Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Novasomes
2.2. Particle Size and Zeta Potential Analysis
2.3. Entrapment Efficiency (EE)
2.4. In Vitro Drug Release
2.5. Release Kinetics
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Differential Scanning Calorimetry (DSC)
2.8. Thermogravimetric Analysis (TGA)
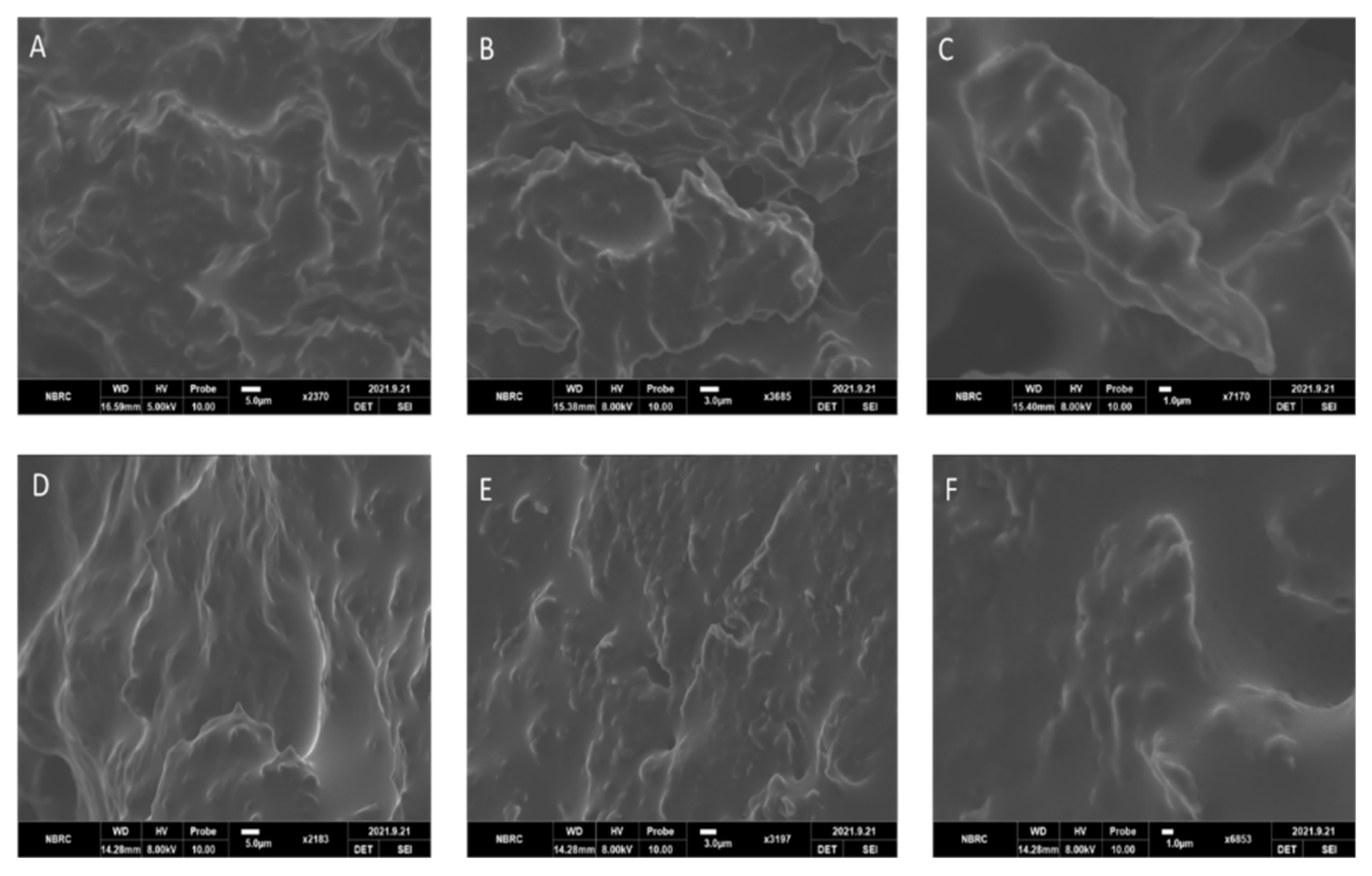
2.9. Scanning Electron Microscopy (SEM)
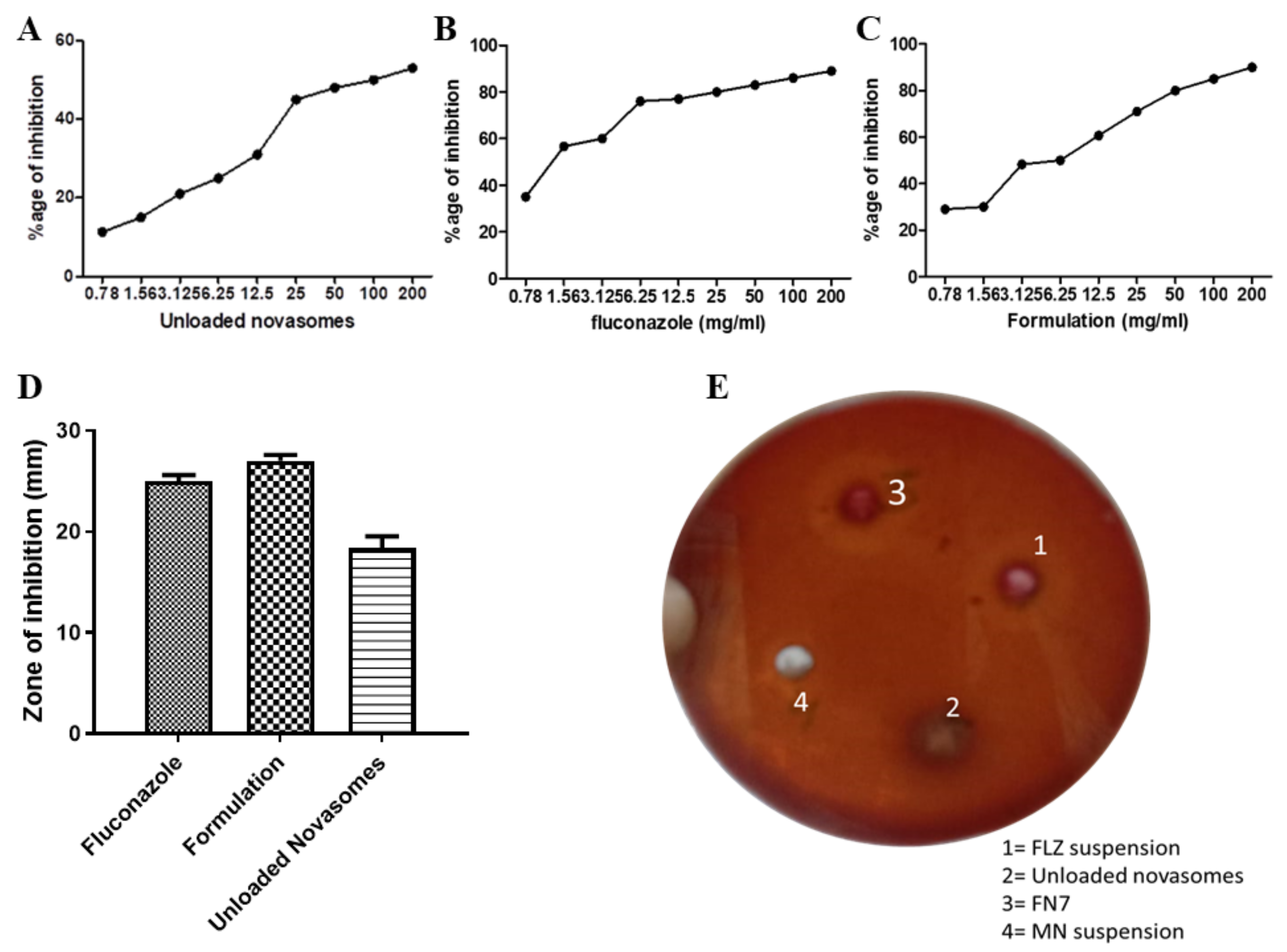
2.10. Antifungal Activity
2.10.1. Minimum Inhibitory Concentration (MIC) Assay via Resazurin Reduction Technique
2.10.2. Agar-Well Diffusion Method
2.11. Effect of Storage Conditions on Nanoparticles
2.12. Toxicity Studies of Novasomes
3. Materials and Methods
3.1. Materials
3.2. Preparation of FLZ-Loaded Novasomes
3.3. Particle Size and Zeta Potential Analysis
3.4. Percent Entrapment Efficiency (EE)
3.5. In Vitro Drug Release
3.6. Release Kinetics
3.7. Fourier-Transform Infrared Spectroscopy (FTIR)
3.8. Differential Scanning Calorimetry (DSC)
3.9. Thermogravimetric Analysis (TGA)
3.10. Scanning Electron Microscopy (SEM)
3.11. Antifungal Activity
3.11.1. Minimum Inhibitory Concentration (MIC) Assay via Resazurin Reduction Technique
3.11.2. Agar-Well Diffusion Method
