Curing Kinetics Modeling of Epoxy Modified by Fully Vulcanized Elastomer Nanoparticles Using Rheometry Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
2.3. Instrumental Analysis
2.4. Cure Kinetics Analysis
3. Result and Discussion
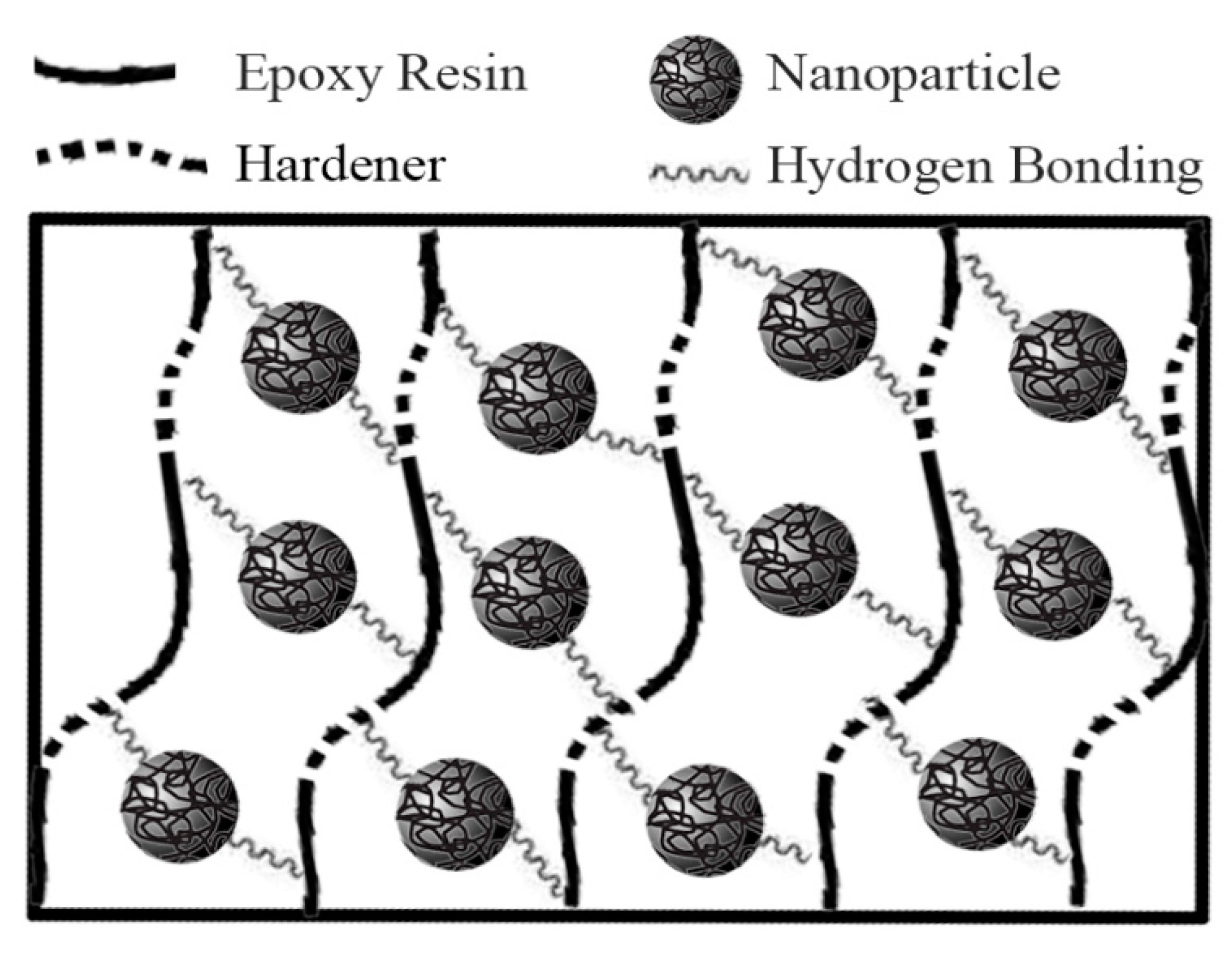
3.1. Nanocomposites FTIR Study
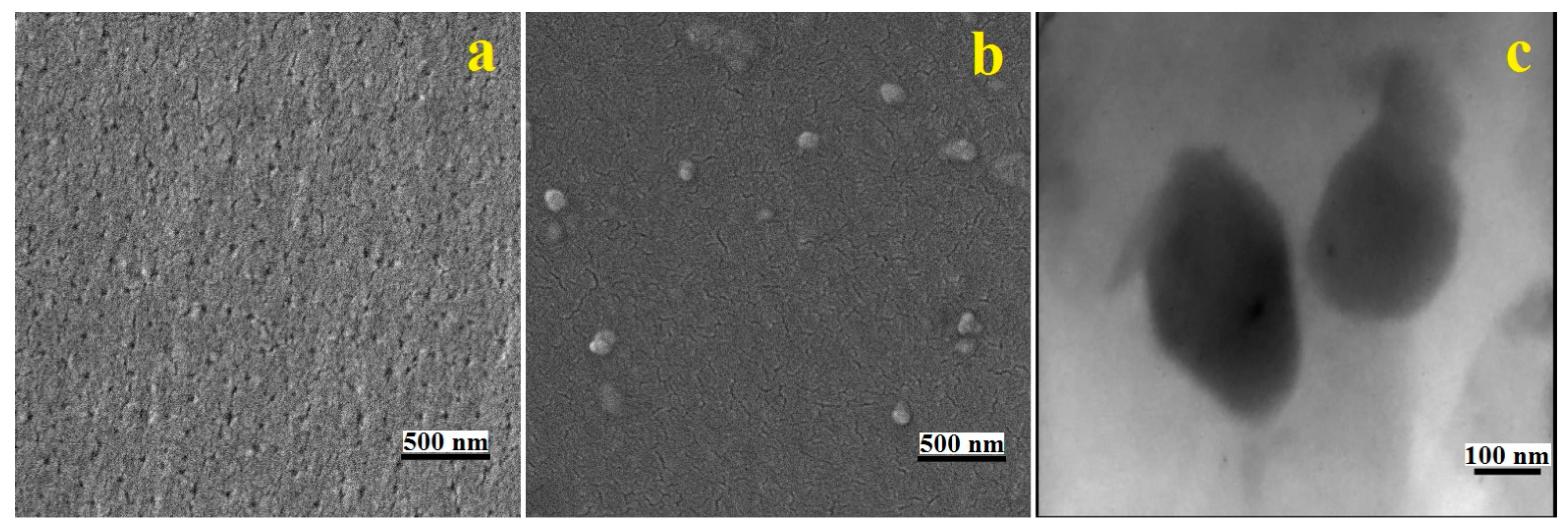
3.2. Morphology of Nanocomposites
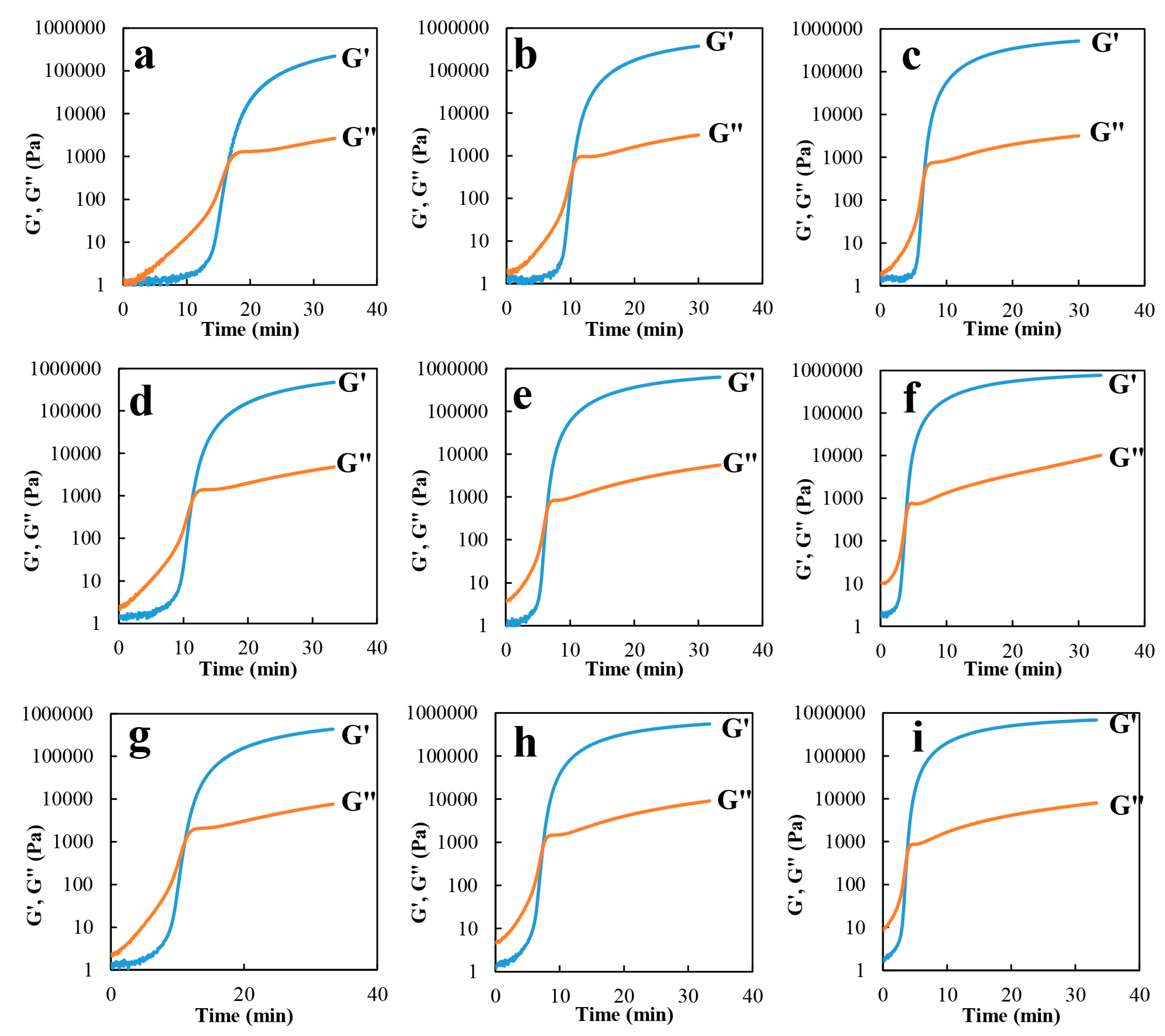
3.3. Reaction Kinetics by Rheometry (Chemorheology Analysis)
3.4. Effect of Elastomeric Nanoparticles on the Complex Viscosity at Different Temperatures
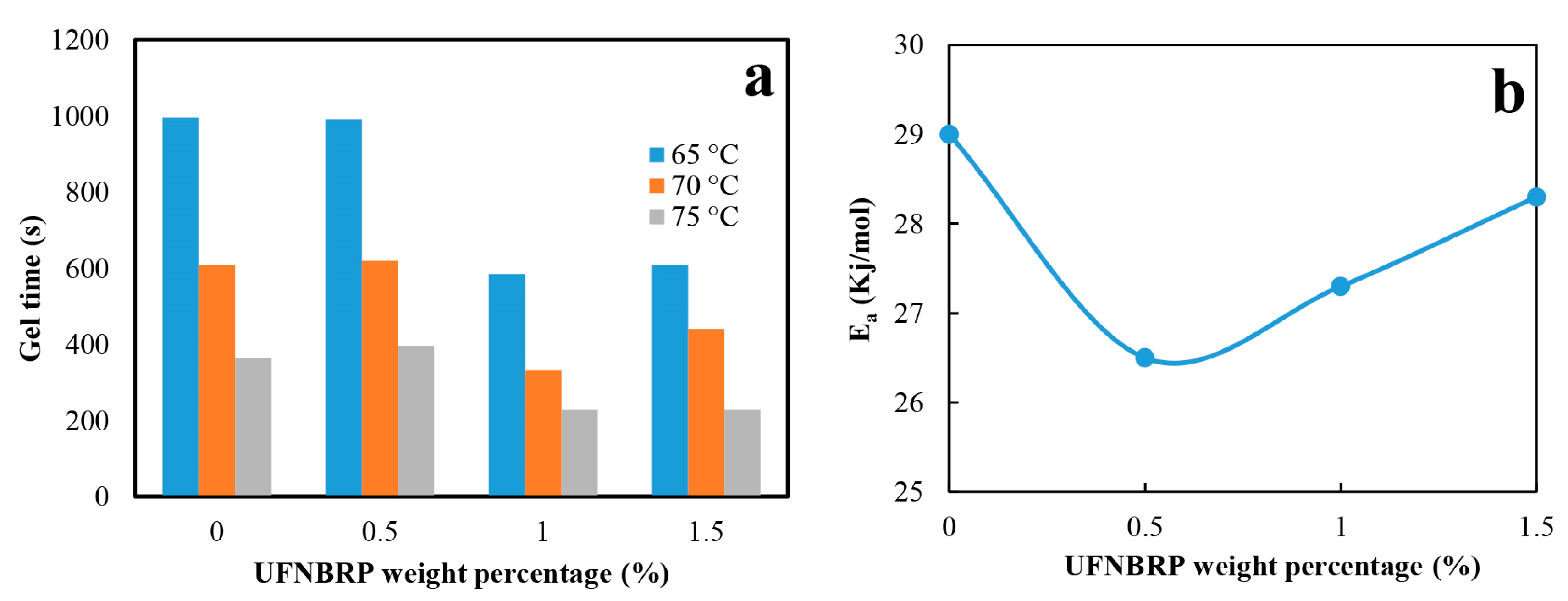
3.5. Evaluation of Gel Point and Activation Energy (Ea) Calculation by Rheometry
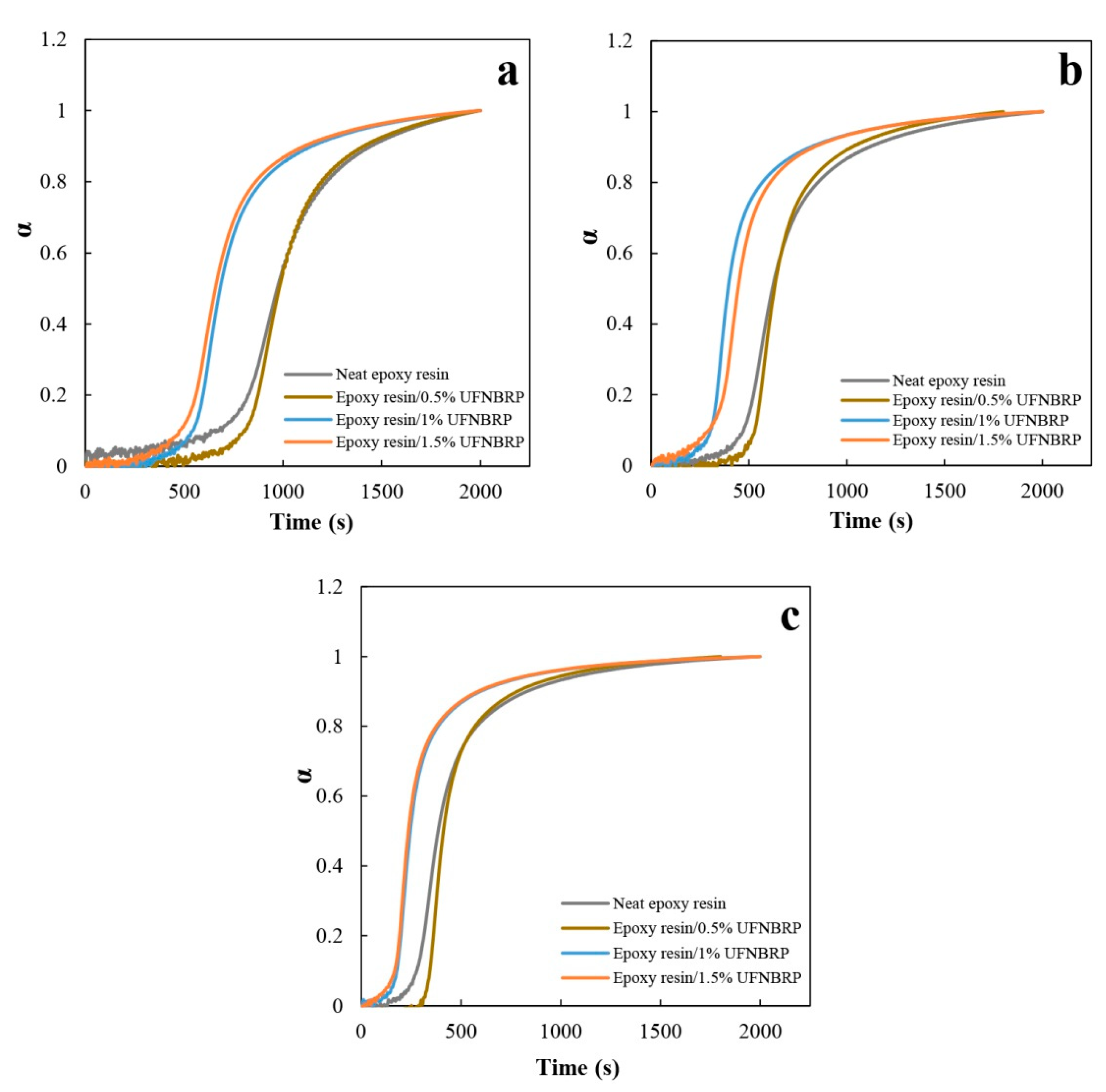
3.6. Effect of Elastomeric Nanoparticles on Degree of Curing at Constant Temperatures
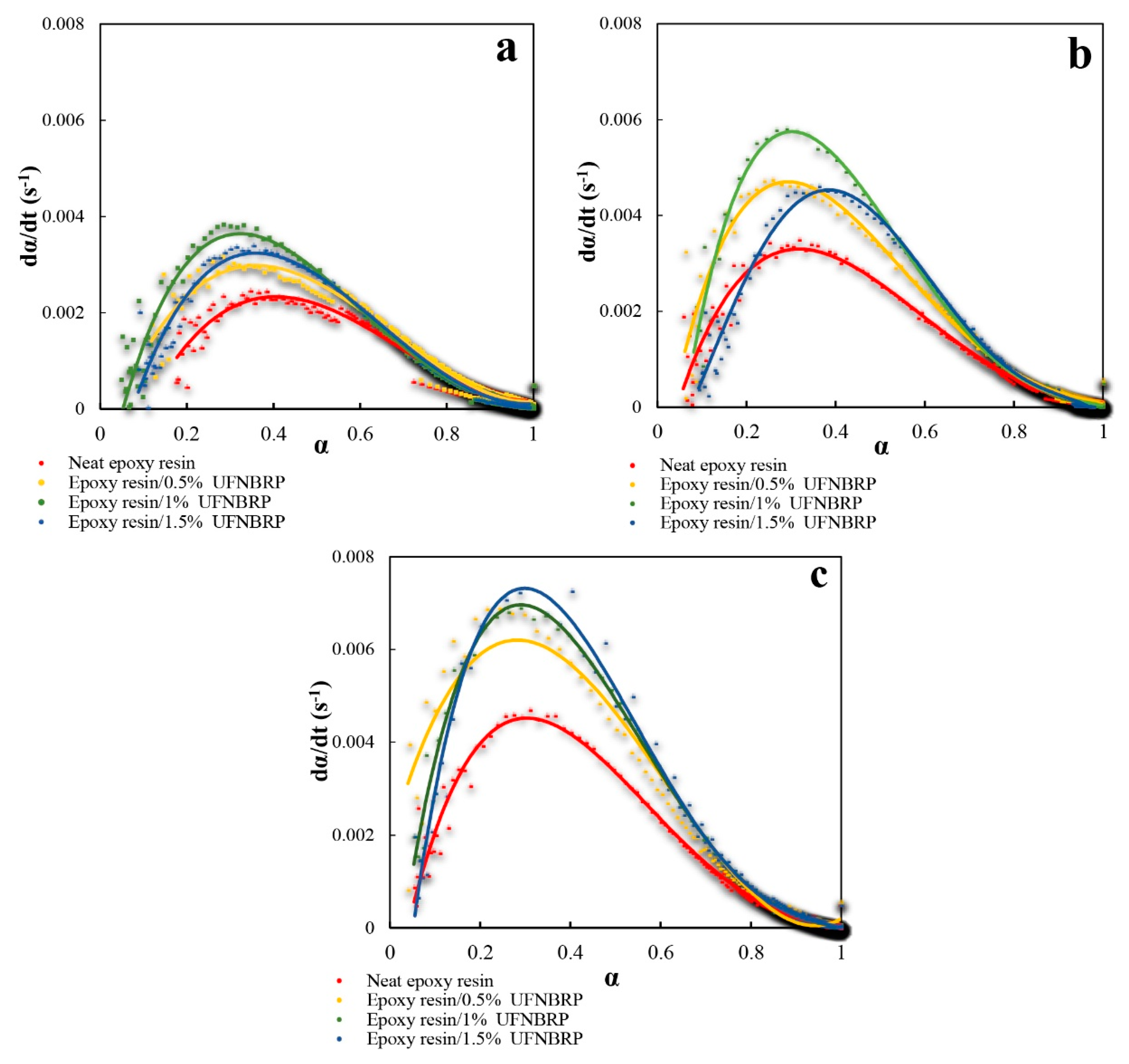
3.7. Kinetics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Compton, B.G.; Hmeidat, N.S.; Pack, R.C.; Heres, M.F.; Sangoro, J.R. Electrical and mechanical properties of 3D-printed graphene-reinforced epoxy. JOM 2018, 70, 292–297. [Google Scholar] [CrossRef]
- Karami, M.H.; Kalaee, M. Study of the effect of graphene oxide nanoplatelets on the thermal degradation kinetics of epoxy nanocomposites. Nano World 2022, 17, 57–66. [Google Scholar]
- Akatsuka, M.; Takezawa, Y.; Amagi, S. Influences of inorganic fillers on curing reactions of epoxy resins initiated with a boron trifluoride amine complex. Polymer 2001, 42, 3003–3007. [Google Scholar] [CrossRef]
- Saad, G.R.; Abd Elhamid, E.E.; Elmenyawy, S.A. Dynamic cure kinetics and thermal degradation of brominated epoxy resin–organoclay based nanocomposites. Thermochim. Acta 2011, 524, 186–193. [Google Scholar] [CrossRef]
- Wetzel, B.; Haupert, F.; Zhang, M.Q. Epoxy nanocomposites with high mechanical and tribological performance. Compos. Sci. Technol. 2003, 63, 2055–2067. [Google Scholar] [CrossRef]
- Thomas, R.; Sinturel, C.; Pionteck, J.R.; Puliyalil, H.; Thomas, S. In-situ cure and cure kinetic analysis of a liquid rubber modified epoxy resin. Ind. Eng. Chem. Res. 2012, 51, 12178–12191. [Google Scholar] [CrossRef]
