Novel Pyridinium Based Ionic Liquid Promoter for Aqueous Knoevenagel Condensation: Green and Efficient Synthesis of New Derivatives with Their Anticancer Evaluation
Abstract
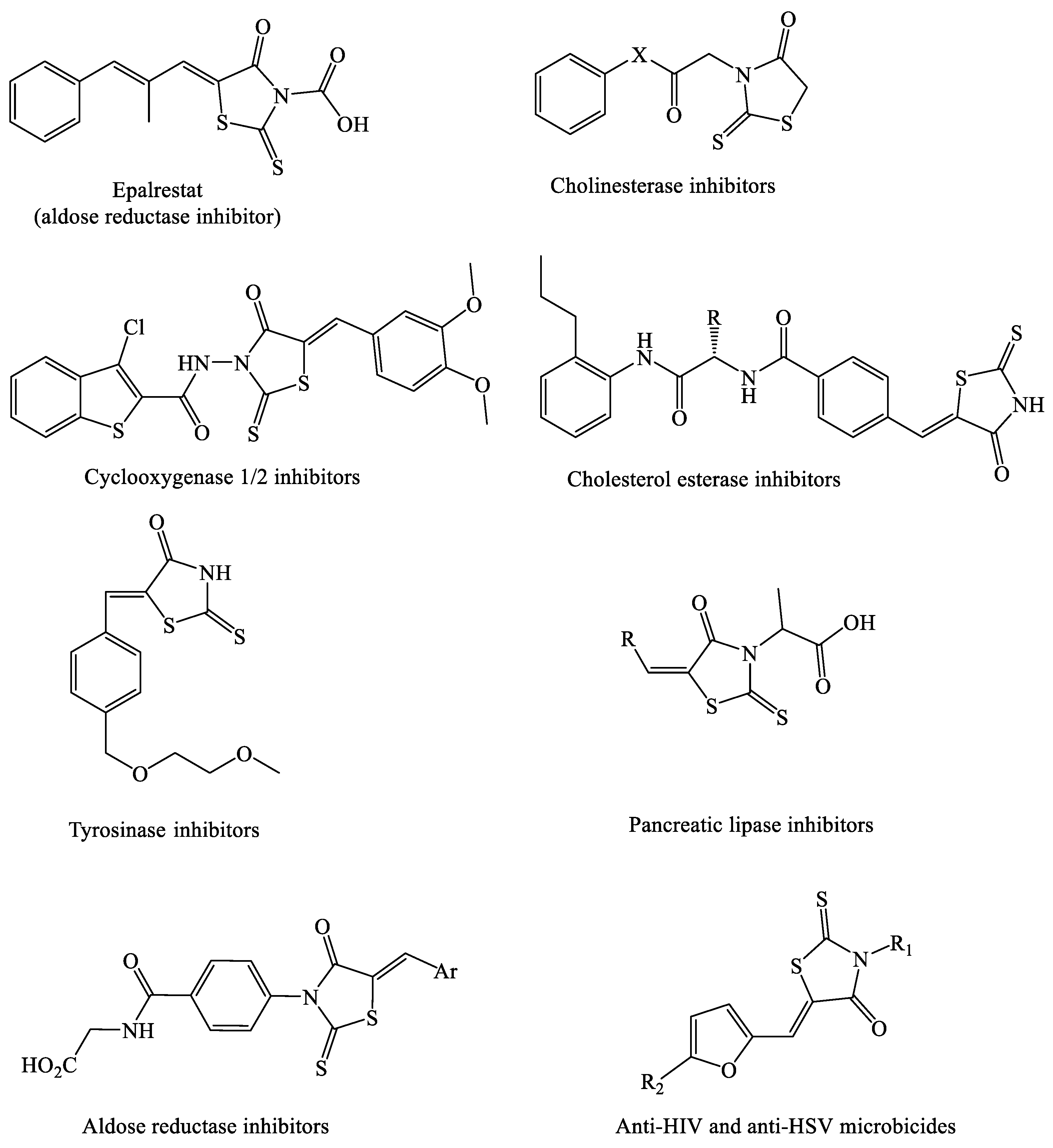
:1. Introduction
2. Experimental
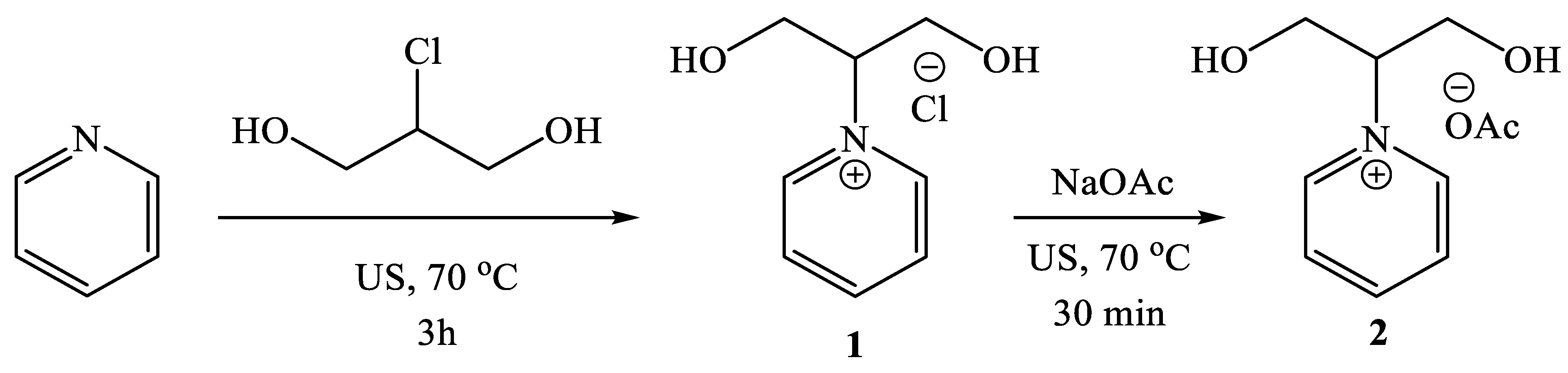
2.1. Synthesis of 1-(1,3-dihydroxypropan-2-yl)pyridin-1-ium Acetate [Py-2OH]OAc
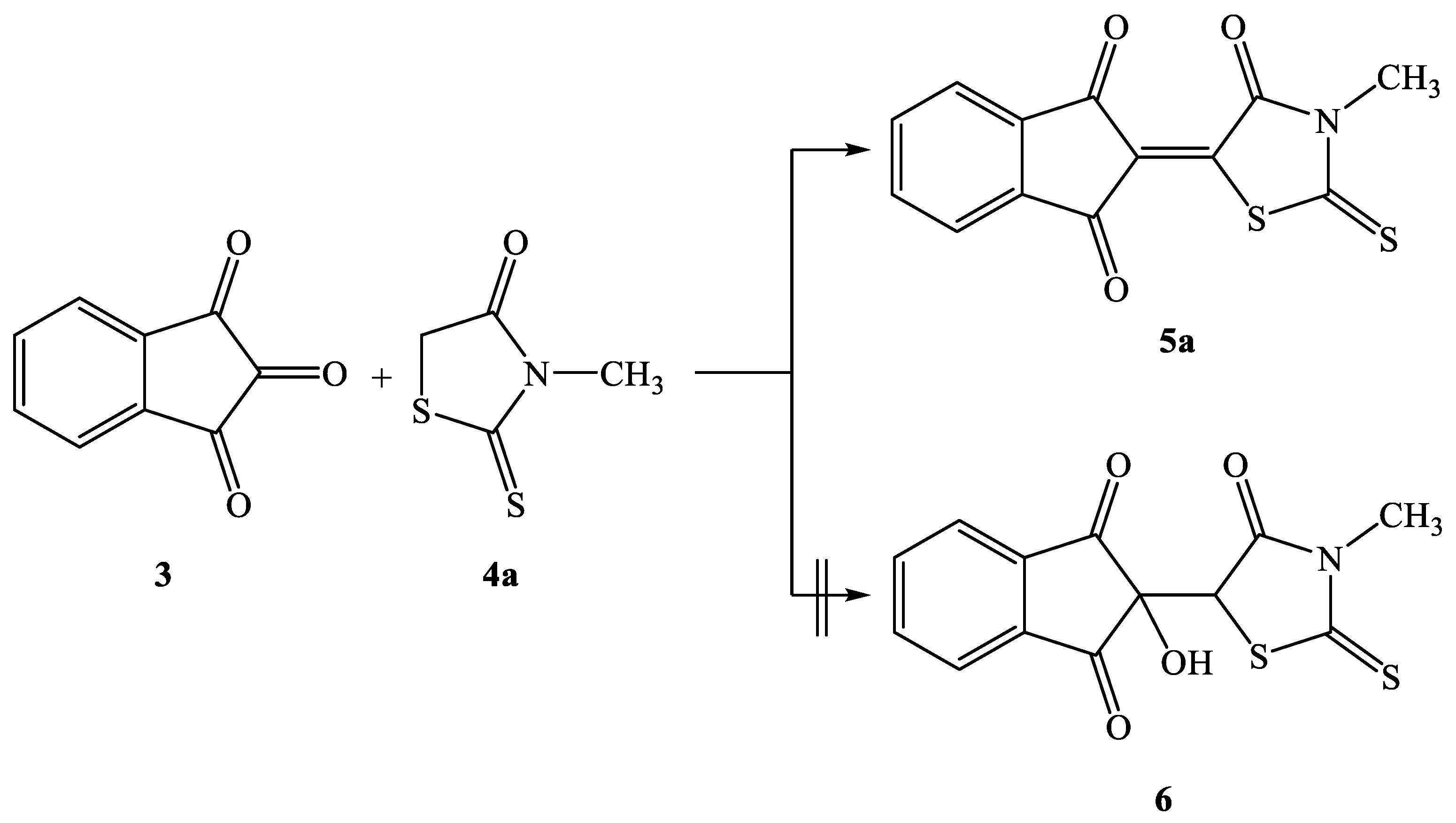
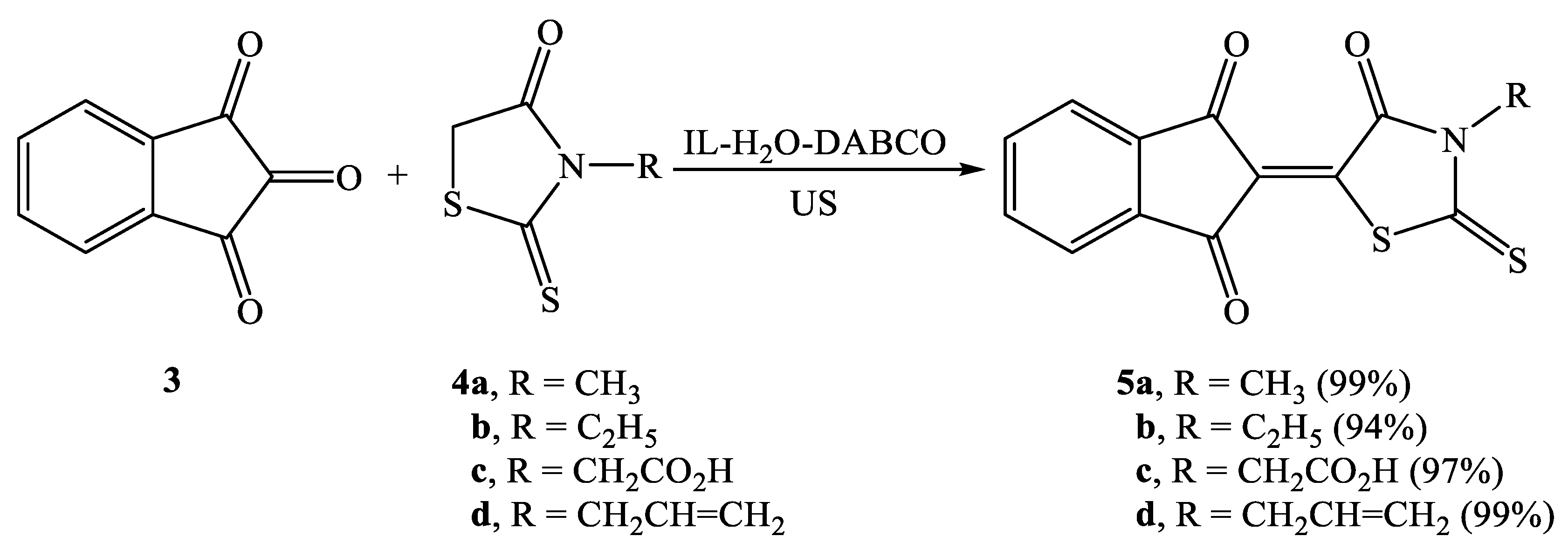
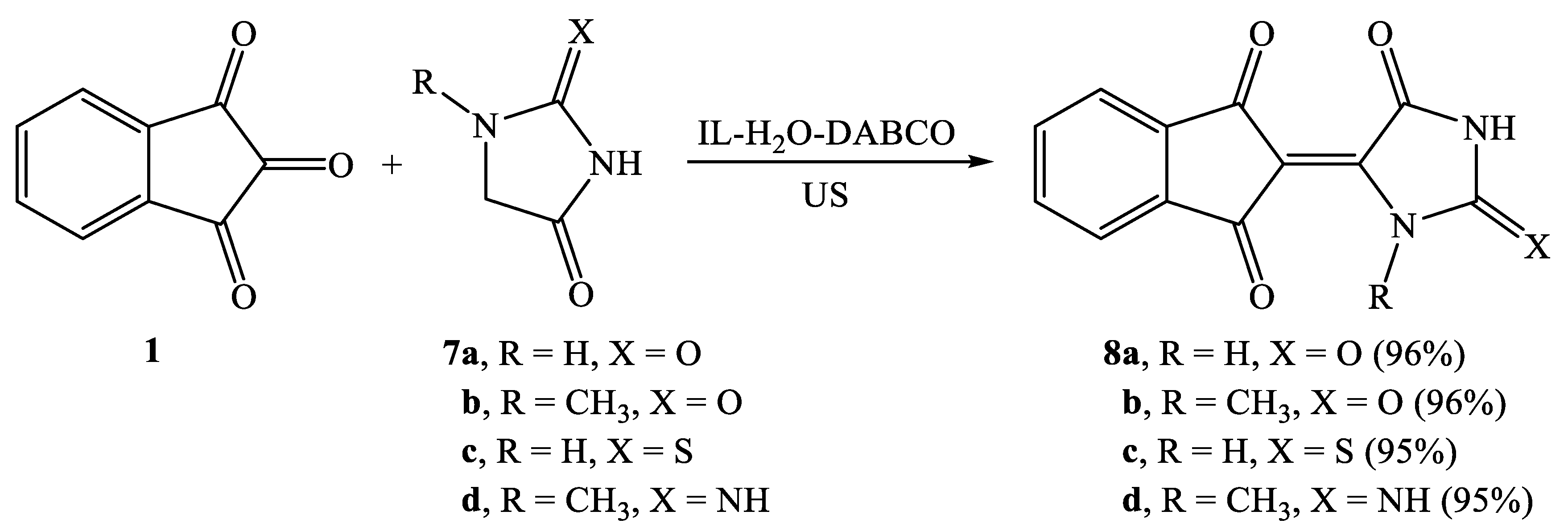
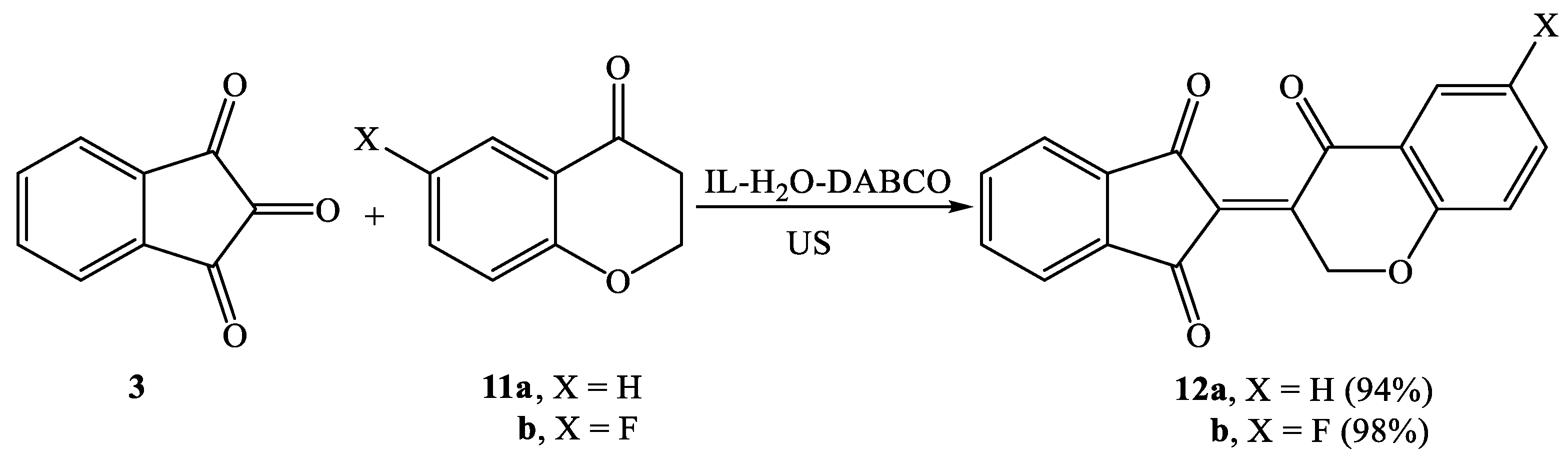
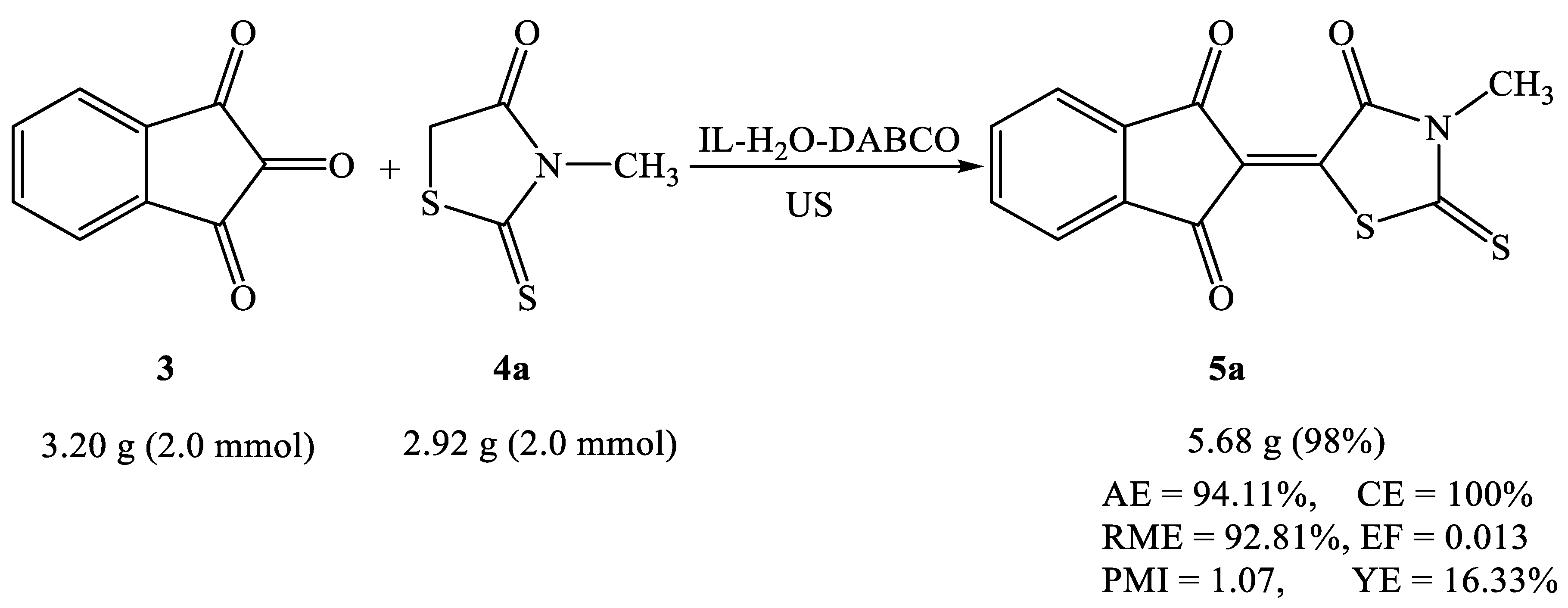
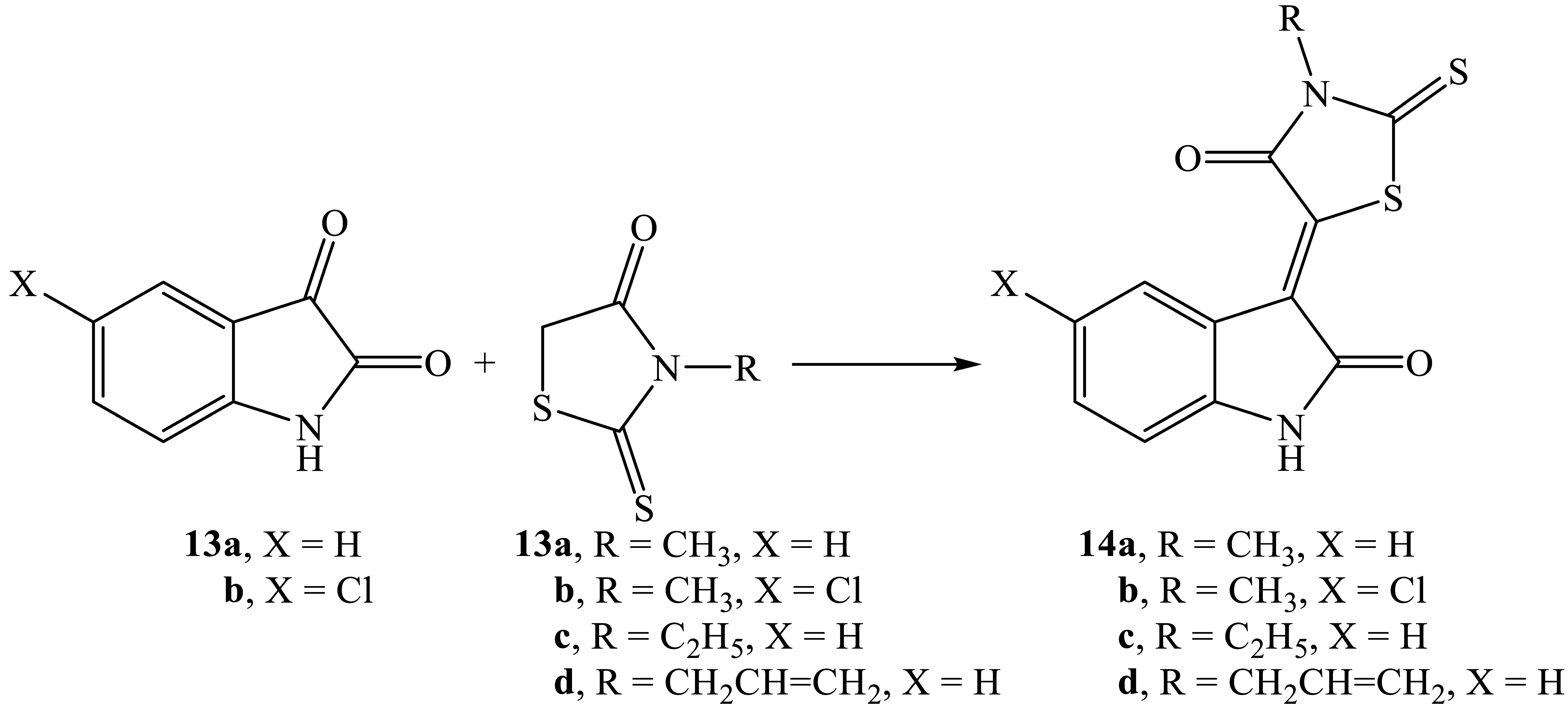
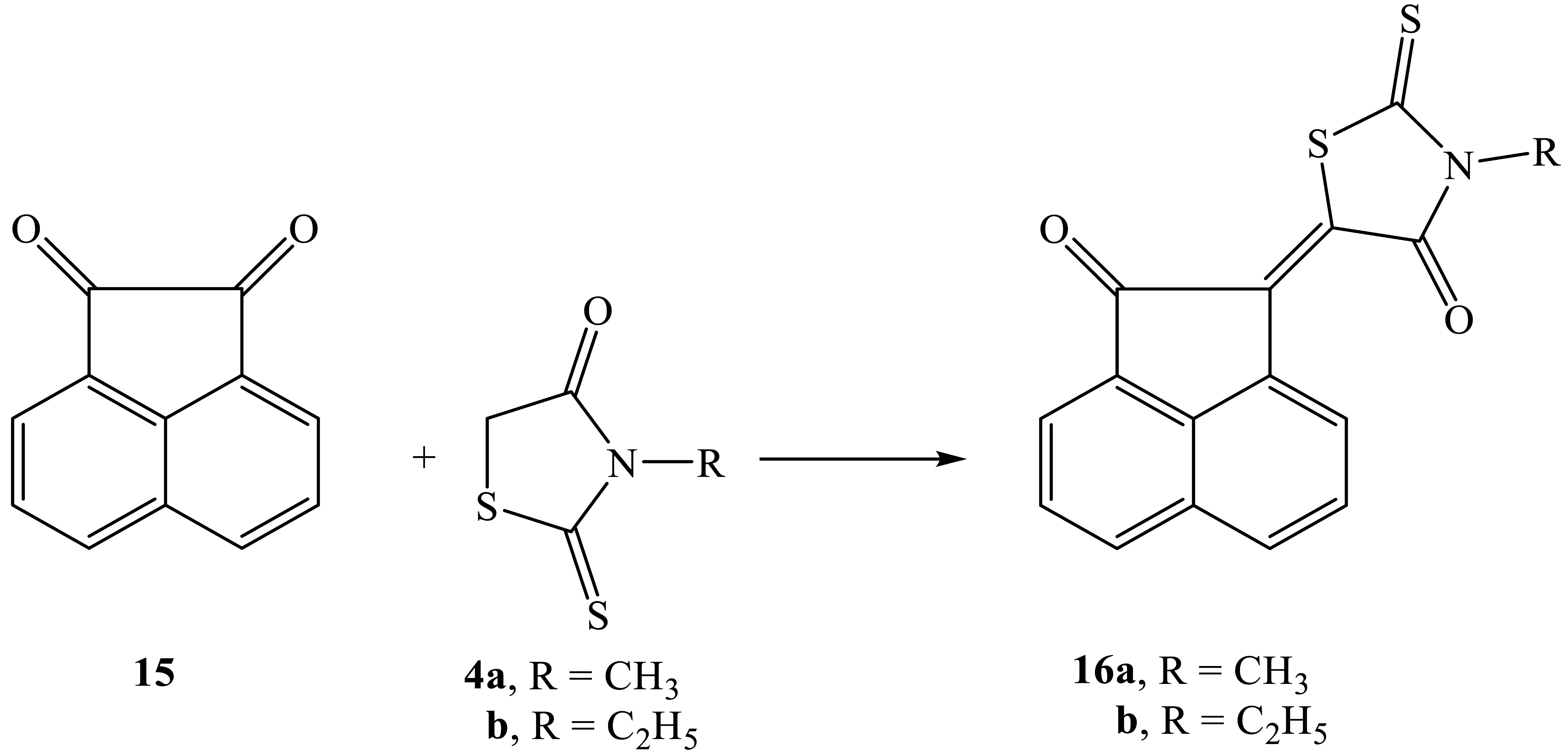
2.2. Synthesis of Knoevenagel Condensation Products (5a–d, 8a–d, 10a,b, 12a,b, 14a–d, and 16a,b)
2.3. MTT Assay for Cell Viability/Proliferation
2.4. Protocol of Docking Studies
3. Results and Discussion
3.1. Cytotoxic Activity
3.2. Structure–Activity Relationship (SARs)
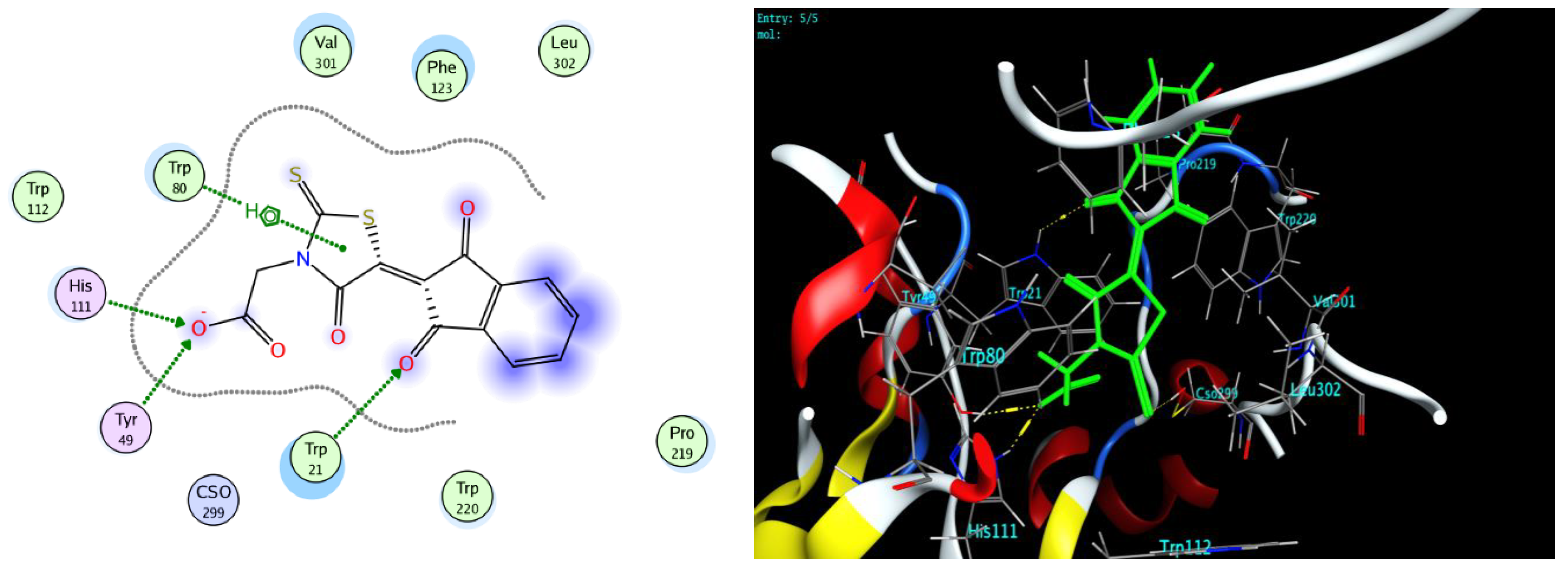
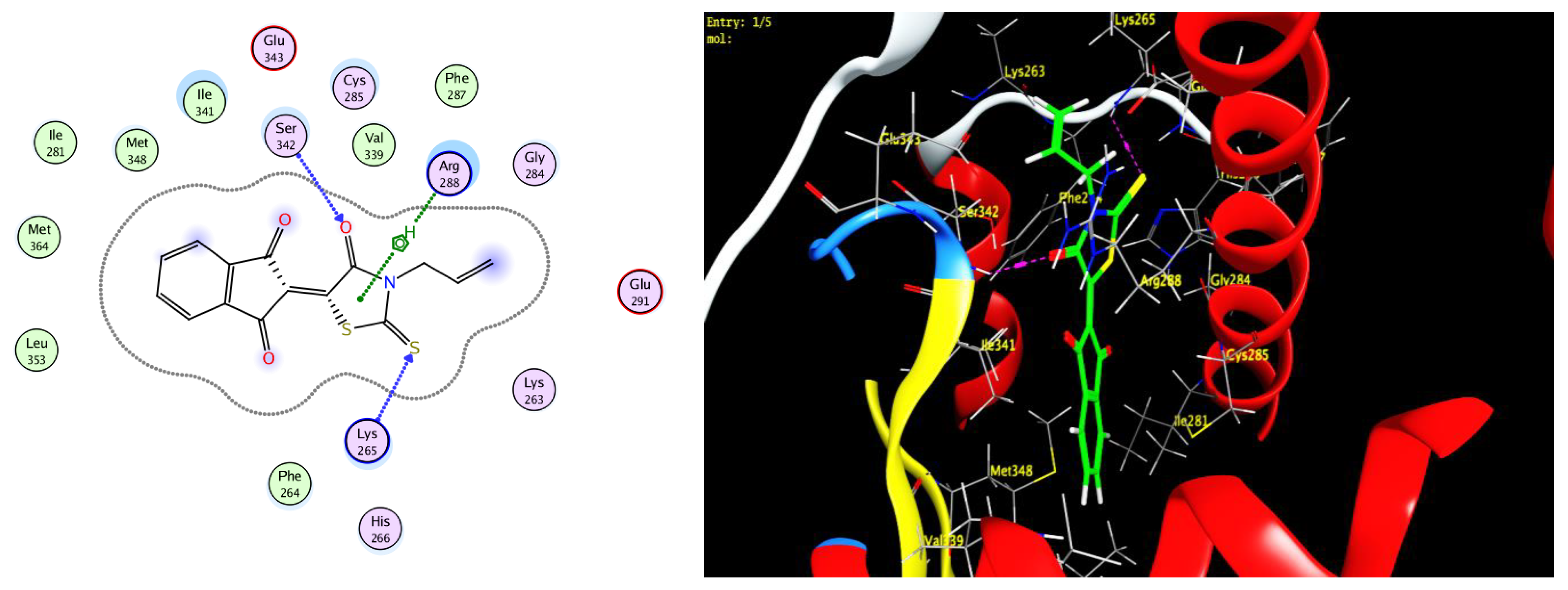
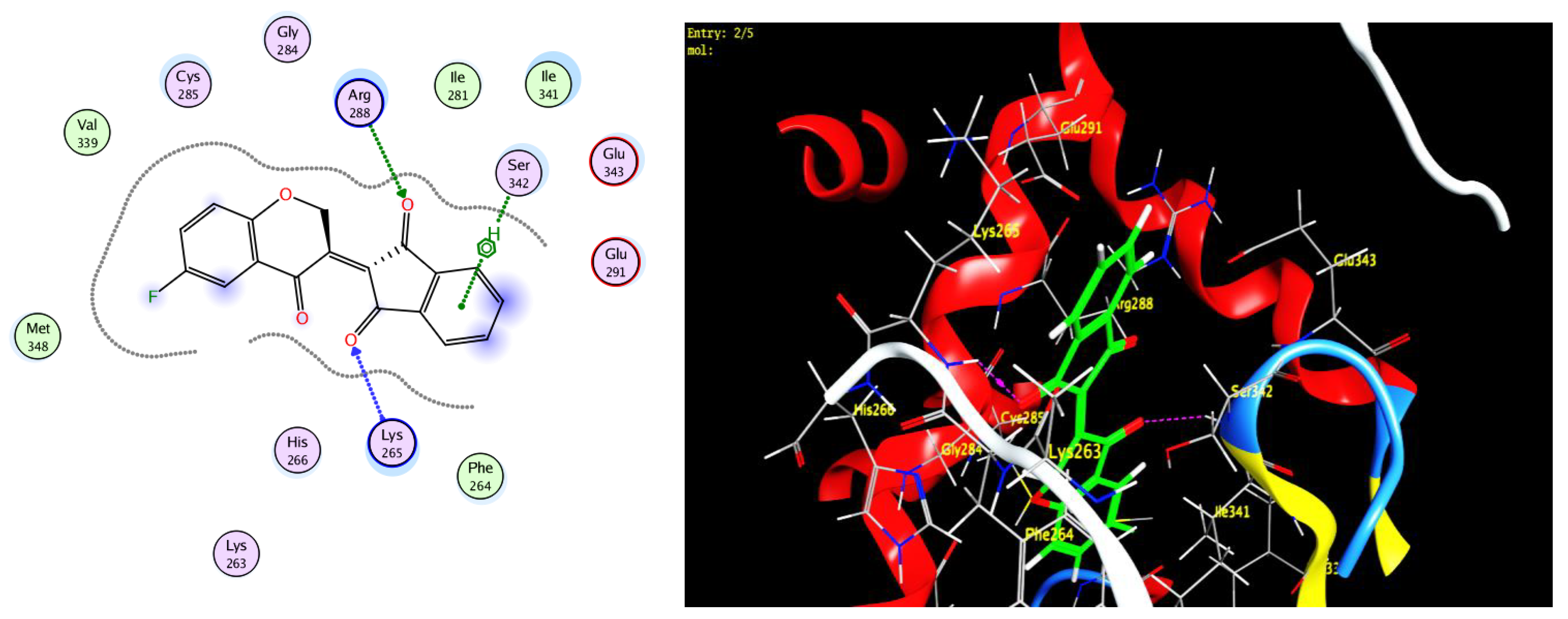
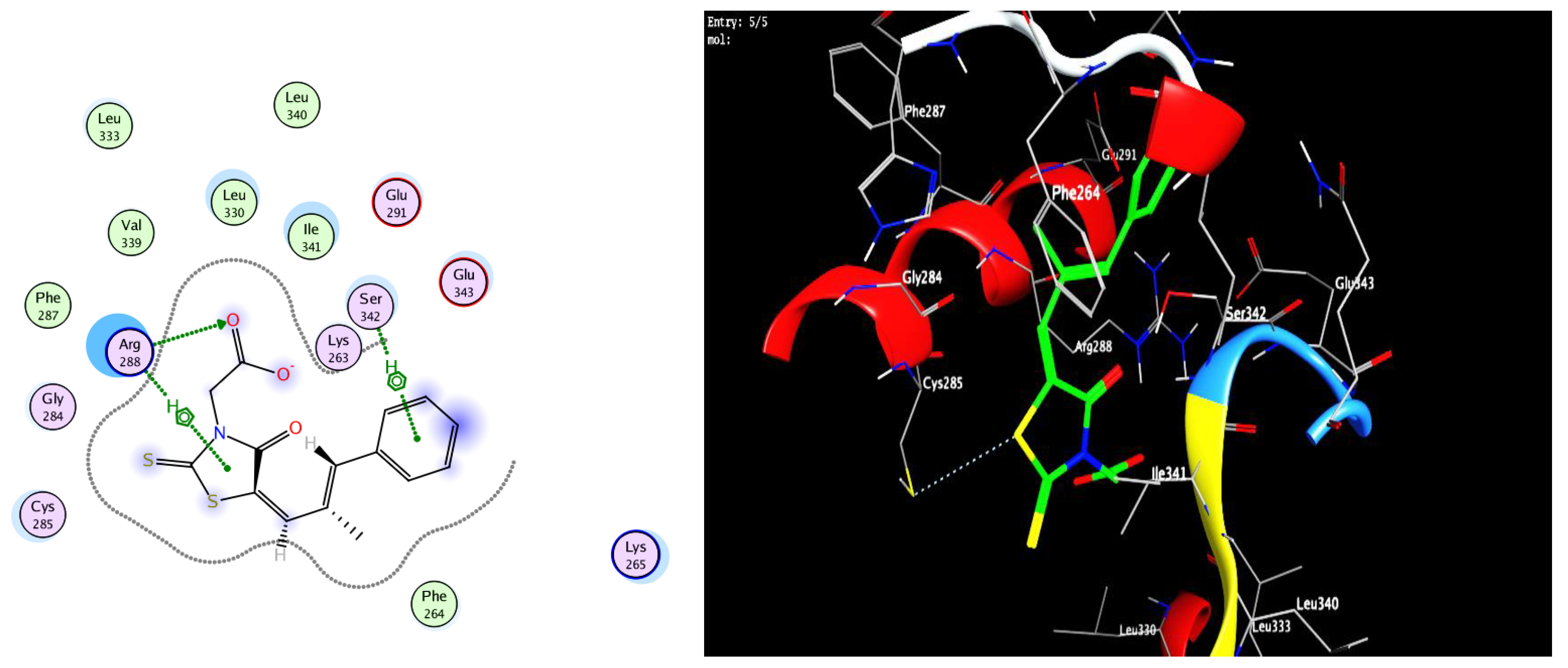
3.3. Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendgen, T.; Steuer, C.; Klein, C. Privileged Scaffolds or Promiscuous Binders: A Comparative Study on Rhodanines and Related Heterocycles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Borja, N. Epalrestat: An Aldose Reductase Inhibitor for the Treatment of Diabetic Neuropathy. Pharmacotherapy 2008, 28, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Enchev, V.; Chorbadjiev, S.; Jordanov, B. Comparative Study of the Structure of Rhodanine, Isorhodanine, Thiazolidine-2,4-dione, and Thiorhodanine. Chem. Heterocycl. Compd. 2002, 38, 1110–1120. [Google Scholar] [CrossRef]
- Krátky’, M.; Štěpánková, S.; Vorčáková, K.; Vinšová, J. Synthesis and in vitro evaluation of novel rhodanine derivatives as potential cholinesterase inhibitors. Bioorg. Chem. 2016, 68, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Afifi, O.S.; Shaaban, O.G.; Abd El Razik, H.A.; Shams El-Dine, A.; Ashour, F.A.; El-Tombary, A.A.; Abu-Serie, M.M. Synthesis and biological evaluation of purine-pyrazole hybrids incorporating thiazole, thiazolidinone or rhodanine moiety as 15-LOX inhibitors endowed with anticancer and antioxidant potential. Bioorg. Chem. 2019, 87, 821–837. [Google Scholar] [CrossRef]
