Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
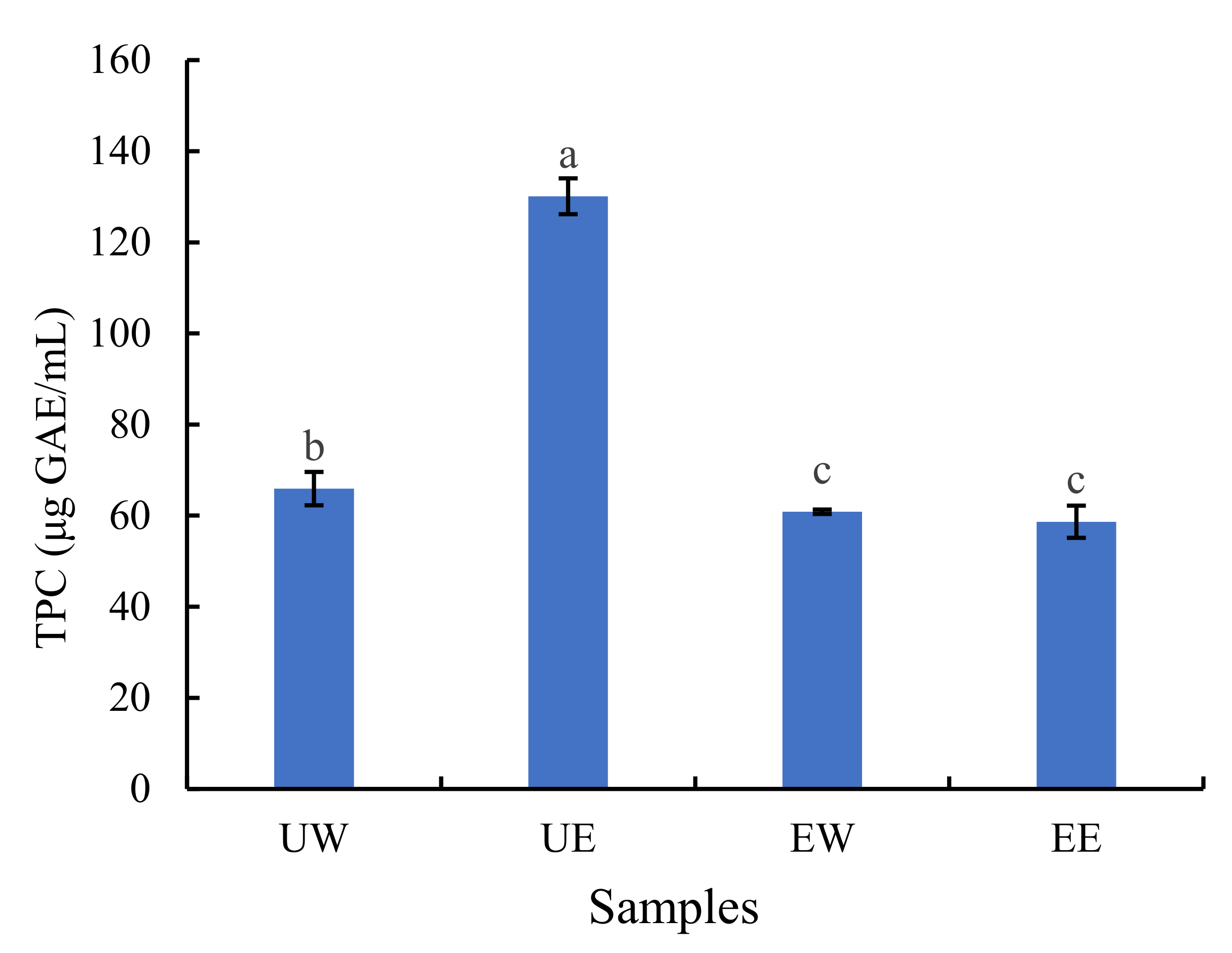
2.1. The Effect of Extraction Method on TPC
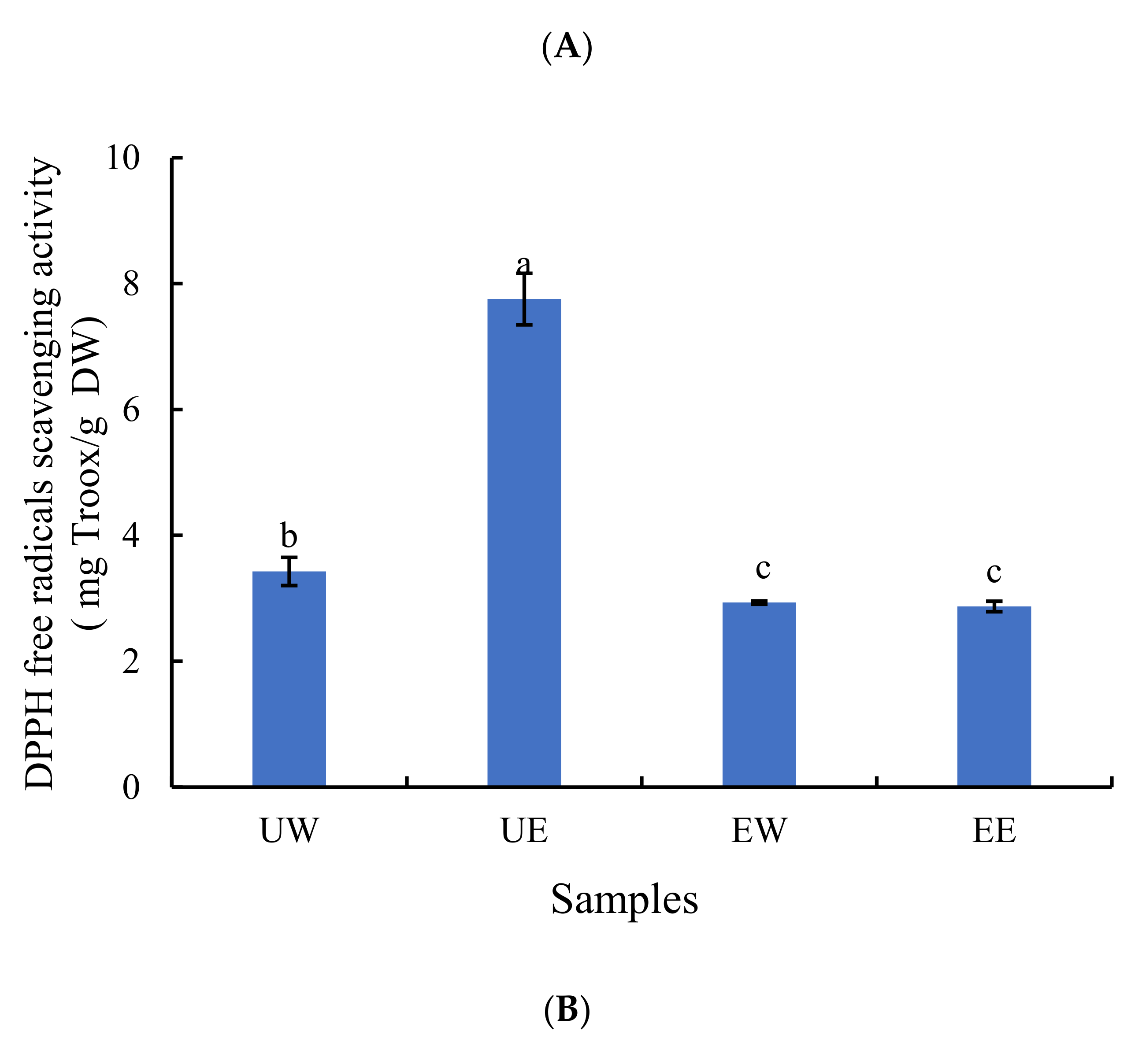
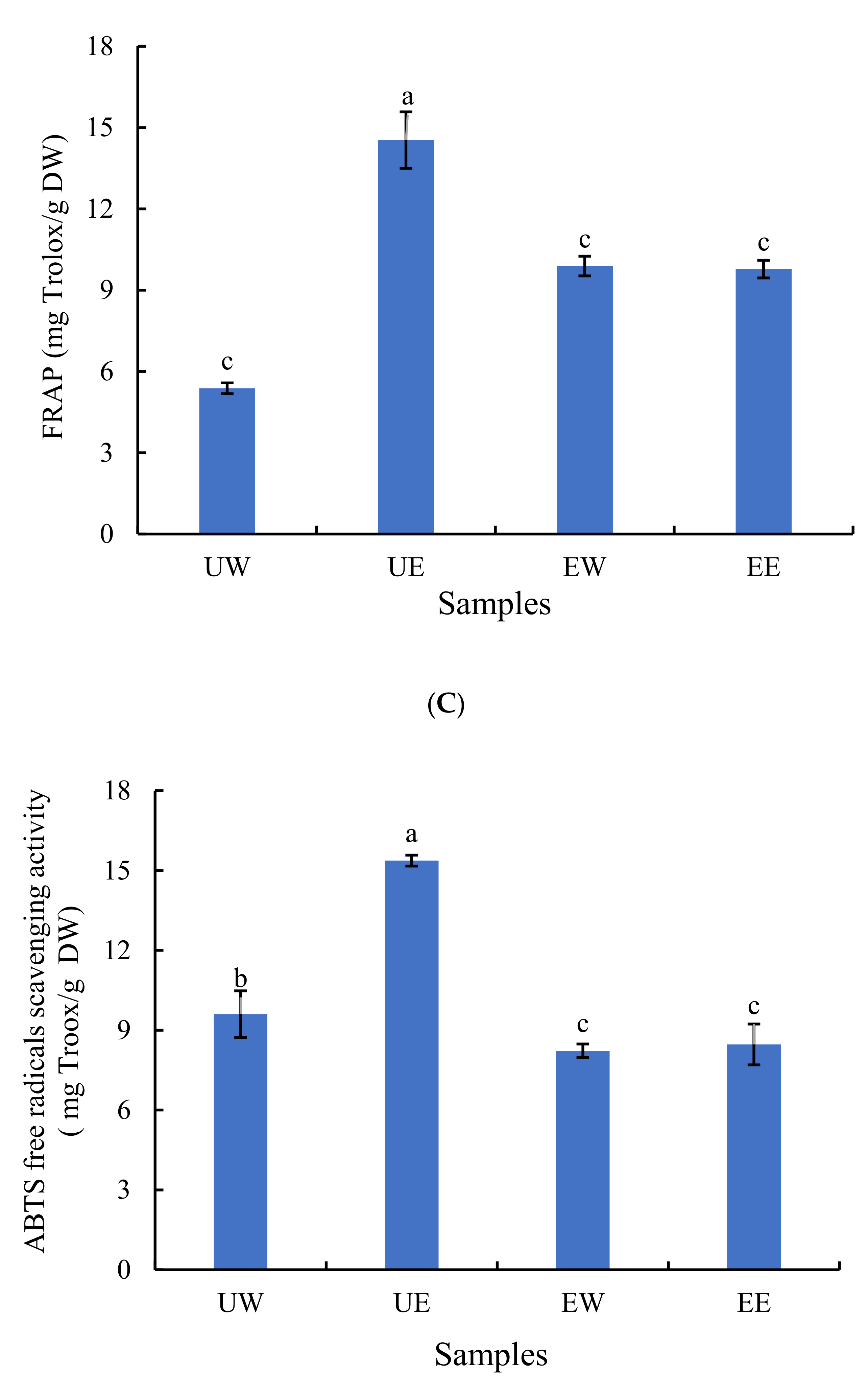
2.2. The Effect of Extraction Method on Antioxidant Activity
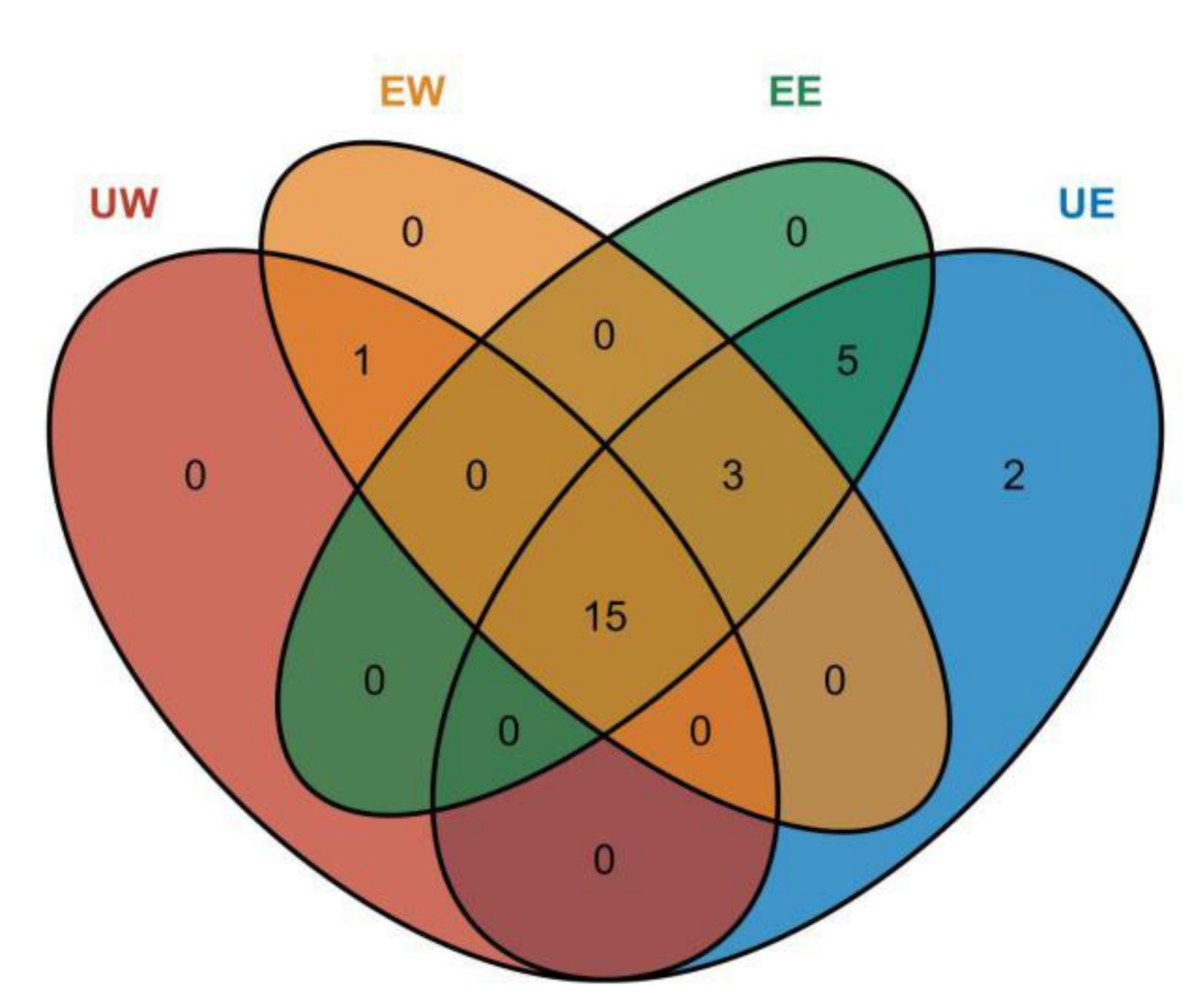
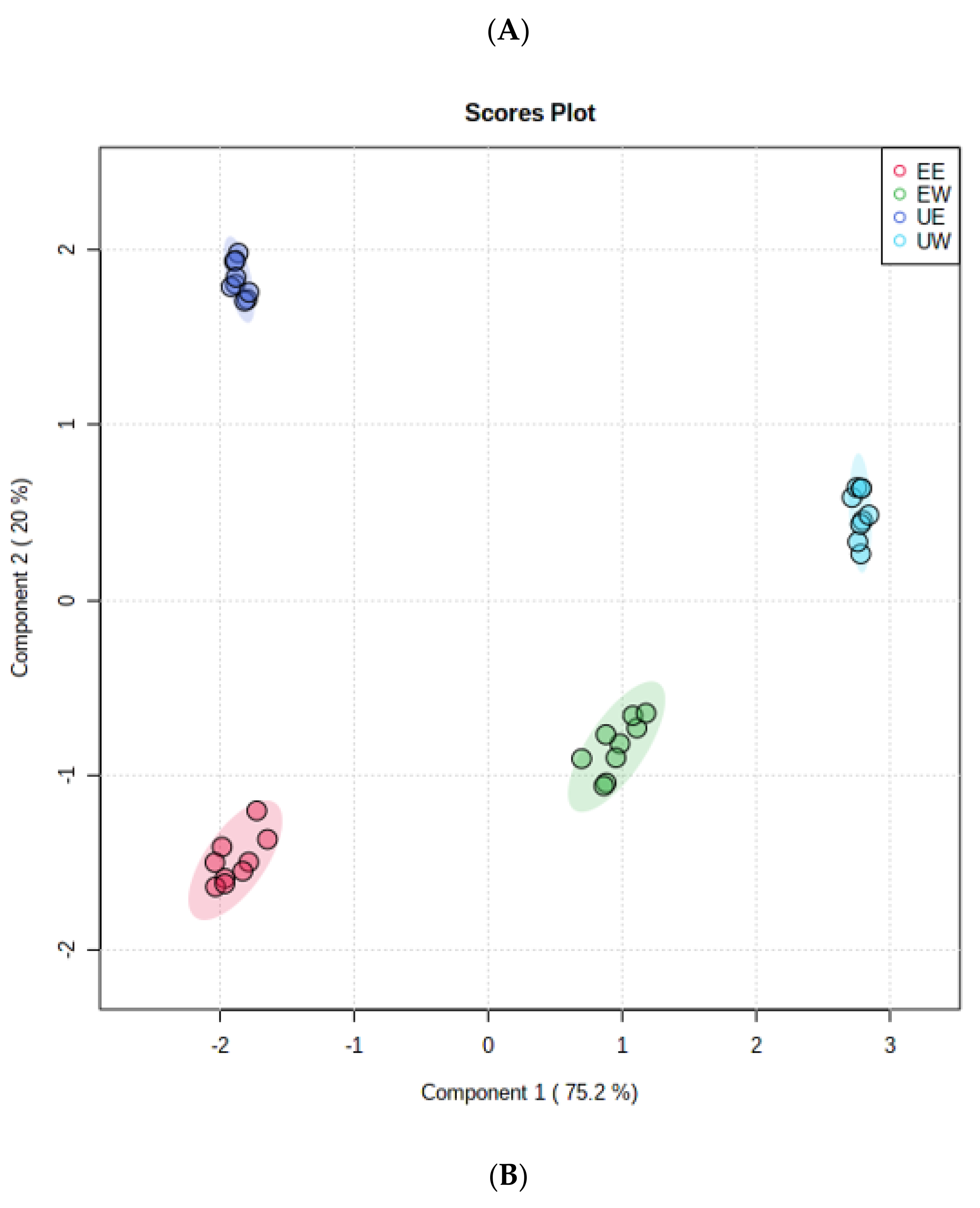
2.3. Identification of Phenolic Compounds
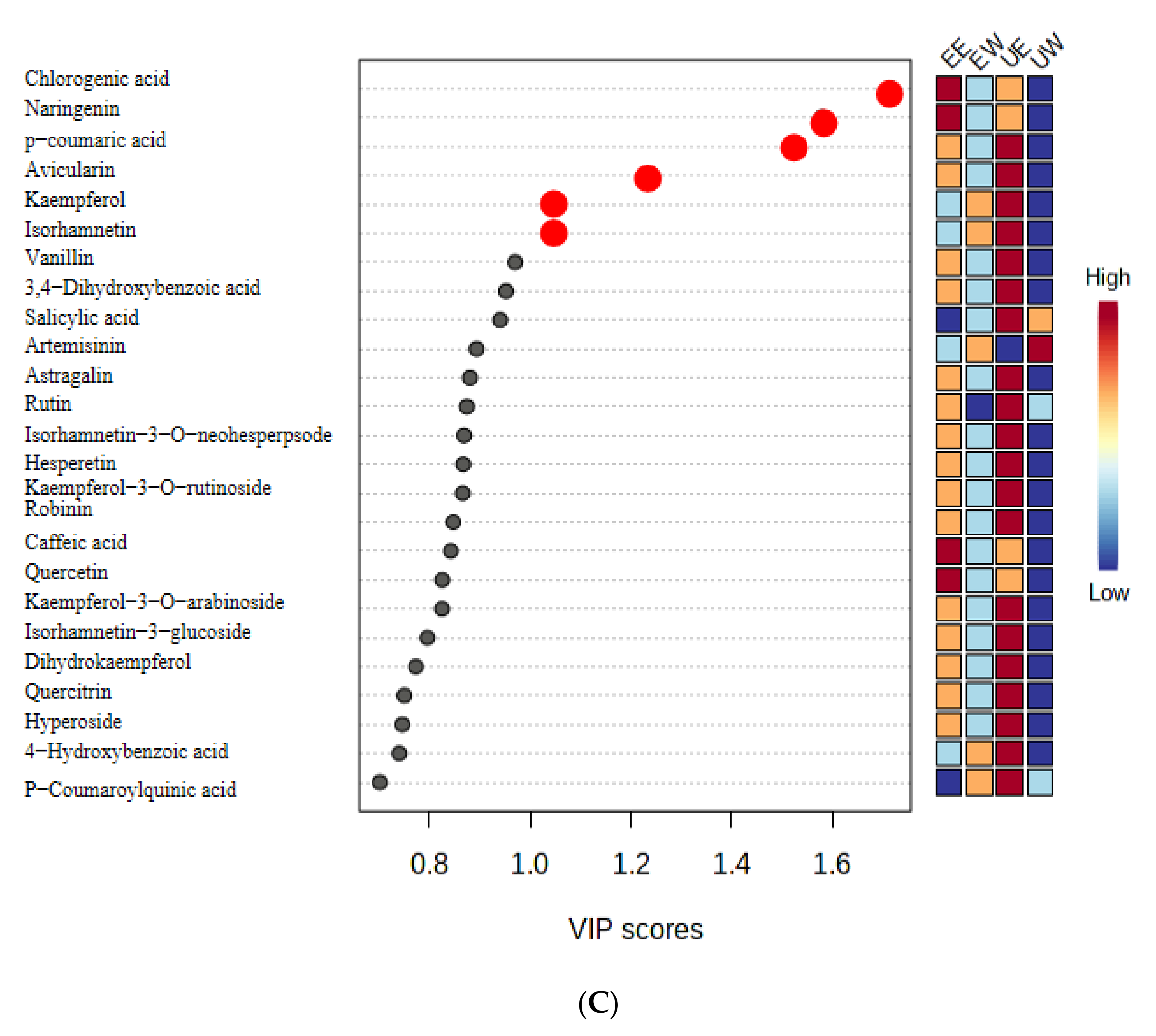
2.4. Analysis of Differential Compounds
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction Methods of Daylily
3.2.1. Ultrasonic-Assisted Extraction (UAE)
3.2.2. Enzymatic-Assisted Extraction (EAE)
3.3. Determination of Total Phenolic Content (TPC)
3.4. Determination of Antioxidant Indexes
3.4.1. Ferric Reducing/Antioxidant Power Assay (FRAP Assay)
3.4.2. DPPH Free Radical-Scavenging Assay
3.4.3. ABTS Free Radical Scavenging Ability
3.5. LC-QTOF-MS/MS Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, A.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovacevic, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.Y.; Chen, B.H. Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis disticha) as affected by various treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Zheng, X. In vitro and vivo antioxidant activities of daylily flowers and the involvement of phenolic compounds. Asia Pac. J. Clin. Nutr. 2007, 16, 196–203. [Google Scholar]
- Fu, M.; He, Z.; Zhao, Y.; Yang, J.; Mao, L. Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem. 2009, 114, 1192–1197. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, S.; Wang, M.; Feng, X.Y. Characterization of free, conjugated, and bound phenolic acids in seven commonly consumed vegetables. Molecules 2017, 22, 1878. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, X.; Wang, M.L.; Cao, J.G.; Xiao, J.B.; Wang, Q.X. Effects of different pretreatments on flavonoids and antioxidant activity of Dryopteris erythrosora leave. PLoS ONE 2019, 14, e0200174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.G.; Kan, H.; Chen, S.X.; Thakur, K.; Wang, S.Y.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.L.; Le, V. Comparison of enzyme-assisted and ultrasound-assisted extraction of vitamin c and phenolic compounds from acerola (malpighia emarginata dc.) fruit. Int. J. Food Sci. Technol. 2012, 47, 1206–1214. [Google Scholar]
- Boonkird, S.; Phisalaphong, C.; Phisalaphong, M. Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab- and pilot-plant scale. Ultrason. Sonochem. 2008, 15, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Chowdhury, P.; Viraraghavan, T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes-a review. Sci. Total Environ. 2009, 407, 2474–2492. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Szewczyk, K.; Miazga-Karska, M.; Pietrzak, W.; Komsta, U.; Krzemińska, B.; Grzywa-Celińska, A. Phenolic composition and skin-related properties of the aerial parts extract of different hemerocallis cultivars. Antioxidants 2020, 9, 690. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Lapornik, B.; Prosek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharmaceut. Biomed. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Calabrone, L.; Larocca, M.; Marzocco, S.; Martelli, G.; Rossano, R. Total Phenols and Flavonoids Content, Antioxidant Capacity and Lipase Inhibition of Root and Leaf Horseradish (Armoracia rusticana) Extracts. Food Nutr. Sci. 2015, 6, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of limnophila aromatica. J. Food Drug. Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Pyo, Y.H.; Lee, T.C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of swiss chard (beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Lopez, C.C.; Ventura-Sobrevilla, J.M.; Gonzalez-Hernandez, M.D.; Rojas, R.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Guillermo, C.G.; Martínez-Ávila, G.C.C. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Wen, L.; He, M.; Yin, C.; Jiang, Y.; Yang, B. Phenolics in citrus aurantium fruit identified by uhplc-ms/ms and their bioactivities. LWT 2021, 147, 111671. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complementary Altern. Med. 2013, 201, 801457. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tošovic, J.; Markovic, S. Antioxidative activity of chlorogenic acid relative to trolox in aqueous solution—DFT study. Food Chem. 2019, 278, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.; Dimitric, J.M.; Mojovic, M.; Milenkovic, D.; Tošovic, J. Antioxidative mechanisms in chlorogenic acid. Food Chem. 2017, 237, 390–398. [Google Scholar]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Cham, T.M.; Lin, C.C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with ccl4-induced acute liver failure. Pharm. Res. 2009, 26, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Hrazdina, G.; Parsons, G.G.; Mattick, L.R. Physiological and Biochemical events during development and maturation of grape berries. Am. J. Enol. Viticul. 1984, 35, 220–227. [Google Scholar]
- Salameh, D.; Brandam, C.; Medawar, W.; Lteif, R.; Strehaiano, P. Highlight on the problems generated by p-coumaric acid analysis in wine fermentations. Food Chem. 2008, 107, 1661–1667. [Google Scholar] [CrossRef] [Green Version]
- Torre, S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 185–193. [Google Scholar] [CrossRef]
- Liang, L.; Duan, W.; Zhang, J.; Huang, Y.; Zhang, Y.; Sun, B. Characterization and molecular docking study of taste peptides from chicken soup by sensory analysis combined with nano-LC-Q-TOF-MS/MS. Food Chem. 2022, 383, 132455. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhou, C.; Zhang, J.; Huang, Y.; Zhao, J.; Sun, B.; Zhang, Y. Characteristics of Umami Peptides Identified from Porcine Bone Soup and Molecular Docking to the Taste Receptor T1R1/T1R3. Food Chem. 2022, 387, 132870. [Google Scholar] [CrossRef] [PubMed]

| No. | RT (min) | Identification | Formula | [M − H]− (m/z) | MS2 (m/z) | Occurrence | |||
|---|---|---|---|---|---|---|---|---|---|
| UW | UE | EW | EE | ||||||
