Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia delavayi Using Supercritical Carbon Dioxide Fluid
Abstract
:1. Introduction
2. Results
2.1. Physical and Chemical Properties of Essential Oils Extracted from Petals of Different Colored Flowers
2.2. Comparison of Volatile Profiles among Essential Oils from Different Colors by Total Ion Chromatograms
2.3. Comparative Analysis of Volatile Compounds in Essential Oil of P. delavayi with Different Flower Colors
2.3.1. Analysis of Common VOLATILE Compounds in Essential Oils of P. delavayi Petals with Three Colors
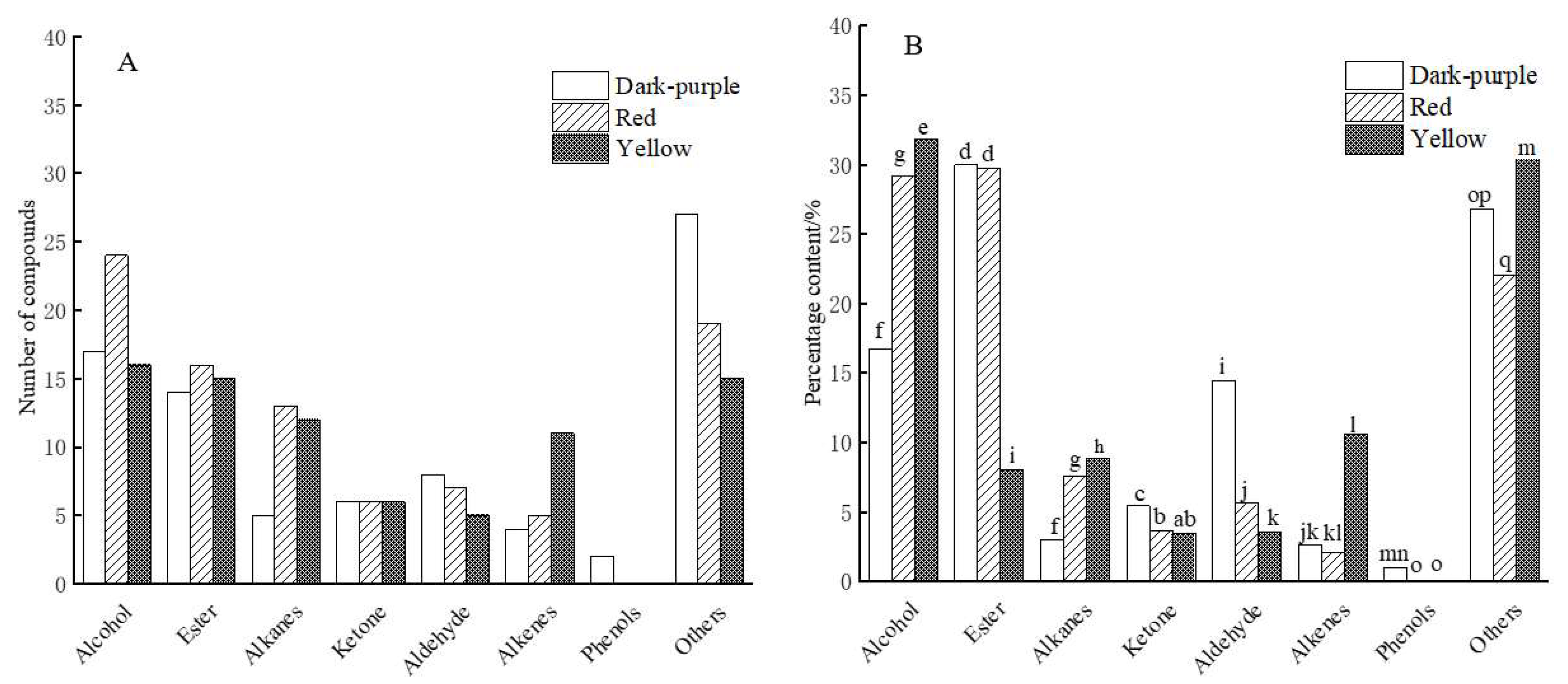
2.3.2. Differences in Chemical Classes of the Essential Oils from the Three Colors of Flower Petals in P. delavayi
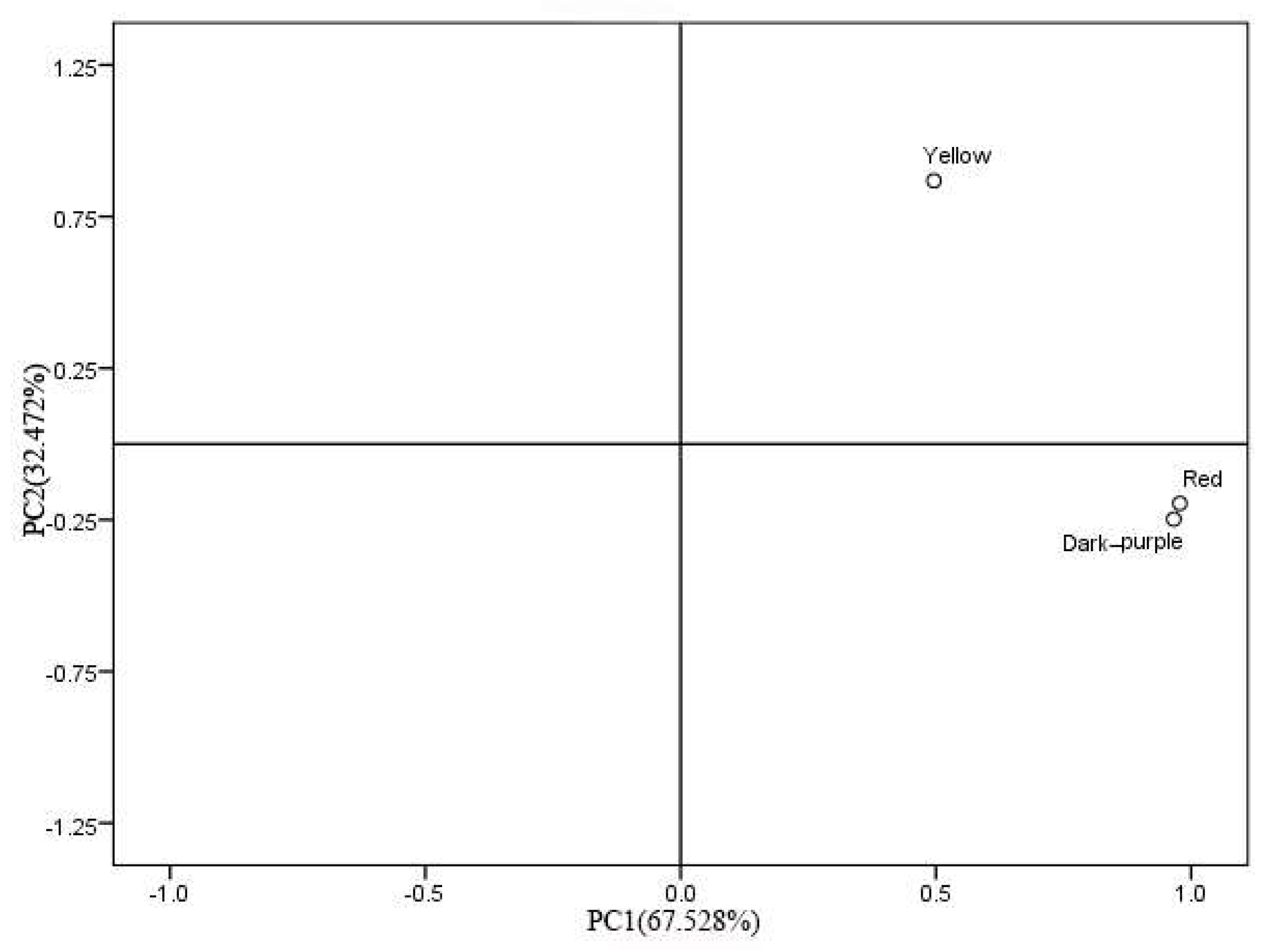
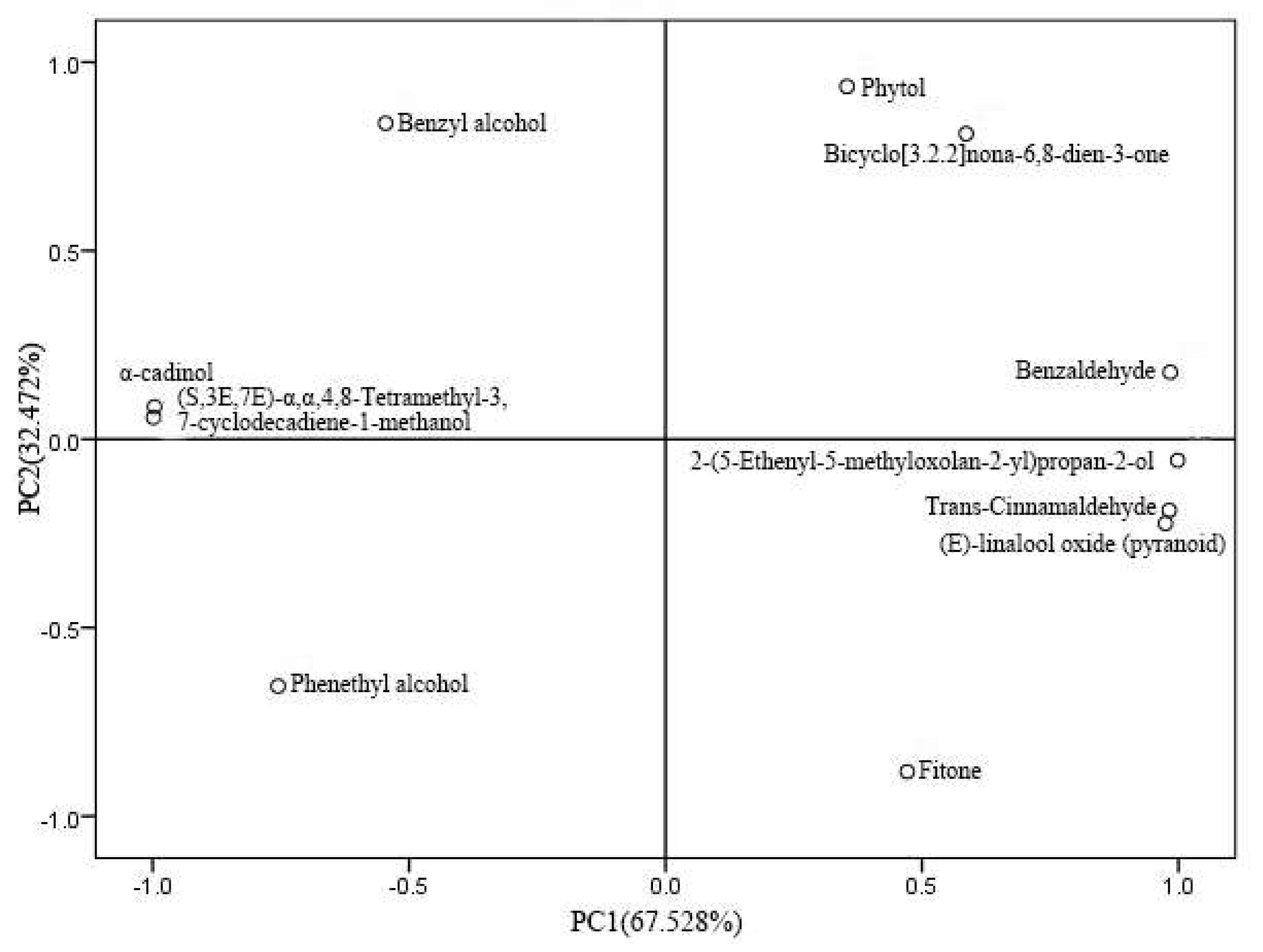
2.3.3. Principal Component Analysis
2.4. Antioxidant Properties
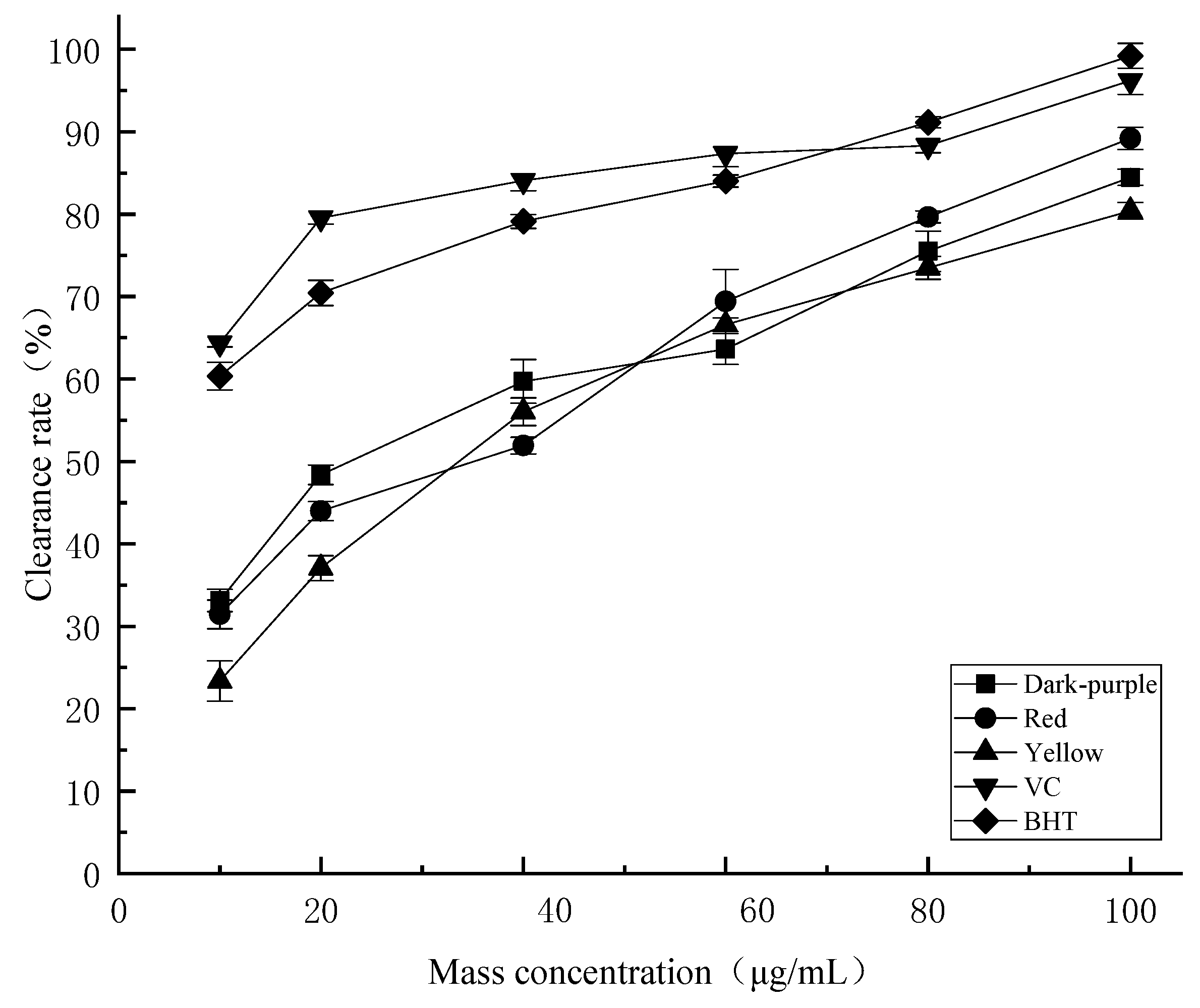
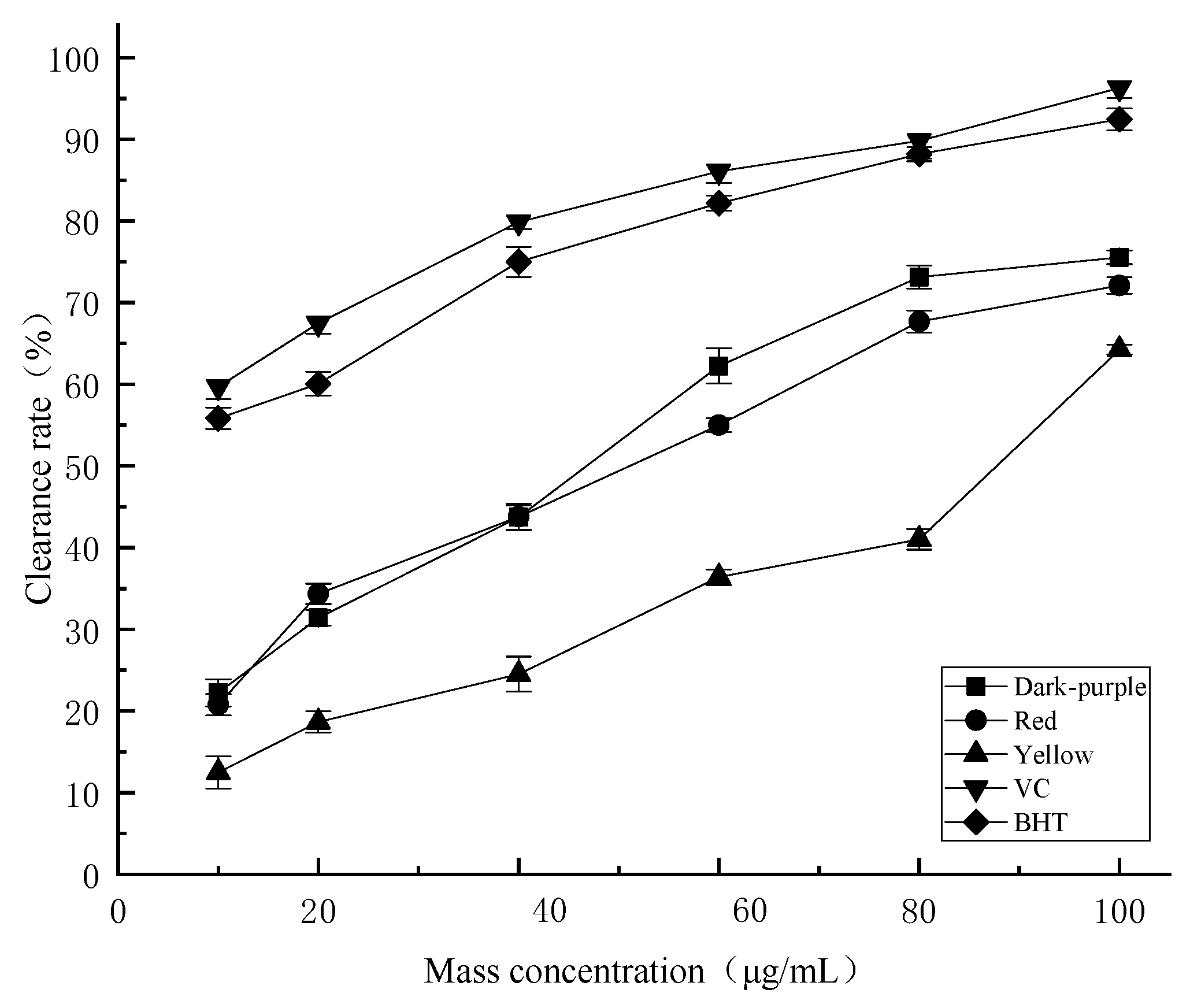
2.4.1. DPPH Radical Scavenging Activity
2.4.2. ABTS Radical Scavenging Activity
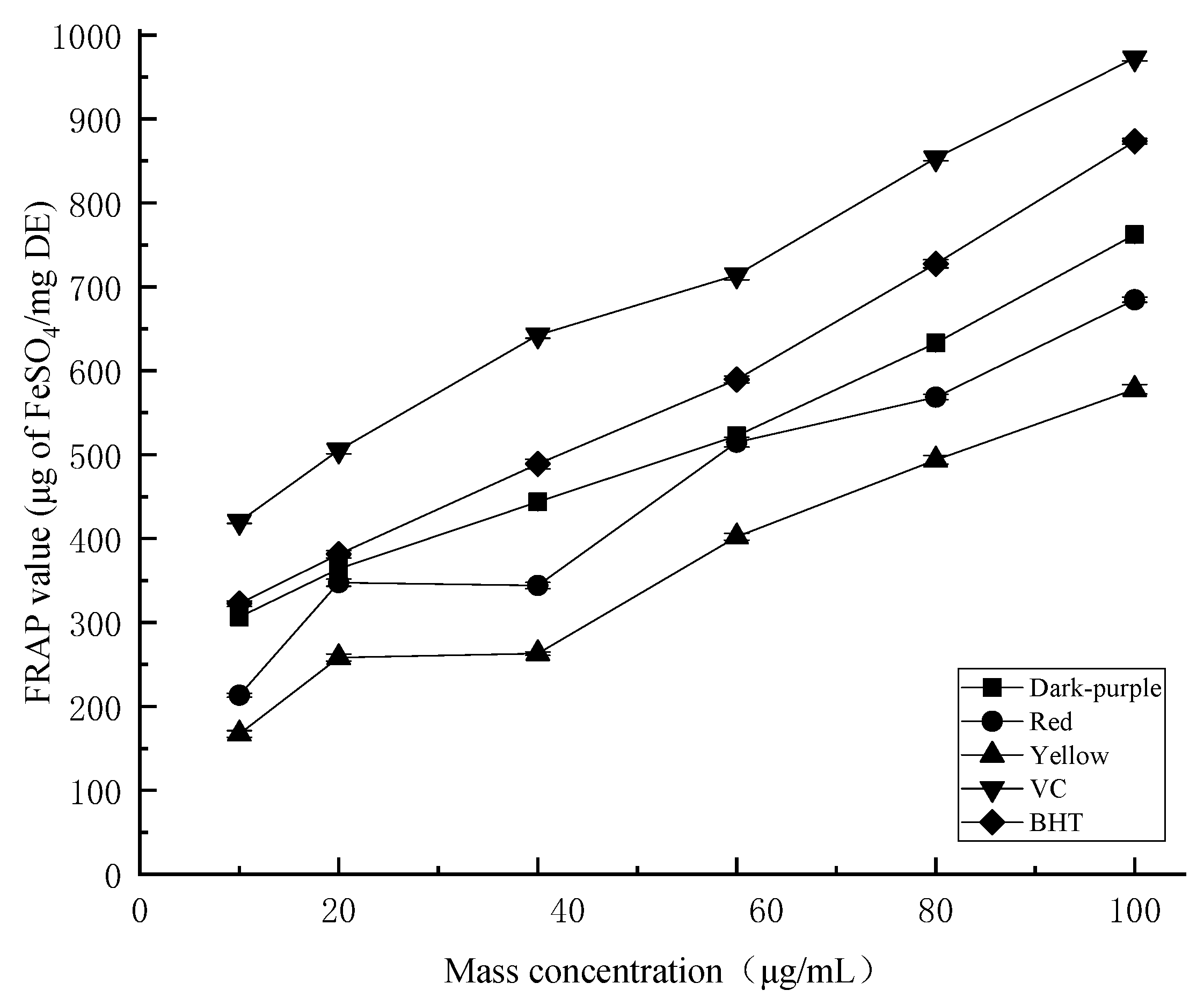
2.4.3. Ferric Reducing Antioxidant Power
2.5. Total Phenolic and Flavonoid Content
3. Discussion
3.1. Chemical Compositions in the Essential Oils Are Differentially Accumulated in Relation to the Flower Colors in P. delavayi
3.2. Essential Oils of P. delavayi Possess Significant Antioxidant Activities Influenced by Polyphenolic and Flavonoids
4. Materials and Methods
4.1. Materials and Reagents
4.2. Main Instruments and Equipment
4.3. Essential Oil Extraction
4.4. GC-MS Composition Detection
4.5. Determination and Analysis of Physical and Chemical Indicators of Essential Oils
4.6. Determination of Antioxidant Capacity of Different Flower Colors
4.6.1. Determination of the Ability to DPPH Free Radical Scavenging
4.6.2. Determination of the Ability to ABTS Free Radical Scavenging
4.6.3. Ferric Reducing Antioxidant Power
4.7. Determination of Bioactive Compounds
4.7.1. Total Phenolic Content
4.7.2. Total Flavonoids Content
