3.1. FTO/TiO2/CdS and FTO/ZrO2/CdS Thin Films: Structural and Optical Properties
Chemical bath deposition (CBD) of CdS on top of either nanostructured ZrO
2 or TiO
2 films (MO
2 films, where M is either Ti(IV) or Zr(IV)) affords a visually homogeneous orange film (Inset in
Figure 1a,b). The overall thickness of the ZrO
2/CdS and TiO
2/CdS layers was of the order of 4 and 8 µm respectively (
Figure S1, Supplementary Materials). No significant increase in the film thickness was observed prior and after deposition of CdS, meaning that we form an interpenetrated junction where the electrolyte, during CBD depositions, percolates within the TiO
2 or ZrO
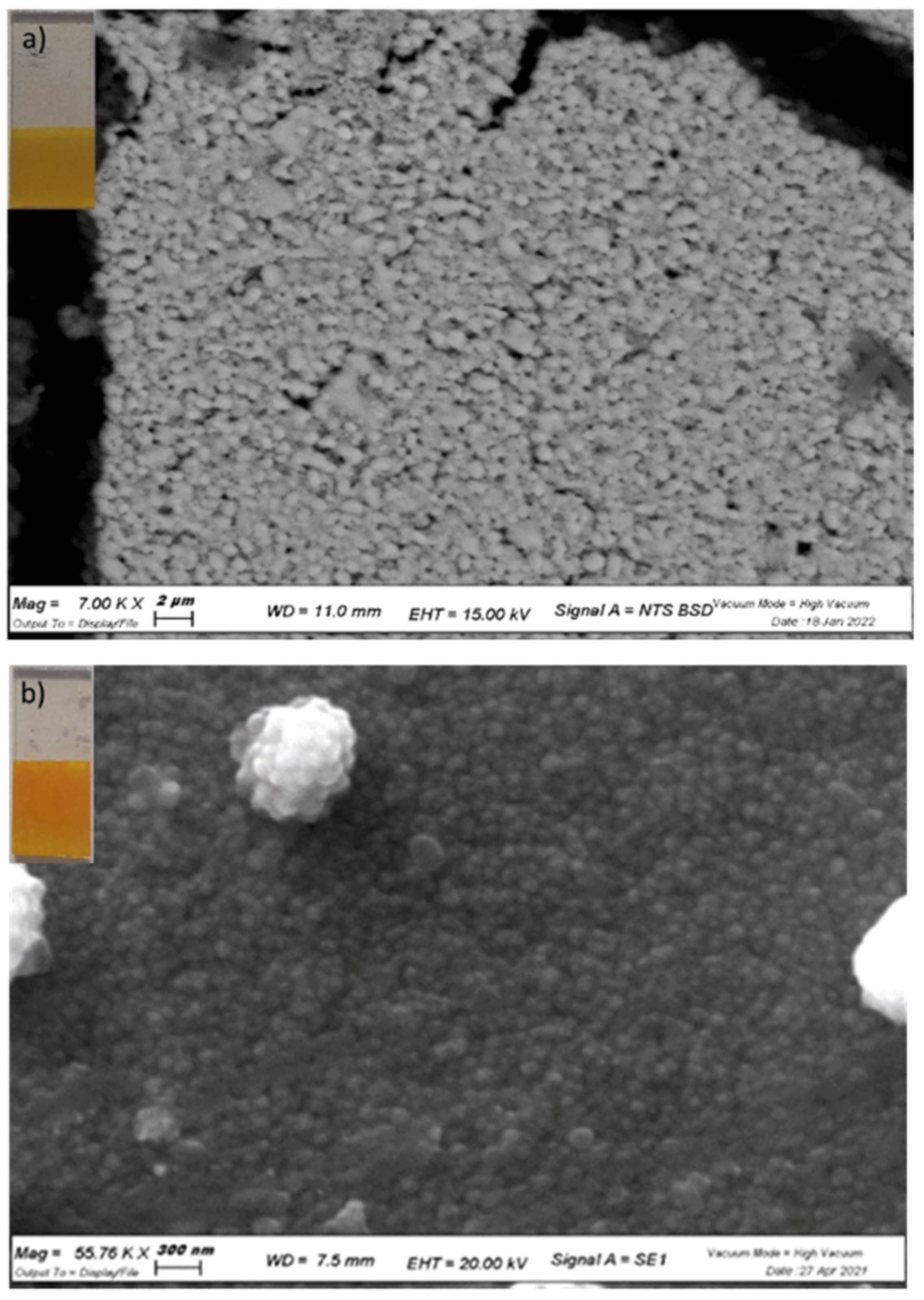
2 mesopores and is conformably deposited on the nanoparticle surface. The SEM imaging reveals a homogeneous network of nanoparticles (
Figure 1a,b), which can be better resolved with AFM (
Figure S2), where spheroidal particles of the approximate size of 25 nm are observed on ZrO
2, while large structures having with a ca. 50 nm diameter are seen on TiO
2. Thus, after CdS deposition the junctions maintain their mesoporous nature, with pores and voids allowing electrolyte permeation.