- El-Miligy, M.M.M.; Hazzaa, A.A.; El-Messmary, H.; Nassra, R.A.; El-Hawash, S.A.M. New hybrid molecules combining benzothiophene or benzofuran with rhodanine as dual COX-1/2 and 5-LOX inhibitors: Synthesis, biological evaluation and docking study. Bioorg. Chem. 2017, 72, 102–115. [Google Scholar] [CrossRef]
- Yang, N.; Ren, Z.; Zheng, J.; Feng, L.; Li, D.; Gao, K.; Zhang, L.; Liu, Y.; Zuo, P. 5-(4-hydroxy-3-dimethoxybenzylidene)-rhodanine (RD-1)-improved mitochondrial function prevents anxiety- and depressive-like states induced by chronic corticosterone injections in mice. Neuropharmacology 2016, 105, 587–593. [Google Scholar] [CrossRef]
- Song, H.; Lee, Y.S.; Roh, E.J.; Seo, J.H.; Oh, K.-S.; Lee, B.H.; Han, H.; Shin, K.J. Discovery of potent and selective rhodanine type IKKβ inhibitors by hit-to-lead strategy. Bioorg. Med. Chem. Lett. 2012, 22, 5668–5674. [Google Scholar] [CrossRef]
- Heng, S.; Tieu, W.; Hautmann, S.; Kuan, K.; Pedersen, D.S.; Pietsch, M.; Gütschow, M.; Abell, A.D. New cholesterol esterase inhibitors based on rhodanine and thiazolidinedione scaffolds. Bioorg. Med. Chem. 2011, 19, 7453–7463. [Google Scholar] [CrossRef]
- Liu, J.; Wu, F.; Chen, L.; Hu, J.; Zhao, L.; Chen, C.; Peng, L. Evaluation of dihydropyrimidin-(2H)-one analogues and rhodanine derivatives as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 2376–2379. [Google Scholar] [CrossRef]
- Chandrappa, S.; Chandru, H.; Sharada, A.C.; Vinaya, K.; Kumar, C.A.; Thimmegowda, N.R.; Nagegowda, P.; Kumar, M.K.; Rangappa, K.S. Synthesis and in vivo anticancer and antiangiogenic effects of novel thioxothiazolidin-4-one derivatives against transplantable mouse tumor. Med. Chem. Res. 2010, 19, 236–249. [Google Scholar] [CrossRef]
- Chauhan, D.; George, G.; Sridhar, S.N.C.; Bhatia, R.; Paul, A.T.; Monga, V. Design, synthesis, biological evaluation, and molecular modeling studies of rhodanine derivatives as pancreatic lipase inhibitors. Arch. Pharm. 2019, 352, 1900029. [Google Scholar] [CrossRef] [PubMed]
- Toumi, A.; Boudriga, S.; Hamden, K.; Sobeh, M.; Cheurfa, M.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L. Synthesis, antidiabetic activity and molecular docking study of rhodanine-substitued spirooxindole pyrrolidine derivatives as novel α-amylase inhibitors. Bioorg. Chem. 2021, 106, 104507. [Google Scholar] [CrossRef] [PubMed]
- Celestina, S.K.; Sundaram, K.; Ravi, S. In vitro studies of potent aldose reductase inhibitors: Synthesis, characterization, biological evaluation and docking analysis of rhodanine-3-hippuric acid derivatives. Bioorg. Chem. 2020, 97, 103640. [Google Scholar] [CrossRef] [PubMed]