4.8. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shi, Q.Q.; Zhou, L.; Wang, Y. Transcriptomic Analysis of Paeonia delavayi Wild Population Flowers to Identify Differentially Expressed Genes Involved in Purple-Red and Yellow Petal Pigmentation. PLoS ONE 2015, 10, e0135038. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.; Pan, K. Taxonomical history and revision of Paeonia sect. Moutan (Paeoniaceae). J. Syst. Evol. 1999, 37, 351–368. [Google Scholar]
- Hua, M.; Ma, H.; Tan, R.; Yuan, X.; Chen, J.; Yang, W. Determination of anthocyanins and flavonols in Paeonia delavayi by high-performance liquid chromatography with diode array and mass spectrometric detection. Anal. Lett. 2018, 51, 2331–2339. [Google Scholar] [CrossRef]
- Wu, S.H.; Chen, Y.W.; Li, Z.Y.; Yang, L.Y.; Li, S.L. Chemical constituents from the root bark of Paeonia delavayi. Chem. Natur. Compd. 2009, 45, 597–598. [Google Scholar] [CrossRef]
- Tao, A.E.; Zhao, F.Y.; Xia, C.L. The complete chloroplast genome sequence of the medicinal plant Paeonia delavayi Franchet. (Paeoniaceae). Mitochondrial DNA Part B-Resour. 2019, 4, 3553–3555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Liu, J.; Sun, H.L.; Yu, J.; Wang, J.X. Nuclear and chloroplast SSR markers in Paeonia delavayi (paeoniaceae) and cross-species amplification in P. ludlowii. Am. J. Bot. 2012, 98, E346–E348. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zheng, B.Q.; Wang, Y.; Guo, X. Study on pollination biology of paeonia delavayi (peaoniaceae). Acta Hortic. 2013, 977, 175–182. [Google Scholar] [CrossRef]
- Tan, S.L.; Peter, M.H.; Qin, H.T.; Ye, L.J.; Zou, J.Y.; Gao, L.M. Development of polymorphic microsatellite markers for tree peony paeonia delavayi (paeoniaceae) using ddrad-seq data. Mol. Biol. Rep. 2019, 46, 4605–4610. [Google Scholar] [CrossRef]