XRD of a pure CdS sample (
Figure S3a) deposited on FTO by CBD does not reveal any diffraction pattern originating from CdS, but only the obvious presence of crystalline FTO giving rise to intense and sharp peaks according to the well crystallized cassiterite structure. XRD of the n–n junctions supported on FTO display a complicated pattern due to several overlapping contributions from FTO and from the nanoparticulate crystalline phases of TiO
2 (anatase [101] at 2θ = 26°) and ZrO
2 ([101], at 2θ = 31° and [201] 2θ = 51°) particularly within the 2θ interval between 25 and 30° (
Figure S3b,c). We can however identify clear diffraction peaks from CdS at 2θ = 44° [220] and 2θ = 52° [311], in a region relatively free from interfering patterns originated by the other compounds [
22,
23]. This indicates that the CBD procedure produces a completely amorphous CdS layer on bare FTO, but the presence of the underlying crystalline structure of ZrO
2 and TiO
2 induces the formation of crystalline CdS domains. The broad diffraction peaks of CdS are however indicative of a small size of the coherent diffraction domains and of a substantial disorder of the CdS semiconductor film grown by CBD on top of both TiO
2 and ZrO
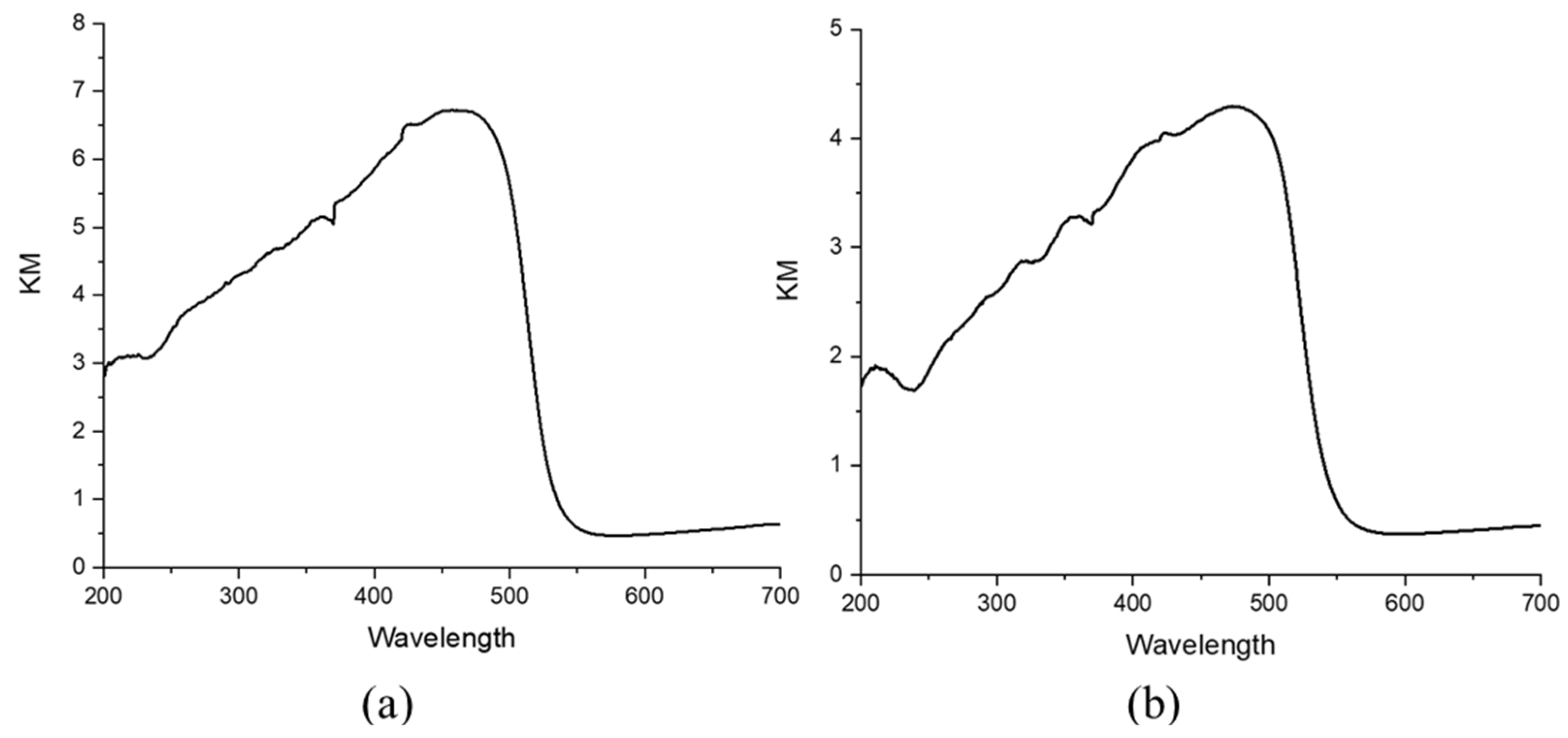
2. The absorption spectra of the CdS modified MO
2 films from diffuse reflectance data in Kubelka Munk (KM) units (
Figure 2) are consistent with the presence of CdS displaying a direct band gap of 2.15 ± 0.3 eV, extracted from Tauc Plots (
Figure S4). From the raw reflectance data, we observe that the light-harvesting efficiency between 400 and 500 nm, where CdS manifests its absorption peak, is ca. 90%.
CdS modified films were found emissive at room temperature upon 450 nm excitation. Compared to a pure CdS film deposited on bare FTO (FTO/CdS), both the FTO/MO
2/CdS films display similarly broadened emission spectra, with a red shift in the emission maximum of approximately 20 nm (
Figure S5). The broadened and red shifted response might be the result of increased heterogeneity in the nanoparticulate films and of some stabilizing interaction between CdS and the mesoporous oxide, which lower the exciton energy. Time resolved analysis of the fluorescence revealed a multiexponential decay, consistent with a distribution of sites on the MO
2/CdS from which the radiative recombination occurs with different rate constants. Typically, the emission decays (
Figures S6 and S7) were satisfactorily fitted with a triexponential function, where the largely dominant (≈98%) component is below the instrumental resolution of our apparatus (<300 ps), while smaller amplitudes (≈1–2%) were much longer lived and decayed in the nanosecond time scale. Interestingly, the ns radiative recombination was faster on FTO/ZrO
2/CdS where amplitudes of 0.48 and 0.31% decayed with a lifetime of 4.01 and 1.44 ns (integrated intensities 15.94 and 3.71, respectively,
Figure S6) compared to FTO/TiO
2/CdS, where amplitudes of 1.04% and 0.11% exhibited a lifetime of 2.52 and 12.9 ns (integrated intensities of 16.5% and 9.15%, respectively,
Figure S7). This indicates that already on such short time scales, the radiative recombination of the carriers is slowed down on TiO
2, probably due to the injection of electrons from the CdS phase to anatase and subsequent recombination occurring at the interface between these two materials (
Scheme 2).
We note that the observation of the kinetics on the ns time scales does not allow to draw sure conclusions about the comparative photocatalytic activity of the FTO/MO
2/CdS films under investigation, given that usually multi-electron heterogeneous processes relevant to photocatalysis occur on much longer time scales (milliseconds/seconds) [
24] and often involve trapped long lived carriers that do not recombine radiatively. Nevertheless, it provides a first indication of different carrier dynamics in the two systems. Photoelectrochemical experiments will be used to gain further indication of a longer lived charge separation in FTO/TiO
2/CdS.