- Tintori, C.; Iovenitti, G.; Ceresola, E.R.; Ferrarese, R.; Zamperini, C.; Brai, A.; Poli, G.; Dreassi, E.; Cagno, V.; Lembo, D. Rhodanine derivatives as potent anti-HIV and anti-HSV microbicides. PLoS ONE 2018, 13, e0198478. [Google Scholar] [CrossRef]
- Nitsche, C.; Schreier, V.N.; Behnam, M.A.; Kumar, A.; Bartenschlager, R.; Klein, C.D. Thiazolidinone–Peptide Hybrids as Dengue Virus Protease Inhibitors with Antiviral Activity in Cell Culture. J. Med. Chem. 2013, 56, 8389–8403. [Google Scholar] [CrossRef]
- Maccari, R.; Ottanà, R. Targeting Aldose Reductase for the Treatment of Diabetes Complications and Inflammatory Diseases: New Insights and Future Directions. J. Med. Chem. 2015, 58, 2047–2067. [Google Scholar] [CrossRef]
- Tammali, R.; Ramana, K.V.; Singhal, S.S.; Awasthi, S.; Srivastava, S.K. Aldose Reductase Regulates Growth Factor-Induced Cyclooxygenase-2 Expression and Prostaglandin E2 Production in Human Colon Cancer Cells. Cancer Res. 2006, 66, 9705–9713. [Google Scholar] [CrossRef] [Green Version]
- Balendiran, G.K.; Martin, H.J.; El-Hawari, Y.; Maser, E. Cancer biomarker AKR1B10 and carbonyl metabolism. Chem.-Biol. Interact. 2009, 178, 134–137. [Google Scholar] [CrossRef]
- Yki-Jarvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef]
- Eucker, J.; Bängeroth, K.; Zavrski, I.; Krebbel, H.; Zang, C.; Heider, U.; Jakob, C.; Elstner, E.; Possinger, K.; Sezer, O. Ligands of peroxisome proliferator-activated receptor γ induce apoptosis in multiple myeloma. Anti-Cancer Drugs 2004, 15, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.; Smith, M.; Sarraf, P.; Kroll, T.; Aiyer, A.; Kaufman, D.S.; Oh, W.; Demetri, G.; Figg, W.D.; Zhou, X.P.; et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. PNAS 2000, 97, 10990–10995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priede, E.; Brica, S.; Bakis, E.; Udris, N.; Zicmanis, A. Ionic liquids as solvents for the Knoevenagel condensation: Understanding the role of solvent–solute interactions. New J. Chem. 2015, 39, 9132–9142. [Google Scholar] [CrossRef]
- Yamazaki, S.; Katayama, K.; Wang, Z.; Mikata, Y.; Morimoto, T.; Ogawa, A. Sequential Knoevenagel Condensation/Cyclization for the Synthesis of Indene and Benzofulvene Derivatives. ACS Omega 2021, 6, 28441–28454. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ratti, R.; Phoon, B.L.; Batagarawa, S.M.; Din, I.U.; Selvaraj, M.; Ramalingam, R.J. A review of the recent progress on heterogeneous catalysts for Knoevenagel condensation. Dalton Trans. 2021, 50, 4445–4469. [Google Scholar] [CrossRef] [PubMed]
