Synthetic Analogs of Marine Alkaloid Aplysinopsin Suppress Anti-Apoptotic Protein BCL2 in Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Aplysinopsin Analogs Induced Cytotoxic Activity against Prostate Cancer
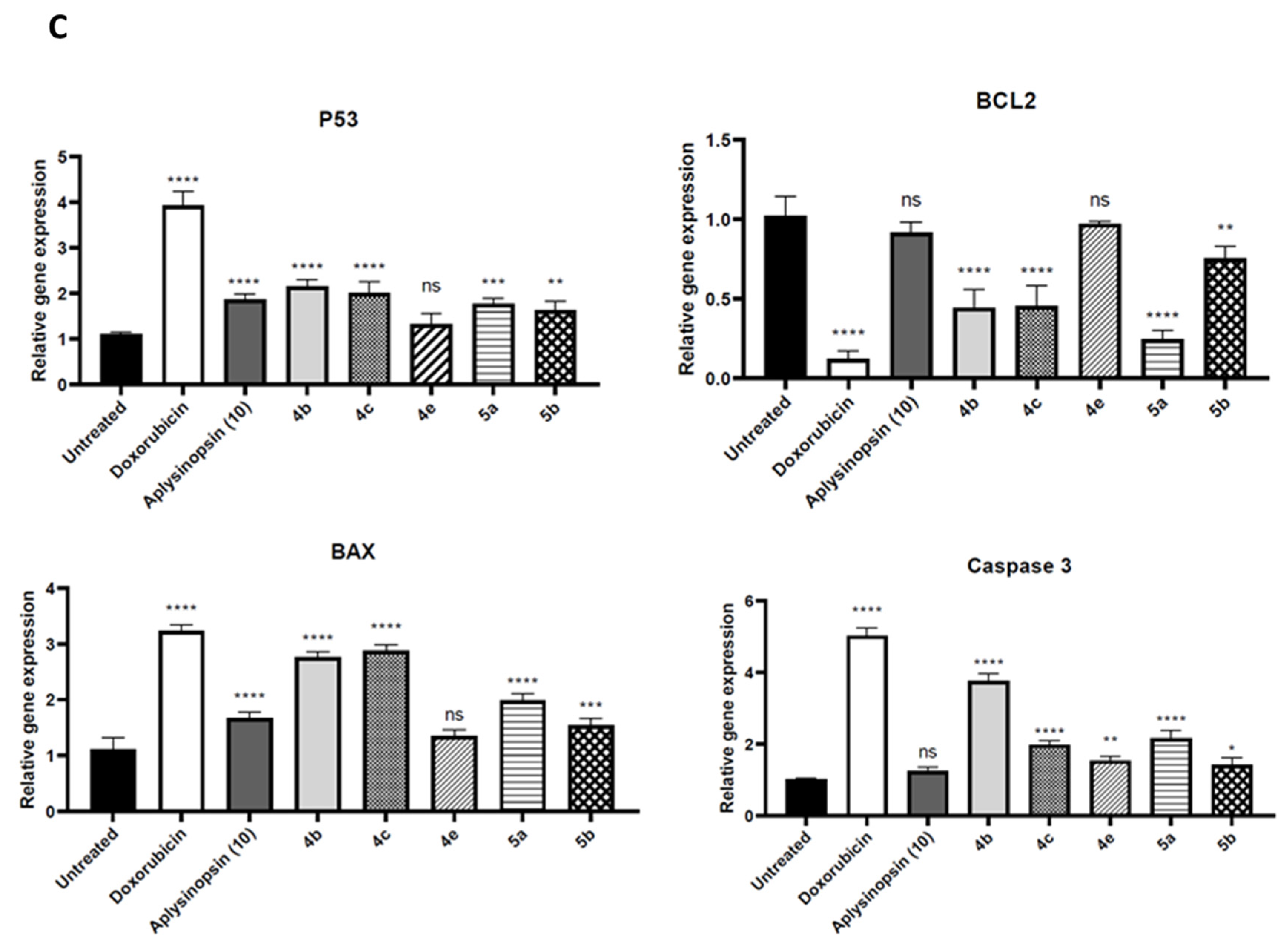
2.2. Aplysinopsin Analogs Induced Apoptosis in Prostate Cancer
2.3. Aplysinopsin Analogs Promoted Cell Cycle Arrest at S-Phase in Prostate Cancer
2.4. Bioavailability of Aplysinopsin Analogs According to Lipinski’s Rule of Five
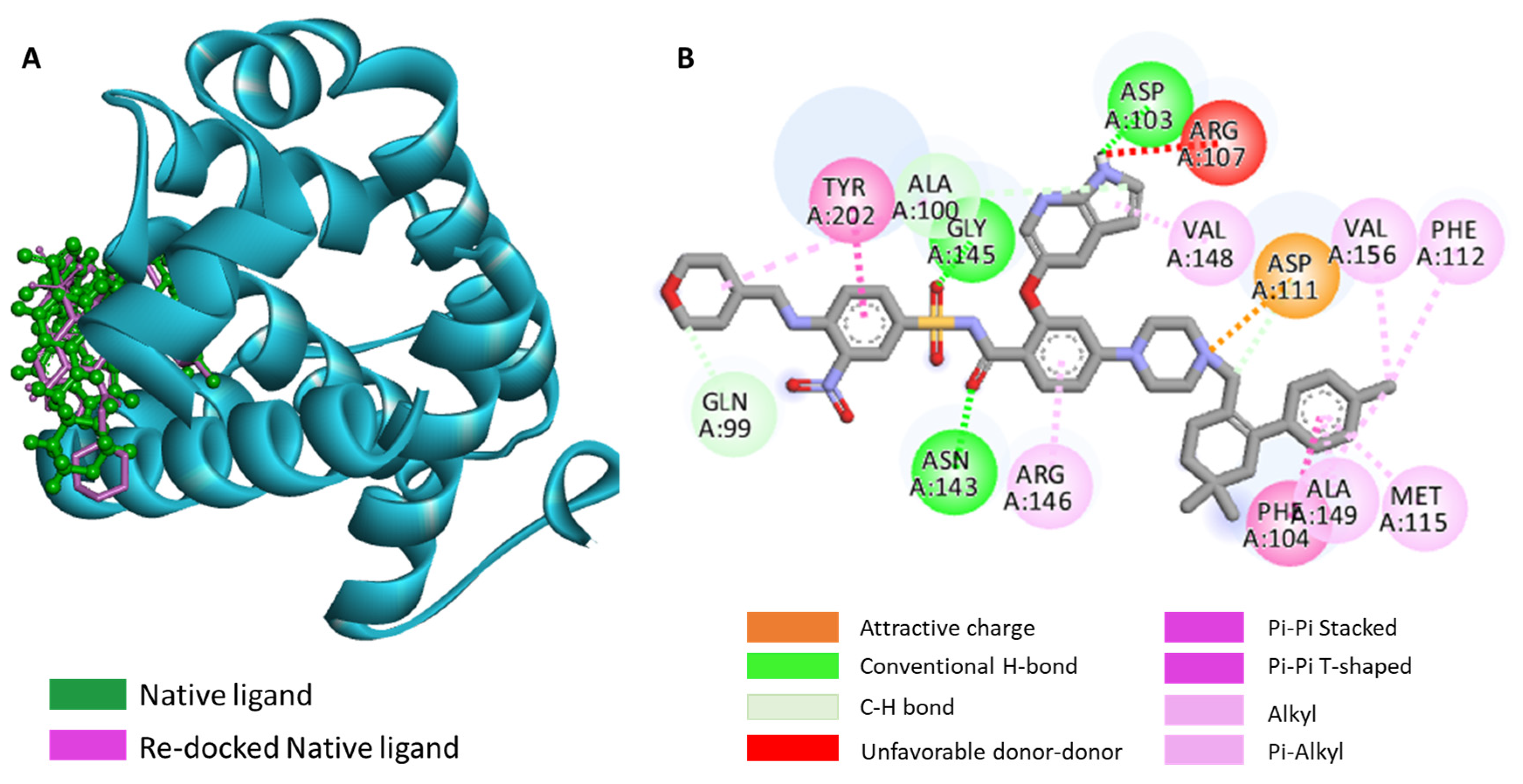
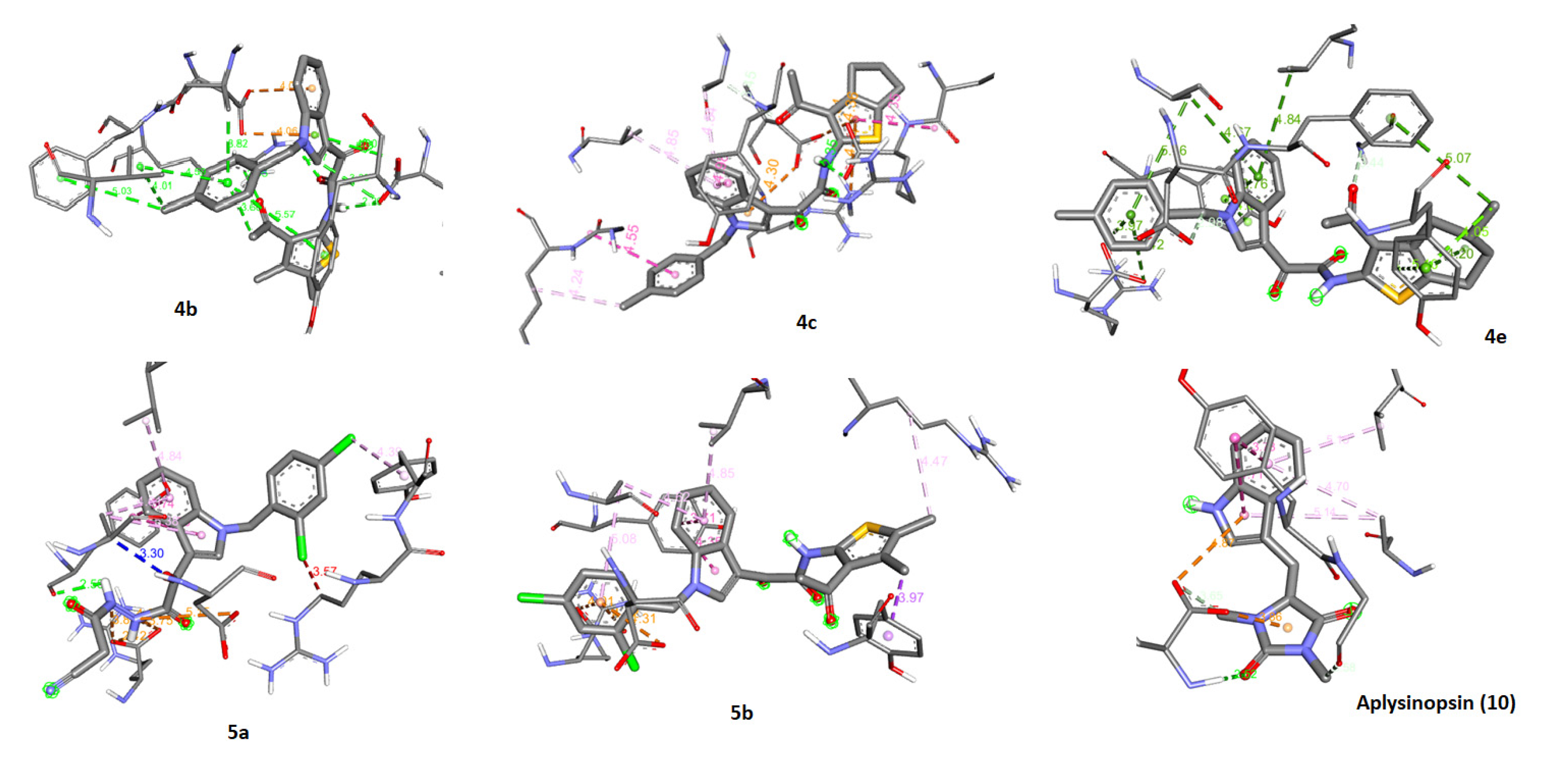
2.5. Molecular Docking of Aplysinopsin (10) and Its Analogs 4b, 4c, 4e, 5a, and 5b towrds Anti-Apoptotic Protein BCL2
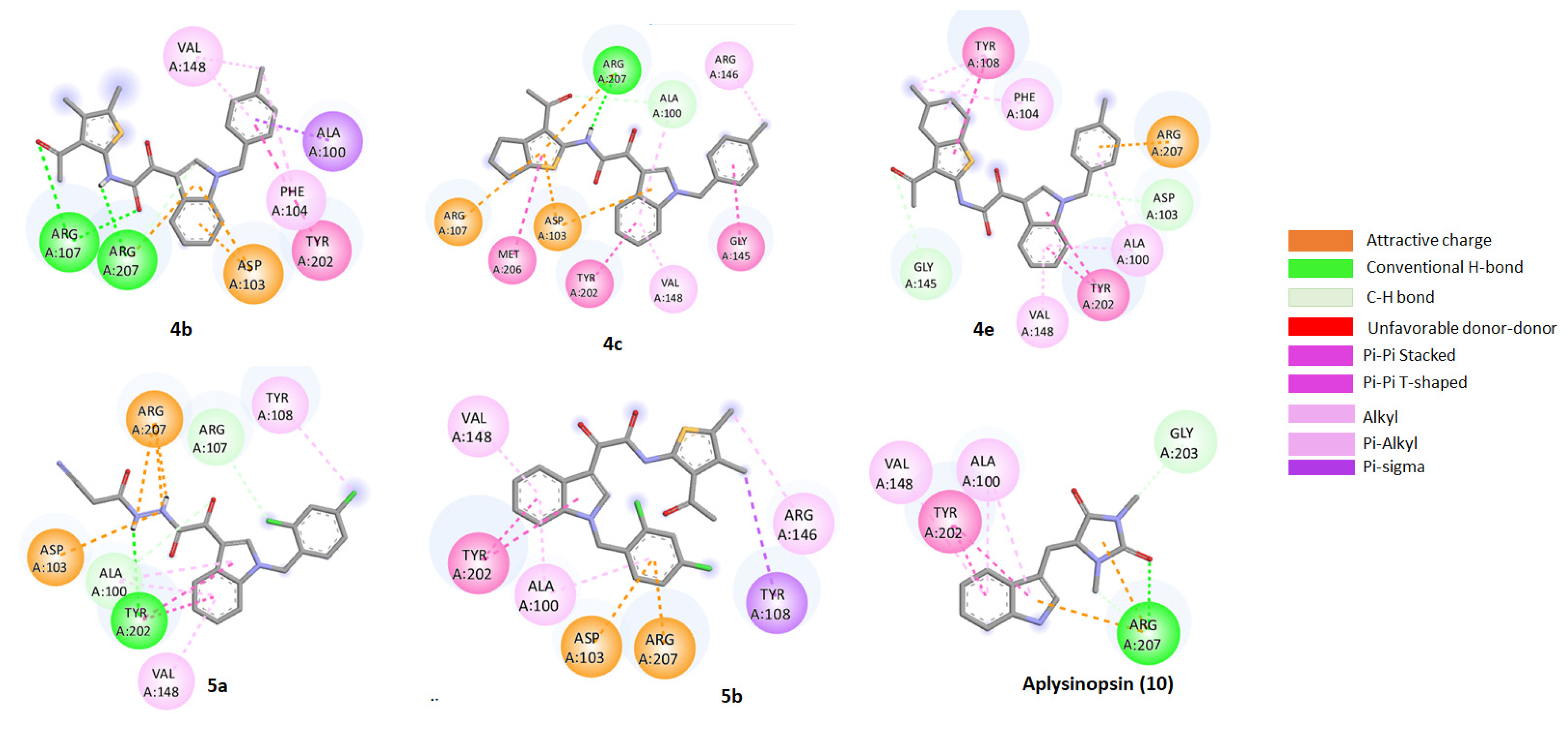
2.6. Assessment of the Binding Mode of the Studied compounds with BCL2 (PDB ID: 6O0K)
3. Discussion
4. Materials and Methods
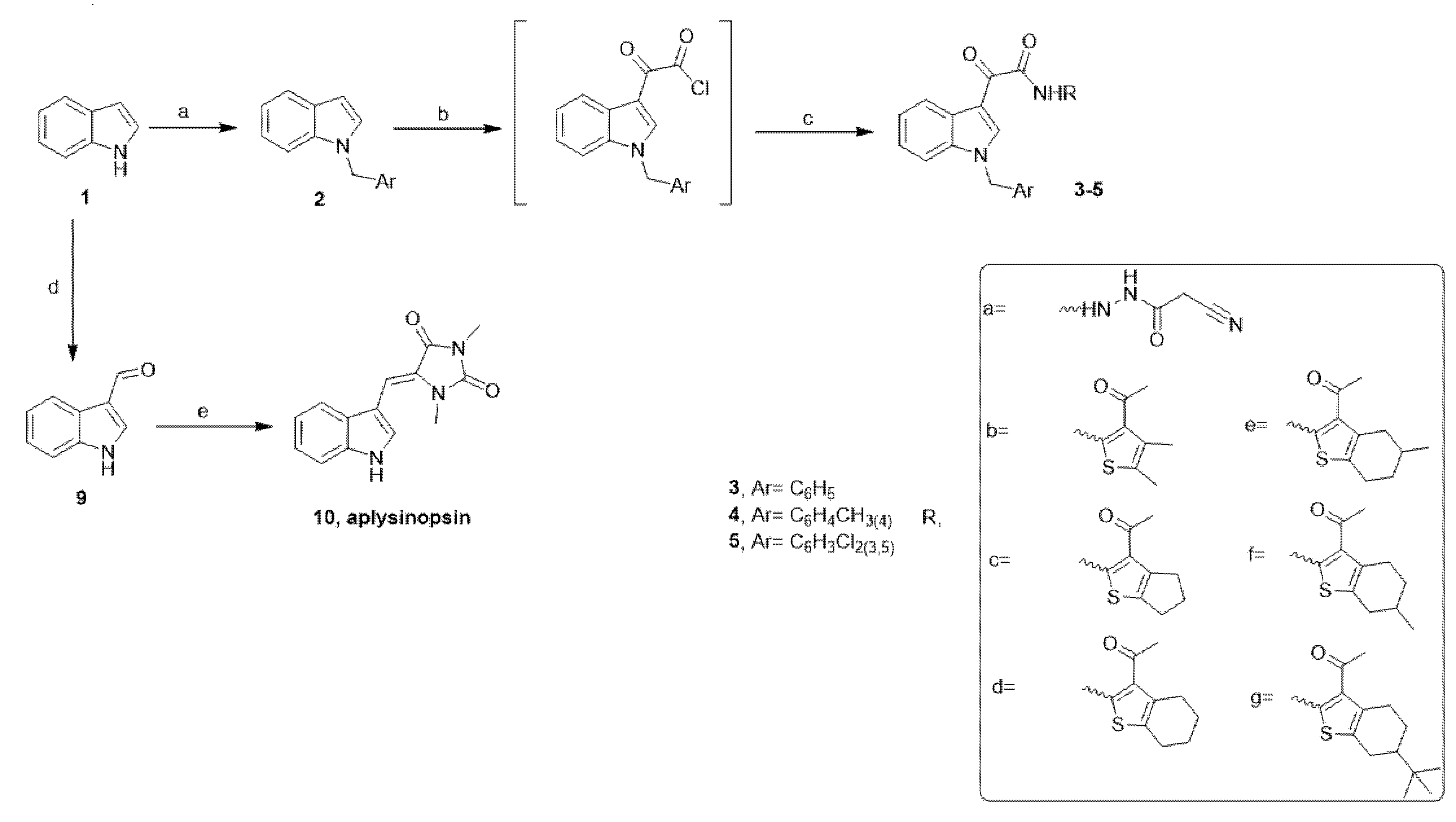
4.1. Chemistry
4.1.1. General Information
4.1.2. General Procedure for the Preparation of Aplysinopsin Analogs (EE) 3c–g, 4b–g and 5a–g
4.1.3. Preparation of (Z)-5-((1H-indol-3-yl)methylene)-1,3-dimethylimidazolidine-2,4-dione (Aplysinopsin, 10) [26,51]
4.2. Biological Assays
Chemicals
4.3. Cell Lines and Cell Cultures
4.4. Cell Proliferation and Viability
4.5. Determination of IC50 Values
4.6. Gene Expression Analysis by Quantitative Real-Time PCR Method (qPCR)
4.7. RNA Isolation
4.8. Reverse Transcription (RT) Reaction
4.9. Cell Cycle Analysis by Flow Cytometry Assay
4.10. In Silico Prediction for the Drug-Likeness
4.11. Molecular Docking