- Shakiba, M.; Kakoei, A.; Jafari, I.; Rezvani Ghomi, E.; Kalaee, M.; Zarei, D.; Abdouss, M.; Shafiei-Navid, S.; Khosravi, F.; Ramakrishna, S. Kinetic modeling and degradation study of liquid polysulfide resin-clay nanocomposite. Molecules 2021, 26, 635. [Google Scholar] [CrossRef]
- Jafari, I.; Shakiba, M.; Khosravi, F.; Ramakrishna, S.; Abasi, E.; Teo, Y.S.; Kalaee, M.; Abdouss, M.; Moradi, O.; Rezvani Ghomi, E. Thermal degradation kinetics and modeling study of ultra high molecular weight polyethylene (UHMWP)/graphene nanocomposite. Molecules 2021, 26, 1597. [Google Scholar] [CrossRef]
- Shamsi, M.; Nabavi, S.R.; Shakiba, M. Fabrication and characterization of polyamide 6@polyaniline core shell nanofibrous composite reinforced via reduced graphene oxide. Polym. Bull. 2021, 1–18. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, X.; Li, B.; Song, Z.; Qiao, J. The study of rubber-modified plastics with higher heat resistance and higher toughness and its application. Polym. Chem. 2011, 2, 1271–1274. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Y.; Zhang, X.; Gao, J.; Song, Z.; Tang, B.; Wei, G.; Qiao, J. Interface and properties of epoxy resin modified by elastomeric nano-particles. Sci. China Ser. B Chem. 2005, 48, 148–155. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, G.; Wang, X.; Li, B.; Song, Z.; Ru, Y.; Zhang, X.; Qiao, J. Novel conductive core–shell particles of elastomeric nanoparticles coated with polypyrrole. RSC Adv. 2015, 5, 98904–98909. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Shafiei-Navid, S.; Shabanian, M.; Vahabi, H. Novel poly (amide-azomethine) nanocomposites reinforced with polyacrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid modified LDH: Synthesis and properties. Appl. Clay Sci. 2018, 157, 165–176. [Google Scholar] [CrossRef]
- Momeni, S.; Rezvani Ghomi, E.; Shakiba, M.; Shafiei-Navid, S.; Abdouss, M.; Bigham, A.; Khosravi, F.; Ahmadi, Z.; Faraji, M.; Abdouss, H. The Effect of Poly (Ethylene glycol) Emulation on the Degradation of PLA/Starch Composites. Polymers 2021, 13, 1019. [Google Scholar] [CrossRef] [PubMed]