3.2. FTO/TiO2/CdS and FTO/ZrO2/CdS Thin Films: Photoelectrochemical Properties
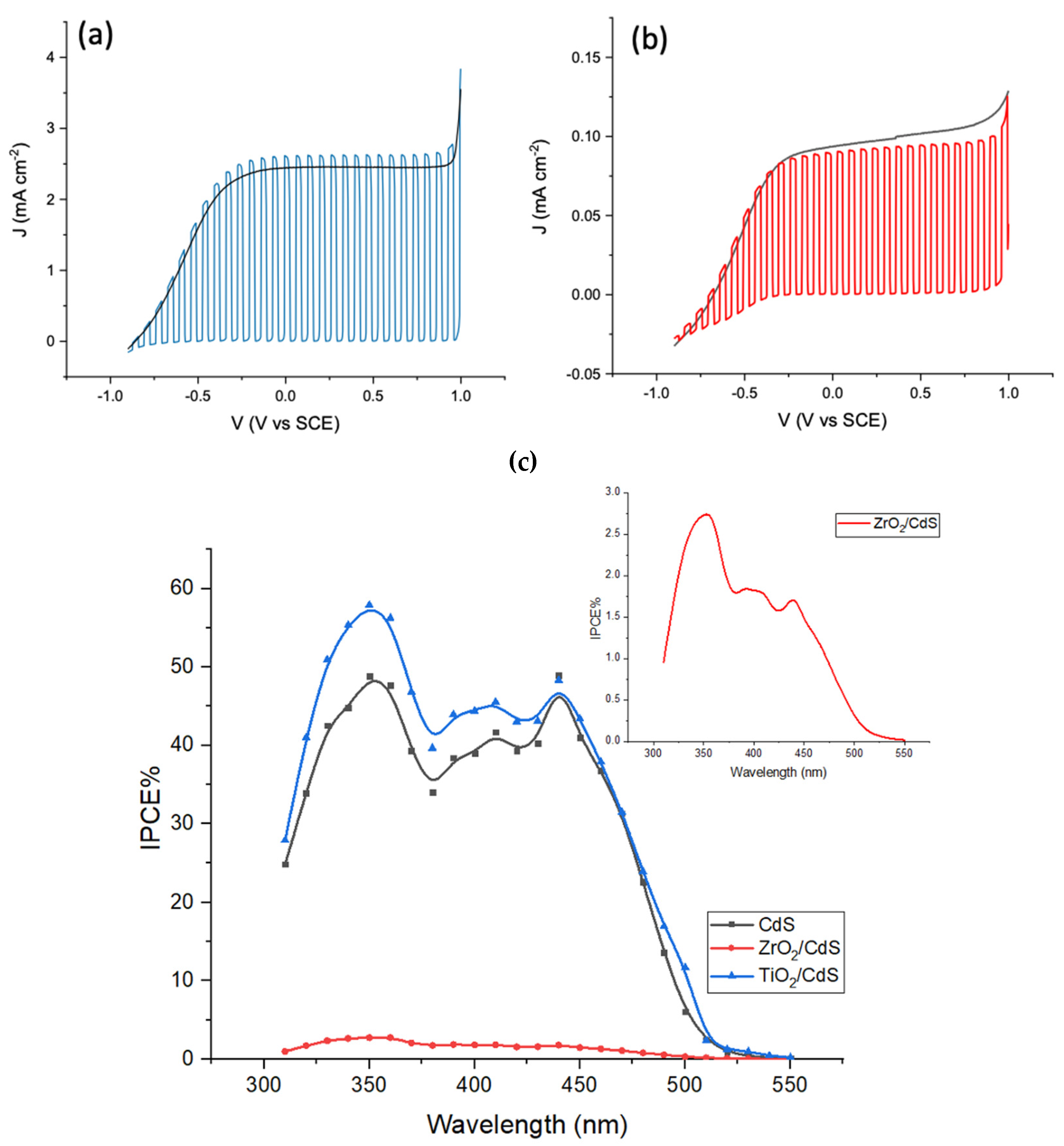
Photoelectrochemical investigations provide a comprehensive picture of the charge separation and recombination dynamics of FTO/MO
2/CdS films in the same electrolyte and similar illumination conditions to those used for photocatalytic experiments. In particular, the photocurrent density and photovoltage decay are related to the efficiency of charge separation, which ultimately affects the photocatalytic performance towards azo-dyes reduction. The photocurrent density/voltage curves of the FTO/MO
2/CdS in 1 M HCOONa are reported in
Figure 3 along with the photoaction spectra (IPCE vs. λ) recorded under 0 V vs. SCE bias.
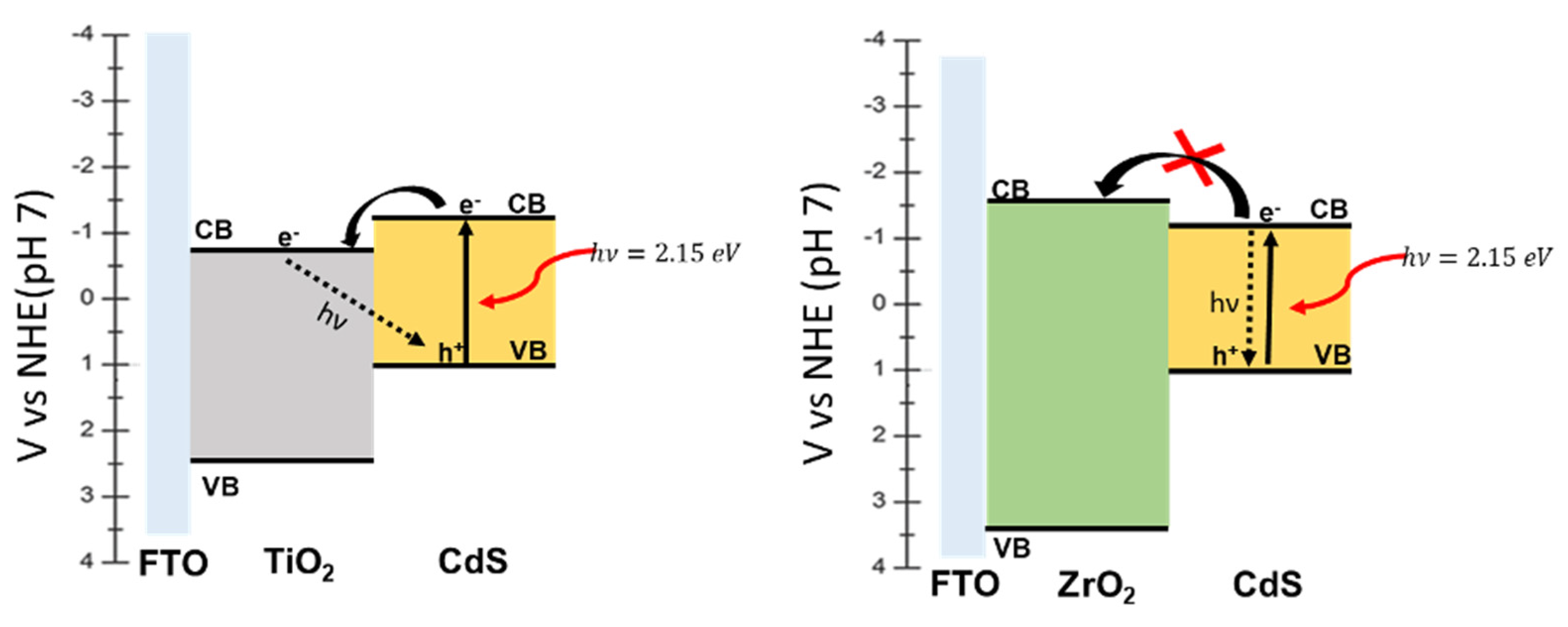
The J/V curves are consistent with a n-type junction where TiO
2 offers an unimpeded electron collection to the electrons generated upon white light illumination of CdS/TiO
2 junction. A photocurrent plateau, extending from +1 and −0.25 V vs. SCE is observed, with a photovoltage close to −1 V vs. SCE. A similar figure, both in terms of photocurrent and photovoltage, is observed for FTO/CdS. A similar photovoltage is observed in the case of FTO/ZrO
2/CdS, which, however, delivers a much lower photocurrent (ca. 0.1 mA/cm
2). This agrees with the prohibitively high conduction band edge of ZrO
2 which precludes electron injection from CdS. Here ZrO
2 behaves as an inert insulating substrate, which only offers support for nucleation of CdS during the CBD growth. Collection of charge is thus possible only through the random direct contact between CdS and FTO, hence the electron collection through the ZrO