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Litwin, M.S.; Tan, H.-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Prim. 2021, 7, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Elancheran, R.; Saravanan, K.; Divakar, S.; Kumari, S.; Maruthanila, V.L.; Kabilan, S.; Ramanathan, M.; Devi, R.; Kotoky, J. Design, Synthesis and Biological Evaluation of Novel 1, 3- Thiazolidine-2, 4-Diones as Anti-Prostate Cancer Agents. Anti-Cancer Agents Med. Chem. 2017, 17, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Sumanasuriya, S.; De Bono, J. Treatment of Advanced Prostate Cancer—A Review of Current Therapies and Future Promise. Cold Spring Harb. Perspect. Med. 2018, 8, a030635. [Google Scholar] [CrossRef]
- Semenas, J.; Allegrucci, C.; Boorjian, S.A.; Mongan, N.P.; Persson, J.L. Overcoming Drug Resistance and Treating Advanced Prostate Cancer. Curr. Drug Targets 2012, 13, 1308–1323. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.A.; Huang, D.; Roberts, A. Targeting BCL2 for the Treatment of Lymphoid Malignancies. Semin. Hematol. 2014, 51, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of Action of Bcl-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef] [Green Version]
- Sobol, B.; Azzam Nieto, O.; Eberlein, E.L.; Scherr, A.-L.; Ismail, L.; Kessler, A.; Nader, L.; Schwab, M.; Hoffmeister, P.; Schmitt, N.; et al. Specific Targeting of Antiapoptotic Bcl-2 Proteins as a Radiosensitizing Approach in Solid Tumors. Int. J. Mol. Sci. 2022, 23, 7850. [Google Scholar] [CrossRef]
- Westphal, D.; Kluck, R.M.; Dewson, G. Building Blocks of the Apoptotic Pore: How Bax and Bak Are Activated and Oligomerize during Apoptosis. Cell Death Differ. 2014, 21, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 Domains Either Sensitize or Activate Mitochondrial Apoptosis, Serving as Prototype Cancer Therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Kuwana, T.; Bouchier-Hayes, L.; Chipuk, J.E.; Bonzon, C.; Sullivan, B.A.; Green, D.R.; Newmeyer, D.D. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol. Cell 2005, 17, 525–535. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Lukić Bilela, L.; Moulin, C.; Taffin-de-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- De Rop, A.-S.; Rombaut, J.; Willems, T.; De Graeve, M.; Vanhaecke, L.; Hulpiau, P.; De Maeseneire, S.L.; De Mol, M.L.; Soetaert, W.K. Novel Alkaloids from Marine Actinobacteria: Discovery and Characterization. Mar. Drugs 2022, 20, 6. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Pateiro, M.; Conte-Junior, C.A.; Domínguez, R.; Nawaz, A.; Walayat, N.; Movilla Fierro, E.; Lorenzo, J.M. Marine Alkaloids: Compounds with In Vivo Activity and Chemical Synthesis. Mar. Drugs 2021, 19, 374. [Google Scholar] [CrossRef]
- Jaromin, A.; Czopek, A.; Parapini, S.; Basilico, N.; Misiak, E.; Gubernator, J.; Zagórska, A. Synthesis and Antiplasmodial Activity of Novel Bioinspired Imidazolidinedione Derivatives. Biomolecules 2021, 11, 33. [Google Scholar] [CrossRef]
- Campos, P.-E.; Pichon, E.; Moriou, C.; Clerc, P.; Trépos, R.; Frederich, M.; De Voogd, N.; Hellio, C.; Gauvin-Bialecki, A.; Al-Mourabit, A. New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis Reticulata. Mar. Drugs 2019, 17, 167. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.C.; Resende, D.I.S.P.; da Costa, P.M.; Pinto, M.M.M.; Sousa, E. Tryptophan Derived Natural Marine Alkaloids and Synthetic Derivatives as Promising Antimicrobial Agents. Eur. J. Med. Chem. 2021, 209, 112945. [Google Scholar] [CrossRef]
- Hong, A.; Tu, L.C.; Yang, I.; Lim, K.-M.; Nam, S.-J. Marine Natural Products with Monoamine Oxidase (MAO) Inhibitory Activity. Pharm. Biol. 2020, 58, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Lewellyn, K.; Bialonska, D.; Loria, M.J.; White, S.W.; Sufka, K.J.; Zjawiony, J.K. In Vitro Structure–Activity Relationships of Aplysinopsin Analogs and Their in Vivo Evaluation in the Chick Anxiety–Depression Model. Bioorganic Med. Chem. 2013, 21, 7083–7090. [Google Scholar] [CrossRef] [PubMed]