- Luo, X.N.; Yuan, M.; Li, B.J.; Li, C.Y.; Zhang, Y.L.; Shi, Q.Q. Variation of floral volatiles and fragrance reveals the phylogenetic relationship among nine wild tree peony species. Flavour Fragr. J. 2020, 35, 2. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Li, L.; Zhou, L.; Wang, Y. Morphological and Biochemical Studies of the Yellow and Purple-red Petal Pigmentation in Paeonia delavayi. Hortscience 2018, 53, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lewis, D.; Shi, R.; McGhie, T.; Wang, L.; Arathoon, S.; Schwinn, K.; Davies, K.; Qian, X.H.; Zhang, H.B. The colour variations of flowers in wild Paeonia delavayi plants are determined by four classes of plant pigments. N. Z. J. Crop. Hortic. Sci. 2022, 50, 1. [Google Scholar] [CrossRef]
- Monahan, A.H. Nonlinear principal component analysis by neural networks: Qheory and application to the lorenz system. J. Clim. 2000, 13, 821–835. [Google Scholar] [CrossRef]
- Yuan, W.Q.; Hu, J.Z.; Wu, Y.; Yin, L.Q.; Han, X.; Wang, X.X.; Lv, Z.L. Essential oil composition of Xanthoceras sorbifolium flower. J. Beijing For. Univ. 2020, 42, 111–121. [Google Scholar]
- Wu, Y.; Hu, J.Z.; Han, X.; Yin, L.Q.; Yuan, W.Q.; Zhao, Q.L.; Lv, Z.L. Comparison in essential oil components of different varieties at varied altitudes of Paeonia rockii. J. Beijing For. Univ. 2020, 42, 150–160. [Google Scholar]
- Zhang, X.Y.; Wu, Y.; Han, X.; Yan, Z.X.; Yuan, W.Q.; Lv, Z.L. Characterization of volatile compounds in five blueberry varieties using purge and trap coupled to gas chromatography-mass spectrometry. Ital. J. Food Sci. 2020, 32, 482–497. [Google Scholar]
- Yu, J.; Chen, G.L.; Yang, L.Q.; Wang, Y.K.; Gao, Y.Q.; Fu, N.L. Study on Purification and Antioxidant Activity of Polyphenols from the Flower of Paeonia lactiflora Palla. Food Res. Dev. 2017, 38, 38–44. [Google Scholar]
- Xue, Y.Q.; Liu, R.; Xue, J.Q.; Wang, S.L.; Zhang, X.X. Genetic diversity and relatedness analysis of nine wild species of tree peony based on simple sequence repeats markers. Hortic. Plant. J. 2021, 7, 579–588. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhang, X.X.; Niu, L.X.; Zhang, Y.L. Chemical compositions and antioxidant activities of essential oil extracted from the petals of three wild tree peony species and eleven cultivars. Chem. Biodivers 2017, 14, e1700282. [Google Scholar]