- Saeb, M.R.; Rastin, H.; Nonahal, M.; Paran, S.M.R.; Khonakdar, H.A.; Puglia, D. Cure kinetics of epoxy/chicken eggshell biowaste composites: Isothermal calorimetric and chemorheological analyses. Prog. Org. Coat. 2018, 114, 208–215. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Y.; Yang, B.; Ning, N.; Fu, Q. Highly efficient toughening effect of ultrafine full-vulcanized powdered rubber on poly (lactic acid)(PLA). Polym. Test. 2013, 32, 299–305. [Google Scholar] [CrossRef]
- Wu, F.; Xie, T.; Yang, G. Properties of toughened poly (butylene terephthalate) by blending with reactive ultra-fine full-vulcanized acrylonitrile butadiene rubber particles (UFNBRP). Polym. Bull. 2010, 65, 731–742. [Google Scholar] [CrossRef]
- Kalaee, M.; Karami, M.H.; Mazinani, S. Chemorheology of epoxy nanocomposites in the presence of elastomeric nanoparticles. In Proceeding of the National Conference on Advanced Technologies in Energy, Water and Environment; Sharif Energy Research Institute: Tehran, Iran, 2021. [Google Scholar]
- Aghajani, A.; Kalaee, M.; Mazinani, S. Physical, Mechanical and Thermal Properties of Epoxy Coatings Modified with Nitrile-Butadiene Nano-rubber. Adv. Mater. New Coat. 2019, 7, 2036–2047. [Google Scholar]
- Karami, M.H.; Kalaee, M.; Khajavi, R.; Moradi, O.; Zaarei, D. Thermal degradation kinetics of epoxy resin modified with elastomeric nanoparticles. Adv. Compos. Hybrid Mater. 2022, 5, 390–401. [Google Scholar] [CrossRef]
- Karami, Z.; Paran, S.M.R.; Vijayan, P.P.; Ganjali, M.R.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; Stadler, J.F.; Saeb, M.R. A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites. J. Compos. Sci. 2020, 4, 111. [Google Scholar] [CrossRef]
- Kalaee, M.; Karami, M.H. Study of thermal degradation kinetics of epoxy composite/carbon nanotubes (In Persian). Polymerization 2021, 11, 65–76. [Google Scholar]
- Kalaee, M.; Karami, M. Degradation Kinetics of Epoxy Nanocomposites in the Presence of Clay Nanoparticles: A Review. Polymerization 2022, 11, 65–76. (In Persian) [Google Scholar]
- Kalaee, M.; Karami, M. Review of Curing Kinetics of Epoxy Nanocomposites in the Presence of Iron Oxide Nanoparticles. Polymerization 2021, 11, 34–43. (In Persian) [Google Scholar]