- Nuthakki, V.K.; Yadav Bheemanaboina, R.R.; Bharate, S.B. Identification of Aplysinopsin as a Blood-Brain Barrier Permeable Scaffold for Anti-Cholinesterase and Anti-BACE-1 Activity. Bioorganic Chem. 2021, 107, 104568. [Google Scholar] [CrossRef]
- Penthala, N.R.; Yerramreddy, T.R.; Crooks, P.A. Synthesis and in Vitro Screening of Novel N-Benzyl Aplysinopsin Analogs as Potential Anticancer Agents. Bioorganic Med. Chem. Lett. 2011, 21, 1411–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirupathi Reddy, Y.; Narsimha Reddy, P.; Koduru, S.; Damodaran, C.; Crooks, P.A. Aplysinopsin Analogs: Synthesis and Anti-Proliferative Activity of Substituted (Z)-5-(N-Benzylindol-3-Ylmethylene)Imidazolidine-2,4-Diones. Bioorganic Med. Chem. 2010, 18, 3570–3574. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Kim, S.; El-Sawy, E.R.; Cerella, C.; Orlikova-Boyer, B.; Kirsch, G.; Christov, C.; Dicato, M.; Diederich, M. Anti-Leukemic Properties of Aplysinopsin Derivative EE-84 Alone and Combined to BH3 Mimetic A-1210477. Mar. Drugs 2021, 19, 285. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Porwal, S.; Chauhan, S.S.; Chauhan, P.M.S.; Shakya, N.; Verma, A.; Gupta, S. Discovery of Novel Antileishmanial Agents in an Attempt to Synthesize Pentamidine−Aplysinopsin Hybrid Molecule. J. Med. Chem. 2009, 52, 5793–5802. [Google Scholar] [CrossRef]
- Passemar, C.; Saléry, M.; Soh, P.N.; Linas, M.-D.; Ahond, A.; Poupat, C.; Benoit-Vical, F. Indole and Aminoimidazole Moieties Appear as Key Structural Units in Antiplasmodial Molecules. Phytomedicine 2011, 18, 1118–1125. [Google Scholar] [CrossRef]