| 1 | 3.51 | Vanillin | C8H8O3 | 151.0401 | 136.0164, 92.0260 | √ | √ | ||
| 2 | 4.71 | Robinin | C33H40O19 | 739.0256 | 285.0312 | √ | √ | √ | √ |
| 3 | 6.68 | Kaempferol-3-O-rutinoside | C27H30O15 | 593.1478 | 327.0507, 285.0379, 255.0287 | √ | √ | √ | √ |
| 4 | 6.74 | Isorhamnetin-3-O-neohesperpsode | C28H32O16 | 623.1583 | 315.0488, 299.0185 | √ | √ | √ | √ |
| 5 | 8.17 | Astragalin | C21H20O11 | 447.0912 | 284.0308, 255.0284, 227.0337 | √ | √ | √ | |
| 6 | 8.24 | Isorhamnetin-3-glucoside | C22H22O12 | 477.1018 | 314.0416, 271.0238 | √ | √ | √ | √ |
| 7 | 8.62 | Hesperetin | C16H14O6 | 301.0711 | 286.0486, 164.0114, 151.0034, 134.0371, 108.0216 | √ | √ | ||
| 8 | 8.66 | Rutin | C27H30O16 | 609.1444 | 300.0266, 255.0298 | √ | √ | √ | √ |
| 9 | 8.68 | Dihydrokaempferol | C15H12O6 | 287.0556 | 259.0604, 177.0553, 125.0234, 57.0341 | √ | √ | √ | √ |
| 10 | 8.74 | Kaempferol-3-O-arabinoside | C20H18O10 | 417.0807 | 284.0308, 255.0286, 227.0337 | √ | √ | √ | √ |
| 11 | 8.83 | Quercitrin | C21H20O11 | 477.0926 | 314.0425, 271.0243 | √ | √ | ||
| 12 | 8.92 | Naringenin | C15H12O5 | 271.0605 | 151.0031, 119.0494, 107.0132, 66.0025 | √ | √ | √ | |
| 13 | 10.14 | Hyperoside | C21H20O12 | 463.089 | 300.0264, 255.0293 | √ | √ | √ | √ |
