Determination of Chiral Impurity of Naproxen in Different Pharmaceutical Formulations Using Polysaccharide-Based Stationary Phases in Reversed-Phased Mode
Abstract
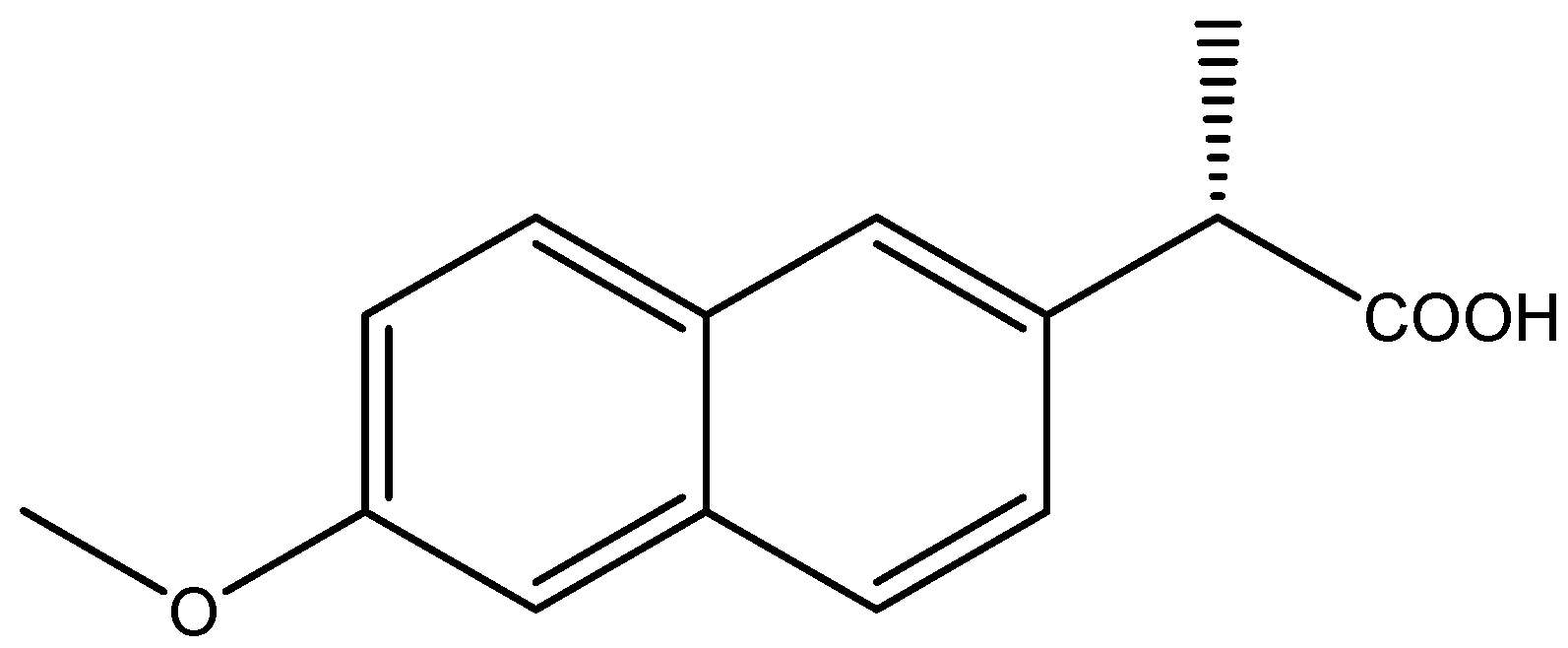
:1. Introduction
2. Results
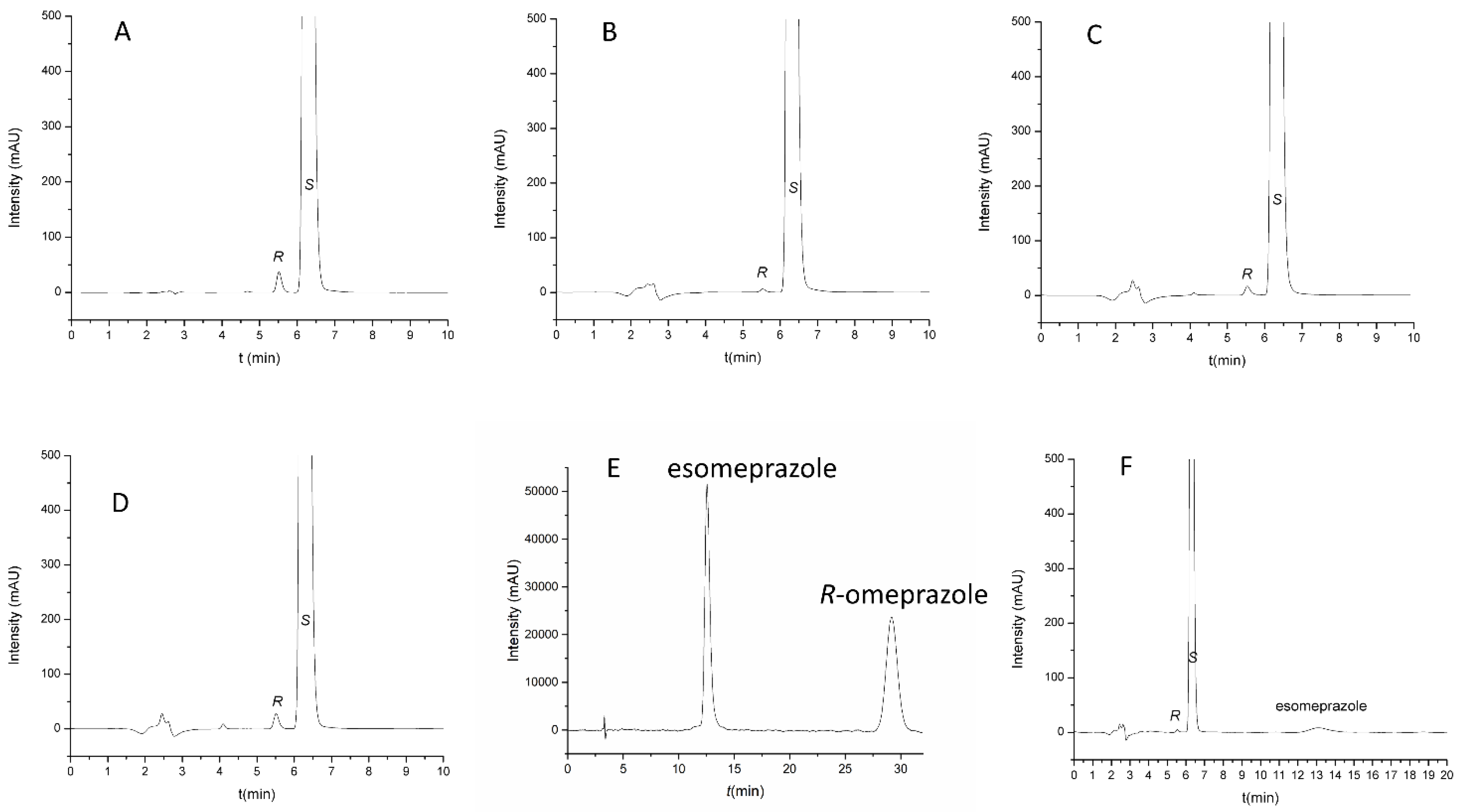
2.1. Screening Phase
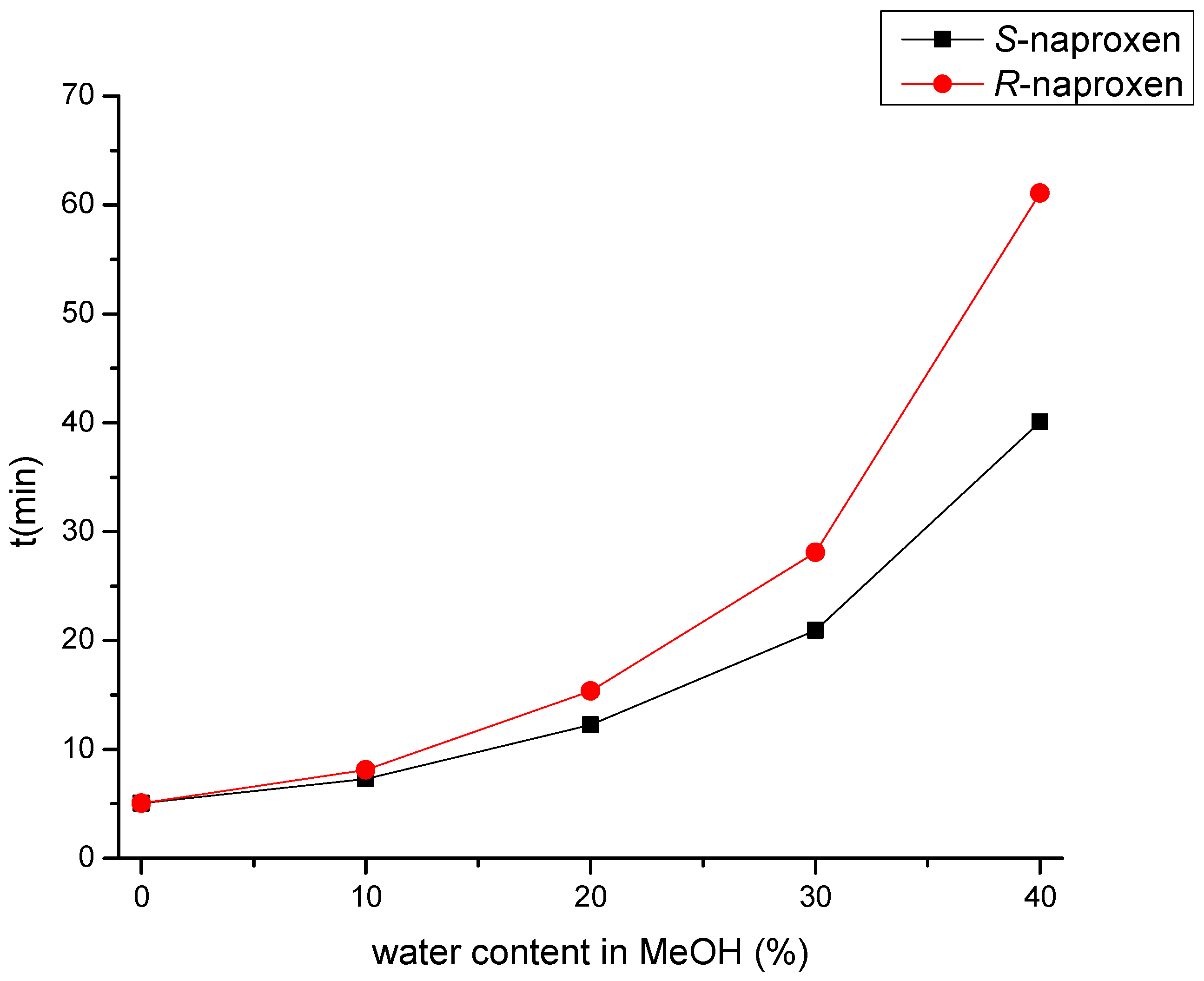
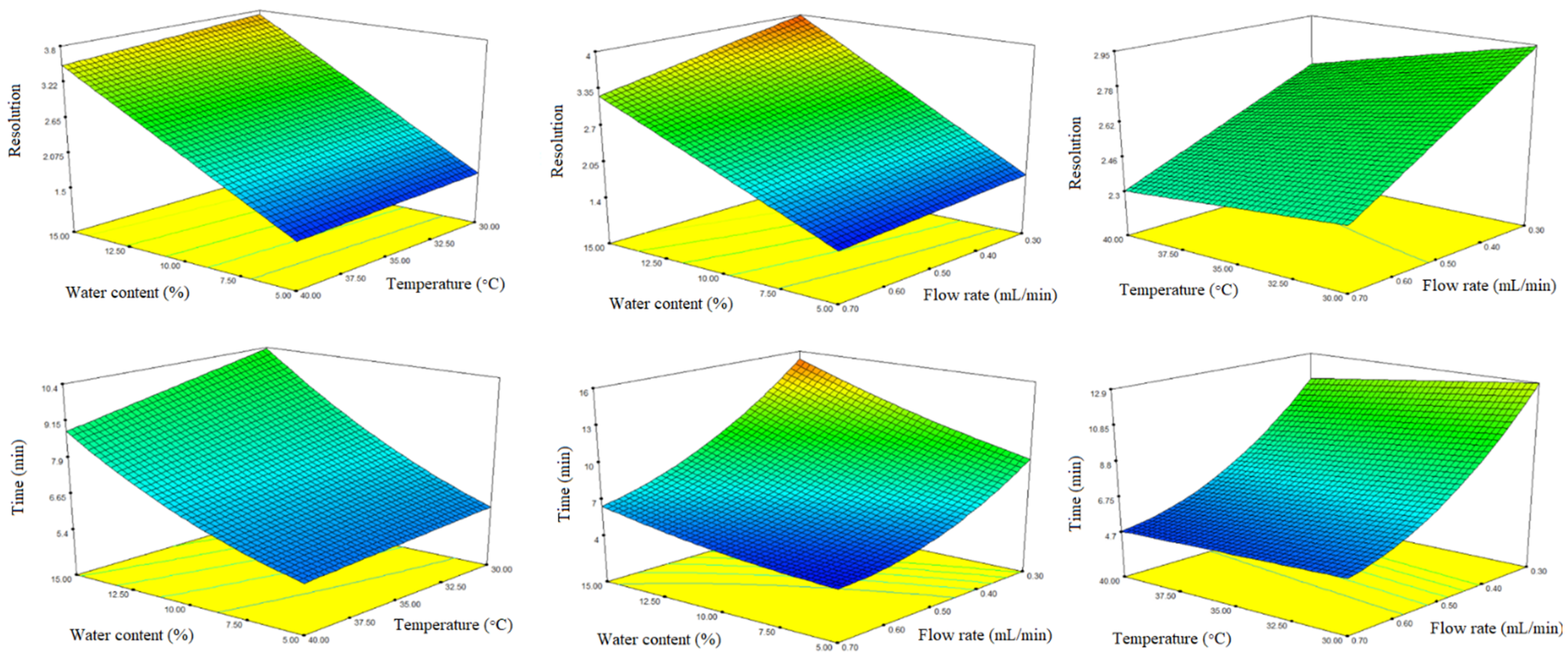
2.2. Method Optimization
+ 1.31 * C2
2.3. Method Validation and Application
3. Materials and Methods
3.1. Materials
3.2. LC-UV Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Almáši, M.; Matiašová, A.A.; Šuleková, M.; Beňová, E.; Ševc, J.; Váhovská, L.; Lisnichuk, M.; Girman, V.; Zeleňáková, A.; Hudák, A.; et al. In vivo study of light-driven naproxen release from gated mesoporous silica drug delivery system. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Clissold, S.P. Naproxen. Drugs 1990, 40, 91–137. [Google Scholar] [CrossRef] [PubMed]
- Naproxen—Drug Usage Statistics, ClinCalc DrugStats Database. Available online: https://clincalc.com/DrugStats/Drugs/Naproxen (accessed on 7 April 2022).
- Guttman, A.; Cooke, N. Practical aspects in chiral separation of pharmaceuticals by capillary electrophoresis: II. Quantitative separation of naproxen enantiomers. J. Chromatogr. A 1994, 685, 155–159. [Google Scholar] [CrossRef]
- Fanali, S.; Desiderio, C.; Aturki, Z. Enantiomeric resolution study by capillary electrophoresis. Selection of the appropriate chiral selector. J. Chromatogr. A 1997, 772, 185–194. [Google Scholar] [CrossRef]