- van Beurdena, K.; de Koning, S.; Molendijk, D.; van Schijndel, J. The Knoevenagel reaction: A review of the unfinished treasure map to forming carbon–carbon bonds. Green Chem. Lett. Rev. 2020, 13, 349–364. [Google Scholar] [CrossRef]
- Keller, T.C.; Isabettini, S.; Verboekend, D.; Rodrigues, E.G.; P´erez-Ram´ırez, J. Hierarchical high-silica zeolites as superior base catalysts. Chem. Sci. 2014, 5, 677. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Mahmudov, K.T.; da Silva, M.F.C.G.; Pombeiro, A.J.L. pH dependent synthesis of Zn(ii) and Cd(ii) coordination polymers with dicarboxyl-functionalized arylhydrazone of barbituric acid: Photoluminescence properties and catalysts for Knoevenagel condensation. New J. Chem. 2016, 40, 1535. [Google Scholar] [CrossRef]
- Li, G.; Xiao, J.; Zhang, W. Efficient and reusable amine-functionalized polyacrylonitrile fiber catalysts for Knoevenagel condensation in water. Green Chem. 2012, 14, 2234. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, H.; Li, X.; Wu, X.; Li, H. Amine-Functionalized GO as an Active and Reusable Acid–Base Bifunctional Catalyst for One-Pot Cascade Reactions. ACS Catal. 2014, 4, 394. [Google Scholar] [CrossRef]
- Panja, S.K.; Dwivedi, N.; Saha, S. First report of the application of simple molecular complexes as organo-catalysts for Knoevenagel condensation. RSC Adv. 2015, 5, 65526. [Google Scholar] [CrossRef]
- Garrabou, X.; Wicky, B.I.M.; Hilvert, D. Fast Knoevenagel Condensations Catalyzed by an Artificial Schiff-Base-Forming Enzyme. J. Am. Chem. Soc. 2016, 138, 6972. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.; Qiu, F.; Wu, C.; Hu, H.; Yang, J. Ionic tagged amine supported on magnetic nanoparticles: Synthesis and application for versatile catalytic Knoevenagel condensation in water. RSC Adv. 2014, 4, 33175. [Google Scholar] [CrossRef]
- Xi, F.-G.; Liu, H.; Yang, N.-N.; Gao, E.-O. Aldehyde-Tagged Zirconium Metal–Organic Frameworks: A Versatile Platform for Postsynthetic Modification. Inorg. Chem. 2016, 55, 4701. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Qiao, Y.; Wang, X.; Wen, W.; Zhao, S. DABCO-catalyzed Knoevenagel condensation of aldehydes with ethyl cyanoacetate using hydroxy ionic liquid as a promoter. RSC Adv. 2018, 8, 30180–30185. [Google Scholar] [CrossRef] [Green Version]