- Li, S.; Wang, C.Z.; Tang, X.X.; Wang, X.H.; Zhou, X. Study on the Physical-chemical Properties and Components of Peony Essential Oil Extracted by Different Extraction Methods. Food Ind. 2015, 36, 170–174. [Google Scholar]
- Jia, P.P.; Wang, X.C. Analysis of odor components derived from Chinese soft-shelled turtle (Trionyx sinensis) calipash cooked by steaming and boiling. Sci. Technol. Food Ind. 2017, 38, 66–89. [Google Scholar]
- Vella, F.M.; Calandrelli, R.; Cautela, D.; Fiume, I.; Laratta, B. Chemometric screening of fourteen essential oils for their composition and biological properties. Molecules 2020, 25, 5126. [Google Scholar] [CrossRef] [PubMed]
- Mischko, W.; Hirte, M.; Fuchs, M.; Mehlmer, N.; Brück, T.B. Identification of sesquiterpene synthases from the basidiomycota coniophora puteana for the efficient and highly selective β-copaene and cubebol production in E. coli. Microb. Cell Factories 2018, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, M.L.; Li, T.T.; Rao, Z.; Zhang, Y.; Sun, W.; Wen, X.; Wang, Z.; Ding, P.; Yuan, W. Composition and content of tobacco (Nicotiana tabacum L.) leaf cuticular waxes. Acta Agric. Boreali-Occident. Sin. 2014, 23, 140–145. [Google Scholar]
- Chen, X.Q.; Zhang, H.Y.; Xu, P.Z. Analysis of content of anthocyanidins, proanthocyanidins and flavone in color rice. Molecular Plant. Breeding. 2008, 6, 245–250. [Google Scholar]
- Dai, S.L.; Hong, Y. Molecular breeding for flower colors modification on ornamental plants based on the mechanism of anthocyanins biosynthesis and coloration. Sci. Agric. Sin. 2016, 49, 128–141. [Google Scholar]
- Guo, X.F.; Jia, X.G.; Ni, H.; Sun, Q.W.; Li, X.J. Study on the extraction and purification technology of the active ingredient from Cistanche tubulosa. J. Xinjiang Med. Univ. 2012, 35, 874–877. [Google Scholar]