3.12. Effect of Storage Conditions on Novasomes
3.13. Toxicity Studies of Novasomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kumar, L.; Verma, S.; Bhardwaj, A.; Vaidya, S.; Vaidya, B. Eradication of superficial fungal infections by conventional and novel approaches: A comprehensive review. Artif. Cells Nanomed. Biotechnol. 2014, 42, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Mehrandish, S.; Mirzaeei, S. A Review on Ocular Novel Drug Delivery Systems of Antifungal Drugs: Functional Evaluation and Comparison of Conventional and Novel Dosage Forms. Adv. Pharm. Bull. 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; Fadda, A.M. Vesicular carriers for dermal drug delivery. Expert Opin. Drug Deliv. 2009, 6, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138. [Google Scholar] [CrossRef]
- Ca, A.A.R.; Krishnan, K.; Sreelekshmi, A.; Arjun, K.; Nair, S.C. Novasome: A pioneering advance-mentin vesicular drug delivery. Int. J. Appl. Pharm. 2021, 13, 59–64. [Google Scholar]
- Liu, X.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Gun’Ko, V.M.; Heinmaa, I.; Kumar, N.; Eränen, K.; Salmi, T.; Murzin, D.Y. Zeta Potential of Beta Zeolites: Influence of Structure, Acidity, pH, Temperature and Concentration. Molecules 2018, 23, 946. [Google Scholar] [CrossRef]