- Wang, X.; Jin, J.; Song, M. Cyanate ester resin/graphene nanocomposite: Curing dynamics and network formation. Eur. Polym. J. 2012, 48, 1034–1041. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Kalaee, M.; Jowdar, E.; Nouri, A.; Mazinani, S.; Afshari, M.; Famili, M.N.; Amini, N.; Behrouz, T. Simultaneous study of cure kinetics and rheology of montmorillonite/vinyl ester resin nanocomposites. Polym. Adv. Technol. 2012, 23, 534–544. [Google Scholar] [CrossRef]
- Kalaee, M.; Akhlaghi, S.; Nouri, A.; Mazinani, S.; Mortezaei, M.; Afshari, M.; Mostafanezhad, D.; Allahbakhsh, A.; Dehaghi, H.A.; Amirsadri, A. Effect of nano-sized calcium carbonate on cure kinetics and properties of polyester/epoxy blend powder coatings. Prog. Org. Coat. 2011, 71, 173–180. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Qi, G.; Wang, X.; Zhang, J.; Han, P.; Ru, Y.; Qiao, J. A rubber-modified epoxy composite with very high toughness and heat resistance. Polym. Polym. Compos. 2019, 27, 582–586. [Google Scholar] [CrossRef]
- Ghaffari, M.; Ehsani, M.; Khonakdar, H.A.; Van Assche, G.; Terryn, H. The kinetic analysis of isothermal curing reaction of an epoxy resin-glassflake nanocomposite. Thermochim. Acta 2012, 549, 81–86. [Google Scholar] [CrossRef]
- Kalaee, M.; Famili, M.; Mahdavi, H. Cure kinetic of poly (alkyltetrasulfide) using a rheological method. Polym.-Plast. Technol. Eng. 2009, 48, 627–632. [Google Scholar] [CrossRef]
- Ferdosian, F.; Ebrahimi, M.; Jannesari, A. Curing kinetics of solid epoxy/DDM/nanoclay: Isoconversional models versus fitting model. Thermochim. Acta 2013, 568, 67–73. [Google Scholar] [CrossRef]
- Koosha, M.; Ebrahimi, N.; Jahani, Y.; Sajjadi, S.A.S. Degradation kinetics of electron beam irradiated poly(propylene-co-ethylene) heterophasic copolymer. Radiat. Phys. Chem. 2011, 80, 810–816. [Google Scholar] [CrossRef]
- Ke, Q.; Wu, C.; Chen, X. Model-free cure kinetics of additional liquid silicone rubber. Thermochim. Acta 2020, 688, 178584. [Google Scholar] [CrossRef]