- Chun, J.Y.; Jo, Y.J.; Bjrapha, P.; Choi, M.J.; Min, S.G. Antimicrobial Effect of alpha- or beta-Cyclodextrin Complexes with Trans-Cinnamaldehyde Against Staphylococcus aureus and Escherichia coli. Dry. Technol. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Bektas, T.; Munevver, S.; Askin, A.; Dimitra, D.; Moschos, P.; Atalay, S. Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J. Food Eng. 2005, 66, 447–454. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species(ROS)and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Saad, D.; Yasser, T.; Muhammad, I.; Mohamed, A.; Mohammed, E. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar]
- Gullón, B.; Gullón, P.; Lú-Chau, T.A. Optimization of solvent extraction of antioxidants from Eucalyptus globulus leaves by response surface methodology: Characterization and assessment of their bioactive properties. Ind. Crops Prod. 2017, 108, 649–659. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bonfim, N.S. Curcuma longa, L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides, and fumonisin production. Food Control. 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufian-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of Melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 21–29. [Google Scholar] [CrossRef]
- Bao, Y.T.; Qu, Y.; Li, J.H.; Li, Y.F.; Ren, X.D.; Maffucci, K.G.; Li, R.P.; Wang, Z.G.; Zeng, R. In Vitro and In Vivo Antioxidant Activities of the Flowers and Leaves from Paeonia rockii and Identification of Their Antioxidant Constituents by UHPLC-ESI-HRMS via Pre-Column DPPH Reaction. Molecules 2018, 23, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.S.; Chen, M.L.; Jin, Y.Z.; Tao, J. In vitro free radical scavenging activities and active constituents from Paeonia lactiflora flowers. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2012, 33, 86–90. [Google Scholar]
- Li, H.L.; Gao, X.; Xu, F.L.; Chen, H.K.; Wang, W.L. Chemical composition and antioxidant activities of Essential oil from Paeonia lactiflora flowers. J. Northwest. AF Univ. (Nat. Sci. Ed.) 2017, 45, 204–210. [Google Scholar]