2/CdS network is considerably suppressed. Consistent with this interpretation, we observe maximum photon to electron quantum yields (measured at the photocurrent plateau value at 0 V vs. SCE) of 50–55% for FTO/TiO
2/CdS > FTO/CdS (40–45%) >> FTO/ZrO
2/CdS (2–2.5%,
Figure 3c inset). The significant quantum yield detected with FTO/TiO
2/CdS also confirms that HCOO
− behaves as an efficient hole scavenger, leading to a >60% conversion of absorbed photons into electrons flowing through the electrochemical cell. Dark cyclic voltammetry (−1 V/1 V vs. SCE) of the FTO/TiO
2/CdS (
Figure S8) reveals two subsequent quasi-reversible waves, a first less intense process centered at approximately −0.6 V vs. SCE, assigned to filling of trap states of TiO
2, followed, at stronger negative polarization, by a second more intense process, assigned to reduction of a higher density of electron acceptor states, probably conduction band states. By contrast CVs of FTO/CdS and FTO/ZrO
2/CdS (
Figures S9 and S10 respectively) exhibit similar features assigned to the irreversible reduction of CdS giving rise to a continuous discharge with a shoulder around −0.7 V vs. SCE. This means that a driving force of at least ≈ 100 meV exists for the transfer of photoexcited electrons of CdS to TiO
2 and that trap states of TiO
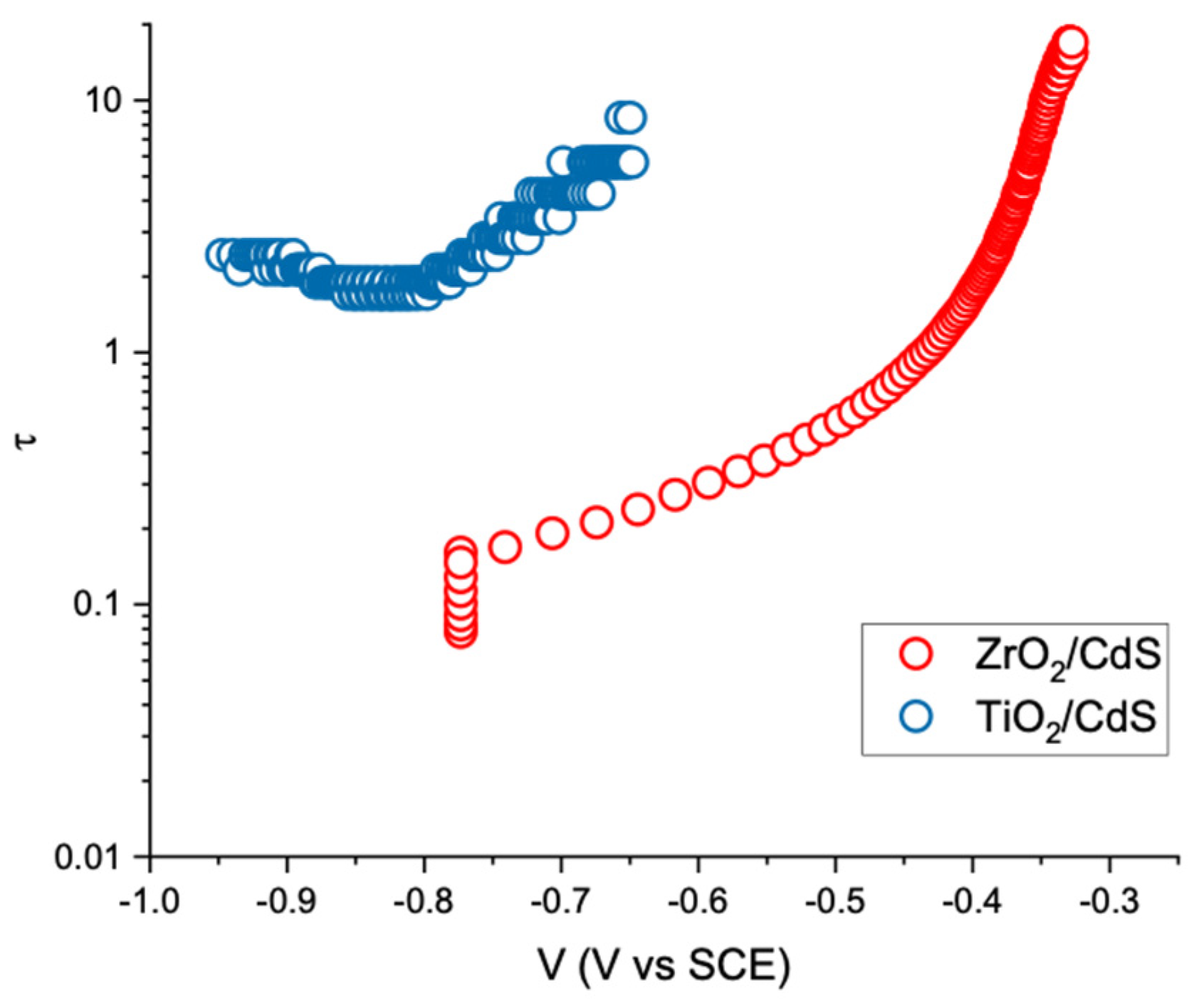
2 can act as a reservoir for long lived charge storage. Photovoltage decay experiments allow to observe the recombination dynamics of long lived charge carriers which are relevant to photocatalysis. First, we observe that under illumination in 1 M sodium formate, the photovoltage of FTO/TiO
2/CdS is ca. 100 mV more negative (−0.9 V vs. SCE) than that of FTO/ZrO
2/CdS (≈ −0.8 V vs. SCE) and that the photovoltage decay upon restoration of the dark conditions occurs with a slower kinetics in the former (