- Pandolfi, F.; Feroci, M.; Chiarotto, I. Role of Anion and Cation in the 1-Methyl-3-butyl Imidazolium Ionic Liquids BMImX: The Knoevenagel Condensation. ChemistrySelect 2018, 3, 4745–4749. [Google Scholar] [CrossRef] [Green Version]
- Goodman, E.D.; Zhou, C.; Cargnello, M. Design of Organic/Inorganic Hybrid Catalysts for Energy and Environmental Applications. ACS Cent. Sci. 2020, 6, 1916–1937. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S. Magnetic (poly) ionic liquids: A promising platform for green chemistry. J. Mol. Liq. 2021, 323, 114994. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743. [Google Scholar] [CrossRef]
- Adam, J.G.; Johan, J.; Christopher, H. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Sandip, K.S.; Anthony, W.S. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Arafa, W.A.A. An eco-compatible pathway to the synthesis of mono and bis-multisubstituted imidazoles over novel reusable ionic liquids: An efficient and green sonochemical process. RSC Adv. 2018, 8, 16392–16399. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-active ionic liquids: A review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Khaligh, N.G.; Mihankhah, T.; Johan, M.R. Synthesis of new low-viscous sulfonic acid-functionalized ionic liquid and its application as a Brönsted liquid acid catalyst for the one-pot mechanosynthesis of 4H-pyrans through the ball milling process. J. Mol. Liq. 2019, 277, 794–804. [Google Scholar] [CrossRef]
- Dalessandro, E.V.; Collin, H.P.; Guimarães, L.G.L.; Valle, M.S.; Pliego, J.R., Jr. Mechanism of the Piperidine-Catalyzed Knoevenagel Condensation Reaction in Methanol: The Role of Iminium and Enolate Ions. J. Phys. Chem. B 2017, 121, 5300–5307. [Google Scholar] [CrossRef]
- Zhao, S.; He, M.; Guo, Z.; Zhou, N.; Wang, D.; Li, J.; Zhang, L. [HyEtPy]Cl–H2O: An efficient and versatile solvent system for the DABCO-catalyzed Morita–Baylis–Hillman reaction. RSC Adv. 2015, 5, 32839–32845. [Google Scholar] [CrossRef]
- Arafa, W.A.A. Deep eutectic solvent for an expeditious sono-synthesis of novel series of bis-quinazolin-4-one derivatives as potential anti-cancer agents. R. Soc. Open Sci. 2019, 6, 182046. [Google Scholar] [CrossRef] [Green Version]
- Arafa, W.A.A.; Mourad, A.K. New dicationic DABCO-based ionic liquids: A scalable metal-free one-pot synthesis of bis-2-amino-5-arylidenethiazol-4-ones. R. Soc. Open Sci. 2019, 6, 190997. [Google Scholar] [CrossRef] [Green Version]