- Fillet, M.; Fotsing, L.; Bonnard, J.; Crommen, J. Stereoselective determination of S-naproxen in tablets by capillary electrophoresis. J. Pharm. Biomed. Anal. 1998, 18, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Ruan, Z.; Kang, J.; Ou, Q. Simultaneous chiral separation and determination of the optical purity of naproxen and methyl naproxen by capillary electrophoresis with dual-cyclodextrin system as chiral selector. Anal. Lett. 2001, 34, 1657–1668. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Zhang, Q.; Lei, Y. Evaluation of vancomycin-based synergistic system with amino acid ester chiral ionic liquids as additives for enantioseparation of non-steroidal anti-inflamatory drugs by capillary electrophoresis. Talanta 2014, 119, 193–201. [Google Scholar] [CrossRef]
- Chen, D.-M.; Fu, Q.; Li, N.; Zhang, S.-X.; Zhang, Q.-Q. Enantiomeric Separation of Naproxen by High Performance Liquid Chromatography Using CHIRALCEL OD as Stationary Phase. Chin. J. Anal. Chem. 2007, 35, 75–78. [Google Scholar] [CrossRef]
- Jin, J.-Y.; Lee, W.; Baek, C.-S. Enantiomer resolution of non-steroidal anti-inflammatory drugs on chiral stationary phases derived from polysaccharide derivatives. Chin. J. Anal. Chem. 2008, 36, 1207–1211. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, G.; Kang, S.; Guo, X.; Yao, B.; Weng, W.; Zeng, Q. Unusual chromatographic enantioseparation behavior of naproxen on an immobilized polysaccharide-based chiral stationary phase. J. Chromatogr. A 2011, 1218, 8718–8721. [Google Scholar] [CrossRef]
- Tanaka, M.; Nagamatsu, K.; Nishi, H. High-performance enantiomer separation of nonsteroidal anti-inflammatory drugs (NSAIDs) by 3 µm reversed-phase chiral columns and application to the optical purity testing of naproxen drug substances and its formulations. Anal. Sci. 2014, 30, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragab, M.A.A.; El-Kimary, E.I. High