- Málek, J. The kinetic analysis of non-isothermal data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Montserrat, S.; Málek, J. A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 1993, 228, 47–60. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.; Criado, J.; La, P.-M.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Granado, L.; Kempa, S.; Gregoriades, L.J.; Brüning, F.; Genix, A.-C.; Fréty, N.; Anglaret, E. Kinetic regimes in the curing process of epoxy-phenol composites. Thermochim. Acta 2018, 667, 185–192. [Google Scholar] [CrossRef]
- Lu, L.; Xia, L.; Zengheng, H.; Xingyue, S.; Yi, Z.; Pan, L. Investigation on cure kinetics of epoxy resin containing carbon nanotubes modified with hyper-branched polyester. RSC Adv. 2018, 8, 29830–29839. [Google Scholar] [CrossRef] [Green Version]
- Allahbakhsh, A.; Mazinani, S.; Kalaee, M.R.; Sharif, F. Cure kinetics and chemorheology of EPDM/graphene oxide nanocomposites. Thermochim. Acta 2013, 563, 22–32. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jubsilp, C.; Kunopast, P.; Bangsen, W. Chemorheology of benzoxazine-based resins. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 143–155. [Google Scholar]
- Hwang, J.G.; Row, C.G.; Hwang, I.; Lee, S.J. A chemorheological study on the curing of thermosetting resins. Ind. Eng. Chem. Res. 1994, 33, 2377–2383. [Google Scholar] [CrossRef]
- Chow, W.; Grishchuk, S.; Burkhart, T.; Karger-Kocsis, J. Gelling and curing behaviors of benzoxazine/epoxy formulations containing 4, 4′-thiodiphenol accelerator. Thermochim. Acta 2012, 543, 172–177. [Google Scholar] [CrossRef]
- Abliz, D.; Artys, T.; Ziegmann, G. Influence of model parameter estimation methods and regression algorithms on curing kinetics and rheological modelling. J. Appl. Polym. Sci. 2017, 134, 45137. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, R.; Farhan, S.; Wang, M.; Zheng, S. Optimization and preparation of an allyl phenoxy-modified bismaleimide resin. High Perform. Polym. 2016, 28, 669–681. [Google Scholar] [CrossRef]
- Mijović, J.; Kenny, J.; Nicolais, L. Comparison of kinetic and rheological evaluation of gel time for an amine-epoxy system. Polymer 1993, 34, 207–209. [Google Scholar] [CrossRef]
- Domínguez, J.; Alonso, M.; Oliet, M.; Rojo, E.; Rodríguez, F. Kinetic study of a phenolic-novolac resin curing process by rheological and DSC analysis. Thermochim. Acta 2010, 498, 39–44. [Google Scholar] [CrossRef]
