Abstract
Nanomedicine is an emerging field with continuous growth and differentiation. Liposomal formulations are a major platform in nanomedicine, with more than fifteen FDA-approved liposomal products in the market. However, as is the case for other types of nanoparticle-based delivery systems, liposomal formulations and manufacturing is intrinsically complex and associated with a set of dependent and independent variables, rendering experiential optimization a tedious process in general. Quality by design (QbD) is a powerful approach that can be applied in such complex systems to facilitate product development and ensure reproducible manufacturing processes, which are an essential pre-requisite for efficient and safe therapeutics. Input variables (related to materials, processes and experiment design) and the quality attributes for the final liposomal product should follow a systematic and planned experimental design to identify critical variables and optimal formulations/processes, where these elements are subjected to risk assessment. This review discusses the current practices that employ QbD in developing liposomal-based nano-pharmaceuticals.
1. Introduction
Nanomedicine and nanoparticle-based therapeutics are gaining increasing interest in both academia and industry. Currently, there are many FDA-approved nanomedicine products with proven clinical outcomes [1]. Liposomes are spherical vesicles of a continuous three-dimensional phospholipids bilayer wrapping an aqueous core [2]. Liposomes have been used to deliver a wide range of therapeutics [3]. For example, liposomes have been successfully loaded with the anticancer agent, doxorubicin, and showed enhanced therapeutic efficacy and decreased unwanted side effects [4]. Moreover, they have been widely investigated as carriers of nucleic acid-based therapies, such as siRNA [5] and DNA, enabling enhanced penetration in targeted cells and protecting drugs from degradation [5]. Liposomes were one of the first nanotechnology-platforms that entered the market early in 1995 and is still one of the major nano-platforms [1]. It is worth mentioning that the first FDA-approved mRNA vaccine for COVID-19 was approved in 2020 utilizes lipidic/liposomal nanocarriers as a delivery system [6]. Despite the outstanding properties of liposomes, the complexity in their formulations, product development and manufacturing are clearly challenging. The explanation of increased complexity in the case of nano-formulations/nanomanufacturing is associated with the unique physics and chemistry at the nanoscale and thus a higher number of variables needed to be understood and optimized [7]. Lack of this understanding and optimization is the reason behind the common sensitivity and poor reproducibility in nano-preparations and manufacturing. For these systems, an experimental approach that facilitates the identification of critical parameters and help in understanding their contributions to the characteristics/quality of the final product is certainly beneficial. For this purpose, the quality by design (QbD) has been proposed and recommended by various industries and regulatory agencies [8,9]. QbD starts by identifying the quality target product profile (QTPP), which is a summary of the quality attributes (QA) of the final product to ensure its efficacy and safety. QA is dependent on critical attributes related to the material attributes (CMA) and process parameters (CPP). QbD follows by identifying and optimizing CMA and CPP and setting their target specifications to ensure the QA and ultimately QTPP for the final product [9,10,11]. Proper experimental design is used to link CMA and CPP to QA [8,12], which then facilitate the establishment of targeted specifications for materials, processes and the final product. Moreover, QbD enables the evaluation of the effect of more than one factor at a time on the QTPP. Additionally, risk assessments are used to prioritize QA [13]. Considering the potent liposomal-based drug products in clinical use and the diverse clinical and pre-clinical applications, there is an unmet need for strategic and systematic development of liposomes as potent drug delivery systems that enable better therapeutic efficacy of the loaded therapies. Although applying QbD liposomal drug delivery systems development have been described in several research, there is more and more need to understand and describe current advances in using QbD in liposomal formulation developments to guarantee liposomal-based drug delivery systems with higher therapeutic outcomes and possible industrial development. Therefore, this review highlights the main strategic points of developing liposomes according to the QbD to reduce the obstacles of using such vehicles in clinical applications in the future.
2. Quality by Design (QbD)
2.1. QbD in Pharmaceutical Products
The production of quality pharmaceutical products is the major goal of pharmaceutical industry [14]. The quality of the pharmaceutical products covers all aspects that may have an impact on the prescribed products which will consequently affect the health of the patients. Previously, the quality by testing method (QbT) was the common method to ensure quality of the manufactured products. QbT is based on an in-process testing of input materials, intermediates and the final product [15]. However, the pharmaceutical quality sectors call for an alternative practice that can ensure the quality before manufacturing in addition to maintaining the required quality control testing suggested by QbT. To this end, the current pharmaceutical industry and regulation firms switch toward what is now known as the QbD, which ensures that pharmaceutical products will be developed and manufactured as per pre-defined quality attributes, thus QbD is expected to minimize intensive testing during or after manufacturing as well as improve reproducibility, manufacturability, efficacy and safety [16]. Therefore, QbD can be defined as a prospective approach to improve product quality [17]. ICH, US FDA and EMA have specified thoroughly the outlines of the QbD key elements to ensure the consistency of high-quality pharmaceutical products (Figure 1), reflecting a continuous interest in QbD implementation by various international regulatory bodies [16,18].
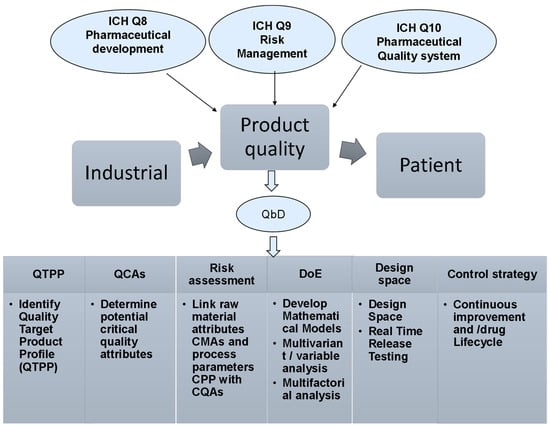
Figure 1.
The pharmaceutical development guidelines suggested by ICH, US FDA and EMA to outline the QbD key elements to ensure the consistency of high-quality pharmaceutical products.
2.2. Tools and Key Elements of QbD
Generally, there are four key elements of the QbD: (i) the quality target product profile (QTPP), (ii) the critical quality attributes (CQAs), (iii) the critical material attributes (CMAs) and (iv) the critical process parameters (CPPs) [12,19,20]. All of these elements are collaborating in a step-by-step approach to draw the framework of the QbD strategy. The recruitment of these key elements in the QbD method needs well-defined experimental design combined to proper statistical analysis (Figure 2) [16,21].
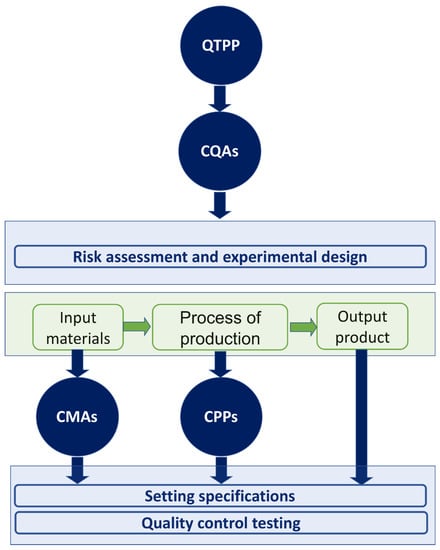
Figure 2.
QbD roadmap, and QTPP and CQAs key elements.