performance liquid chromatography with photo diode array for separation and analysis of naproxen and esomeprazole in presence of their chiral impurities: Enantiomeric purity determination in tablets. J. Chromatogr. A 2017, 1497, 110–117. [Google Scholar] [CrossRef]
- Maisuradze, M.; Sheklashvili, G.; Chokheli, A.; Matarashvili, I.; Gogatishvili, T.; Farkas, T.; Chankvetadze, B. Chromatographic and thermodynamic comparison of amylose tris(3-chloro-5-methylphenylcarbamate) coated or covalently immobilized on silica in high-performance liquid chromatographic separation of the enantiomers of select chiral weak acids. J. Chromatogr. A 2019, 1602, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Qu, H.; Wang, B.; Wang, F.; Yu, Y.; Yu, G. Simultaneous enantiomeric analysis of non-steroidal anti-inflammatory drugs in environment by chiral LC-MS/MS: A pilot study in Beijing, China. Ecotoxicol. Environ. Saf. 2019, 174, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, X.; Guo, X.; Di, X.; Jiang, Z. Enantiomeric separation and enantioselective determination of some representive non-steroidal anti-inflammatory drug enantiomers in fish tissues by using chiral liquid chromatography coupled with tandem mass spectrometry. Microchem. J. 2020, 153, 104511. [Google Scholar] [CrossRef]
- Camilo, K.; Foley, J.P. Simultaneous Achiral/Chiral HPLC Separation of Ketoprofen, Ibuprofen, Flurbiprofen, and Naproxen. Chromatographia 2021, 84, 371–379. [Google Scholar] [CrossRef]