| 14 | 10.65 | Avicularin | C20H18O11 | 433.0776 | 300.0263, 271.0249 | √ | √ | √ | √ |
| 15 | 11.26 | Salicylic acid | C7H6O3 | 137.0231 | 93.0342, 65.0389 | √ | √ | √ | √ |
| 16 | 11.53 | Isorhamnetin | C16H12O7 | 315.0541 | 300.0266, 151.0025 | √ | |||
| 17 | 11.63 | Kaempferol | C15H10O6 | 285.041 | 255.0884 | √ | |||
| 18 | 14.5 | Quercetin | C15H10O7 | 301.034 | 158.0423, 138.0283 | √ | √ | √ | √ |
| 19 | 14.85 | Artemisinin | C16H12O6 | 299.2023 | 183.0123 | √ | √ | ||
| 20 | 15.99 | P-Coumaroylquinic acid | C16H18O8 | 191.054 | 173.0438, 163.0380, 119.0485 | √ | √ | √ | √ |
| 21 | 17.45 | 4-Hydroxybenzoic acid | C7H6O3 | 137.0228 | 93.0335, 65.0386 | √ | √ | √ | √ |
| 22 | 17.52 | Vanillic acid | C8H8O4 | 167.035 | 152.0091, 108.0203, 91.0199 | √ | √ | √ | √ |
| 23 | 19.28 | P-coumaric acid | C9H8O3 | 104.0406 | 119.0493, 93.0338 | √ | √ | √ | √ |
| 24 | 26.53 | Caffeic acid | C9H8O4 | 179.0358 | 135.0440, 91.0549 | √ | √ | ||
| 25 | 26.59 | 3,4-Dihydroxybenzoic acid | C7H6O4 | 153.0168 | 108.0205, 91.0186 | √ | √ | ||
| 26 | 27.37 | Chlorogenic acid | C16H18O9 | 353.0819 | 191.0555, 179.0339, 135.0445, 111.0082 | √ | √ | √ | |