- Sedyakina, N.; Kuskov, A.; Velonia, K.; Feldman, N.; Lutsenko, S.; Avramenko, G. Modulation of Entrapment Efficiency and In Vitro Release Properties of BSA-Loaded Chitosan Microparticles Cross-Linked with Citric Acid as a Potential Protein-Drug Delivery System. Materials 2020, 13, 1989. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Flucona-zole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef]
- Salama, A.; Badran, M.; Elmowafy, M.; Soliman, G.M. Spironolactone-Loaded LeciPlexes as Potential Topical Delivery Systems for Female Acne: In Vitro Appraisal and Ex Vivo Skin Permeability Studies. Pharmaceutics 2019, 12, 25. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Zhao, J. Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate-Oleic Acid Composite Film. Materials 2015, 8, 2346–2360. [Google Scholar] [CrossRef]
- Nowak, M.; Gajda, M.; Baranowski, P.; Szymczyk, P.; Karolewicz, B.; Nartowski, K.P. Stabilisation and Growth of Metastable Form II of Fluconazole in Amorphous Solid Dispersions. Pharmaceutics 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Alam Shah, M.K.; Azad, A.K.; Nawaz, A.; Ullah, S.; Latif, M.S.; Rahman, H.; Alsharif, K.F.; Alzahrani, K.J.; El-Kott, A.F.; Albrakati, A.; et al. Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers 2021, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.A.; Ryu, H.J.; Seong, N.-W.; So, B.J.; Seo, H.-S.; Kim, J.-H.; Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Kim, Y.-R.; et al. Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int. J. Nanomed. 2014, 9, 127–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ajrin, M.; Anjum, F. Proniosome: A promising approach for vesicular drug delivery. Turk. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of Novasomes as a Vesicular Carrier for Improving the Topical Delivery of Terconazole: In Vitro Characterization, In Vivo Assessment and Exploratory Clinical Experimentation. Int. J. Nanomed. 2021, 16, 119. [Google Scholar] [CrossRef]
- Baig, M.S.; Ahad, A.; Aslam, M.; Imam, S.S.; Aqil, M.; Ali, A. Application of Box–Behnken design for preparation of levofloxacin-loaded stearic acid solid lipid nanoparticles for ocular delivery: Optimization, in vitro release, ocular tolerance, and antibacterial activity. Int. J. Biol. Macromol. 2016, 85, 258–270. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate micro-dilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Tunney, M.M.; Ramage, G.; Field, T.R.; Moriarty, T.F.; Storey, D.G. Rapid Colorimetric Assay for Antimicrobial Susceptibility Testing of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004, 48, 1879–1881. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays: Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MA, USA, 2016. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic antifungal activity of chitosan with fluconazole against Candida albicans, Candida tropicalis, and fluconazole-resistant strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Plyduang, T.; Atipairin, A.; Yoon, A.S.; Sermkaew, N.; Sakdiset, P.; Sawatdee, S. Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers. Cosmetics 2021, 9, 3. [Google Scholar] [CrossRef]
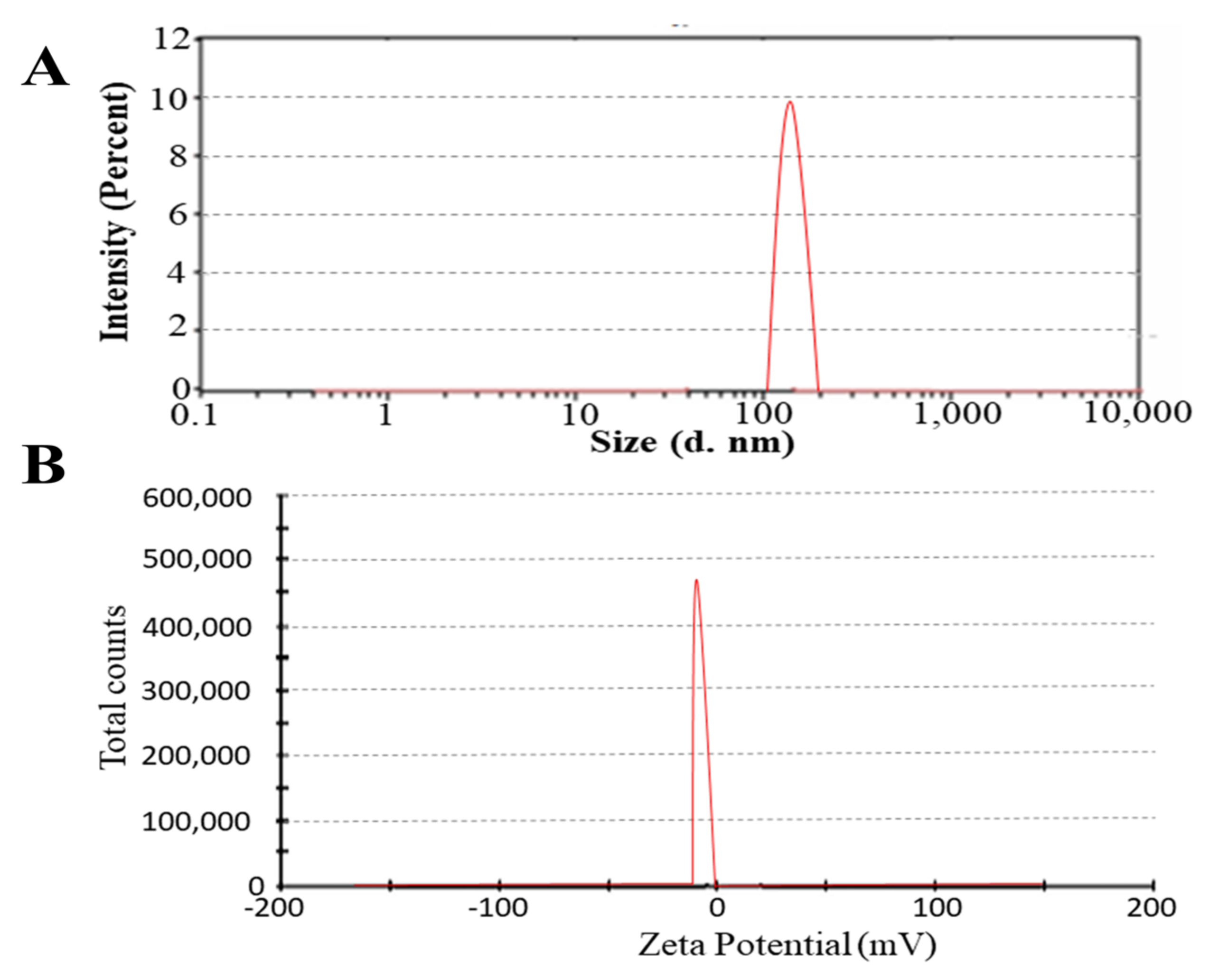
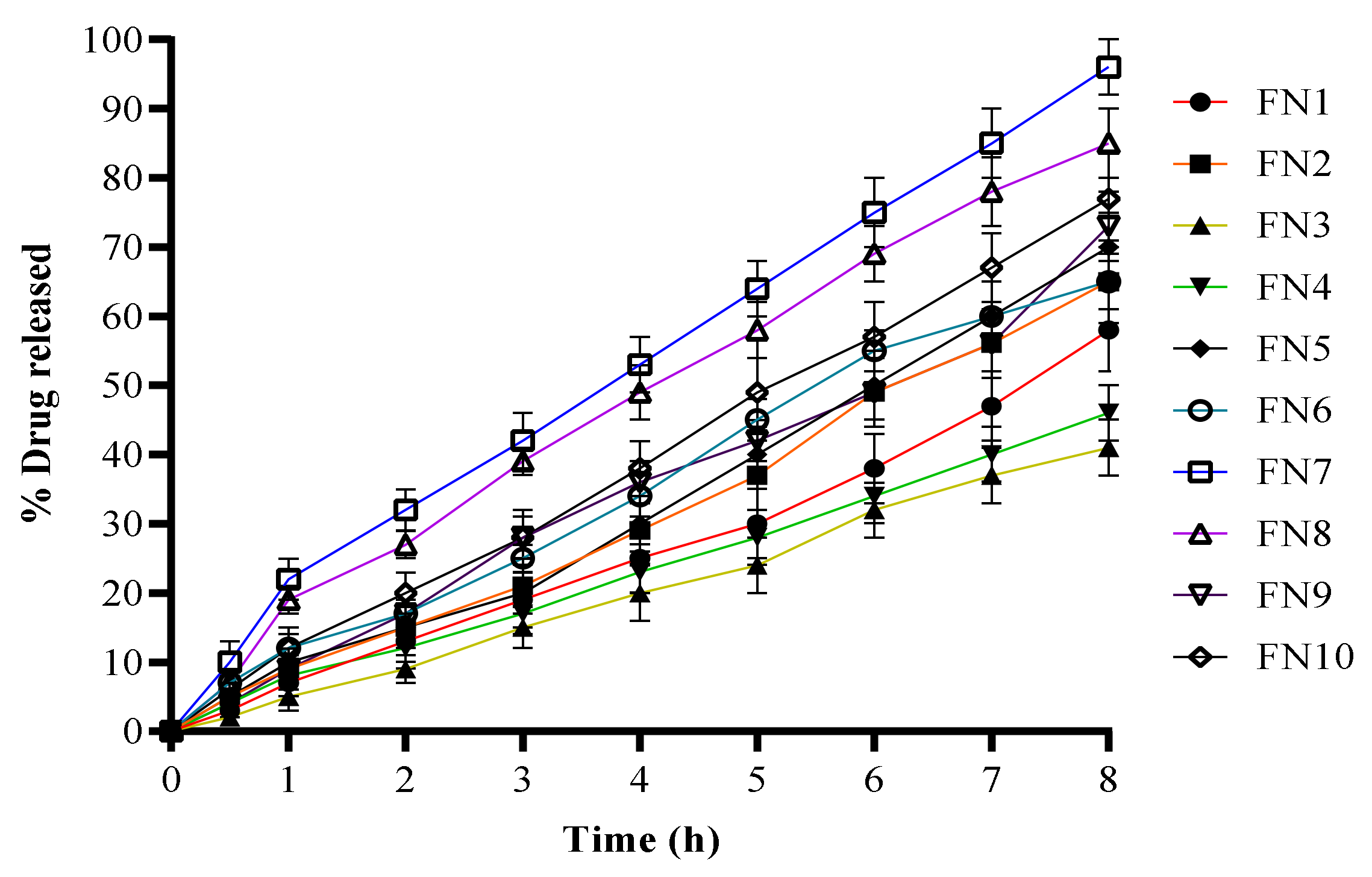
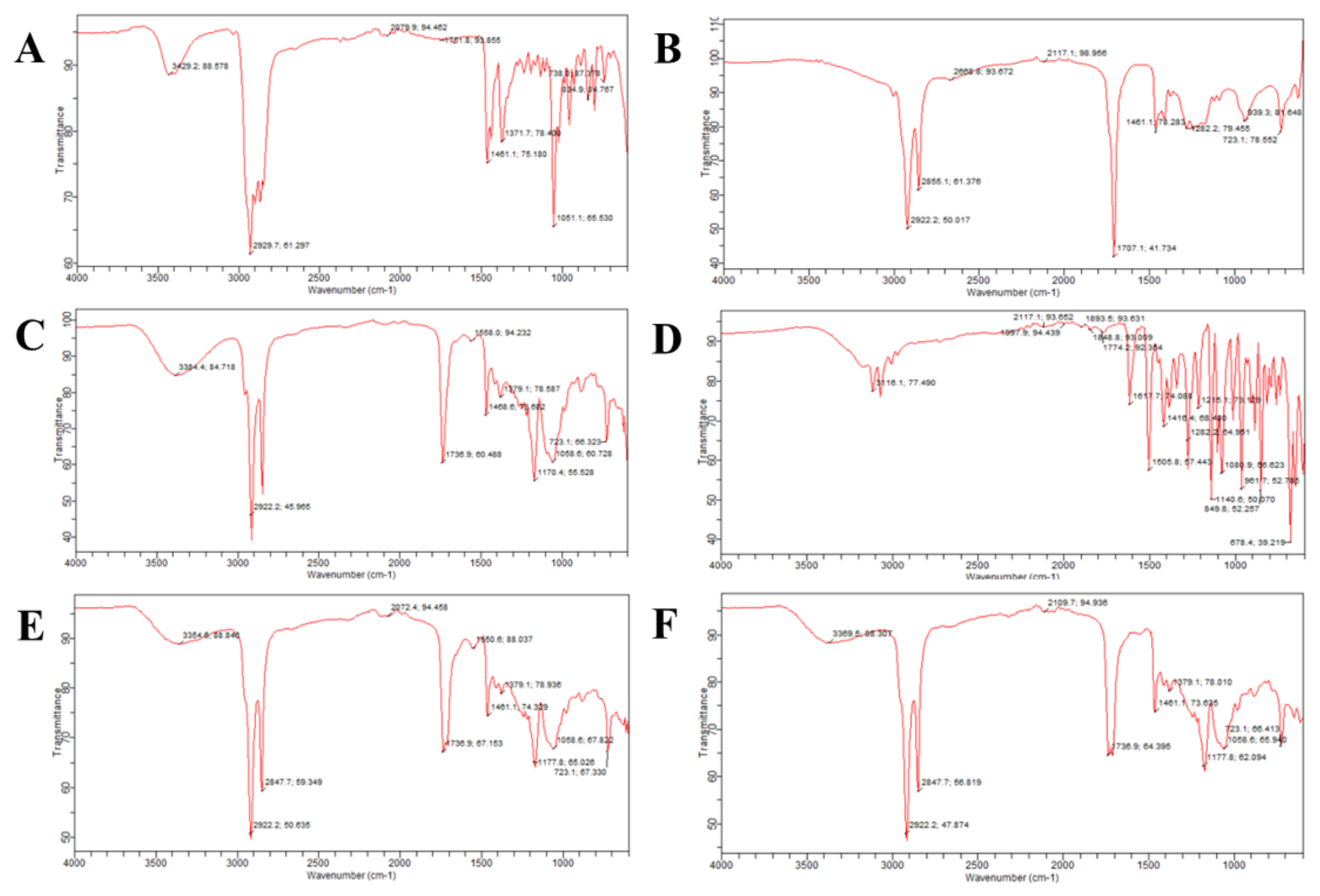
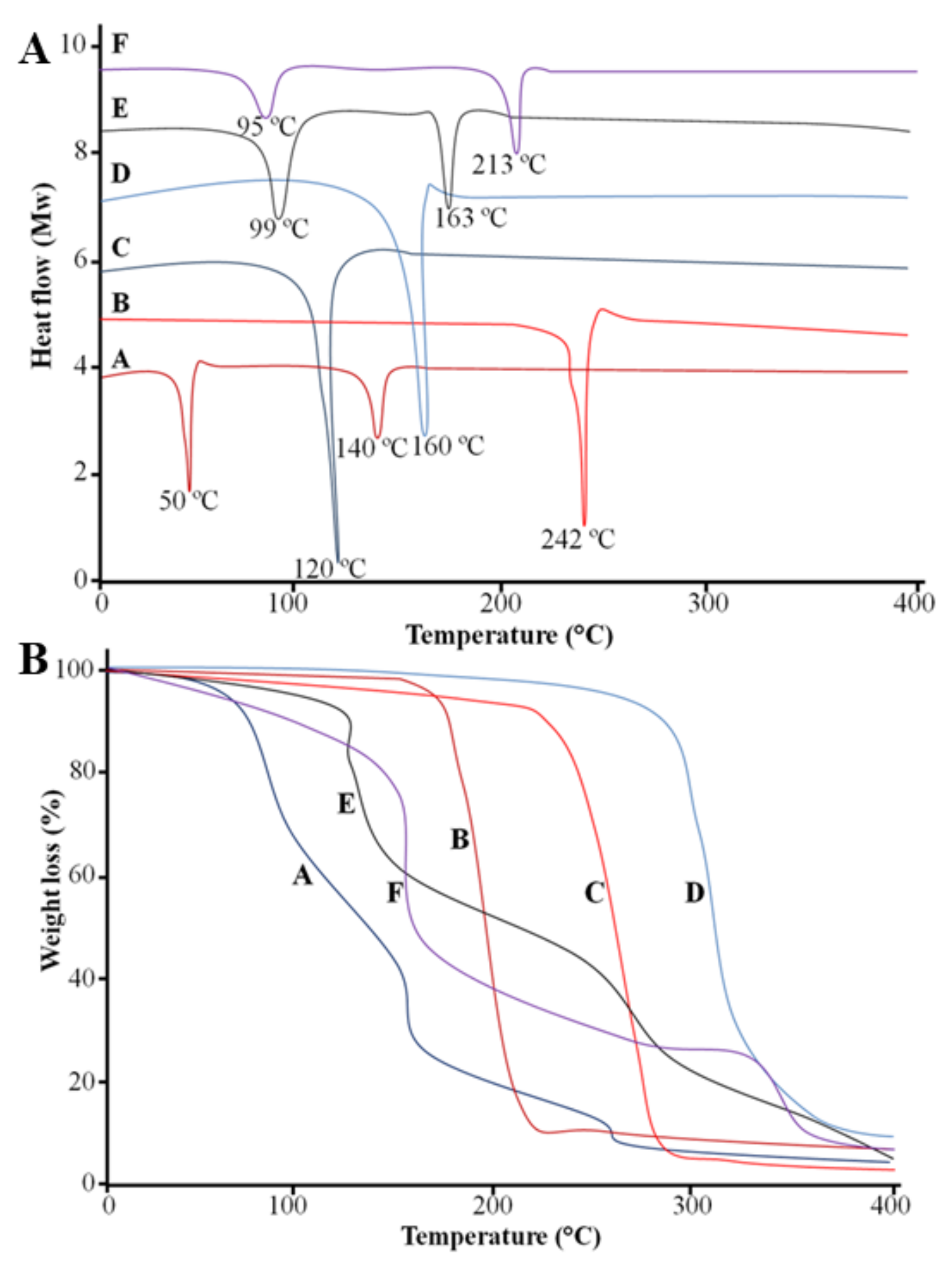
| Code | PS (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|
| FN1 | 145 | 0.120 | −22 | 76.34 |
| FN2 | 178 | 0.230 | −19 | 70.23 |
| FN3 | 123 | 0.345 | −23 | 56.12 |
| FN4 | 149 | 0.319 | −17 | 80.20 |
| FN5 | 236 | 0.287 | −20 | 45.09 |
| FN6 | 298 | 0.067 | −18 | 89.18 |
| FN7 | 110 | 0.023 | −24 | 94.45 |
| FN8 | 120 | 0.069 | −21 | 49.82 |
