Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes
Abstract
:1. Introduction
2. Results
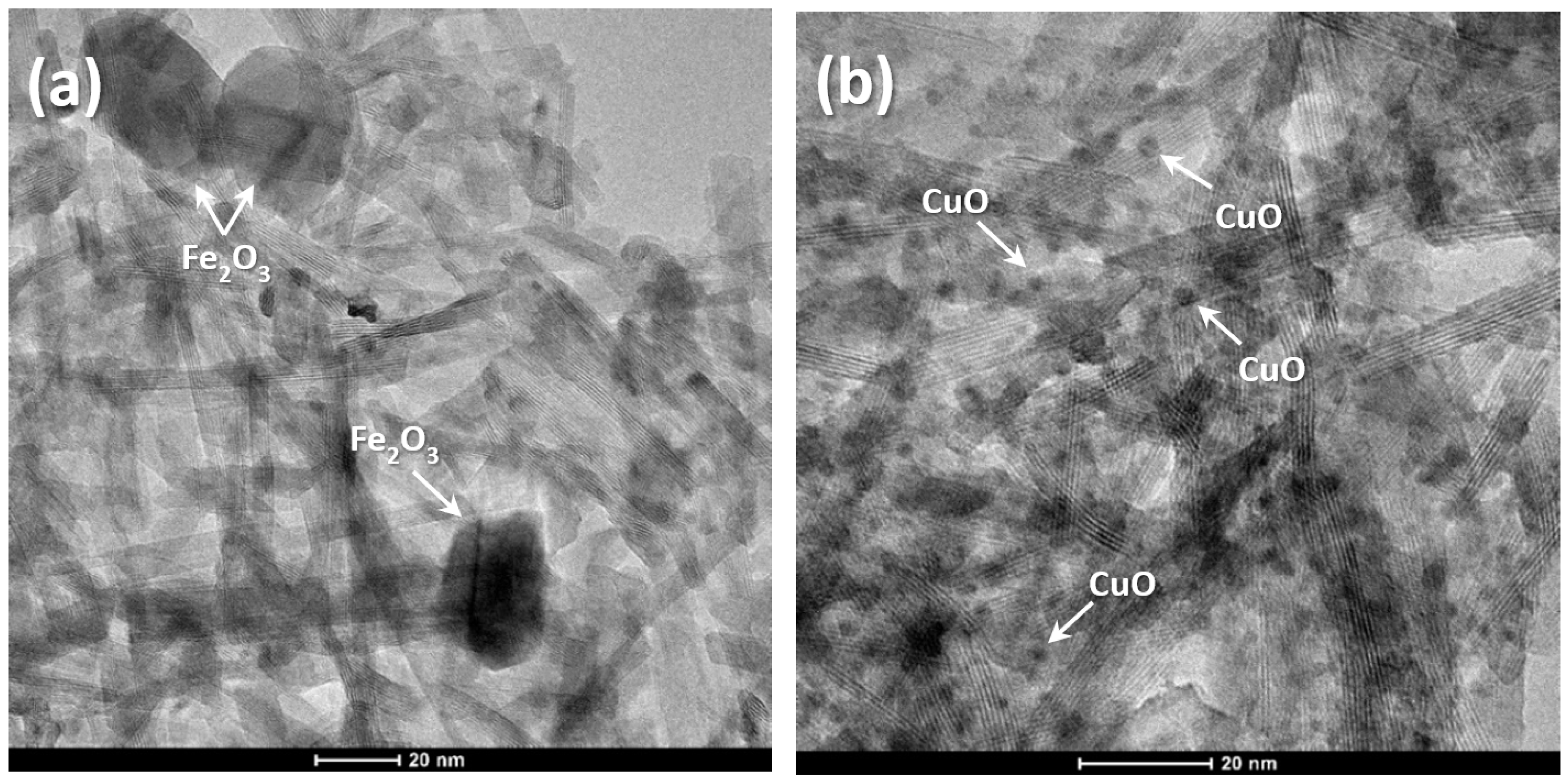
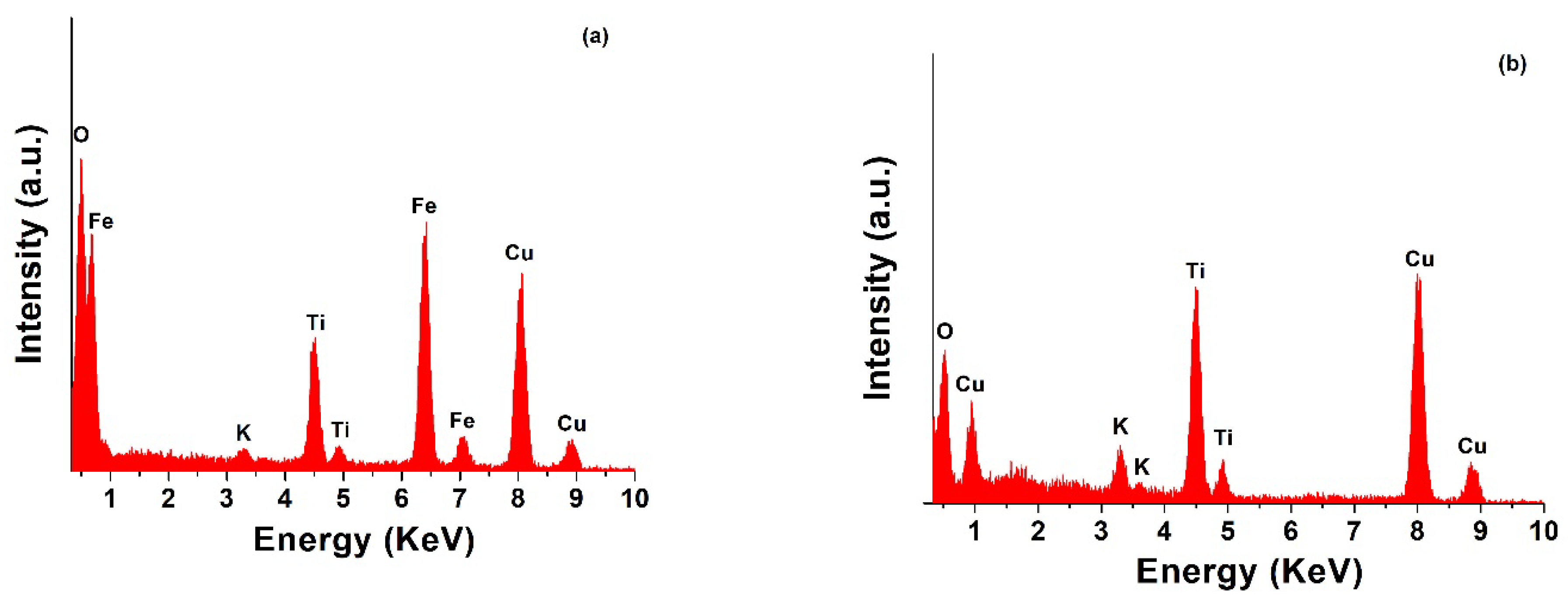
2.1. HRTEM and EDS Analysis of TiO2 NW@Fe2O3 and TiO2 NW@CuO Nanocomposites
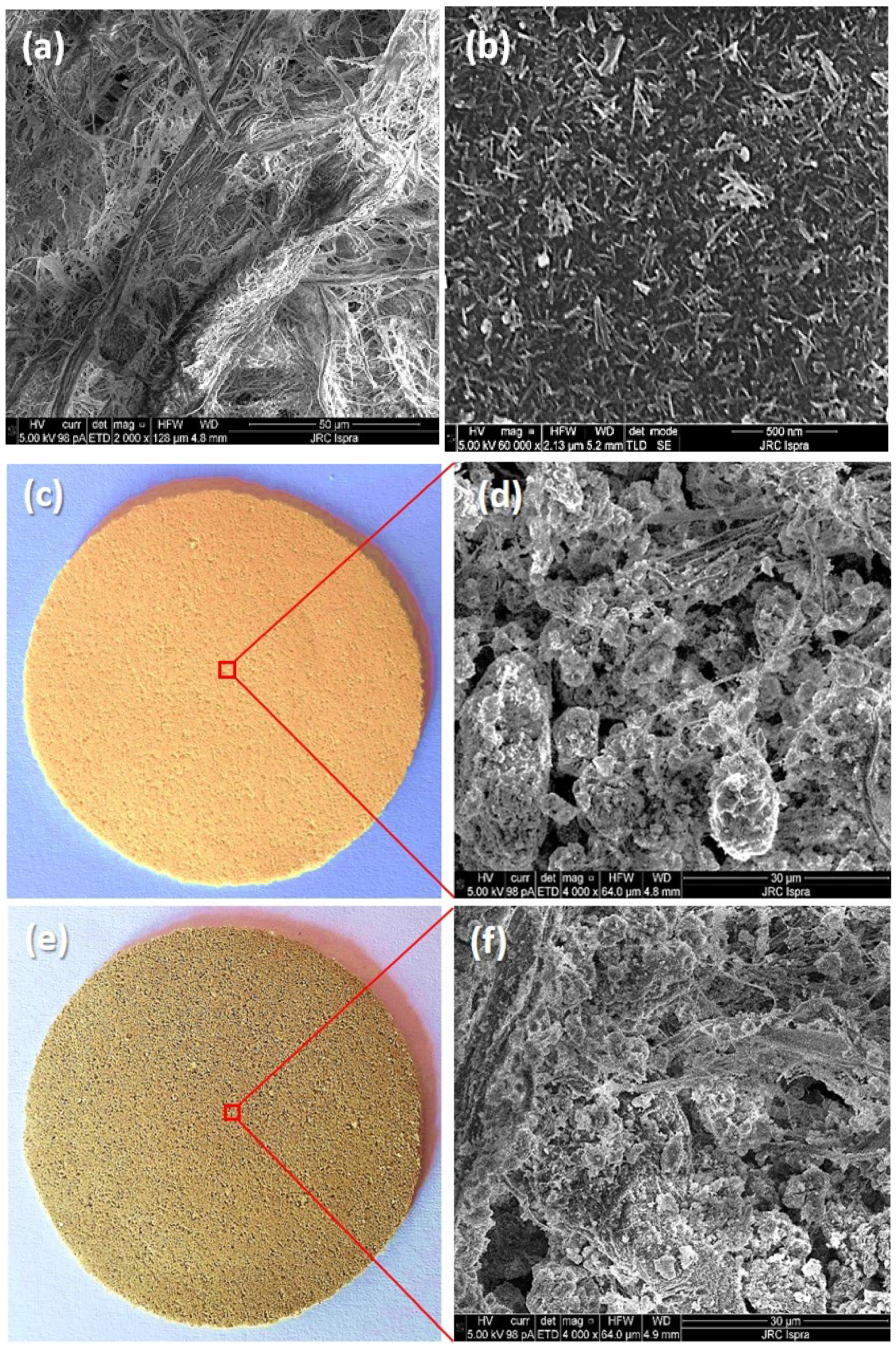
2.2. SEM and EDS Analysis of the Hybrid Membranes
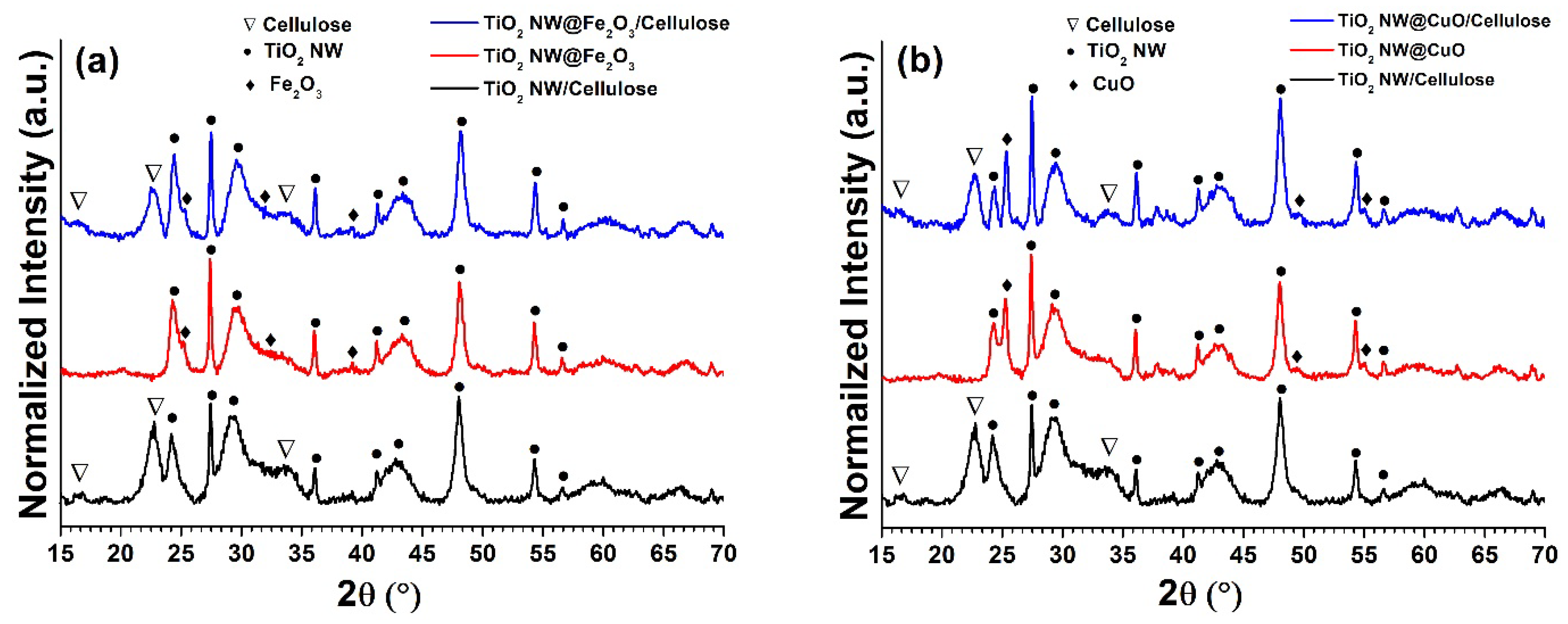
2.3. XRD and Specific Surface Area Analysis of the Hybrid Membranes
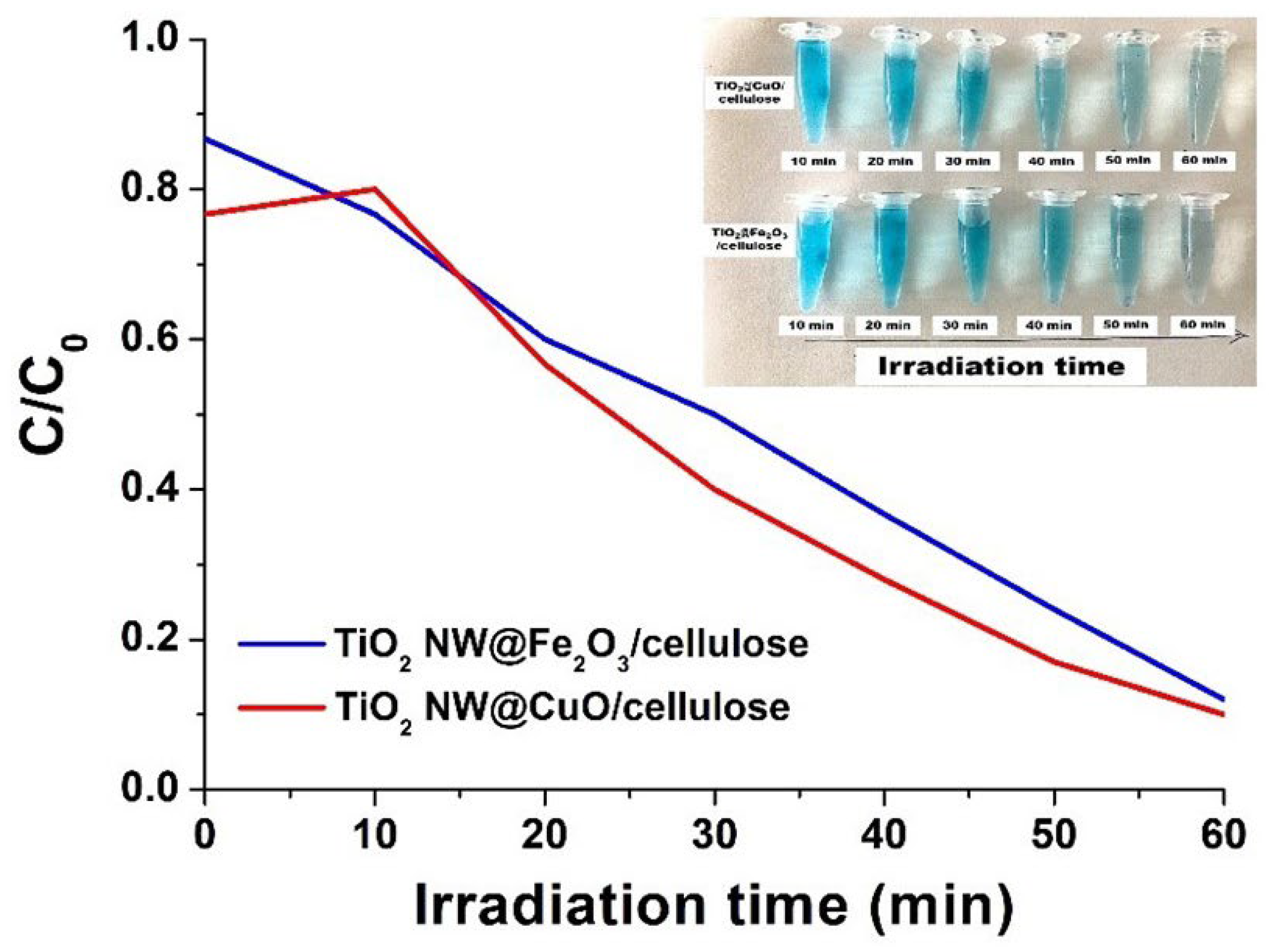
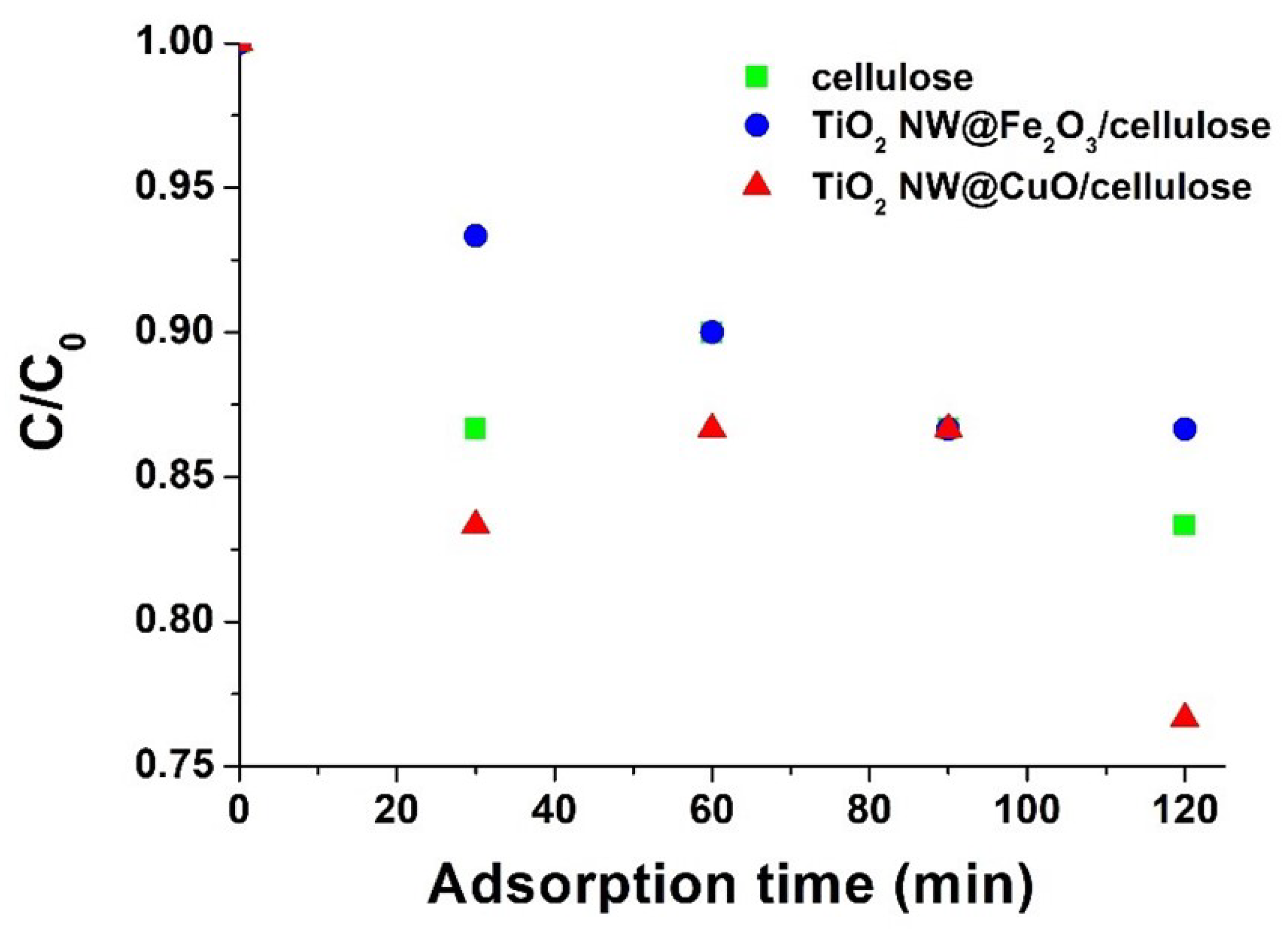
2.4. Photocatalytic Efficiency of the Hybrid Membranes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2 Nanowires (TiO2 NW)
3.3. Synthesis of TiO2 NW@Fe2O3/Cellulose Membranes
3.4. Synthesis of TiO2 NW@CuO/Cellulose Membranes
3.5. Characterization Techniques
3.6. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddique, K.; Rizwan, M.; Shahid, M.J.; Ali, S.; Ahmad, R.; Rizvi, H. Textile wastewater treatment options: A critical review. In Enhancing Cleanup of Environmental Pollutants; Springer International Publishing: Cham/Basel, Switzerland, 2017; pp. 183–207. [Google Scholar]
- di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Duo, S.; Li, Y.; Liu, Z.; Zhong, R.; Liu, T.; Xu, H. Preparation of ZnO from 2D nanosheets to diverse 1D nanorods and their structure, surface area, photocurrent, optical and photocatalytic properties by simple hydrothermal synthesis. J. Alloys Compd. 2017, 695, 2563–2579. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide-properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- He, R.A.; Cao, S.W.; Yu, J.G. Recent advances in morphology control and surface modification of Bi-based photocatalysts. Acta Phys.-Chim. Sin. 2016, 32, 2841–2870. [Google Scholar] [CrossRef]
- Dong, H.; Sun, J.; Chen, G.; Li, C.; Hu, Y.; Lv, C. An advanced Ag-based photocatalyst Ag2Ta4O11 with outstanding activity, durability and universality for removing organic dyes. Phys. Chem. Chem. Phys. 2014, 16, 23915–23921. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-chemical preparation of TiO2-based composites with different morphologies and photocatalytic properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [Green Version]
- Mandal, R.K.; Kundu, S.; Sain, S.; Pradhan, S.K. Enhanced photocatalytic performance of V2O5-TiO2 nanocomposites synthesized by mechanical alloying with morphological hierarchy. New J. Chem. 2019, 43, 2804–2816. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; el kacem Qaiss, A.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Liu, H.; Huang, J. Design, modification and application of semiconductor photocatalysts. J. Taiwan Inst. Chem. Eng. 2018, 93, 590–602. [Google Scholar] [CrossRef]
- Samokhvalov, A. Hydrogen by photocatalysis with nitrogen codoped titanium dioxide. Renew. Sustain. Energy Rev. 2017, 72, 981–1000. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahimi, K. Adverse effects of graphene incorporated in TiO2 photocatalyst on minuscule animals under solar light irradiation. J. Mater. Chem. 2012, 22, 23260–23266. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Fua, J.; Wanga, X.; Mac, Z.; Wenmingd, H.; Lie, J.; Wanga, Z.; Wanga, L. Photocatalytic ultrafiltration membranes based on visible light responsive photocatalyst: A review. Desalin. Water Treat. 2019, 168, 42–55. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Hanaor, D.A.H. The effects of copper doping on photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Endo-Kimura, M.; Karabiyik, B.; Wang, K.; Wei, Z.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Vis-responsive copper-modified titania for decomposition of organic compounds and microorganisms. Catalysts 2020, 10, 1194. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, G.; Rogers, J.; Zhao, C.; Liu, L.; Li, Y. Novel anti-fouling Fe2O3/TiO2 nanowire membranes for humic acid removal from water. Chem. Eng. J. 2015, 271, 180–187. [Google Scholar] [CrossRef]
- Saber, O.; Kotb, H.M.; Osama, M.; Khater, H.A. An effective photocatalytic degradation of industrial pollutants through converting titanium oxide to magnetic nanotubes and hollow nanorods by kirkendall effect. Nanomaterials 2022, 12, 440. [Google Scholar] [CrossRef]
- Akhavan, O. Thickness dependent activity of nanostructured TiO2/α-Fe2O3 photocatalyst thin films. Appl. Surf. Sci. 2010, 257, 1724–1728. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of synthesized Magnetic TiO2-based membranes for wastewater treatment: A review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Rossi, L.; Mercier, C.; Lehmann, C.; Sienkiewicz, A.; Forró, L. Photocatalytic nanowires-based air filter: Towards reusable protective masks. Adv. Funct. Mater. 2020, 30, 2004615. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Sun, M.; Ding, W.; Ding, Z.; Liu, W. Decoration of γ-graphyne on TiO2 nanotube arrays: Improved photoelectrochemical and photoelectrocatalytic properties. Appl. Catal. B Environ. 2021, 281, 119492. [Google Scholar] [CrossRef]
- Gyulavári, T.; Kovács, K.; Kovács, Z.; Bárdos, E.; Kovács, G.; Baán, K.; Magyari, K.; Veréb, G.; Pap, Z.; Hernadi, K. Preparation and characterization of noble metal modified titanium dioxide hollow spheres new insights concerning the light trapping efficiency. Appl. Surf. Sci. 2020, 534, 147327. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Rosales, M.; Zoltan, T.; Yadarola, C.; Mosquera, E.; Gracia, F.; García, A. The influence of the morphology of 1D TiO2 nanostructures on photogeneration of reactive oxygen species and enhanced photocatalytic activity. J. Mol. Liq. 2019, 281, 59–69. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Jitputti, J.; Suzuki, Y.; Yoshikawa, S. Synthesis of TiO2 nanowires and their photocatalytic activity for hydrogen evolution. Catal. Commun. 2008, 9, 1265–1271. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef]
