, W. Dual Fluorescence and Excited-State Structural Relaxations in Donor–Acceptor Stilbenes. J. Phys. Chem. A 2003, 107, 236. [Google Scholar] [CrossRef]





| Compound | Methylene Chloride | Acetonitrile | Methanol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| λabs/nm | λem/nm | Φfl | λabs/nm | λem/nm | Φfl | λabs/nm | λem/nm | Φfl | |
| 1 | 421 | 686 | 0.34 | 397 | 690 | 0.19 | 401 | 695 | 0.18 |
| 2 | 413 | 678 | 0.27 | 391 | 683 | 0.28 | 394 | 693 | 0.14 |
| 3 | 447 | 683 | 0.32 | 423 | 704 | 0.21 | 423 | 707 | 0.15 |
| 4 | 436 | 675 | 0.28 | 417 | 700 | 0.19 | 416 | 692 | 0.15 |
| 5 | 447 | 580 | 0.009 | 416 | 568 | 0.008 | 425 | 576 | 0.007 |
| 6 | 399 | 587/660 | 0.0021 | 379 | 575/696 | 0.0020 | 382 | 572/690 | 0.0015 |
| Pyridinium-Based Stilbenes a | Lifetime (ps) | Transient |
|---|---|---|
 | τ1 = 0.70 | Solv |
| τ2 = 52 | 1LE b | |
| τ3 = 134 | 1ICT | |
 | τ1 = 0.68 | Solv |
| τ2 = 10 | 1LE | |
| τ3 = 172 | 1ICT |
| Compound | Lifetime (ns) | Normalized Pre-Exponential | Fractional Intensities | (ns) | χ2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| τ1 | τ2 | τ3 | α1 | α2 | α3 | f1 | f2 | f3 | |||
| 1 | 1.05 | 3.38 | 0.17 | 0.83 | 5.84% | 94.16% | 2.99 | 1.02 | |||
| 2 | 1.27 | 3.61 | 0.14 | 0.86 | 5.52% | 94.48% | 3.27 | 1.03 | |||
| 3 | 2.04 | 2.75 | 0.20 | 0.80 | 15.84% | 84.16% | 2.60 | 1.14 | |||
| 4 | 1.70 | 3.07 | 0.68 | 0.32 | 53.97% | 46.03% | 2.14 | 1.02 | |||
| 5 | 0.10 | 0.24 | 1.77 | 0.53 | 0.47 | 0.00 | 32.04% | 66.62% | 1.33% | 0.17 | 1.00 |
| 6 * | 0.11 | 1.41 | 2.61 | 0.45 | 0.43 | 0.12 | 5.30% | 63.24% | 31.47% | 0.96 | 1.12 |
| 6 ** | 0.10 | 1.35 | 2.67 | 0.27 | 0.47 | 0.26 | 1.99% | 46.74% | 51.27% | 1.35 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Dahal, D.; Pang, Y. Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer. Molecules 2023, 28, 125. https://doi.org/10.3390/molecules28010125
Li Y, Dahal D, Pang Y. Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer. Molecules. 2023; 28(1):125. https://doi.org/10.3390/molecules28010125
Chicago/Turabian StyleLi, Yonghao, Dipendra Dahal, and Yi Pang. 2023. "Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer" Molecules 28, no. 1: 125. https://doi.org/10.3390/molecules28010125
APA StyleLi, Y., Dahal, D., & Pang, Y. (2023). Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer. Molecules, 28(1), 125. https://doi.org/10.3390/molecules28010125