- Chen, G.L.; Chen, S.G.; Xie, Y.Q.; Chen, F.; Zhao, Y.Y.; Luo, C.X.; Gao, Y.Q. Total phenolic, fl avonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Shang, P.P.; Jia, M.X.; Liu, A.Q.; Jiang, X.R.; Liu, Y. Reaserch on the Bioactive Compounds and Antioxidant Activity of Petals from Different Cultivars of Paeonia lactiflora. Plant. Physiol. J. 2016, 52, 234–240. [Google Scholar]
- Maham, I.; Bushra, A.; Faqir, M.; Ali, S.; Muhammad, F.A.; Irfan, H.; Kashif, S.; Hosh, M. Antioxidant and Wound Healing Potential of Essential Oil from Citrus reticulata Peel and its Chemical Characterization. Curr. Pharm. Biotechnol. 2021, 22, 1114–1121. [Google Scholar]
- Jang, H.D.; Chang, K.S.; Chang, T.C.; Hsu, C.L. Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chem. 2010, 118, 554–558. [Google Scholar] [CrossRef]
- Gürkan, S.; Gurbet, Ç.; Erhan, G.; Aslı, S. Essential oil composition, antioxidant activity and phenolic content of endemic Teucrium alyssifolium Staph. (Lamiaceae). Nat. Prod. Res. 2016, 30, 2225–2229. [Google Scholar]
- Li, D.X.; Qian, J.; Huang, D.Z.; Guo, X.; Tian, M.; Zeng, D.Q. Analysis of the Antioxidant and Antifungal Activities of the Essential oil of Laurocerasus phaeosticta. Chin. J. Trop. Agric. 2018, 38, 72–80. [Google Scholar]
- Antonella, R.; Andrea, M.; Danilo, P.; Angela, A.; Benedetta, E.; Antonella, F.; Cinzia, S.; Piras, A. Chemical composition of Lycium europaeum fruit oil obtained by supercritical CO2 extraction and evaluation of its antioxidant activity, cytotoxicity and cell absorption. Food Chem. 2017, 230, 82–90. [Google Scholar]
- Qiu, W.; Su, W.; Cai, Z.; Dong, L.; Wu, Z. Combined Analysis of Transcriptome and Metabolome Reveals the Potential Mechanism of Coloration and Fruit Quality in Yellow and Purple Passiflora edulis Sims. J. Agric. Food Chem. 2020, 68, 12096–12106. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.L.; Zhu, W.X.; Kang, H.B.; Ma, H.L.; Tao, G.J. Flavonoid constituents and antioxidant capacity in flowers of different Zhongyuan tree penoy cultivars. J. Funct. Foods 2012, 4, 147–157. [Google Scholar] [CrossRef]