ICH guidelines define QTPP as “a prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficiency of the drug product” [16,18]. To identify QTPPs and define the desired performance of the product, the manufacturer should consider complex variables, such as drug pharmacokinetic parameters, product stability, sterility and drug release [22]. The critical quality attributes (CQAs) were defined by ICH Q8 guideline as “physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality.” In light of this definition, the CQAs are derived from the QTPP, regulatory requirements, or available literature knowledge. Thus, the critical Quality attribute (CQA) of the drug product and its QTPP is the basis of its dosage form, excipient and manufacturing process selection [23].
The critical process parameters (CPPs) are the process-related parameters that significantly affect the QTPP [16]. The identification of CPPs, an in-depth understanding of the developed standards/specifications, and linking CMAs and CPPs to CQAs are crucial to ensure quality products [24]. Furthermore, both critical material attributes (CMAs) and critical process parameters (CPPs) are generally defined as “A material or process whose variability has an impact on a critical quality attribute and should be monitored or controlled to ensure the desired drug product quality” [23]. It is worth mentioning that CMAs are for the input materials including drug substances, excipients, in-process materials, while CQAs are for output materials, i.e., the product.
Implementing a risk assessment is vital to identify formulations, ingredients, or process parameters that can impact CQAs after the risk analysis appraises the impact of these parameters on the CQAs. Additionally, a qualitative or quantitative scale is used to rate the risk of each identified factor for the desired CQAs. For this reason, a risk assessment scale has to be established based on the severity and dubiety of the impact on efficacy and safety. Effect analysis and the Failer mode can be used to identify CQAs. After the risk evaluation process a few of these parameters become potentially critical for the CMAs, which must have certain properties and must be selected within a reasonable range to guarantee the CQAs of the final product [25,26].
3. Development of Liposomes Using QbD
3.1. QbD in Liposomal Formulation
The quality of liposomal pharmaceutical products is affected by their contents, preparation, properties and manufacturing key variables [12]. Therefore, QbD involves designing the final liposomal products by optimizing input material and manufacturing processes to acquire a pharmaceutical product with superior quality [27]. Moreover, QbD classifies and translates the critical parameters and key variables to produce a high-quality drug product with the most desired characteristics [28]. Indeed, several liposomal products have been developed using QbD approach, as summarized in Table 1.

Table 1.
Examples of pharmaceutical liposomes developed by QbD.
To certify the desired quality of the final pharmaceutical product, a quality target product profile (QTPP) should be established [27]. QTPP is usually performed based on the available scientific data and proper in vivo significance [25]. To identify the QTPP and the process key parameters that can influence the liposomal product’s quality attributes (CQAs), the following principal CQAs generally should be recognized/optimized: average particle size, particle size distribution, zeta potential, drug content, in vivo stability and drug release [25,38].
Although there are many benefits of applying QbD to liposomal-based products, there are many challenges that limit the application of QbD liposomal-based product development. Benefits and challenges are summarized in Table 2.

Table 2.
Benefit and challenges of applying QbD in of liposomal-based products.
3.2. QbD Process Key Parameters for Liposomal Products
3.2.1. Lipid Type and Content
The integrity and stability of liposomes mainly rely on the lipid type. Lipids with unsaturated fatty acids are susceptible to degradation by hydrolysis or oxidation, while saturated fatty acids are more stable and have higher transition temperature (Tm) [39]. Moreover, liposomes fluidity, permeability and surface charge also count on the lipid type and the liposomal lipidic composition [40]. For example, cholesterol typically increases liposome stability but should be optimized and not exceed 50% [41]. Generally, the carbon chain length of the formulated lipids may affect the drug encapsulation efficiency of both hydrophilic and hydrophobic drugs [40]. For example, a large aqueous core can be obtained using short fatty acid lipids that can enable a high internal volume for hydrophilic drugs. In contrast, long carbon chain lipids are more suitable to encapsulate the hydrophobic drugs within the hydrophobic lipid bilayer [42,43]. Furthermore, the loaded material has a great influence on the morphological features of the particles. The concentration of nucleic acids impacts the change from a multilamellar to an electron-dense morphology in lipidic-based particles [44].
Since 1978, liposomes have been used for the selective insertion of exogenous RNA into cells [45]. Many liposomes have been optimized and fabricated to encapsulate nucleic acids with low toxicity and high efficiency [46]. However, ionized lipids, especially cationic lipids, are still the most used for this purpose [47,48]. Unfortunately, cationic lipids produce many changes in the cell and proteins, such as cell shrinking, reduction in mitoses and changes in protein kinase C and cytoplasm vacuoles [49,50]. On the other hand, compared with viral vectors for gene delivery into cells, cationic lipids are easy to fabricate, simple and possess lower immunogenicity [51]
Both hydrophobic and hydrophilic parts of the cationic lipids have a toxic effect, especially if they contain a quaternary amine that acts as a protein kinase C inhibitor [52]. A new approach to decrease the effect of the positive charge was proposed to spread the charge by delocalizing it into a heterocyclic ring imidazolium [53] and a pyridinium [54]. Chang et. al., developed cationic lipids with a cyclen headgroup and revealed that this novel lipid is safer and possesses lower cytotoxicity than the commonly used lipid to deliver gene therapy [55].
3.2.2. Manufacturing Process
The most commonly used manufacturing process for liposome preparation is the thin-film hydration method (Figure 3) [56,57]. Other approaches such as reverse-phase evaporation, ethanol injection and emulsification have also been applied [58]. The thin-film hydration method produces multilamellar structure liposomes with an average diameter in micrometers [42]. Thus, resizing liposomes to less than 200 nm is required to improve the surface area to volume ratio for superior encapsulation and drug loading efficiency. Improving the size distribution of the prepared liposomes, extrusion, sonication (probe or bath) and freeze–thaw cycling have been used for liposomes size reduction [59].
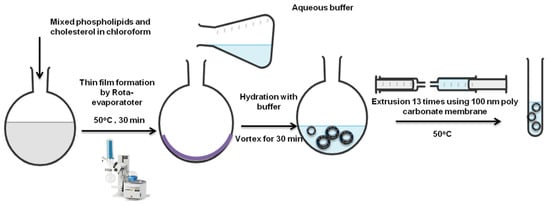
Figure 3.
Liposomes preparation via thin-film hydration extrusion technique [57].
Various parameters can be optimized to achieve a uniform multilamellar thin film followed by proper size reduction [43]. Rota-evaporator temperature, rotation speed and gradual pressure reduction, in addition to membrane pore size, can result in unilamellar, monodispersed liposomes with high encapsulation efficiency [33,60].
3.2.3. Average Particle Size and Nanoparticles Distribution
Average particle size and nanoparticle distribution are considered the main CQAs for all nano-formulations [61]. These parameters play major roles in determining the nanoparticle in vivo distribution, drug loading ability, drug release and targeting capacity [62]. For better biodistribution, the ideal nanocarrier particle size should be in the range of 10 to 100 nm to avoid kidney elimination, escape the reticuloendothelial system (RES) and provide an effective enhanced permeability and retention (EPR) effect [63,64]. A small particle size means a high surface area to volume ratio. This leads to fast drug release due to more drugs close to the surface of the nanoparticles compared to larger ones [65]. However, it is important to keep in mind that for inhaled drug particles to be therapeutically useful, they should be smaller than 2 µm, which is most suitable for deposition in the alveolar [66]. Moreover, liposome delivery through the skin is dependent on size. Liposomes up to 600 nm penetrate through the skin easily, whereas liposomes larger than 1000 nm remain interiorized in the stratum corneum [67]. The polydispersity index (PDI) reflects the homogeneity and size distribution of the nano-dispersions. PDI values of less than 0.3 indicate homogeneous, stable and well-dispersed liposomes [68]. Generally, increasing lipids concentrations can lead to increased liposomal size and PDI values simultaneously [69].