- Cao, S.; Xie, C.; Ma, Q.; Wang, S.; Zhang, J.; Wang, Z. Enantioselective separation of nonsteroidal anti-inflammatory drugs with amylose tris(3-chloro-5-methylphenylcarbamate) stationary phase in HPLC with a focus on enantiomeric quality control in six pharmaceutical formulations containing racemic mixtures or single stereoisomers. Chirality 2021, 33, 938–950. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of enantiomers. J. Chromatogr. A 2012, 1269, 26–51. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Matarashvili, I.; Ghughunishvili, D.; Chankvetadze, L.; Takaishvili, N.; Khatiashvili, T.; Tsintsadze, M.; Farkas, T.; Chankvetadze, B. Separation of enantiomers of chiral weak acids with polysaccharide-based chiral columns and aqueous-organic mobile phases in high-performance liquid chromatography: Typical reversed-phase behavior? J. Chromatogr. A 2017, 1483, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Ferencz, E.; Kovács, B.; Boda, F.; Foroughbakhshfasaei, M.; Kelemen, É.K.; Tóth, G.; Szabó, Z.I. Simultaneous determination of chiral and achiral impurities of ivabradine on a cellulose tris(3-chloro-4-methylphenylcarbamate) chiral column using polar organic mode. J. Pharm. Biomed. Anal. 2020, 177, 112851. [Google Scholar] [CrossRef] [PubMed]
- Köteles, I.; Foroughbakhshfasaei, M.; Dobó, M.; Ádám, M.; Boldizsár, I.; Szabó, Z.I.; Tóth, G. Determination of the Enantiomeric Purity of Solriamfetol by High-Performance Liquid Chromatography in Polar Organic Mode Using Polysaccharide-Type Chiral Stationary Phases. Chromatographia 2020, 83, 909–913. [Google Scholar] [CrossRef]
- Dobó, M.; Foroughbakhshfasaei, M.; Horváth, P.; Szabó, Z.I.; Tóth, G. Chiral separation of oxazolidinone analogues by liquid chromatography on polysaccharide stationary phases using polar organic mode. J. Chromatogr. A 2022, 1662, 462741. [Google Scholar] [CrossRef] [PubMed]