Figure S11). Electron lifetime can be obtained from the open circuit photovoltage decay (V = (E*
F − E
SCE)/(−e), where –e is the electronic charge, E*
F is the quasi Fermi energy of the photoanode and E
SCE is the reference potential) as a function of the voltage as [
25]:
The resulting plot is reported in
Figure 4, from which we can observe that the electron lifetime in FTO/TiO
2/CdS is almost two orders of magnitude longer than in FTO/ZrO
2/CdS within the potential range −0.9/−0.6 V vs. SCE. This is a further confirmation of transfer and storage of electrons from CdS to the TiO
2 phase. Based on this experimental piece of evidence we expect the FTO/TiO
2/CdS films to be able to outperform FTO/ZrO
2/CdS films in terms of long lived charge separation ability.
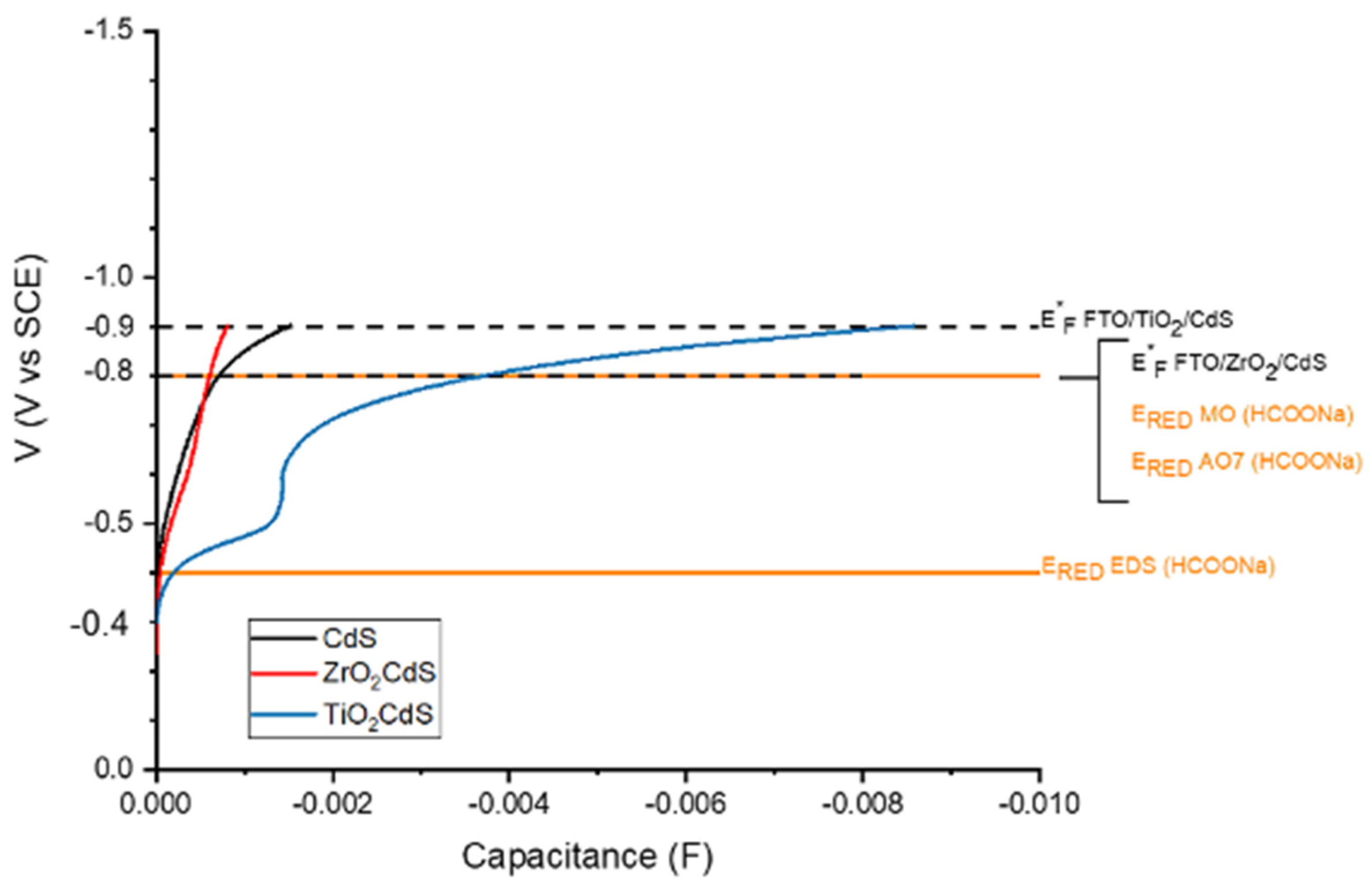
Reduction of two out of three of the azo-dyes (MO and AO7) under consideration occurs according to an irreversible process centered at ca. −0.8 V vs. SCE and is thus exoergonic by approximately 100 meV with respect to the quasi Fermi potential achieved by FTO/TiO
2/CdS, whereas it is almost isoergonic with that of FTO/ZrO
2/CdS. Reductive cleavage of EDS should be thermodynamically easier with both photocatalysts, according to its irreversible wave at −0.4 V vs. SCE (
Figure S12). The differential capacitance of the semiconductor films
C (
, where
Q is the charge and
V the applied voltage) is obtained from the voltammetric curves of the junctions in 1 M sodium formate according to
C = i/v, where
i is the current and
v is the scan rate, which is reported in
Figure 5, vs. the applied potential (−E
F/e), together with the relevant redox levels for the reductive chemistry under consideration.