3.2.4. Zeta Potential (ZP)
ZP evaluates the nano-dispersion stability. Neutral nanoparticles have decreased stability and tend to aggregate [70]. A charge greater than +30 or less than −30 mV indicates good stability due to the high electrostatic repulsions [71]. The ZP of the nano-system affects their systemic circulation, interactions with body tissues and cell recognition. For example, the cellular uptake of cationic liposomes is higher compared than anionic liposomes due to the negatively charged cell membrane [72]. Moreover, charged liposomes can exhibit a high encapsulation efficiency for drugs with opposite charges [73]. In order to control the ZP values to achieve maximum stability, fatty acids and hydrophilic polymers of varying change can be incorporated into the liposome formulations [40].
3.2.5. Drug Content
Liposomal drug content can be expressed in three ways: weight per volume (w/v); percentage encapsulation efficiency (EE%, weight of drug entrapped into the liposomes compared to the initial amount of drug used %); and drug loading (DL%, the amount of drug entrapped into the liposomes relative to the initial mass of the lipid used; drug-to-lipid ratio) [62,74]. Improved EE% preserves high concentrations of the precious pharmaceutical agent in liposomes and may reduce the manufacturing cost, thus resulting in enhanced pharmacokinetics and improved patient compliance [75].
Several parameters may influence the drug EE, such as the lipid-to-drug ratio, nature of phospholipids, cholesterol molar ratio and the manufacturing process parameters [76,77]. Increasing the lipid-to-drug ratio leads to an increase in the number of nano-vesicles that are able to entrap more hydrophilic drugs in their aqueous cores [78]. Cholesterol and unsaturated lipids create more pockets within the lipid bilayer, thereby entrapping more hydrophobic drugs [79,80]. Freeze–thaw resizing cycles have also been proven to enhance the EE [81]. Moreover, remote loading approaches into preformed liposomes have been able to raise the EE of ionizable drugs compared to conventional passive loading [82,83].
3.2.6. In Vivo Stability
The hydrophobic/hydrophilic characteristics of the liposomes surface affect liposome interaction with blood components [84]. These interactions are responsible for the in vivo stability of liposomes. Liposomal in vivo stability causes prolonged drug release and enhanced drug localization in the targeted tissue [42]. For example, hydrophobic nanoparticles are easily cleared from blood circulation due to their high ability to bind blood proteins [38]. Moreover, stealth liposomes, usually coated with hydrophilic polymers, show higher in vivo stability with prolonged circulation time that leads to improved therapeutic potential of the encapsulated drug [70].
3.2.7. Drug Release Kinetics
The kinetics of releasing drugs from liposomes is a critical parameter for liposome formulation design and considered a key factor to accomplish optimal efficacy and to minimize drug toxicity [85]. The optimal therapeutic activity of the drug can be achieved when the whole drug delivery system enters the target cells via endocytosis or the drug is released at the proper rate at the site of action for enough time [86]. Furthermore, the liposomes surface can be functionalized with targeting ligands for active drug targeting [87]. These targeting ligands can selectively bind to certain receptors or biomarkers that are overexpressed on cancerous or diseased tissues. These ligands could be antibodies, peptides, oligonucleotides, small carbohydrates, or small organic molecules [88].
Triggered drug release from liposomes could be achieved by incorporating sensitive excipients within liposome structures [89]. These excipients produce a liposomal destabilizing effect upon exposure to specific stimuli, such as light, temperature, radiation or different pH [90,91].
3.3. Product and Process Design Space
For the effective implementation of QbD in liposomal formulation, QTPP should be first defined, then the formulae and manufacturing processes can be selected and designed to ensure achievement of the pre-defined QTPP. Identification of CQAs and CPPs is achieved by an experimental design that is capable of assessing their contribution to the CQAs [62].
DS is performed to assure a high-quality product through demonstrating a range of process and/or formulation parameters [62,75]. DS involves the product and process DS. The product DS is established with the products CQAs as scopes, while the process DS is presented as CQAs related to CPPs [92].
The DS for liposome preparation is established by understanding and controlling the formulations, materials and manufacturing variables. Alina et al. established a DS for lyophilized liposomes with the drug simvastatin [32]. Their DS approach was based on both formulation factors and CPPs. Their results showed that cholesterol molar ratio, the PEG proportion, the cryoprotectant to phospholipids amounts and the number of extrusion cycles were designated as the most significant factors for lyophilized liposome CQAs [32]. These parameters were proven to directly affect the QTPP, including proper particle size, high drug entrapment, proper lyophilization process and minimum changes in phospholipid transition temperature. This DS approach was validated and considered a valuable approach for designing stable high-quality lyophilized liposomes [32].
This DS methodology was also applied to the prednisolone-loaded long-circulating liposomes using the thin-film hydration-extrusion method. The selected formulation parameters were drug concentration and PEG ratio in the bilayer membrane, and the process parameters were the number of extrusion cycles, temperature and rotation speed [33]. The same DS strategy was used to encapsulate tenofovir into liposomes with high EE [62]. Pandey et al. established a DS for chitosan-coated nanoliposomes using the ethanol injection method as a function of drug and chitosan concentration, and the organic phase-to-aqueous phase ratio to achieve the best design, in terms of average particle size, EE and coating efficiency [60].
Several factors may affect CQAs in the DS strategy. For example, the co-encapsulation of two drugs in the same liposome expands the studied attributes that are related to both drugs which are usually independent of each other. These variations may not lead to enhanced product quality [37]. Moreover, liposome drying process parameters are considered major CQAs that should be involved in the DS process study to obtain long-term stable liposomes [93]. Drying steps, such as pre-freezing, lyophilization and/or spray drying or even the type and ratio of the used cryoprotectants should be managed to reach a high drug content after lyophilization, maintaining the same particle size and ZP with minimal moisture content [94]. For example, the DS for the freeze-drying process of pravastatin-loaded long-circulating liposomes was developed as a function of the freezing rate and the shelf temperature during the initial drying. The two processing factors were found to have a great influence on the product’s CQAs [34].
3.4. The Control Strategy
Although liposomes have been shown to have many advantages as a stable and effective drug delivery system, they present many challenges in analytical and bioanalytical characterization due to their distinctive preparation processes and complex physicochemical properties. According to the FDA guidelines, numerous critical quality attributes (CQAs) have been reported that need full characterization for liposome drug products (Table 3).

Table 3.
Critical quality attributes (CQAs) needed for full liposome drug product characterization.
3.4.1. Lipid Content Identification and Quantification
The quality of the ultimate product is affected by the source of lipids and also by the nature of the lipids: synthetic, semi-synthetic or natural. Phospholipids are the major lipid component of liposome formulations. These lipids can be identified by nuclear magnetic resonance (NMR). 31P-NMR can differentiate phospholipid types according to their unique 31P shifts [95]. 1H- and 13C-NMR can also be used to clarify the molecular chemical structures of alkyl chains and lipid polar head groups. NMR analysis usually requires expensive instruments [96]. Liquid chromatography (LC) coupled with mass spectrometry (MS) is widely used for lipid identification and profiling [136]. MS is a powerful tool to determine the molecular mass of lipids especially when soft ionization approaches such as electrospray ionization (ESI) MS are used [137]. Raman spectroscopy can be used to characterize the vibrational modes of the lipid carbon skeleton. They are characterized by the C-C backbone vibrations (1000−1150 cm−1) and C-H stretching (2800−2900 cm−1) [138].