- Al-Hajji, L.A.; Ismail, A.A.; Al-Hazza, A.; Ahmed, S.A.; Alsaidi, M.; Almutawa, F.; Bumajdad, A. Impact of calcination of hydrothermally synthesized TiO2 nanowires on their photocatalytic efficiency. J. Mol. Struct. 2020, 1200, 127153. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Cheng, W.; Hu, J. Photocatalytic membrane in water purification: Is it stepping closer to be driven by visible light? J. Membr. Sci. 2019, 584, 364–392. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A. review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Madhura, L.; Kanchi, S.; Sabela, M.I.; Singh, S.; Bisetty, K. Inamuddin. Membrane technology for water purification. Environ. Chem. Lett. 2018, 16, 2343–2365. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Duan, C.; Liu, C.; Zhang, Y.; Meng, X.; Dai, L.; Wang, W.; Yu, H.; Ni, Y. A self-cleaning and photocatalytic cellulose-fiber- supported “Ag@AgCl@MOF- cloth’’ membrane for complex wastewater remediation. Carbohydr. Polym. 2020, 247, 116691. [Google Scholar] [CrossRef]
- Azmi, A.; Lau, K.S.; Chin, S.X.; Zakaria, S.; Chia, C.H. Regenerated cellulose membrane incorporating photocatalytic zinc oxide as a bifunctional membrane for decoloration of methylene blue. Polym. Adv. Technol. 2022, 33, 3843–3850. [Google Scholar] [CrossRef]
- Rabiee, N.; Fatahi, Y.; Asadnia, M.; Daneshgar, H.; Kiani, M.; Ghadiri, A.M.; Amin, M.A.; Mashhadzadeh, H.; Akhavan, O.; Bagherzadeh, M.; et al. Green porous benzamide-like nanomembranes for hazardous cations detection, separation, and concentration adjustment. J. Hazard. Mater. 