| FN9 | 141 | 0.267 | −21 | 52.98 |
| FN10 | 192 | 0.345 | −20 | 90.06 |
| Code | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | Hixon–Crowell | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | R2 | |
| FN1 | 0.9878 | 0.9581 | 0.831 | 0.993 | 1.141 | 0.9693 |
| FN2 | 0.9935 | 0.9599 | 0.8456 | 0.9959 | 1.091 | 0.9737 |
| FN3 | 0.9961 | 0.984 | 0.8497 | 0.9974 | 1.066 | 0.9893 |
| FN4 | 0.9967 | 0.9898 | 0.8859 | 0.9973 | 0.958 | 0.9938 |
| FN5 | 0.9888 | 0.9439 | 0.8285 | 0.9948 | 1.152 | 0.9612 |
| FN6 | 0.9898 | 0.9797 | 0.892 | 0.9925 | 0.916 | 0.9884 |
| FN7 | 0.978 | 0.9666 | 0.9356 | 0.9975 | 0.793 | 0.9834 |
| FN8 | 0.9805 | 0.9818 | 0.9308 | 0.9978 | 0.806 | 0.9931 |
| FN9 | 0.9903 | 0.9656 | 0.8668 | 0.9903 | 0.995 | 0.9773 |
| FN10 | 0.9984 | 0.9685 | 0.8809 | 0.9987 | 0.973 | 0.9836 |
| Phases | Hemoglobin g/dL | Red Blood Cells (RBCs) (103/µL) | White Blood Cells (WBCs) (106/µL) | Platelet Count (103/µL) |
|---|---|---|---|---|
| Control group | ||||
| Administration | 14.2 ± 1.35 ** | 7.99 ± 2.19 ** | 5.27 ± 1.80 * | 1023 ± 3.45 ** |
| Recovery | 14.3 ± 1.94 * | 8.01 ± 2.31 * | 5.29 ± 1.67 * | 1022 ± 3.28 ** |
| Experimental group | ||||
| Administration | 14.3 ± 1.18 ** | 7.96 ± 2.17 ** | 5.26 ± 1.21 ** | 1019 ± 3.29 * |