- García-Martínez, V.; Gude, M.; Calvo, S.; Martínez-Miranda, M.; Ureña, A. Influence of graphene nanoplatelets on curing kinetics and rheological properties of a benzoxazine resin. Mater. Today Commun. 2020, 24, 100990. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Mathew, L.; Hassan, P.; Mozetic, M.; Thomas, S. Rheological behaviour of nanocellulose reinforced unsaturated polyester nanocomposites. Int. J. Biol. Macromol. 2014, 69, 274–281. [Google Scholar] [CrossRef]
- Kim, J.-T.; Martin, D.; Halley, P.; Kim, D.S. Chemorheological studies on a thermoset PU/clay nanocomposite system. Compos. Interfaces 2007, 14, 449–465. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Ivankovic, M.; Incarnato, L.; Kenny, J.M.; Nicolais, L. Curing kinetics and chemorheology of epoxy/anhydride system. J. Appl. Polym. Sci. 2003, 90, 3012–3019. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Bolgov, S.; Begishev, V.; Mansurov, V. Evolution of viscoelastic properties of polyurethane in the course of curing. Rheol. Acta 1992, 31, 345–350. [Google Scholar] [CrossRef]
- García-Martínez, V.; Gude, M.; Ureña, A. Understanding the curing kinetics and rheological behaviour of a new benzoxazine resin for carbon fibre composites. React. Funct. Polym. 2018, 129, 103–110. [Google Scholar] [CrossRef]
- Ivankovic, M.; Brnardic, I.; Ivankovic, H.; Mencer, H.J. DSC study of the cure kinetics during nanocomposite formation: Epoxy/poly (oxypropylene) diamine/organically modified montmorillonite system. J. Appl. Polym. Sci. 2006, 99, 550–557. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Zeng, K.; Li, G.; Zhao, X.; Zhao, C. Curing kinetics and thermal stability of novel siloxane-containing benzoxazines. Thermochim. Acta 2019, 671, 119–126. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, X. Autocatalytic curing kinetics of thermosetting polymers: A new model based on temperature dependent reaction orders. Polymer 2010, 51, 3814–3820. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.; Shim, M.; Kim, S. Kinetic studies of an epoxy cure reaction by isothermal DSC analysis. Thermochim. Acta 2000, 343, 111–117. [Google Scholar] [CrossRef]