Liquid chromatography techniques have been widely applied in quantitative lipid analysis [139]. First, liposomes should be disrupted using organic solvents followed by chromatographic separation; then, lipids can be sensed and quantified by different detectors, including diode array ultraviolet (UV), refractive index (RI) [97], evaporative light scattering detector (ELSD) [98] and charged aerosol detector (CAD) [99]. Singh et al. quantified the phospholipids and cholesterol from six different liposomal preparations using isocratic, reversed-phase liquid chromatography (RP-HPLC) with UV and ELSD detectors [100].
Gas chromatography (GC) has also been applied for lipid analysis [102]. Lipid fatty acids should be first converted into volatile methyl esters prior to GC analysis [140]. Recently, supercritical fluid chromatography (SFC) has also been used for lipid analysis [103,141].
Many colorimetric assays have been stated to evaluate phospholipids. A blue-color is produced when reacting phosphorus with molybdate. Diphenylhexatriene (DPH) is usually used to identify bilayer membranes. Moreover, DPH fluorescence-based detection has improved the phospholipid concentration detection limits [101]. Additionally, several commercial kits have been designed to quantify unsaturated phospholipids based on the sulfo-phospho-vanillin reaction [142] or based on enzymatic assay [143,144].
3.4.2. Quantification of Drug Encapsulation
Liposomes provide lipid bilayers and an aqueous core to entrap hydrophobic and/or hydrophilic drugs, respectively. To evaluate the drug encapsulation, the unloaded drug is first removed from the nanocarriers through ultrafiltration, ultracentrifugation, dialysis or solid-phase extraction. The loaded or unloaded drug amount can then be quantified with respect to the total drug amount, yielding the percent drug encapsulation [99].
RP-HPLC has shown high efficiency for both the separation and quantification of free drugs and drug-loaded liposomes [104]. RP-HPLC connected to a UV-detector has been used for fast quantification of doxorubicin-loaded into Doxil® with a linear correlation [105,106]. Capillary electrophoresis (CE) has also been used to separate loaded drugs into liposomes of different change [107]. Oxaliplatin-loaded, anionic PEGylated liposomes have been purified from unloaded oxaliplatin and calculated for EE using a CE-UV detector [108]. Moreover, cisplatin has also been analyzed from loaded liposomes using CE connected to inductively coupled plasma mass spectrometry (ICP-MS) [109]. Flow-based field-flow fractionation (FFF) has been developed to overcome the restrictions of traditional chromatography [110,111]. Size exclusion chromatography (SEC) has also been used to separate unloaded drugs from drug-loaded liposomes based on their size differences [112].
3.4.3. Liposomes Size and Morphology Characterization
Direct particle size and morphology can be evaluated by electron microscopy, such as scanning or transition electron microscopy (SEM and TEM, respectively) [113]. Cryogenic TEM (Cryo-TEM) does not require a drying process because it solidifies the aqueous sample by rapid freezing and thus drying-related artifacts are minimal. Cryo-TEM has been developed to provide high-resolution morphology and comprehensive structural information about the lipid layers and encapsulation mechanisms (Figure 4) [114,145]. SEM can penetrate the particle surfaces and is not commonly used for liposomal imaging due to the destructive manner of sample preparation. In addition, atomic fluoresce microscopy (AFM) has also been used to explore the three-dimensional structure of liposomes [115].
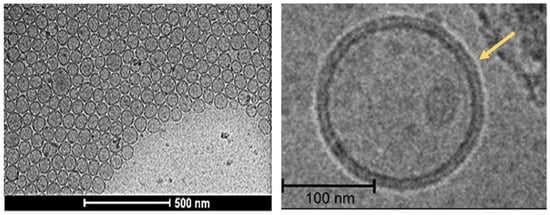
Figure 4.
High-resolution Cryo-TEM images of liposomes [146].
Liposome lamellarity can be evaluated by 31P-NMR [116]. Phospholipids in unilamellar liposomes can be characterized by a narrow-line spectrum, whereas multilamellar liposomes displayed wider peaks due to the restricted anisotropic molecular motions within multiple lipid layers [117].
Dynamic light scattering (DLS) has been applied to characterize nanoparticle size distribution. DLS has become the conventional strategy for the simple quantitative analysis of nanoparticle size distributions [118]. DLS measures time-dependent fluctuations in the scattered light from particles in Brownian motions. Variable sample parameters for DLS measurements include temperature, solvent viscosity and solvent refractive index, should all be pre-determined to precisely estimate the hydrodynamic particle size [119].
3.4.4. Nanoparticle Surface Charge (Zeta Potential, ZP)
Liposomal surface charges are usually reflected by the polar head groups of the phospholipids, tertiary amines or negatively charged carboxylate functional groups. This factor is most often expressed by the ZP [120,121]. It is an important physicochemical property that is responsible for the strength of liposome interactions, adsorption and therefore nanoparticle stability. ZP can be determined from the electrophoretic mobility of particles measured by the phase analysis light scattering (PALS) or electrophoretic light scattering (ELS) technique [122]. Significant medium properties including the phase nature, refractive index, and viscosity, as well as temperature, all have to be pre-determined to obtain exact measurements. ZP values outside ±30 mV maintain sufficient stable nanosuspensions [123]. The surface potential of liposomes can also be determined by several techniques including fluorescent labeling [124], electron paramagnetic resonance [125] and the second harmonic generation from optical analyses [126].
3.4.5. Physical and Chemical Stability
The physical and chemical stability of liposome formulations should be examined to meet the criteria for high product quality [147]. Spectroscopic methods and DLS measurements provide simple tests to measure liposome fusion and aggregation, respectively, while liposome disruption can be determined by chromatographic methods equipped with suitable detectors [42]. Liposomal fusion has been examined mainly using differential scanning calorimetry (DSC) and fluorescence-based lipid mixing assays [127]. Liposome aggregation can be envisaged by microscopic techniques and quantified by UV–Vis spectroscopy or DLS [128]. Lipid degradation rates can be affected by lipid composition, storage temperature, buffers and pH. The precursor lipid classes and their hydrolyzed derivatives can be separated and measured by several chromatographic approaches [129].
3.4.6. In Vitro Drug Release
Several in vitro release testing methods to predict the in vivo behaviors of liposome formulations have been developed [130]. These methods can be classified into sampling and separate (SS), dialysis membrane (DM) and continuous flow (CF) [131,132]. The SS method involves incubating the samples in the release media, sampling and separating the released drug from integral liposomes, usually by stand-alone ultracentrifugation or filtration, followed by drug quantification [42,133]. Low-efficiency ultracentrifugation or filtration separation process for submicron nanoparticles has been observed upon using this method. DM is more common for studying the in vitro drug release of most nano-formulations. DM approaches mainly include dialysis sac (regular or tube dialysis) and reverse dialysis [134]. The dialysis sac keeps nano-formulations inside, attaining simultaneous release and separation, and then quantifying the released drug. Key factors for this approach include the type and cut-off of the dialysis membrane, volume ratios between the sample and release solvent, and mixing procedures [135].