C is proportional to the electronic density of states
DOS (variation of the number of electronic states
N as a function of the Fermi energy) according to:
By comparing CCdS with CTiO2/CdS, we see that electron transfer from CdS to TiO2 will be efficient, in agreement with the photoelectrochemical evidence discussed before. This is due to the fact that electronic states of CdS and TiO2 have a large energy overlap. We also observe that the deposition of CdS on ZrO2 has a quite minor impact on the energy distribution of CdS states: CCdS/ZrO2 only tends to be slightly larger than that of pure CdS at lower potential (<−0.6 V vs. SCE). We note that we limited the potential scan to −1 V vs. SCE, due to the interception of electrolyte reduction occurring beyond that value. Nevertheless, such potential range embraces the maximum open circuit value recorded under our conditions. Electronic transfer from CdS to TiO2, resulting in the confinement of electrons and holes on different phases, retards recombination, as evidenced from photovoltage decay analysis and improves the electronic build up in the n–n junction. As a result, the quasi-Fermi potential of FTO/TiO2/CdS under illumination increases to ca. −0.9 V vs. SCE. At such equilibrium potential, the overlap between the TiO2 and CdS states is very large, since the TiO2 capacitance increases very rapidly above −0.6 V vs. SCE, and we expect that electrons will be able to cross back (from CdS to TiO2) and forth (from TiO2 to CdS) the boundaries between CdS and TiO2 with a negligible activation energy. This will result in the population of both CdS and TiO2 states under dynamic equilibrium and in the possibility to drive simultaneously the azo-dye reduction both at the surface of CdS and of exposed TiO2 particles, making irrelevant the fact that in the FTO/TiO2/CdS architecture TiO2 is substantially covered by CdS, a fact that, at first glance, may preclude an efficient contact between the photocatalyst and the molecular electron acceptors dissolved in the electrolyte.
3.3. Open Circuit Photocatalysis
An FTO/TiO
2/CdS slide, having a CdS coated area of 1 cm
2, immersed in a deaerated aqueous solution containing the dye of interest (MO, AO7 or EDS, C
0 = 10 ppm) and HCOONa (1 M), was irradiated from the CdS side (λ
> 420 nm). This illumination geometry is referred to as front illumination mode. FTO/TiO
2/CdS slide displays a nearly quantitative light absorption and from AFM measurements (see
Section 3.1) we estimated that its surface area is much larger (by a factor of 10
4) than that required to saturate the surface with dye molecule present in our photocatalytic cell.
During irradiation, a loss of the solution color that corresponds to a decrease of the absorption band of the azo-dye is observed.
Figure 6 reports the C/C
0 ratio of MO, AO7, or EDS as a function of irradiation time.
It is observed that after 180 min irradiation about 90% of the starting MO and AO7 has disappeared from the solution (full circles and triangles), while the decrease of C/C
0 relative to EDS is slower and is around 70% despite the larger driving force that we foresee for such process. Control experiments (in
Figure 6 are reported only those relative to MO) emphasize that this result is exclusively ascribable to the photocatalytic activity of CdS in the FTO/TiO
2/CdS system. In fact, visible irradiation of the dye solution in the absence of any semiconductor (empty triangle in
Figure 6) did not result in a dye concentration decrease. In addition, no decrease was observed when FTO/TiO
2/CdS is placed in contact with the dye solution and kept in the dark (empty rhombus in
Figure 6), also indicating that adsorption of MO on FTO/TiO
2/CdS was negligible. Finally, irradiation (λ > 420 nm) of FTO/TiO
2 system immersed in the MO solution did not cause any photosensitization effect on the dye, since TiO
2 is not photochemically active above 420 nm (data not shown but superimposed with empty rhombus).
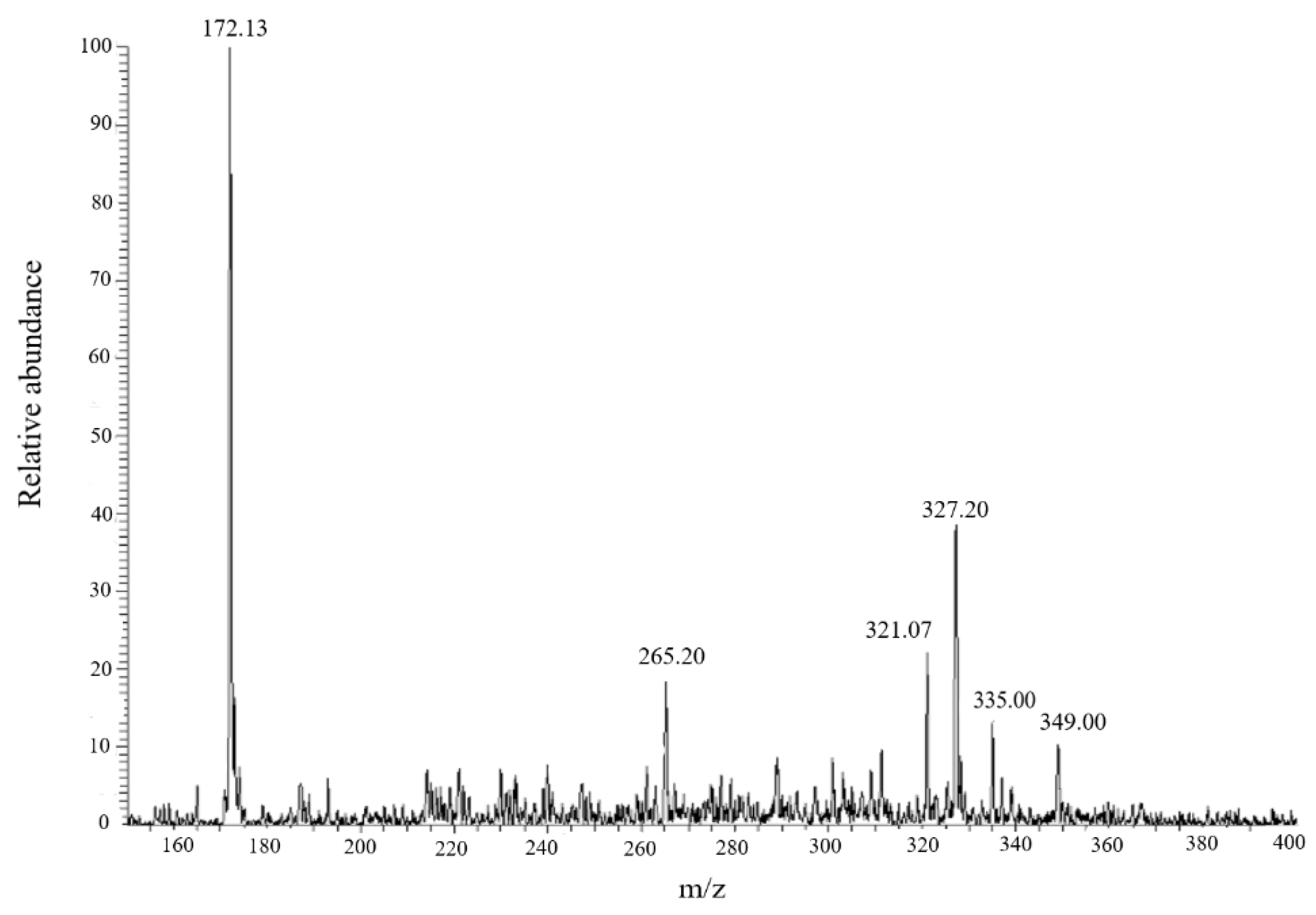
Considering that fading of the dye solution is only an indication of the interruption of conjugation and that monitoring the disappearance rate of the target dye is not the most appropriate way to establish the nature of the photocatalytic reaction, we recorded the negative ion mode ESI-MS spectrum of the irradiated solutions.