- Yu, H.; Ma, W.P.; Liu, Y.P.; Li, J.X.; Liu, J.M. Analysis of Volatile Components in Peony Essence Oil by Headspace Gas Chromatography-Mass Spectrometry. Food Sci. 2015, 36, 167–171. [Google Scholar]
- Liu, J.Y.; Yin, G.Y.; Zhang, H.J.; Wu, Y.S.; Xu, S.T.; Wei, J. Comparison of essential oils from Macadamia ternifolia flowers by supercritical carbon dioxide fluid extraction and simultaneous distillation extraction. J. Yunnan Univ. 2013, 35, 678–684. [Google Scholar]
- Lin, L.J.; Huang, X.B.; Liu, M.J.; Li, J.H. Composition of Essential oils and Hydrosols Acquired from Alpinia officinarum Hance by Supercritical CO2 Extraction and Steam Extraction. Chin. J. Trop. Crops 2019, 40, 2498–2504. [Google Scholar]
- Ghane, S.G.; Attar, U.A.; Yadav, P.B.; Lekhak, M.M. Antioxidant, anti-diabetic, acetylcholinesterase inhibitory potential and estimation of alkaloids (lycorine and galanthamine) from Crinum species: An important source of anticancer and anti-Alzheimer drug. Ind. Crops Prod. 2018, 125, 168–177. [Google Scholar] [CrossRef]
- Liao, H.; Banbury, L. Different Proportions of Huangqi (Radix Astragali Mongolici) and Honghua (Flos Carthami) Injection on α-Glucosidase and a α-Amylase Activities. Evid. -Based Complementray Altern. Med. 2015, 1, 785193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.X.; Lin, Q.Q.; Tu, Z.C.; Zhang, L. The influence of in vitro gastrointestinal digestion on the Perilla frutescens leaf extract: Changes in the active compounds and bioactivities. J. Food Biochem. 2020, 44, e13530. [Google Scholar] [CrossRef] [PubMed]
| Number | Compounds | Relative Content/% | ||
|---|---|---|---|---|
| Dark-Purple | Red | Yellow | ||
| 1 | Benzyl alcohol | 2.24 a ± 0.97 | 3.24 a ± 1.00 | 3.44 a ± 0.86 |
| 2 | Phenethyl alcohol | 1.76 ab ± 0.51 | 0.80 a ± 0.15 | 2.06 bc ± 0.92 |