3.4.7. Liposomes Safety and Toxicity
The fact that liposomes are biocompatible, biodegradable and relatively easy to fabricate have led to an exponential increase in their use [148]. However, liposomes as a vehicle for drugs might be vulnerable to safety issues related to their lipid type, charge and concentrations. One of the most toxic effects of liposomes is the activation of the immune system of the patient that leads to drug sequestering in the mononuclear phagocytic system which might influence the function of the liver and spleen [149]. Therefore, strategies to improve the safety should be developed in the early stages of product design. Many strategies to improve drug safety and decrease the toxicity of the nanocarriers have been developed, such as increasing the encapsulation efficiency of drug into liposomes to decrease the lipid concentration needed to give the patient the recommended therapeutic dose [149]. The liposomes particle size, morphology, lipid content, charge, polydispersity and cholesterol content are key factors in toxicity. Consequently, precise design of all these factors will increase the loading capacity of liposomes and decrease the toxicity [150]. Recently approved were the PEGylated and surface-engineered liposomes having a lesser effect on the immune system. The combination of lipids with polymers should be designed and optimized. Therefore, the type of materials used for liposomal functionalization and their concentration should be minimized [148,151].
Finally, as the risk assessment is the backbone of the QbD process connecting all the key elements together, the liposomal biocompatibility and toxicity should be assessed using in vitro cell lines, ex vivo and in animals [152]. Many in vitro approaches have been used to test nanoparticle toxicity, including liposomes such as two-dimensional monolayer cell culture [153] and three-dimensional cell culture [154]. Additionally, ex vivo models are valuable tests systems in which slices of complete tissue can be used similar to organ slice cultures [155]. Finally, the most relevant evaluation is in vivo [156]. In conclusion, to minimize liposomal toxicity, it is important to start with the safety by design approach to ensure a low toxicity and voluble drug delivery system.
4. Conclusions and Future Perspectives
The application of QbD in pharmaceutical manufacturing has become an essential approach for the pharmaceutical industry to ensure the efficacy and safety of pharmaceutical products. The implementation of commercial nanomedicines as drug delivery systems to the site of action with limited systemic toxicities is an emerging concept that unfortunately, has not reached its full potential yet. Nano-pharmaceuticals are still in the initial stages of their development. Therefore, the implementation of QbD could create great value and benefits. Particularly, nano-pharmaceuticals is faced with many challenges related to structural stability and the lack of in-depth understanding of the manufacturing processes.
Liposomes are biocompatible and biodegradable drug delivery systems that have shown important successes in their clinical use. However, there are a lot of regulatory and technical challenges connected with the production and quality control strategies of liposomal products. There is a wide range of variability in liposomal preparations that include their morphology, size, fabricating materials, spatial configuration and manufacturing methods. Consequently, the application of a QbD approach in developing liposomes is critical and challenging compared to traditional dosage forms. Therefore, for the successful development of quality liposomal products, manufacturers need to consider employing QbD to identify and classify product attributes as well as material/process parameters with a deeper understanding of their complex interplay using proper experimental design and statistical analysis. QbD implementation is vital to ensure the final product attributes and the intended therapeutic and safety profiles.
Author Contributions
Conceptualization, W.A. and A.M.A.; writing—original draft preparation, W.A., H.N., Z.L., O.M.H., A.A.-K. and E.E.; writing—review and editing, W.A. and A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Hatmal, M.m.M.; Alshaer, W.; Rahman, E.N.S.E.A.; Mohd-Zahid, M.H.; Alhaj-Qasem, D.M.; Yean, C.Y.; Alias, I.Z.; Jaafar, J.; Ferji, K. COVID-19 infection and nanomedicine applications for development of vaccines and therapeutics: An overview and future perspectives based on polymersomes. Eur. J. Pharmacol. 2021, 896, 173930. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Tefas, L.R.; Rus, L.M.; Achim, M.; Vlase, L.; Tomuță, I. Application of the quality by design concept in the development of quercetin-loaded polymeric nanoparticles. Farmacia 2018, 66, 798–810. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Deshpande, M.; Zaheer, Z.; Shinde, D.B.; Arote, R. Quality by design approach: Regulatory need. Arab. J. Chem. 2017, 10, S3412–S3425. [Google Scholar] [CrossRef]
- Wu, H.; Khan, M.A. Quality-by-Design (QbD): An integrated process analytical technology (PAT) approach for real-time monitoring and mapping the state of a pharmaceutical coprecipitation process. J. Pharm. Sci. 2010, 99, 1516–1534. [Google Scholar] [CrossRef]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Application of the Quality by Design Approach to the Drug Substance Manufacturing Process of An Fc Fusion Protein: Towards a Global Multi-step Design Space ALEX. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int. J. Pharm. 2017, 526, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, W.P. Reputation and product quality. Bell J. Econ. 1983, 14, 508–516. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency; Committee for Human Medicinal Products. ICH Guideline Q10 on Pharmaceutical Quality System; European Medicines Agency: Amsterdam, The Netherlands, 2008; Volume 44. [Google Scholar]
- European Medicines Agency. ICH Guideline Q9 on Quality Risk Management; European Medicines Agency: Amsterdam, The Netherlands, 2006; Volume 44. [Google Scholar]
- Kamemura, N. Ich Harmonised Tripartite Guideline Pharmaceutical Development Q8(R2). Comput. Toxicol. 2009, 6, 32–38. [Google Scholar] [CrossRef]
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef]
- Tomba, E.; Facco, P.; Bezzo, F.; Barolo, M. Latent variable modeling to assist the implementation of Quality-by-Design paradigms in pharmaceutical development and manufacturing: A review. Int. J. Pharm. 2013, 457, 283–297. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Visser, J.C.; Dohmen, W.M.; Hinrichs, W.L.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef]
- Kumar, V.P.; Vishal Gupta, N. A review on quality by design approach (QBD) for pharmaceuticals. Int. J. Drug Dev. Res. 2015, 7, 52–60. [Google Scholar]