2022, 423, 127130. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Y.; Zhu, J. Photocatalytic antifouling graphene oxide-mediated hierarchical filtration membranes with potential applications on water purification. ACS Appl. Mater. Interfaces 2014, 6, 16117–16123. [Google Scholar] [CrossRef]
- Bai, H.; Zan, X.; Zhang, L.; Sun, D.D. Multi-functional CNT/ZnO/TiO2 nanocomposite membrane for concurrent filtration and photocatalytic degradation. Sep. Purif. Technol. 2015, 156, 922–930. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.; Rodríguez, J.M.D.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Santos, É.N.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac. J. Chem. Eng. 2020, 15, 5. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, X.; Oakes, K.D.; Pemg, P.; Zhou, Y.N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef]
- Garcia, R.A.; Stevanovic, T.; Berthier, J.; Njamen, G.; Tolnai, B.; Achim, A. Cellulose, nanocellulose, and antimicrobial materials for the manufacture of disposable face masks: A review. BioResources 2021, 16, 4321–4353. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 137–149. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Fan, J.; Sun, D.; Tang, W.; Yang, X. Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J. Hazard. Mater. 2011, 189, 377–383. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef]
- Liu, G.Q.; Pan, X.J.; Li, J.; Li, C.; Ji, C.L. Facile preparation and characterization of anatase TiO2/nanocellulose composite for photocatalytic degradation of methyl orange. J. Saudi Chem. Soc. 2021, 25, 101383. [Google Scholar] [CrossRef]
- Cao, X.; Huang, M.; Ding, B.; Yu, J.; Sun, G. Robust polyacrylonitrile nanofibrous membrane reinforced with jute cellulose nanowhiskers for water purification. Desalination 2013, 316, 120–126. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Yang, Y.; Zheng, F.; Ni, H.; Zhang, M.; Guo, M. Synthesis of potassium hexatitanate whiskers with high thermal stability from Ti-bearing electric arc furnace molten slag. Ceram. Int. 2016, 42, 11294–11302. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mater. Devices 2016, 1, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Li, Z.; Zhang, Y.; Wang, Q.; Xu, Q. Synthesis of α-Fe2O3/TiO2 nanotube arrays for photoelectro-fenton degradation of phenol. Chem. Eng. J. 2012, 191, 356–363. [Google Scholar] [CrossRef]
- Shehab, M.A.; Sharma, N.; Karacs, G.; Kristaly, F.; Mrabate, B.E.; Chauhan, T.; Koós, T.; Leskó, A.K.; Palotás, Á.B.; Hernádi, K.; et al. Effect of the synthesis parameters on the formation of TiO2 nanostructures: Controllable synthesis and adsorption properties of nanowires and nanotubes. Circ. Econ. Environ. Prot. 2021, 5, 16–38. [Google Scholar]
| Sample Name | C | O | Ti | Na | F | K | Fe | Cu |
|---|---|---|---|---|---|---|---|---|
| Cellulose | 45 | 55 | - | - | - | - | - | - |
| TiO2 NW | - | 64 | 36 | - | - | - | - | - |
| TiO2 NW@Fe2O3/cellulose | 20 | 50 | 23 | 3 | - | 3 | 1 | - |