| Differential Compounds | Content (mg/100 g DW) | |||
|---|---|---|---|---|
| UW | UE | EW | EE | |
| Chlorogenic acid | nd | 6.713 ± 0.097 a | 6.17 ± 0.153 c | 6.406 ± 0.174 b |
| P-coumaric acid | 0.950 ± 0 b | 1.091 ± 0.012 a | 0.950 ± 0 b | 0.952 ± 0 b |
| Kaempferol | nd | 9.592 ± 0.167 | nd | nd |
| Avicularin | nd | 0.598 ± 0.001 a | 0.589 ± 0 c | 0.592 ± 0 b |
| Naringenin | nd | 2.759 ± 0.075 a | 2.059 ± 0.020 c | 2.456 ± 0.034 b |
| Isorhamnetin | nd | 2.126 ± 0.015 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Liang, L.; Liu, H.; Yan, Y.; Zhang, Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules 2022, 27, 2964. https://doi.org/10.3390/molecules27092964
Hao Z, Liang L, Liu H, Yan Y, Zhang Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules. 2022; 27(9):2964. https://doi.org/10.3390/molecules27092964
Chicago/Turabian StyleHao, Zhilin, Li Liang, He Liu, Yi Yan, and Yuyu Zhang. 2022. "Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity" Molecules 27, no. 9: 2964. https://doi.org/10.3390/molecules27092964
APA StyleHao, Z., Liang, L., Liu, H., Yan, Y., & Zhang, Y. (2022). Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules, 27(9), 2964. https://doi.org/10.3390/molecules27092964