Figure 7 reports the spectrum obtained in the case of AO7, while the analogous spectra for MO and EDS are reported in the
supplementary material (Figure S13).
Moreover, the
m/z peak at 327 related to the residual AO7, the dominant base peak was at
m/z 172. The same result was obtained also with the other two investigated dyes (
Figure S13). In agreement with our previous work [
16],
m/z 172 corresponds to the anionic form of sulfanilic acid, which is the common part in the structures of the studied dyes (
Scheme 1).
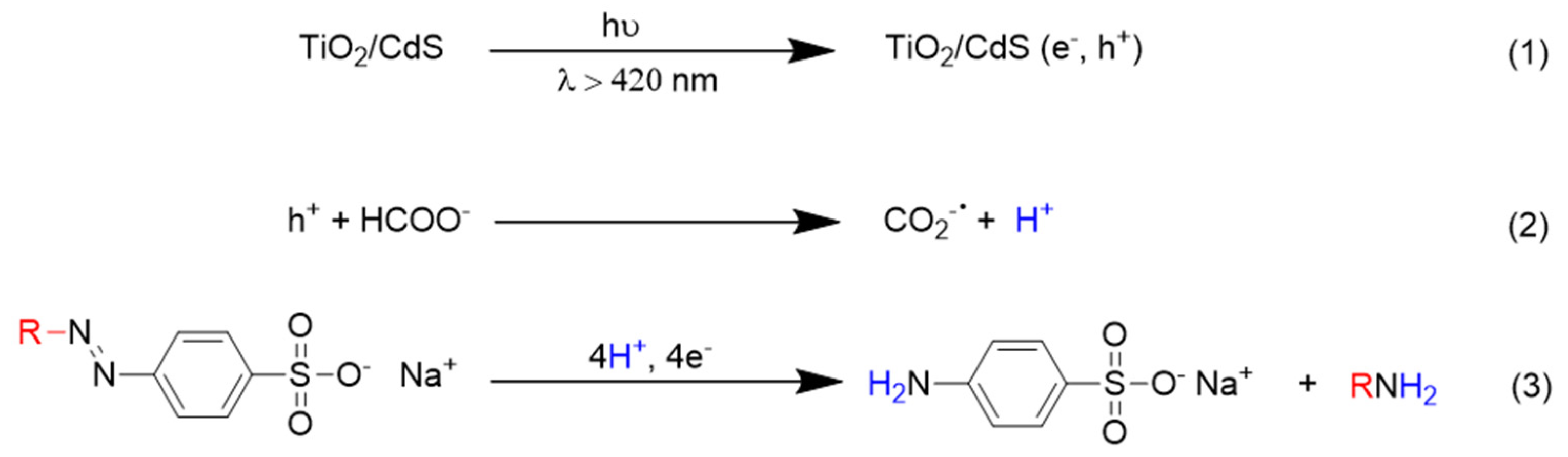
These results give evidence that the N=N bond undergoes a reductive cleavage (via protons and electrons addition). In fact, electron-hole pairs are generated on the surface of the composite material under visible light irradiation (
Scheme 3, Reaction (1)). Photogenerated holes effectively react with formate, which acts as a hole scavenger and as the proton source (
Scheme 3, Reaction (2)). The formation of CO
2−• radical has been recently evidenced by some of us [
16]. At the same time, electrons promoted in the conduction band of CdS or those subsequently injected in that of TiO
2 can be efficiently captured by dyes since we are operating in the absence of di-oxygen (
Scheme 3, Reaction (3)).
Compared to our previous work in which FTO/TiO
2 plates were used [
16], this result is a step forward in overall sustainability because we use visible light. We emphasize that the use of thin films on glass substrates circumvents all the difficulties related to the use of photocatalytic powder slurries; the films are easy to handle and to re-cover and no separation of the powder from the solution should be carried out at the end of irradiation. Usually, thin films also show excellent stability allowing very good recyclability [
16].