- Pagadala, R.; Kasi, V.; Shabalala, N.G.; Jonnalagadda, S.B. Ultrasound-assisted multicomponent synthesis of heterocycles in water—A review. Arabian J. Chem. 2022, 15, 103544. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem. 2003, 2, 361–363. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Arafa, W.A.A.; Mohamed, A.M.; Abdel-Magied, A.F. Ultrasound-Mediated Three-Component Reaction on-Water Protocol for the Synthesis of Novel Mono- and Bis-1,3-Thiazin-4-One Derivatives. Heterocycles 2017, 94, 1439–1455. [Google Scholar] [CrossRef]
- Safer, A.; Rahmouni, M.; Carreaux, F.; Paquin, L.; Lozach, O.; Meijer, L.; Bazureau, J.P. An expeditious, environment-friendly, and microwave-assisted synthesis of 5-isatinylidenerhodanine derivatives. Chem. Pap. 2011, 65, 332–337. [Google Scholar] [CrossRef]
- Pardasani, R.T.; Pardasani, P.; Jain, A.; Kohli, S. Synthesis and Semiempirical Calculations of Thiazolidinone and Imidazolidinone Derivatives of A-Diones. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 1569–1575. [Google Scholar] [CrossRef]
- Shvets, A.A.; Kurbatov, S.V. Synthesis of bis-β,β′-spiro-pyrrolidinyl-oxindoles, containing a rhodanine fragment. Chem. Heterocycl. Compd. 2012, 48, 799–806. [Google Scholar] [CrossRef]
- Pardasani, R.T.; Pardasani, P.; Sharma, I.; Saxena, A.; Kohli, S. Synthesis and semiempirical calculations of imidazolidine, isoxazolone and thiazoline thiol derivatives of acenaphthylene-1,2-dione. Indian J. Chem. 2003, 42B, 3075–3080. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Joshi, G.; Kalra, S.; Wani, A.A.; Bharatam, P.V.; Kumar, P.; Kumar, R. Knoevenagel/Tandem Knoevenagel and Michael Adducts of Cyclohexane-1,3-dione and Aryl Aldehydes: Synthesis, DFT Studies, Xanthine Oxidase Inhibitory Potential, and Molecular Modeling. ACS Omega 2019, 4, 4604–4614. [Google Scholar] [CrossRef]
- Tsuji, M.; Murota, S.; Morita, I. Docosapentaenoic acid (22:5, n-3) suppressed tube forming activity in endothelial cells induced by vascular endothelial growth factor. Prostaglandins Leukot. Essent. Fatty Acids 2003, 68, 337–342. [Google Scholar] [CrossRef]
- Arafa, W.A.A.; Hussein, M.F. Design, Sonosynthesis, Quantum-Chemical Calculations, and Evaluation of New Mono- and Bis-pyridine Dicarbonitriles as Antiproliferative Agents. Chin. J. Chem. 2020, 38, 501–508. [Google Scholar] [CrossRef]
- Shao, J.-W.; Dai, Y.-C.; Xue, J.-P.; Wang, J.-C.; Lin, F.-P.; Guo, Y.-G. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