- Ryu, S.H.; Sin, J.; Shanmugharaj, A. Study on the effect of hexamethylene diamine functionalized graphene oxide on the curing kinetics of epoxy nanocomposites. Eur. Polym. J. 2014, 52, 88–97. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 5, 90437–90450. [Google Scholar] [CrossRef]
- Fu, Y.; Zhong, W.-H. Cure kinetics behavior of a functionalized graphitic nanofiber modified epoxy resin. Thermochim. Acta 2011, 516, 58–63. [Google Scholar] [CrossRef]
- Schawe, J. A description of chemical and diffusion control in isothermal kinetics of cure kinetics. Thermochim. Acta 2002, 388, 299–312. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, X.; Lu, C.; Zhang, X.; Ji, Y.; Bai, C.; Chen, Y.; Qiao, Y. Studies on curing kinetics and tensile properties of silica-filled phenolic amine/epoxy resin nanocomposite. Polymers 2019, 11, 680. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Panda, B.P.; Mohanty, S.; Nayak, S.K.; Gupta, M.K. Thermokinetics behavior of epoxy adhesive reinforced with low viscous aliphatic reactive diluent and nano-fillers. Korean J. Chem. Eng. 2017, 34, 3028–3040. [Google Scholar] [CrossRef]


| Model | Kinetic Model (or theory) | R2 |
|---|---|---|
| nth-order | Not fitted | |
| Sěsták-Berggren | 0.95–0.97 | |
| Horie | , m = 1 | 0.91–0.95 |
| Kamal | Not fitted |
| Sample | Curing Temperature (°C) | Onset of Cure (s) | |
|---|---|---|---|
| Epoxy resin | 65 | 76,800 | 1050 |
| Epoxy resin | 70 | 182,000 | 656 |
| Epoxy resin | 75 | 223,000 | 370 |
| Epoxy resin/0.5%UFNBRP | 65 | 221,000 | 1060 |
| Epoxy resin/0.5%UFNBRP | 70 | 375,000 | 692 |
| Epoxy resin/0.5%UFNBRP | 75 | 520,000 | 472 |
| Epoxy resin/1%UFNBRP | 65 | 471,000 | 728 |
| Epoxy resin/1%UFNBRP | 70 | 629,000 | 460 |
| Epoxy resin/1%UFNBRP | 75 | 768,000 | 300 |
| Epoxy resin/1.5%UFNBRP | 65 | 434,000 | 728 |
| Epoxy resin/1.5%UFNBRP | 70 | 553,000 | 524 |
| Epoxy resin/1.5%UFNBRP | 75 | 687,000 | 280 |
| Sample | T (°C) | m | n | R2 | ||
|---|---|---|---|---|---|---|
| Epoxy resin | 65 | 1.103 | 1.642 | 0.01252 | 0.90 | 139.80 |
| Epoxy resin | 70 | 1.258 | 2.373 | 0.03344 | 0.94 | |
| Epoxy resin | 75 | 1.282 | 2.607 | 0.0521 | 0.97 | |