| Recovery | 14.4 ± 1.03 * | 8.21 ± 2.11 * | 5.28 ± 1.39 * | 1020 ± 3.12 ** |
| Phases | Albumin (g/dL) | Globulin (mg/dL) | Creatinine (mg/dL) | Glucose (mg/dL) | Cholesterol (mg/dL) | Triglycerides (mg/dL) |
|---|---|---|---|---|---|---|
| Control group | ||||||
| Administration | 2.3 ± 0.23 * | 157 ± 2.15 ** | 0.60 ± 0.04 * | 161 ± 3.78 ** | 56 ± 1.32 ** | 38 ± 1.02 ** |
| Recovery | 2.4 ± 0.21 * | 156 ± 2.65 ** | 0.58 ± 0.05 * | 158 ± 4.09 * | 55 ± 1.54 ** | 37 ± 1.11 * |
| Experimental group | ||||||
| Administration | 2.2 ± 0.67 ** | 158 ± 2.98 * | 0.58 ± 0.07 ** | 163 ± 4.01 * | 63 ± 1.76 ** | 42 ± 1.20 * |
| Recovery | 2.5 ± 0.45 * | 160 ± 2.56 * | 0.56 ± 0.06 * | 160 ± 3.21 ** | 54 ± 1.96 * | 36 ± 1.32 * |
| Formulation | Type of SAA | Type of FFA | Ratio of SAA: FFA | Cholesterol (mg) |
|---|---|---|---|---|
| FN1 | Span 80 | Oleic acid | 2:1 | 30 |
| FN2 | Span 80 | Oleic acid | 1:1 | 30 |
| FN3 | Span 80 | Stearic acid | 2:1 | 30 |
| FN4 | Span 80 | Stearic acid | 1:1 | 30 |
| FN5 | Tween 80 | Oleic acid | 2:1 | 30 |
| FN6 | Tween 80 | Oleic acid | 1:1 | 30 |
| FN7 | Span 60 | Oleic acid | 2:1 | 30 |
| FN8 | Span 60 | Oleic acid | 1:1 | 30 |
| FN9 | Span 60 | Stearic acid | 2:1 | 30 |