- Singh, S.N.; Bhatnagar, S.; Fatma, N.; Chauhan, P.M.; Chatterjee, R.K. Antifilarial Activity of a Synthetic Marine Alkaloid, Aplysinopsin (CDRI Compound 92/138). Trop. Med. Int. Health 1997, 2, 535–543. [Google Scholar] [CrossRef]
- Lewellyn, K.; Bialonska, D.; Chaurasiya, N.D.; Tekwani, B.L.; Zjawiony, J.K. Synthesis and Evaluation of Aplysinopsin Analogs as Inhibitors of Human Monoamine Oxidase A and B. Bioorganic Med. Chem. Lett. 2012, 22, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.F.; Canseco, D.C.; Sheth, P.; Johnson, J.E.; Schetz, J.A. Synthesis and Structure-Affinity Relationships of Novel Small Molecule Natural Product Derivatives Capable of Discriminating between Serotonin 5-HT1A, 5-HT2A, 5-HT2C Receptor Subtypes. Bioorganic Med. Chem. 2010, 18, 4783–4792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penthala, N.R.; Yerramreddy, T.R.; Crooks, P.A. Microwave Assisted Synthesis and in Vitro Cytotoxicities of Substituted (Z)-2-Amino-5-(1-Benzyl-1H-Indol-3-Yl)Methylene-1-Methyl-1H-Imidazol-4 (5H)-Ones Against Human Tumor Cell Lines. Bioorganic Med. Chem. Lett. 2010, 20, 591–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcante, G.; Schaan, A.; Cabral, G.; Santana-da-Silva, M.; Pinto, P.; Vidal, A.; Ribeiro-Dos-Santos, A. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [Green Version]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Fridman, J.S.; Lowe, S.W. Control of Apoptosis by P53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [Green Version]
- Vousden, K.H.; Lane, D.P. P53 in Health and Disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Raimondi, M.V.; Bivacqua, R.; Giuffrida, S.; Montalbano, A.; Cavalli, A.; Bertoni, F.; Barraja, P. GPCR Inhibition in Treating Lymphoma. ACS Med. Chem. Lett. 2022, 13, 358–364. [Google Scholar] [CrossRef]