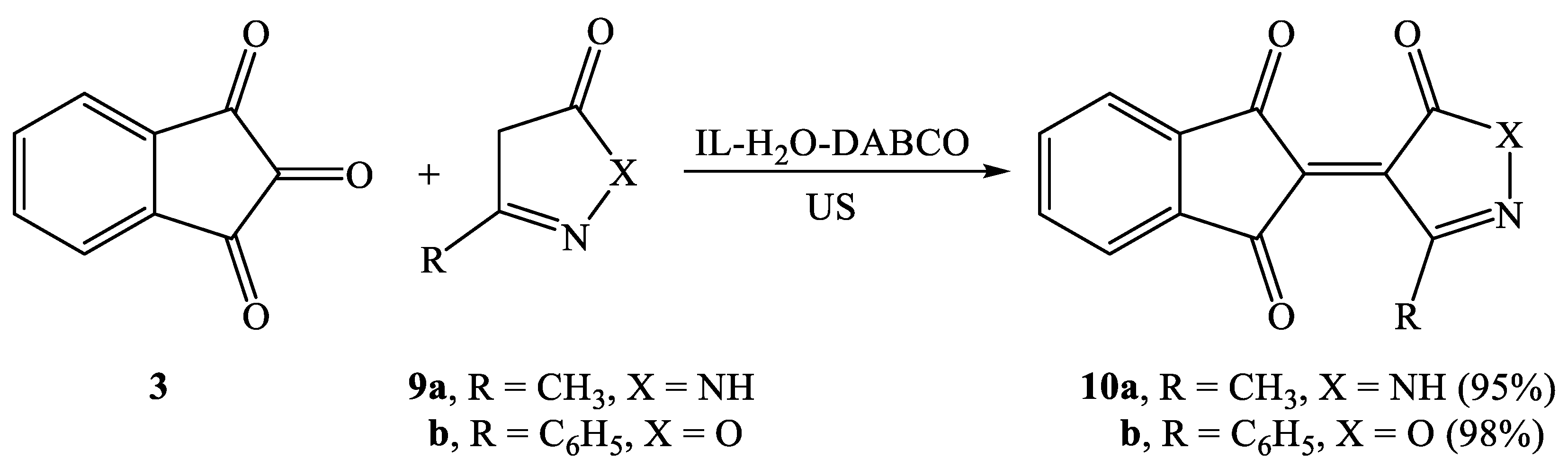





| Entry | Composite System | Equivalent | Conditions | Time (min) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|---|
| 1. | IL-H2O | 50%:50% | US a | 60 | 50 | 62 |
| 2. | H2O | - | US | 60 | 50 | NR |
| 3. | IL-H2O-DABCO | 50%:50%:1.0 mmol | US | 8 | 30 | 93 |
| 4. | IL-H2O-Piperidine | 50%:50%:1.0 mmol | US | 60 | 30 | 75 |
| 5. | IL-H2O-TEA | 50%:50%:1.0 mmol | US | 60 | 30 | 78 |
| 6. | IL-H2O-HMTA | 50%:50%:1.0 mmol | US | 60 | 30 | 83 |
| 7. | IL-H2O-K2CO3 | 50%:50%:1.0 mmol | US | 60 | 30 | 68 |
| 8. | IL-H2O-KOH | 50%:50%:1.0 mmol | US | 60 | 30 | 56 |
| 9. | H2O-DABCO | 3 mL:1.0 mmol | US | 60 | 30 | 81 |
| 10. | IL-EtOH-DABCO | 50%:50%:1.0 mmol | US | 60 | 30 | 77 |
| 11. | IL-DCM-DABCO | 50%:50%:1.0 mmol | US | 60 | 30 | 35 |
| 12. | IL-MeCN-DABCO | 50%:50%:1.0 mmol | US | 60 | 30 | 38 |
| 13. | IL-H2O-DABCO | 50%:50%:1.0 mmol | US | 25 | 23 | 87 |
| 14. | IL-H2O-DABCO | 50%:50%:1.0 mmol | US | 15 | 40 | 88 |
| 15. | IL-H2O-DABCO | 50%:50%:1.0 mmol | US | 25 | 60 | 90 |
| 16. | IL-H2O-DABCO | 50%:50%:1.0 mmol | US | 25 | 80 | 90 |
| 17. | IL-H2O-DABCO | 60%:40%:1.0 mmol | US | 5 | 30 | 99 |
| 18. | IL-H2O-DABCO | 40%:60%:1.0 mmol | US | 10 | 30 | 89 |
| 19. | IL-H2O-DABCO | 70%:30%:1.0 mmol | US | 5 | 30 | 99 |
| 20. | IL-H2O-DABCO | 60%:40%:1.0 mmol | Stirring | 60 | 80 | 83 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Derivatives | Conditions | Time (min) | Yield (%) | Yield Economy (%) | Ref. |
| 1 | 14a | MW/160 °C | 34 | 65 | 0.52 | [51] |
| 2 | 14b | MW/160 °C | 34 | 80 | 2.35 | [51] |
| 3 | 14c | EtOH/Ref. | 300 | 75 | 0.25 | [52] |
| 4 | 14d | MeOH/KOH | 20 | 91 | 4.55 | [53] |
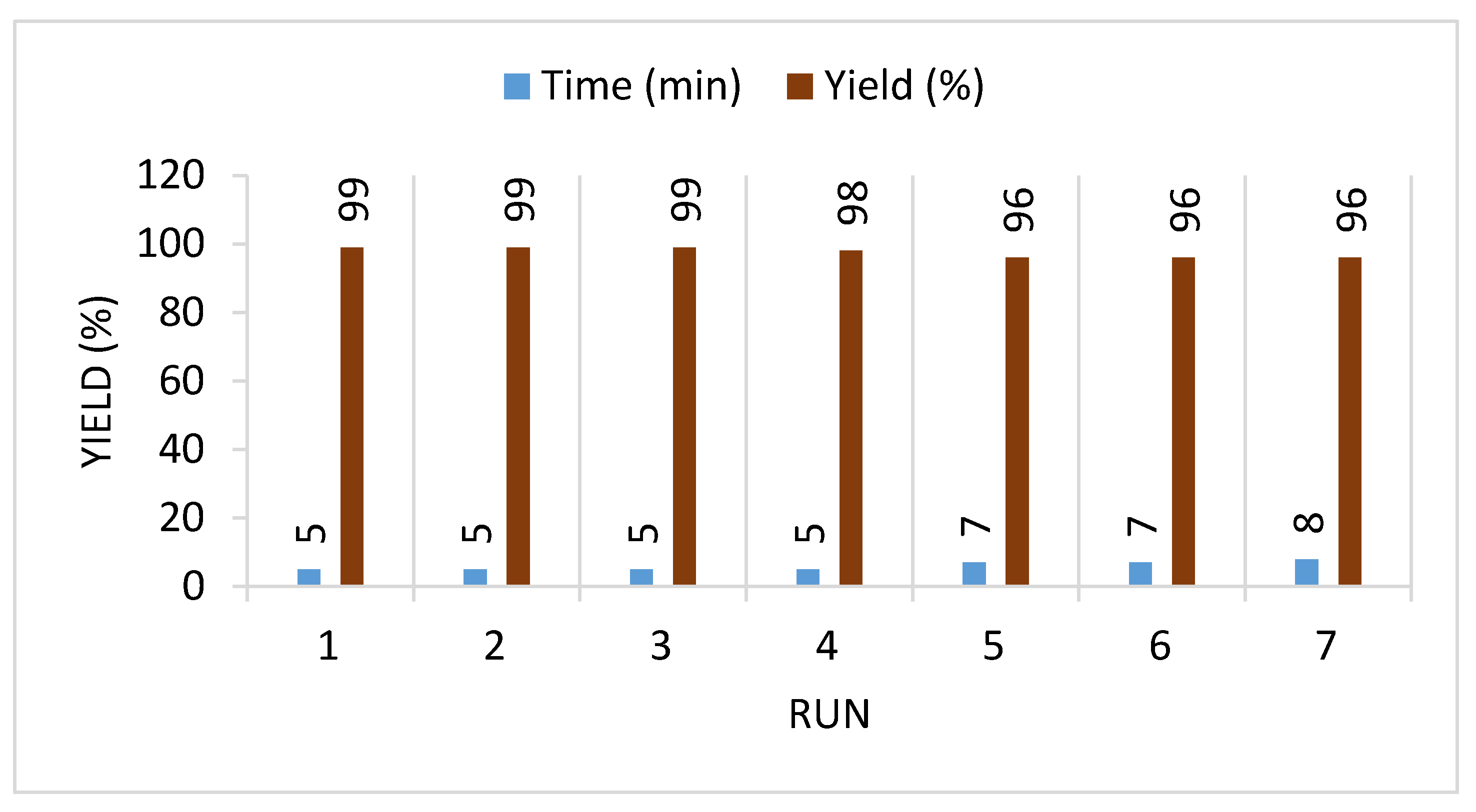
| 5 | 14a | [Py-2OH]AcO | 5 | 99 | 19.8 | Current work |
| 6 | 14b | 5 | 99 | 19.8 | ||
| 7 | 14c | 6 | 98 | 16.3 | ||
| 8 | 14d | 7 | 99 | 14.1 | ||
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Derivatives | Conditions | Time (min) | Yield (%) | Yield Economy (%) | Ref. |
| 1 | 16a | EtOH/Ref. | 300 | 80 | 0.26 | [54] |
| 2 | 16b | EtOH/Ref. | 480 | 74 | 0.15 | [52] |
| 3 | 16a | [Py-2OH]AcO | 5 | 98 | 19.60 | Current work |
| 4 | 16b | 6 | 98 | 16.33 | ||
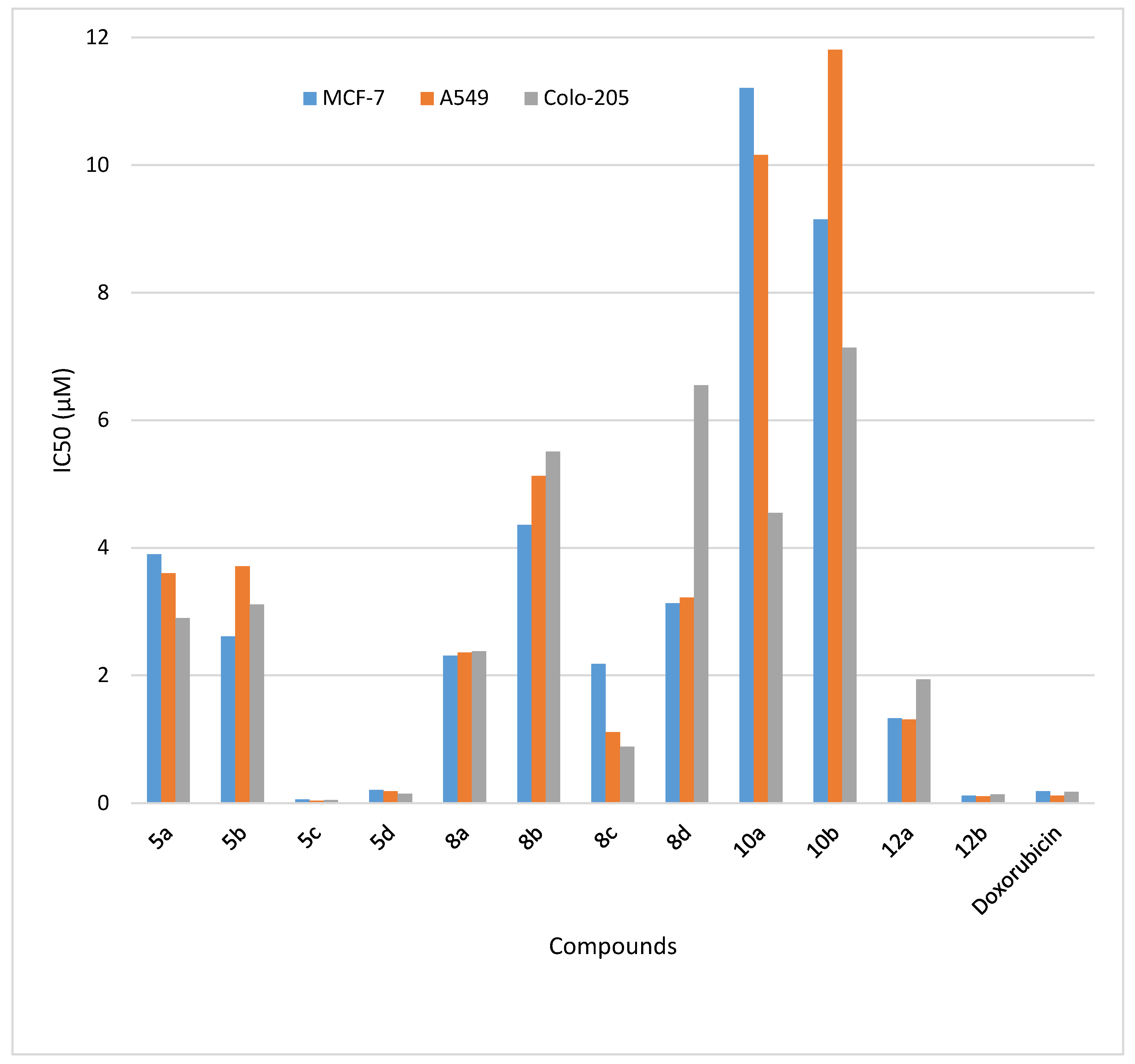
| Entry | Compounds | IC50 (μM) a | ||
|---|---|---|---|---|
| MCF-7 | A549 | Colo-205 | ||
| 1. | 5a | 3.9 ± 2.18 | 3.6 ± 3.54 | 2.9 ± 3.36 |
| 2. | 5b | 2.61 ± 1.22 | 3.71 ± 2.13 | 3.11 ± 1.35 |
| 3. | 5c | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.02 |