- Foroughbakhshfasaei, M.; Dobó, M.; Boda, F.; Szabó, Z.I.; Tóth, G. Comparative chiral separation of thalidomide class of drugs using polysaccharide-type stationary phases with emphasis on elution order and hysteresis in polar organic mode. Molecules 2022, 27, 111. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Kartozia, I.; Yamamoto, C.; Okamoto, Y. Comparative enantioseparation of selected chiral drugs on four different polysaccharide-type chiral stationary phases using polar organic mobile phases. J. Pharm. Biomed. Anal. 2002, 27, 467–478. [Google Scholar] [CrossRef]
- Varfaj, I.; Protti, M.; Cirrincione, M.; Carotti, A.; Mercolini, L.; Sardella, R. Original enantioseparation of illicit fentanyls with cellulose-based chiral stationary phases under polar-ionic conditions. J. Chromatogr. A 2021, 1643, 462088. [Google Scholar] [CrossRef]
- Varfaj, I.; Di Michele, A.; Ianni, F.; Saletti, M.; Anzini, M.; Barola, C.; Chankvetadze, B.; Sardella, R.; Carotti, A. Enantioseparation of novel anti-inflammatory chiral sulfoxides with two cellulose dichlorophenylcarbamate-based chiral stationary phases and polar-organic mobile phase(s). J. Chromatogr. Open 2021, 1, 100022. [Google Scholar] [CrossRef]
- El-Behairy, M.F.; Hassan, R.M.; Abdallah, I.A. Enantioselective Separation of Chiral N1-Substituted-1 H-pyrazoles: Greenness Profile Assessment and Chiral Recognition Analysis. ACS Omega 2021, 6, 25835–25841. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Pierini, M.; Carradori, S.; Menta, S.; Secci, D.; Cirilli, R. 3-(Phenyl-4-oxy)-5-phenyl-4,5-dihydro-(1H)-pyrazole: A fascinating molecular framework to study the enantioseparation ability of the amylose (3,5-dimethylphenylcarbamate) chiral stationary phase. Part II. Solvophobic effects in enantiorecognition process. J. Chromatogr. A 2017, 1499, 140–148. [Google Scholar] [CrossRef]