- Spanò, V.; Rocca, R.; Barreca, M.; Giallombardo, D.; Montalbano, A.; Carbone, A.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Bai, R.; et al. Pyrrolo[2′,3′:3,4]Cyclohepta[1,2-d][1,2]Oxazoles, a New Class of Antimitotic Agents Active against Multiple Malignant Cell Types. J. Med. Chem. 2020, 63, 12023–12042. [Google Scholar] [CrossRef]
- Wu, M.-K.; Man, R.-J.; Liao, Y.-J.; Zhu, H.-L.; Zhou, Z.-G. Discovery of Novel Indole-1,2,4-Triazole Derivatives as Tubulin Polymerization Inhibitors. Drug Dev. Res. 2021, 82, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct Activation of Bax by P53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasul, A.; Yu, B.; Zhong, L.; Khan, M.; Yang, H.; Ma, T. Cytotoxic Effect of Evodiamine in SGC-7901 Human Gastric Adenocarcinoma Cells via Simultaneous Induction of Apoptosis and Autophagy. Oncol. Rep. 2012, 27, 1481–1487. [Google Scholar] [CrossRef]
- Masuelli, L.; Pantanella, F.; La Regina, G.; Benvenuto, M.; Fantini, M.; Mattera, R.; Di Stefano, E.; Mattei, M.; Silvestri, R.; Schippa, S.; et al. Violacein, an Indole-Derived Purple-Colored Natural Pigment Produced by Janthinobacterium Lividum, Inhibits the Growth of Head and Neck Carcinoma Cell Lines Both in Vitro and in Vivo. Tumour Biol. 2016, 37, 3705–3717. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Berneman, Z.N.; Van Bockstaele, D.R. Cell Cycle and Apoptosis. Cell Prolif. 2003, 36, 165–175. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Cappadone, C.; Stefanelli, C.; Malucelli, E.; Zini, M.; Onofrillo, C.; Locatelli, A.; Rambaldi, M.; Sargenti, A.; Merolle, L.; Farruggia, G.; et al. P53-Dependent and P53-Independent Anticancer Activity of a New Indole Derivative in Human Osteosarcoma Cells. Biochem. Biophys. Res. Commun. 2015, 467, 348–353. [Google Scholar] [CrossRef]