| Epoxy resin/0.5%UFNBRP | 65 | 0.9885 | 1.784 | 0.01718 | 0.93 | 81.23 |
| Epoxy resin/0.5%UFNBRP | 70 | 1.045 | 2.297 | 0.03559 | 0.96 | |
| Epoxy resin/0.5%UFNBRP | 75 | 0.7977 | 2.348 | 0.02928 | 0.97 | |
| Epoxy resin/1%UFNBRP | 65 | 1.513 | 2.676 | 0.05607 | 0.92 | 22.72 |
| Epoxy resin/1%UFNBRP | 70 | 1.399 | 2.875 | 0.08207 | 0.97 | |
| Epoxy resin/1%UFNBRP | 75 | 1.167 | 2.624 | 0.07049 | 0.99 | |
| Epoxy resin/1.5%UFNBRP | 65 | 1.575 | 2.451 | 0.04728 | 0.96 | 114.67 |
| Epoxy resin/1.5%UFNBRP | 70 | 2.03 | 3.01 | 0.1323 | 0.96 | |
| Epoxy resin/1.5%UFNBRP | 75 | 1.565 | 3.139 | 0.1442 | 0.96 |
| Sample | Cure Kinetic Model |
|---|---|
| Neat epoxy resin | |
| Epoxy resin/0.5% UFNBRP | |
| Epoxy resin/1% UFNBRP | |
| Epoxy resin/1.5% UFNBRP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, M.H.; Kalaee, M.R.; Mazinani, S.; Shakiba, M.; Shafiei Navid, S.; Abdouss, M.; Beig Mohammadi, A.; Zhao, W.; Koosha, M.; Song, Z.; et al. Curing Kinetics Modeling of Epoxy Modified by Fully Vulcanized Elastomer Nanoparticles Using Rheometry Method. Molecules 2022, 27, 2870. https://doi.org/10.3390/molecules27092870
Karami MH, Kalaee MR, Mazinani S, Shakiba M, Shafiei Navid S, Abdouss M, Beig Mohammadi A, Zhao W, Koosha M, Song Z, et al. Curing Kinetics Modeling of Epoxy Modified by Fully Vulcanized Elastomer Nanoparticles Using Rheometry Method. Molecules. 2022; 27(9):2870. https://doi.org/10.3390/molecules27092870
Chicago/Turabian StyleKarami, Mohammad Hossein, Mohammad Reza Kalaee, Saeideh Mazinani, Mohamadreza Shakiba, Saied Shafiei Navid, Majid Abdouss, Alireza Beig Mohammadi, Weisong Zhao, Mojtaba Koosha, Ziyue Song, and et al. 2022. "Curing Kinetics Modeling of Epoxy Modified by Fully Vulcanized Elastomer Nanoparticles Using Rheometry Method" Molecules 27, no. 9: 2870. https://doi.org/10.3390/molecules27092870
APA StyleKarami, M. H., Kalaee, M. R., Mazinani, S., Shakiba, M., Shafiei Navid, S., Abdouss, M., Beig Mohammadi, A., Zhao, W., Koosha, M., Song, Z., & Li, T. (2022). Curing Kinetics Modeling of Epoxy Modified by Fully Vulcanized Elastomer Nanoparticles Using Rheometry Method. Molecules, 27(9), 2870. https://doi.org/10.3390/molecules27092870