| TiO2 NW@CuO/cellulose | 25 | 58 | 11 | - | 3 | 2 | - | 1 |
| Sample Name | Surface Area (m2/g) | Pore Diameter (nm) |
|---|---|---|
| TiO2 NW | 168 | - |
| cellulose membrane | 6 | 18 |
| Fe2O3 | 62 | - |
| CuO | 141 | - |
| TiO2 NW@Fe2O3 | 146 | - |
| TiO2 NW@CuO | 139 | - |
| TiO2 NW@Fe2O3/cellulose | 122 | 16 |
| TiO2 NW@CuO/cellulose | 117 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehab, M.A.; Sharma, N.; Valsesia, A.; Karacs, G.; Kristály, F.; Koós, T.; Leskó, A.K.; Nánai, L.; Hernadi, K.; Németh, Z. Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Molecules 2022, 27, 2951. https://doi.org/10.3390/molecules27092951
Shehab MA, Sharma N, Valsesia A, Karacs G, Kristály F, Koós T, Leskó AK, Nánai L, Hernadi K, Németh Z. Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Molecules. 2022; 27(9):2951. https://doi.org/10.3390/molecules27092951
Chicago/Turabian StyleShehab, Mohammed Ahmed, Nikita Sharma, Andrea Valsesia, Gábor Karacs, Ferenc Kristály, Tamás Koós, Anett Katalin Leskó, Lilla Nánai, Klara Hernadi, and Zoltán Németh. 2022. "Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes" Molecules 27, no. 9: 2951. https://doi.org/10.3390/molecules27092951
APA StyleShehab, M. A., Sharma, N., Valsesia, A., Karacs, G., Kristály, F., Koós, T., Leskó, A. K., Nánai, L., Hernadi, K., & Németh, Z. (2022). Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Molecules, 27(9), 2951. https://doi.org/10.3390/molecules27092951