- Fonteyne, M.; Vercruysse, J.; Diaz, D.C.; Gildemyn, D.; Vervaet, C.; Remon, J.P.; De Beer, T. Real-time assessment of critical quality attributes of a continuous granulation process. Pharm. Dev. Technol. 2013, 18, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Kohli, K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov. Today 2019, 24, 717–725. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Figueiras, A.; Vitorino, C. A practical framework for implementing Quality by Design to the development of topical drug products: Nanosystem-based dosage forms. Int. J. Pharm. 2018, 548, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Tahir, M.A.; Charoo, N.A.; Ansari, S.H.; Ali, J. Pharmaceutical product development: A quality by design approach. Int. J. Pharm. Investig. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- De Beer, T.R.; Wiggenhorn, M.; Hawe, A.; Kasper, J.C.; Almeida, A.; Quinten, T.; Friess, W.; Winter, G.; Vervaet, C.; Remon, J.P. Optimization of a pharmaceutical freeze-dried product and its process using an experimental design approach and innovative process analyzers. Talanta 2011, 83, 1623–1633. [Google Scholar] [CrossRef]
- Dhoble, S.; Patravale, V. Development of anti-angiogenic erlotinib liposomal formulation for pulmonary hypertension: A QbD approach. Drug Deliv. Transl. Res. 2019, 9, 980–996. [Google Scholar] [CrossRef]
- Ghodake, V.; Vishwakarma, J.; Vavilala, S.L.; Patravale, V. Cefoperazone sodium liposomal formulation to mitigate P. aeruginosa biofilm in Cystic fibrosis infection: A QbD approach. Int. J. Pharm. 2020, 587, 119696. [Google Scholar] [CrossRef]
- Pallagi, E.; Jójárt-Laczkovich, O.; Németh, Z.; Szabó-Révész, P.; Csóka, I. Application of the QbD-based approach in the early development of liposomes for nasal administration. Int. J. Pharm. 2019, 562, 11–22. [Google Scholar] [CrossRef]
- Porfire, A.; Muntean, D.M.; Rus, L.; Sylvester, B.; Tomuţă, I. A quality by design approach for the development of lyophilized liposomes with simvastatin. Saudi Pharm. J. 2017, 25, 981–992. [Google Scholar] [CrossRef]
- Sylvester, B.; Porfire, A.; Muntean, D.M.; Vlase, L.; Lupuţ, L.; Licarete, E.; Sesarman, A.; Alupei, M.C.; Banciu, M.; Achim, M.; et al. Optimization of prednisolone-loaded long-circulating liposomes via application of Quality by Design (QbD) approach. J. Liposome Res. 2018, 28, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, B.; Porfire, A.; Achim, M.; Rus, L.; Tomuţă, I. A step forward towards the development of stable freeze-dried liposomes: A quality by design approach (QbD). Drug Dev. Ind. Pharm. 2018, 44, 385–397. [Google Scholar] [CrossRef]
- Kesharwani, P.; Md, S.; Alhakamy, N.A.; Hosny, K.M.; Haque, A. QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and Its In Vitro Evaluation. Polymers 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Bonde, S.; Tambe, K. Lectin coupled liposomes for pulmonary delivery of salbutamol sulphate for better management of asthma: Formulation development using QbD approach. J. Drug Deliv. Sci. Technol. 2019, 54, 101336. [Google Scholar] [CrossRef]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Dev. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.M.; Shelat, P.K.; Lalwani, A.N. QbD based development of proliposome of lopinavir for improved oral bioavailability. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 108, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Xu, X.; Costa, A.P.; Khan, M.A.; Burgess, D.J. Application of quality by design to formulation and processing of protein liposomes. Int. J. Pharm. 2012, 434, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Tam, Y.Y.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Ostro, M.J.; Giacomoni, D.; Lavelle, D.O.N.; Paxton, W.; Dray, S. Evidence for translation of rabbit globin mRNA after liposomemediated insertion into a human cell line. Nature 1978, 274, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Dow, S. Liposome-nucleic acid immunotherapeutics. Expert Opin. Drug Deliv. 2008, 5, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Vigneshkumar, P.N.; George, E.; Joseph, J.; John, F.; George, J. Chapter 12—Liposomal bionanomaterials for nucleic acid delivery. In Fundamentals of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–362. [Google Scholar] [CrossRef]
- Lappalainen, K.; Jääskeläinen, I.; Syrjänen, K.; Urtti, A.; Syrjänen, S. Comparison of cell proliferation and toxicity assays using two cationic liposomes. Pharm. Res. 1994, 11, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Aberle, A.M.; Tablin, F.; Zhu, J.; Walker, N.J.; Gruenert, D.C.; Nantz, M.H. A novel tetraester construct that reduces cationic lipid-associated cytotoxicity. Implications for the onset of cytotoxicity. Biochemistry 1998, 37, 6533–6540. [Google Scholar] [CrossRef] [PubMed]
- El-Aneed, A. Current strategies in cancer gene therapy. Eur. J. Pharmacol. 2004, 498, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bottega, R.; Epand, R.M. Inhibition of protein kinase C by cationic amphiphiles. Biochemistry 1992, 31, 9025–9030. [Google Scholar] [CrossRef] [PubMed]
- Bhadani, A.; Kataria, H.; Singh, S. Synthesis, characterization and comparative evaluation of phenoxy ring containing long chain gemini imidazolium and pyridinium amphiphiles. J. Colloid Interface Sci. 2011, 361, 33–41. [Google Scholar] [CrossRef]
- Ilies, M.A.; Seitz, W.A.; Ghiviriga, I.; Johnson, B.H.; Miller, A.; Thompson, E.B.; Balaban, A.T. Pyridinium Cationic Lipids in Gene Delivery: A Structure−Activity Correlation Study. J. Med. Chem. 2004, 47, 3744–3754. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-C.; Zhang, Y.-M.; Zhang, J.; Liu, Y.-H.; Yu, X.-Q. Cationic lipids with a cyclen headgroup: Synthesis and structure–activity relationship studies as non-viral gene vectors. RSC Adv. 2017, 7, 18681–18689. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Zhang, T.; Song, Y.; She, Z.; Li, J.; Deng, Y. Progress involving new techniques for liposome preparation. Asian J. Pharm. Sci. 2014, 9, 176–182. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 2011, 591325. [Google Scholar] [CrossRef]
- Lujan, H.; Griffin, W.C.; Taube, J.H.; Sayes, C.M. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int. J. Nanomed. 2019, 14, 5159–5173. [Google Scholar] [CrossRef]
- Pandey, A.P.; Karande, K.P.; Sonawane, R.O.; Deshmukh, P.K. Applying quality by design (QbD) concept for fabrication of chitosan coated nanoliposomes. J. Liposome Res. 2014, 24, 37–52. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. Int. J. Pharm. 2012, 423, 543–553. [Google Scholar] [CrossRef]
- Aftab, S.; Shah, A.; Nadhman, A.; Kurbanoglu, S.; Aysıl Ozkan, S.; Dionysiou, D.D.; Shukla, S.S.; Aminabhavi, T.M. Nanomedicine: An effective tool in cancer therapy. Int. J. Pharm. 2018, 540, 132–149. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A. Stealth liposomes and tumor targeting: One step further in the quest for the magic bullet. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 223–225. [Google Scholar]
- Rudokas, M.; Najlah, M.; Alhnan, M.A.; Elhissi, A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2016, 25 (Suppl. S2), 60–72. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.B.R.; dos Santos Miranda Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607. [Google Scholar] [CrossRef] [PubMed]
- Amasya, G.; Badilli, U.; Aksu, B.; Tarimci, N. Quality by design case study 1: Design of 5-fluorouracil loaded lipid nanoparticles by the W/O/W double emulsion—Solvent evaporation method. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 84, 92–102. [Google Scholar] [CrossRef]
- Azhar Shekoufeh Bahari, L.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharm. 2015, 6, 286. [Google Scholar] [CrossRef]