| 4. | 5d | 0.21 ± 0.02 | 0.19 ± 0.03 | 0.15 ± 0.01 |
| 5. | 8a | 2.31 ± 1.01 | 2.36 ± 0.69 | 2.38 ± 1.95 |
| 6. | 8b | 4.36 ± 1.59 | 5.13 ± 5.38 | 5.51 ± 3.11 |
| 7. | 8c | 2.18 ± 1.56 | 1.11 ± 1.27 | 0.89 ± 0.17 |
| 8. | 8d | 3.13 ± 0.97 | 3.22 ± 0.25 | 4.08 ± 1.67 |
| 9. | 10a | 11.21 ± 2.42 | 10.16 ± 1.62 | 6.55 ± 2.92 |
| 10. | 10b | 9.15 ± 4.24 | 11.81 ± 7.92 | 7.14 ± 5.32 |
| 11. | 12a | 1.33 ± 0.53 | 1.31 ± 1.75 | 1.94 ± 1.21 |
| 12. | 12b | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.14 ± 0.04 |
| 13. | Doxorubicin | 0.19 ± 0.02 | 0.12 ± 0.01 | 0.18 ± 0.11 |
| Compound | Binding Score ΔK (KJ/mole) | |
|---|---|---|
| AKR1B10 | PPARγ | |
| 5c | −7.11 | −6.38 |
| 5d | −6.93 | −6.62 |
| 12b | −6.40 | −6.42 |
| Epalrestat | −7.40 | −6.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayl, A.A.; Arafa, W.A.A.; Ahmed, I.M.; Abd-Elhamid, A.I.; El-Fakharany, E.M.; Abdelgawad, M.A.; Gomha, S.M.; Ibrahim, H.M.; Aly, A.A.; Bräse, S.; et al. Novel Pyridinium Based Ionic Liquid Promoter for Aqueous Knoevenagel Condensation: Green and Efficient Synthesis of New Derivatives with Their Anticancer Evaluation. Molecules 2022, 27, 2940. https://doi.org/10.3390/molecules27092940
Nayl AA, Arafa WAA, Ahmed IM, Abd-Elhamid AI, El-Fakharany EM, Abdelgawad MA, Gomha SM, Ibrahim HM, Aly AA, Bräse S, et al. Novel Pyridinium Based Ionic Liquid Promoter for Aqueous Knoevenagel Condensation: Green and Efficient Synthesis of New Derivatives with Their Anticancer Evaluation. Molecules. 2022; 27(9):2940. https://doi.org/10.3390/molecules27092940
Chicago/Turabian StyleNayl, AbdElAziz A., Wael A. A. Arafa, Ismail M. Ahmed, Ahmed I. Abd-Elhamid, Esmail M. El-Fakharany, Mohamed A. Abdelgawad, Sobhi M. Gomha, Hamada M. Ibrahim, Ashraf A. Aly, Stefan Bräse, and et al. 2022. "Novel Pyridinium Based Ionic Liquid Promoter for Aqueous Knoevenagel Condensation: Green and Efficient Synthesis of New Derivatives with Their Anticancer Evaluation" Molecules 27, no. 9: 2940. https://doi.org/10.3390/molecules27092940
APA StyleNayl, A. A., Arafa, W. A. A., Ahmed, I. M., Abd-Elhamid, A. I., El-Fakharany, E. M., Abdelgawad, M. A., Gomha, S. M., Ibrahim, H. M., Aly, A. A., Bräse, S., & Mourad, A. K. (2022). Novel Pyridinium Based Ionic Liquid Promoter for Aqueous Knoevenagel Condensation: Green and Efficient Synthesis of New Derivatives with Their Anticancer Evaluation. Molecules, 27(9), 2940. https://doi.org/10.3390/molecules27092940