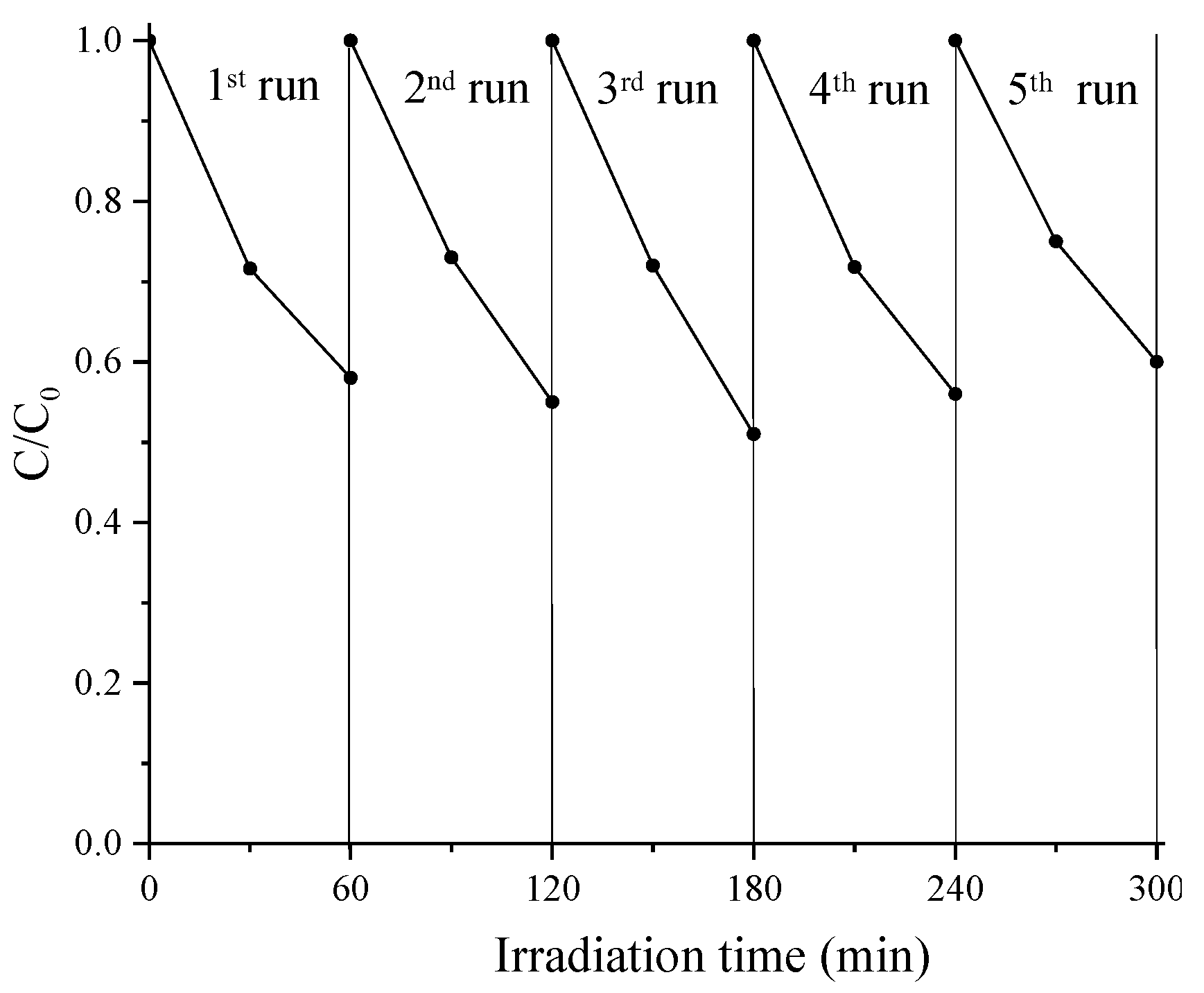
Regarding the FTO/TiO
2/CdS system studied here, we evaluated the photocatalytic performance in MO transformation in repeated cycles using the same plate.
Figure 8 shows that there is no loss of photocatalytic activity after five repeated cycles, indicating a very good stability of the composite system.
Since it is known that the photostability of CdS is low, thus limiting its use as photo-catalyst, the result obtained with FTO/TiO
2/CdS film is noteworthy and in line with the literature that reports that the photo-corrosion of CdS can be decreased when coupling with another semiconductor is carried out [
26,
27,
28]. Atomic absorption spectroscopy measurements of irradiated samples report a release of 220 μg/L of Cd
2+ ions into the solution after each cycle. In any case, we emphasize that this loss does not adversely affect the photocatalytic activity as shown by the results in
Figure 8.
In addition,
Figure 8 highlights that the presence of the underlying TiO
2 layer in the FTO/TiO
2/CdS films also improves the adhesion of CdS to FTO. In fact, we could not evaluate either the photocatalytic performance or the recyclability in the case of FTO/CdS films due to the very poor adhesion of CdS on FTO.
It has been highlighted in
Section 3.1 and
Section 3.2 that TiO
2 plays an important role in the composite system by increasing the lifetime of the charge separated state and improving electron build up in the junction. From the photocatalytic point of view, this could result in high photocatalytic efficiency. This effect was confirmed by the comparative analysis of the photocatalytic performance of FTO/ZrO
2/CdS films where zirconia is used as an electrochemically inert (with respect to electron transfer from photoexcited CdS) nanostructured support for CdS. Irradiation (λ
> 420 nm) of a FTO/ZrO
2/CdS slide, with a CdS coated area of 1 cm
2, immersed in a deaerated aqueous solution containing the dye of interest (MO, AO7 or EDS, C
0 = 10 ppm) and HCOONa (1 M), led qualitatively to the same process discussed above for FTO/TiO
2/CdS. A decrease of C/C
0 ratio of the dye as a function of irradiation time is observed (
Figure S14a), and ESI-MS spectra showed the same peak at
m/z 172 related to sulfanilic acid formation (
Figure S14b).
Figure 9 reports comparative azo-dye decay kinetics observed with the two FTO/MO
2/CdS systems. It is observed that after 180 min of irradiation, C/C
0 values are about 8–10% higher than those observed for FTO/TiO
2/CdS. In addition, kinetic constants reported in
Table S1 relative to the dye disappearance are always lower with FTO/ZrO
2/CdS than for FTO/TiO
2/CdS. These results are consistent with the fact that FTO/TiO
2/CdS achieves, under illumination a ca. 100 meV larger driving force for azo-dye reduction with respect to FTO/ZrO
2/CdS and that the partial coverage of TiO
2 by CdS does not preclude the photocatalytic performance of the junction, once the stationary state under constant illumination is achieved.
Similarly to the FTO/TiO2/CdS system, the presence of ZrO2 underlayer has a positive effect on adhesion of CdS. This result affects the possibility to recycle the same plate several times: 60 min irradiation (λ > 420 nm) of the same FTO/ZrO2/CdS system immersed in an aqueous solution containing the dye (10 ppm) during five repeated experiments showed no decrease of the efficiency (data not shown). If consideration is made for the thicker titania film compared to ZrO2, the overall Cd2+ release appears similar to that observed with TiO2 (around 200 μg/L), confirming that the release is sufficiently low not to adversely affect the photocatalytic activity.