- Suleiman, E.; Damm, D.; Batzoni, M.; Temchura, V.; Wagner, A.; Überla, K.; Vorauer-Uhl, K. Electrostatically Driven Encapsulation of Hydrophilic, Non-Conformational Peptide Epitopes into Liposomes. Pharmaceutics 2019, 11, 619. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Khan, M.A.; Burgess, D.J. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. Int. J. Pharm. 2011, 419, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Haeri, A.; Alinaghian, B.; Daeihamed, M.; Dadashzadeh, S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran J. Pharm. Res. 2014, 13, 3–14. [Google Scholar]
- Johnston, M.J.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Gomez, A.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal Nanovesicles for Efficient Encapsulation of Staphylococcal Antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Porfire, A.; Tomuta, I.; Muntean, D.; Luca, L.; Licarete, E.; Alupei, M.C.; Achim, M.; Vlase, L.; Banciu, M. Optimizing long-circulating liposomes for delivery of simvastatin to C26 colon carcinoma cells. J. Liposome Res. 2015, 25, 261–269. [Google Scholar] [CrossRef]
- Conrard, L.; Tyteca, D. Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment. Biomolecules 2019, 9, 513. [Google Scholar] [CrossRef]
- Wang, T.; Deng, Y.; Geng, Y.; Gao, Z.; Zou, J.; Wang, Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsions. Biochim. Et Biophys. Acta (BBA) Biomembr. 2006, 1758, 222–231. [Google Scholar] [CrossRef]
- Sur, S.; Fries, A.C.; Kinzler, K.W.; Zhou, S.; Vogelstein, B. Remote loading of preencapsulated drugs into stealth liposomes. Proc. Natl. Acad. Sci. USA 2014, 111, 2283–2288. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Alsotari, S.; Buqaien, R.; Ismail, S.; Awidi, A.; Al Bawab, A. Remote loading of curcumin-in-modified β-cyclodextrins into liposomes using a transmembrane pH gradient. RSC Adv. 2019, 9, 37148–37161. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Mahmoud, I.S.; Odeh, F.; Abuarqoub, D.; Al-Azzawi, H.; Zaza, R.; Qadri, M.I.; Ismail, S.; Al Bawab, A.; Awidi, A.; et al. Grafting of anti-nucleolin aptamer into preformed and remotely loaded liposomes through aptamer-cholesterol post-insertion. RSC Adv. 2020, 10, 36219–36229. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef]
- Franco, M.S.; Gomes, E.R.; Roque, M.C.; Oliveira, M.C. Triggered Drug Release From Liposomes: Exploiting the Outer and Inner Tumor Environment. Front. Oncol. 2021, 11, 623760. [Google Scholar] [CrossRef]
- Rehman, A.U.; Omran, Z.; Anton, H.; Mély, Y.; Akram, S.; Vandamme, T.F.; Anton, N. Development of doxorubicin hydrochloride loaded pH-sensitive liposomes: Investigation on the impact of chemical nature of lipids and liposome composition on pH-sensitivity. Eur. J. Pharm. Biopharm. 2018, 133, 331–338. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.; Yu, X.; Li, Y.; Fu, Y.; Zhou, X.; Zhang, D.; Li, J. Design and evaluation of pH-sensitive liposomes constructed by poly(2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur. J. Pharm. Biopharm. 2015, 91, 66–74. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Control. Release Off. J. Control. Release Soc. 2017, 256, 9–18. [Google Scholar] [CrossRef]
- Németh, Z.; Pallagi, E.; Dobó, D.G.; Csóka, I. A Proposed Methodology for a Risk Assessment-Based Liposome Development Process. Pharmaceutics 2020, 12, 1164. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Sotirhos, N.; Herslöf, B.; Kenne, L. Quantitative analysis of phospholipids by 31P-NMR. J. Lipid Res. 1986, 27, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Balsgart, N.M.; Mulbjerg, M.; Guo, Z.; Bertelsen, K.; Vosegaard, T. High Throughput Identification and Quantification of Phospholipids in Complex Mixtures. Anal. Chem. 2016, 88, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Grit, M.; Crommelin, D.J.A.; Lang, J. Determination of phosphatidylcholine, phosphatidylglycerol and their lyso forms from liposome dispersions by high-performance liquid chromatography using high-sensitivity refractive index detection. J. Chromatogr. A 1991, 585, 239–246. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakata, M.; Matsunuma, J.; Mizuochi, T. Simultaneous quantification of components of neoglycolipid-coated liposomes using high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 754, 127–133. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Singh, R.; Ajagbe, M.; Bhamidipati, S.; Ahmad, Z.; Ahmad, I. A rapid isocratic high-performance liquid chromatography method for determination of cholesterol and 1,2-dioleoyl-sn-glycero-3-phosphocholine in liposome-based drug formulations. J. Chromatogr. A 2005, 1073, 347–353. [Google Scholar] [CrossRef]
- London, E.; Feligenson, G.W. A convenient and sensitive fluorescence assay for phospholipid vesicles using diphenylhexatriene. Anal. Biochem. 1978, 88, 203–211. [Google Scholar] [CrossRef]
- Wu, X.; Tong, Y.; Shankar, K.; Baumgardner, J.N.; Kang, J.; Badeaux, J.; Badger, T.M.; Ronis, M.J. Lipid fatty acid profile analyses in liver and serum in rats with nonalcoholic steatohepatitis using improved gas chromatography-mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 747–754. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M. High-Throughput and Comprehensive Lipidomic Analysis Using Ultrahigh-Performance Supercritical Fluid Chromatography-Mass Spectrometry. Anal. Chem. 2015, 87, 7187–7195. [Google Scholar] [CrossRef]
- Itoh, N.; Santa, T.; Kato, M. Rapid evaluation of the quantity of drugs encapsulated within nanoparticles by high-performance liquid chromatography in a monolithic silica column. Anal. Bioanal. Chem. 2015, 407, 6429–6434. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Kimoto, A.; Yamamoto, E.; Higashi, T.; Santa, T.; Funatsu, T.; Kato, M. High performance liquid chromatography analysis of 100-nm liposomal nanoparticles using polymer-coated, silica monolithic columns with aqueous mobile phase. J. Chromatogr. A 2017, 1484, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Hyodo, K.; Ohnishi, N.; Suzuki, T.; Ishihara, H.; Kikuchi, H.; Asakawa, N. Direct, simultaneous measurement of liposome-encapsulated and released drugs in plasma by on-line SPE-SPE-HPLC. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3620–3625. [Google Scholar] [CrossRef] [PubMed]
- Franzen, U.; Østergaard, J. Physico-chemical characterization of liposomes and drug substance-liposome interactions in pharmaceutics using capillary electrophoresis and electrokinetic chromatography. J. Chromatogr. A 2012, 1267, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Franzen, U.; Nguyen, T.T.; Vermehren, C.; Gammelgaard, B.; Ostergaard, J. Characterization of a liposome-based formulation of oxaliplatin using capillary electrophoresis: Encapsulation and leakage. J. Pharm. Biomed. Anal. 2011, 55, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.N.; Østergaard, J.; Stürup, S.; Gammelgaard, B. Determination of platinum drug release and liposome stability in human plasma by CE-ICP-MS. Int. J. Pharm. 2013, 449, 95–102. [Google Scholar] [CrossRef]
- Wagner, M.; Holzschuh, S.; Traeger, A.; Fahr, A.; Schubert, U.S. Asymmetric flow field-flow fractionation in the field of nanomedicine. Anal. Chem. 2014, 86, 5201–5210. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Ruysschaert, T.; Marque, A.; Duteyrat, J.L.; Lesieur, S.; Winterhalter, M.; Fournier, D. Liposome retention in size exclusion chromatography. BMC Biotechnol. 2005, 5, 11. [Google Scholar] [CrossRef]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Ensor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladár, A.E.; Hight Walker, A.R.; et al. Particle size distributions by transmission electron microscopy: An interlaboratory comparison case study. Metrologia 2013, 50, 663–678. [Google Scholar] [CrossRef]