- ICH. Q2(R1) Validation of Analytical Procedures: Text and Methodology; International Council for Harmonisation (ICH): Geneva, Switzerland, 2005; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 7 April 2022).
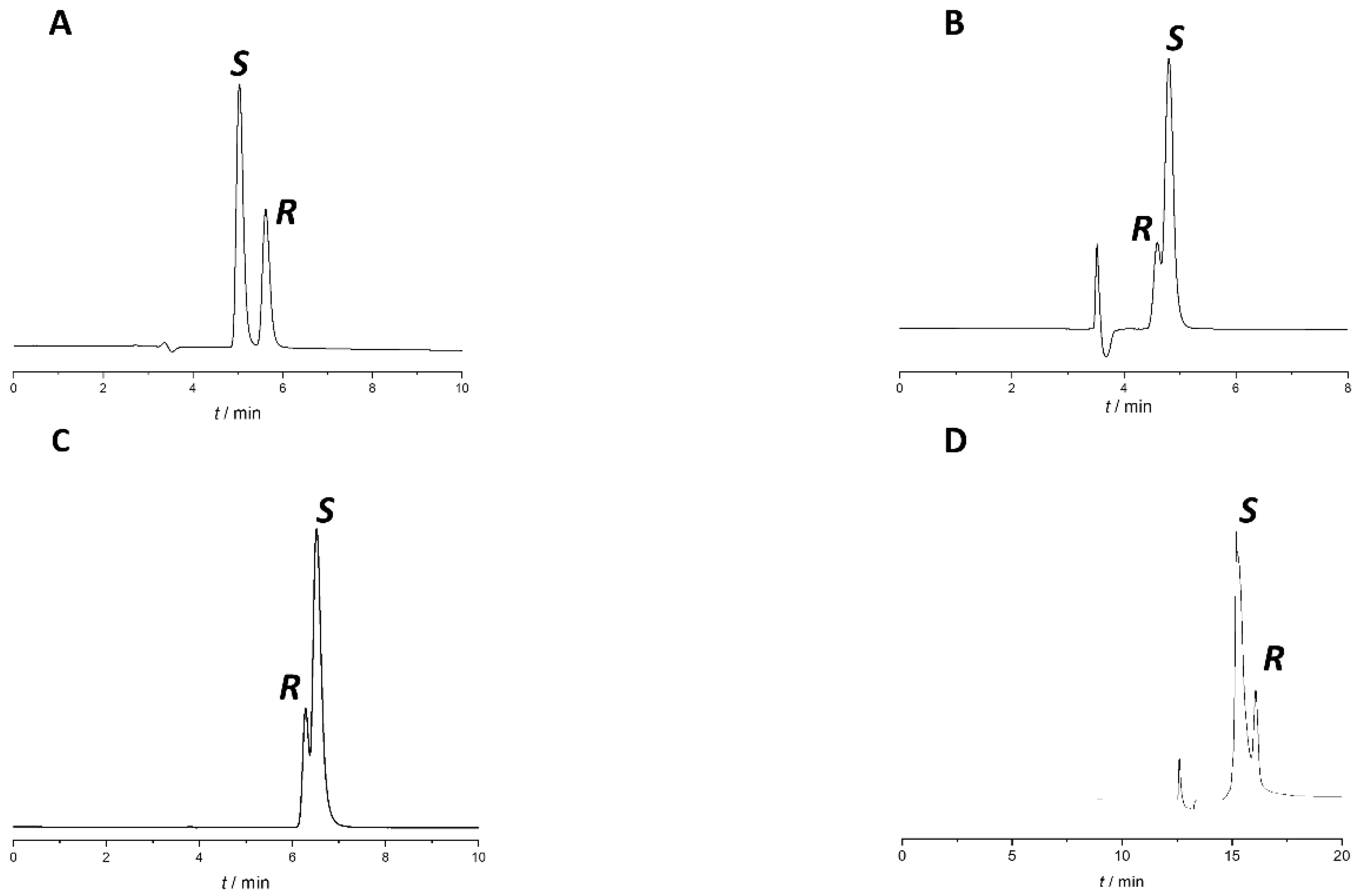

| CSP | Mobile Phase * | tr2 (min) | Rs | EEO |
|---|---|---|---|---|
| Lux Amylose-1 | MeOH | 5.16 | - | - |
| EtOH | 5.25 | 1.24 | S > R | |
| IPA | 5.68 | - | - | |
| ACN | 6.02 | 0.52 | R < S | |
| Lux i-Amylose-1 | MeOH | 4.42 | - | - |
| EtOH | 4.46 | - | - | |
| IPA | 5.00 | - | - | |
| ACN | 4.52 | - | - | |
| Lux Amylose-2 | MeOH | 5.51 | - | - |
| EtOH | 5.10 | - | - | |
| IPA | 6.43 | - | - | |
| ACN | 7.20 | - | - | |
| Lux Cellulose-1 | MeOH | 6.52 | 0.41 | R > S |
| EtOH | 5.36 | - | - | |
| IPA | 7.05 | - | - | |
| ACN | 7.00 | - | - | |
| Lux Cellulose-2 | MeOH | 5.17 | - | - |
| EtOH | 5.23 | - | - | |
| IPA | 7.74 | - | - | |
| ACN | 9.30 | - | - | |
| Lux Cellulose-3 | MeOH | 7.85 | - | - |
| EtOH | 7.17 | - | - | |
| IPA | 9.20 | 0.33 | S > R | |
| ACN | 6.34 | - | - | |
| Lux Cellulose-4 | MeOH | 6.73 | - | - |
| EtOH | 6.77 | - | - | |
| IPA | 16.07 | 0.60 | S > R | |
| ACN | 9.23 | - | - |
| Water Content (v/v %) | Column Temperature (°C) | Flow Rate (mL/min) | Acetic Acid (v/v %) | t2 (min) | Rs | |
|---|---|---|---|---|---|---|
| 1 | 15 | 40 | 0.7 | 0.05 | 6.14 | 3.2 |
| 2 | 10 | 35 | 0.5 | 0.1 | 7.24 | 2.6 |
| 3 | 10 | 35 | 0.5 | 0.05 | 7.24 | 2.6 |
| 4 | 5 | 30 | 0.3 | 0.05 | 10.07 | 1.7 |
| 5 | 15 | 40 | 0.3 | 0.05 | 14.17 | 3.8 |
| 6 | 10 | 40 | 0.5 | 0.1 | 6.91 | 2.5 |
| 7 | 10 | 35 | 0.3 | 0.1 | 11.92 | 2.8 |