- Bondock, S.; Tarhoni, A.E.-G.; Fadda, A.A. Utility of Cyanoacetic Acid Hydrazide in Heterocyclic Synthesis. ChemInform 2007, 38, 113–156. [Google Scholar] [CrossRef]
- Ottoni, O.; Cruz, R.; Alves, R. Efficient and Simple Methods for the Introduction of the Sulfonyl, Acyl and Alkyl Protecting Groups on the Nitrogen of Indole and Its Derivatives. Tetrahedron 1998, 54, 13915–13928. [Google Scholar] [CrossRef]
- EJames, P.N.; Snyder, H.R.; Boekelheide, V.; Knowles, R.N. Indole-3-Aldehyde. Org. Synth. 1959, 39, 30. [Google Scholar] [CrossRef]
- Tymiak, A.A.; Rinehart, K.L.; Bakus, G.J. Constituents of Morphologically Similar Sponges: Aplysina and Smenospongia Species. Tetrahedron 1985, 41, 1039–1047. [Google Scholar] [CrossRef]
- Thabrew, M.I.; Hughes, R.D.; McFarlane, I.G. Screening of Hepatoprotective Plant Components Using a HepG2 Cell Cytotoxicity Assay. J. Pharm. Pharmacol. 1997, 49, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- De Oliveira, S.F.V.; Ganzinelli, M.; Chilà, R.; Serino, L.; Maciel, M.E.; Urban, C.d.A.; Lima, R.S.d.; Cavalli, I.J.; Generali, D.; Broggini, M.; et al. Characterization of MTAP Gene Expression in Breast Cancer Patients and Cell Lines. PLoS ONE 2016, 11, e0145647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.J.; Majumdar, A.P.N.; Nechvatal, J.M.; Ram, J.L.; Basson, M.D.; Heilbrun, L.K.; Kato, I. Exfoliated Cells in Stool: A Source for Reverse Transcription-PCR–Based Analysis of Biomarkers of Gastrointestinal Cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 455–458. [Google Scholar] [CrossRef] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef] [PubMed]



| Lipinski’s rule of five | aplysinopsin (10) | 4b | 4c | 4e | 5a | 5b |
| MW (g/mol) | 255.27 | 444.55 | 456.56 | 484.61 | 429.26 | 499.41 |
| Log Po/w | 0.79 | 2.57 | 2.77 | 3.17 | 1.71 | 3.31 |
| HBA | 1 | 3 | 3 | 3 | 4 | 3 |
| HBD | 2 | 1 | 1 | 1 | 2 | 1 |