- Meister, A.; Blume, A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers 2017, 9, 521. [Google Scholar] [CrossRef]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharm. 2018, 9, 80. [Google Scholar] [CrossRef]
- Pinheiro, T.J.; Vaz, W.L.; Geraldes, C.F.; Prado, A.; da Costa, M.S. A 31P-NMR study on multilamellar liposomes formed from the lipids of a thermophilic bacterium. Biochem. Biophys. Res. Commun. 1987, 148, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, M.; Brecht, V.; Peschka-Süss, R. Parameters influencing the determination of liposome lamellarity by 31P-NMR. Chem. Phys. Lipids 2001, 109, 103–112. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release Off. J. Control. Release Soc. 2019, 299, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.A.; Müller-Goymann, C.C. Characterisation of surface-modified solid lipid nanoparticles (SLN): Influence of lecithin and nonionic emulsifier. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. 2005, 61, 77–86. [Google Scholar] [CrossRef]
- Sathappa, M.; Alder, N.N. Ionization Properties of Phospholipids Determined by Zeta Potential Measurements. Bio-Protocol 2016, 6, e2030. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.C.; Collins, L.R.; Sponsler, E.; Kossovsky, N.; Papahadjopoulos, D.; Martin, F.J. Sterically stabilized liposomes. Reduction in electrophoretic mobility but not electrostatic surface potential. Biophys. J. 1992, 61, 902–910. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef]
- Fernández, M.S. Determination of surface potential in liposomes. Biochim. Et Biophys. Acta (BBA)—Biomembr. 1981, 646, 23–26. [Google Scholar] [CrossRef]
- Cafiso, D.S.; Hubbell, W.L. EPR determination of membrane potentials. Annu. Rev. Biophys. Bioeng. 1981, 10, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, E.C.Y.; Zhao, X.; Eisenthal, K.B. Surface Potential of Charged Liposomes Determined by Second Harmonic Generation. Langmuir 2001, 17, 2063–2066, https://doi.org/10.1021/la0011634; correction in Langmuir2008, 24, 11322. [Google Scholar] [CrossRef][Green Version]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [CrossRef]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Kiełbowicz, G.; Smuga, D.; Gładkowski, W.; Chojnacka, A.; Wawrzeńczyk, C. An LC method for the analysis of phosphatidylcholine hydrolysis products and its application to the monitoring of the acyl migration process. Talanta 2012, 94, 22–29. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In Vitro Dissolution Testing Strategies for Nanoparticulate Drug Delivery Systems: Recent Developments and Challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In Vitro Release Study of the Polymeric Drug Nanoparticles: Development and Validation of a Novel Method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef]
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of In Vitro Release Methods in Liposomal Formulation Development: Challenges and Regulatory Perspective. AAPS J. 2017, 19, 1669–1681. [Google Scholar] [CrossRef]
- Mehn, D.; Iavicoli, P.; Cabaleiro, N.; Borgos, S.E.; Caputo, F.; Geiss, O.; Calzolai, L.; Rossi, F.; Gilliland, D. Analytical ultracentrifugation for analysis of doxorubicin loaded liposomes. Int. J. Pharm. 2017, 523, 320–326. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting drug release kinetics from nanocarriers inside dialysis bags. J. Control. Release Off. J. Control. Release Soc. 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Böttcher, A.; Liebisch, G. Lipid profiling of lipoproteins by electrospray ionization tandem mass spectrometry. Biochim. Et Biophys. Acta 2011, 1811, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C.; Fiedler, J.; Hevko, J. Analysis of nonvolatile lipids by mass spectrometry. Chem. Rev. 2001, 101, 479–526. [Google Scholar] [CrossRef]
- Gaber, B.P.; Peticolas, W.L. On the quantitative interpretation of biomembrane structure by Raman spectroscopy. Biochim. Et Biophys. Acta 1977, 465, 260–274. [Google Scholar] [CrossRef]
- Oswald, M.; Platscher, M.; Geissler, S.; Goepferich, A. HPLC analysis as a tool for assessing targeted liposome composition. Int. J. Pharm. 2016, 497, 293–300. [Google Scholar] [CrossRef]
- Xu, Z.; Harvey, K.; Pavlina, T.; Dutot, G.; Zaloga, G.; Siddiqui, R. An improved method for determining medium- and long-chain FAMEs using gas chromatography. Lipids 2010, 45, 199–208. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Yang, J.; Ye, F.; Zhou, T.; Gongke, L. Advances of supercritical fluid chromatography in lipid profiling. J. Pharm. Anal. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Frings, C.S.; Fendley, T.W.; Dunn, R.T.; Queen, C.A. Improved Determination of Total Serum Lipids by the Sulfo-Phospho-Vanillin Reaction. Clin. Chem. 1972, 18, 673–674. [Google Scholar] [CrossRef]
- Visnovitz, T.; Osteikoetxea, X.; Sódar, B.W.; Mihály, J.; Lőrincz, P.; Vukman, K.V.; Tóth, E.; Koncz, A.; Székács, I.; Horváth, R.; et al. An improved 96 well plate format lipid quantification assay for standardisation of experiments with extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565263. [Google Scholar] [CrossRef]
- Sen Roy, S.; Nguyen, H.C.X.; Angelovich, T.A.; Hearps, A.C.; Huynh, D.; Jaworowski, A.; Kelesidis, T. Cell-free Biochemical Fluorometric Enzymatic Assay for High-throughput Measurement of Lipid Peroxidation in High Density Lipoprotein. J. Vis. Exp. JoVE 2017, 128, e56325. [Google Scholar] [CrossRef]
- Fox, C.; Mulligan, S.; Sung, J.; Dowling, Q.; Fung, H.W.; Vedvick, T.; Coler, R. Cryogenic transmission electron microscopy of recombinant tuberculosis vaccine antigen with anionic liposomes reveals formation of flattened liposomes. Int. J. Nanomed. 2014, 9, 1367–1377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotoucek, J.; Hubatka, F.; Masek, J.; Kulich, P.; Velinska, K.; Bezdekova, J.; Fojtikova, M.; Bartheldyova, E.; Tomeckova, A.; Straska, J.; et al. Preparation of nanoliposomes by microfluidic mixing in herring-bone channel and the role of membrane fluidity in liposomes formation. Sci. Rep. 2020, 10, 5595. [Google Scholar] [CrossRef] [PubMed]
- Parot, J.; Caputo, F.; Mehn, D.; Hackley, V.A.; Calzolai, L. Physical characterization of liposomal drug formulations using multi-detector asymmetrical-flow field flow fractionation. J. Control. Release Off. J. Control. Release Soc. 2020, 320, 495–510. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, H.; Pang, S.; Ya, M.; Qiu, F.; Qin, P.; Zeng, C.; Lu, Y. Liposomes as Multifunctional Nano-Carriers for Medicinal Natural Products. Front. Chem. 2022, 10, 963004. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Imran, M.; Sharma, N. Precision Nanotoxicology in Drug Development: Current Trends and Challenges in Safety and Toxicity Implications of Customized Multifunctional Nanocarriers for Drug-Delivery Applications. Pharmaceutics 2022, 14, 2463. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Rundén, E.; Seglen, P.O.; Haug, F.M.; Ottersen, O.P.; Wieloch, T.; Shamloo, M.; Laake, J.H. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: Evidence for a MAP kinase-dependent mechanism. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 7296–7305. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).