| 3 | (S,3E,7E)-α,α,4,8-Tetramethyl-3,7-cyclodecadiene-1-methanol | 0.42 ab ± 0.13 | 0.38 ab ± 0.17 | 2.50 c ± 0.35 |
| 4 | α-cadinol | 1.11 a ± 0.38 | 0.94 ab ± 0.26 | 4.30 c ± 0.85 |
| 5 | Phytol | 2.15 a ± 0.58 | 3.48 ac ± 0.73 | 2.51 ab ± 0.62 |
| 6 | 2-(5-Ethenyl-5-methyloxolan-2-yl)propan-2-ol | 20.88 ab ± 1.64 | 21.81 ab ± 1.04 | 4.06 c ± 0.82 |
| 7 | Bicyclo[3.2.2]nona-6,8-dien-3-one | 0.60 a ± 0.17 | 0.79 a ± 0.08 | 0.60 a ± 0.13 |
| 8 | Fitone | 4.34 c ± 0.52 | 2.20 ab ± 0.37 | 2.02 ab ± 0.28 |
| 9 | Benzaldehyde | 0.15 a ± 0.04 | 0.17 a ± 0.07 | 0.10 a ± 0.02 |
| 10 | Trans-Cinnamaldehyde | 4.92 a ± 0.64 | 4.31 a ± 0.81 | 0.33 b ± 0.06 |
| 11 | (E)-linalool oxide (pyranoid) | 11.90 a ± 1.45 | 10.85 ac ± 1.26 | 0.97 b ± 0.14 |
| Petal Color | Total Phenolic Content (mg/g) | Total Flavonoids Content (mg/g) |
|---|---|---|
| Dark-purple | 28.71 ab ± 0.42 | 10.8 a ± 0.06 |
| Red | 23.68 b ± 0.76 | 9.43 bc ± 0.21 |
| Yellow | 18.89 c ± 0.36 | 5.45 c ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhang, H.; Wang, J.; Wang, J.; Wang, Z.; Li, J. Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia delavayi Using Supercritical Carbon Dioxide Fluid. Molecules 2022, 27, 3000. https://doi.org/10.3390/molecules27093000
Yu X, Zhang H, Wang J, Wang J, Wang Z, Li J. Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia delavayi Using Supercritical Carbon Dioxide Fluid. Molecules. 2022; 27(9):3000. https://doi.org/10.3390/molecules27093000
Chicago/Turabian StyleYu, Xiao, Huaibi Zhang, Juan Wang, Junming Wang, Zhenxing Wang, and Jinbo Li. 2022. "Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia delavayi Using Supercritical Carbon Dioxide Fluid" Molecules 27, no. 9: 3000. https://doi.org/10.3390/molecules27093000
APA StyleYu, X., Zhang, H., Wang, J., Wang, J., Wang, Z., & Li, J. (2022). Phytochemical Compositions and Antioxidant Activities of Essential Oils Extracted from the Flowers of Paeonia delavayi Using Supercritical Carbon Dioxide Fluid. Molecules, 27(9), 3000. https://doi.org/10.3390/molecules27093000