| 8 | 10 | 35 | 0.7 | 0.1 | 5.11 | 2.3 |
| 9 | 5 | 35 | 0.5 | 0.1 | 5.85 | 1.6 |
| 10 | 10 | 35 | 0.5 | 0.1 | 7.24 | 2.6 |
| 11 | 10 | 35 | 0.5 | 0.1 | 7.23 | 2.6 |
| 12 | 10 | 35 | 0.5 | 0.1 | 7.23 | 2.6 |
| 13 | 5 | 30 | 0.7 | 0.05 | 4.30 | 1.5 |
| 14 | 10 | 35 | 0.5 | 0.1 | 7.26 | 2.6 |
| 15 | 10 | 30 | 0.5 | 0.1 | 7.62 | 2.6 |
| 16 | 15 | 35 | 0.5 | 0.1 | 9.37 | 3.6 |
| 17 | 15 | 30 | 0.7 | 0.15 | 6.84 | 3.3 |
| 18 | 10 | 35 | 0.5 | 0.15 | 7.12 | 2.6 |
| 19 | 5 | 40 | 0.3 | 0.15 | 9.38 | 1.6 |
| 20 | 5 | 40 | 0.7 | 0.15 | 4.04 | 1.4 |
| 21 | 15 | 30 | 0.3 | 0.15 | 16.69 | 4.2 |
| Parameter | Result |
|---|---|
| Linearity | |
| Range (%) | 0.05–3% |
| Range (μg/mL) | 2.5–150 |
| Regression equation | 11.932x + 0.2905 |
| Coefficient of determination (r2) | 0.9998 |
| Sensitivity | |
| LOD (μg/mL) (S/N = 3) | 0.6 |
| LOQ (μg/mL) (S/N = 10) | 2.0 |
| Repeatability (six injections on the same day) RSD% | |
| Level I (5 μg/mL) | 1.02 |
| Level II (25 μg/mL) | 0.75 |
| Level III (125 μg/mL) | 0.63 |
| Intermediate precision (Analysis on three consecutive days, each sample analyzed in triplicate) (RSD%) | |
| Level I (5 μg/mL) | 1.25 |
| Level II (25 μg/mL) | 0.89 |
| Level III (125 μg/mL) | 1.19 |
| Accuracy (Expressed as mean recovery ± 95% confidence interval (n = 3, α = 0.05)) (%). | |
| Level I (5 μg/mL) | 99.06 ± 0.71 |
| Level II (25 μg/mL) | 100.42 ± 0.84 |
| Level III (125 μg/mL) | 98.97 ± 0.77 |
| Column | Mobile Phase | Separation Mode | Enantiomeric Quality Control | Rs | t (min) | Ref. |
|---|---|---|---|---|---|---|
| Lux Amylose-1 | MeOH:H2O:acetic acid mixture | RP | Yes (0.1% limit) | 3.2 (optimized by FCCD) | ~7 | Current work |