| MR | 79.91 | 129.88 | 132.57 | 142.18 | 108.07 | 134.93 |
| No. Lipinski violation | 0 | 0 | 0 | 0 | 0 | 0 |
| Gene | Forward Strand | Reverse Strand |
|---|---|---|
| p53 | 5′-CCCCTCCTGGCCCCTGTCATCTTC-3′ | 5′-GCAGCGCCTCACAACCTCCGTCAT-3′ |
| Bcl2 | 5′-CCTGTG GAT GAC TGA GTA CC-3′ | 5′-GAGACA GCC AGG AGA AAT CA-3′ |
| Bax | 5′-GTTTCA TCC AGG ATC GAG CAG-3′ | 5′-CATCTT CTT CCA GAT GGT GA-3′ |
| Caspase-3 | 5′-GGAAGCGAATCAATGGACTCTGG-3′ | 5′-GCATCGACATCTGTACCAGACC-3′ |
| β -actin | 5′-GTGACATCCACACCCAGAGG-3′ | 5′-ACAGGATGTCAAAACTGCCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sawy, E.R.; El-Shahid, Z.A.; Soliman, A.A.F.; Nassrallah, A.; Abdelwahab, A.B.; Kirsch, G.; Abdelmegeed, H. Synthetic Analogs of Marine Alkaloid Aplysinopsin Suppress Anti-Apoptotic Protein BCL2 in Prostate Cancer. Molecules 2023, 28, 109. https://doi.org/10.3390/molecules28010109
El-Sawy ER, El-Shahid ZA, Soliman AAF, Nassrallah A, Abdelwahab AB, Kirsch G, Abdelmegeed H. Synthetic Analogs of Marine Alkaloid Aplysinopsin Suppress Anti-Apoptotic Protein BCL2 in Prostate Cancer. Molecules. 2023; 28(1):109. https://doi.org/10.3390/molecules28010109
Chicago/Turabian StyleEl-Sawy, Eslam R., Zeinab A. El-Shahid, Ahmed A. F. Soliman, Amr Nassrallah, Ahmed B. Abdelwahab, Gilbert Kirsch, and Heba Abdelmegeed. 2023. "Synthetic Analogs of Marine Alkaloid Aplysinopsin Suppress Anti-Apoptotic Protein BCL2 in Prostate Cancer" Molecules 28, no. 1: 109. https://doi.org/10.3390/molecules28010109
APA StyleEl-Sawy, E. R., El-Shahid, Z. A., Soliman, A. A. F., Nassrallah, A., Abdelwahab, A. B., Kirsch, G., & Abdelmegeed, H. (2023). Synthetic Analogs of Marine Alkaloid Aplysinopsin Suppress Anti-Apoptotic Protein BCL2 in Prostate Cancer. Molecules, 28(1), 109. https://doi.org/10.3390/molecules28010109