| FN10 | Span 60 | Stearic acid | 1:1 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, I.; Rasul, A.; Shah, S.; Saadullah, M.; Islam, N.; Khames, A.; Salawi, A.; Ahmed, M.M.; Almoshari, Y.; Abbas, G.; et al. Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules 2022, 27, 2936. https://doi.org/10.3390/molecules27092936
Fatima I, Rasul A, Shah S, Saadullah M, Islam N, Khames A, Salawi A, Ahmed MM, Almoshari Y, Abbas G, et al. Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules. 2022; 27(9):2936. https://doi.org/10.3390/molecules27092936
Chicago/Turabian StyleFatima, Iman, Akhtar Rasul, Shahid Shah, Malik Saadullah, Nayyer Islam, Ahmed Khames, Ahmad Salawi, Muhammad Masood Ahmed, Yosif Almoshari, Ghulam Abbas, and et al. 2022. "Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections" Molecules 27, no. 9: 2936. https://doi.org/10.3390/molecules27092936
APA StyleFatima, I., Rasul, A., Shah, S., Saadullah, M., Islam, N., Khames, A., Salawi, A., Ahmed, M. M., Almoshari, Y., Abbas, G., Abourehab, M. A. S., Mehmood Khan, S., Chauhdary, Z., Alshamrani, M., Namazi, N. I., & Naguib, D. M. (2022). Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules, 27(9), 2936. https://doi.org/10.3390/molecules27092936