| Silica gel π-acceptor/π-donor for chiral separations | Hexane:IPA:ACN:acetic acid | NP | Yes (2.5% limit) | ~3 | ~7.5 | EurPh |
| Chiralcel OD | Hexane:IPA:acetic acid | NP | No | 1.7 | ~20 | [9] |
| Chiralpak IC | Hexane:EtOH:TFA | NP | N | 3.4 | - | [10] |
| Chiralpak AS-3R | ACN:phosphate buffer | RP | Yes (not complete validation) | 2.55 | ~7 | [12] |
| FLM Chiral NQ(2)-RH | ACN:formic acid in water | RP | Yes | 2.38 | ~14 | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, L.-A.; Krizbai, S.; Dobó, M.; Hancu, G.; Szabó, Z.-I.; Tóth, G. Determination of Chiral Impurity of Naproxen in Different Pharmaceutical Formulations Using Polysaccharide-Based Stationary Phases in Reversed-Phased Mode. Molecules 2022, 27, 2986. https://doi.org/10.3390/molecules27092986
Papp L-A, Krizbai S, Dobó M, Hancu G, Szabó Z-I, Tóth G. Determination of Chiral Impurity of Naproxen in Different Pharmaceutical Formulations Using Polysaccharide-Based Stationary Phases in Reversed-Phased Mode. Molecules. 2022; 27(9):2986. https://doi.org/10.3390/molecules27092986
Chicago/Turabian StylePapp, Lajos-Attila, Sarolta Krizbai, Máté Dobó, Gabriel Hancu, Zoltán-István Szabó, and Gergő Tóth. 2022. "Determination of Chiral Impurity of Naproxen in Different Pharmaceutical Formulations Using Polysaccharide-Based Stationary Phases in Reversed-Phased Mode" Molecules 27, no. 9: 2986. https://doi.org/10.3390/molecules27092986
APA StylePapp, L.-A., Krizbai, S., Dobó, M., Hancu, G., Szabó, Z.-I., & Tóth, G. (2022). Determination of Chiral Impurity of Naproxen in Different Pharmaceutical Formulations Using Polysaccharide-Based Stationary Phases in Reversed-Phased Mode. Molecules, 27(9), 2986. https://doi.org/10.3390/molecules27092986








