Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Bark Extracts
2.2. Total Phenolic Content
2.3. Antioxidant Activity
2.4. HPLC Analysis
2.5. Microorganisms Tested
2.6. Inoculum Preparation
2.7. Agar Diffusion Assay
2.8. Resazurin-Based Broth Microdilution Assay
2.9. Preparation of Heat-Killed C. acnes
2.10. Cell Culture
2.11. Cell Viability Assay
2.12. ELISA
2.13. Scratch Assay
2.14. Statistical Analysis
3. Results
3.1. Extract Characterization
3.1.1. Total Phenolic Content and Antioxidant Activity
3.1.2. High-Pressure Liquid Chromatography (HPLC) Analysis
3.2. Antimicrobial Activity of Birch, Beech and Larch Extracts
3.2.1. Agar Diffusion Test
3.2.2. Broth Microdilution Assay
3.3. Cellular Assays
3.3.1. Viability Assay
3.3.2. Pro-Inflammatory Cytokine Measure
3.3.3. Scratch Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Association of the Austrian Wood Industries. Publikationen-FVHI Website Industry Report 2020. 2021. Available online: https://www.holzindustrie.at/infothek/publikationen/ (accessed on 7 February 2022).
- Kożuch, A.; Banaś, J. The dynamics of beech roundwood prices in selected central European markets. Forests 2020, 11, 902. [Google Scholar] [CrossRef]
- Hansmann, C.; Stingl, R.; Teischinger, A. Inquiry in beech wood processing industry concerning red heartwood. Wood Res. 2009, 54, 1–12. [Google Scholar]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of birch (Betula pendula Roth and B. pubescens Ehrh.) for forestry and forest-based industry sector within the changing climatic and socio-economic context of western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Issarachot, P.; Sangkaew, W.; Sianglum, W.; Saeloh, D.; Limsuwan, S.; Voravuthikunchai, S.P.; Joycharat, N. α-Glucosidase inhibitory, antibacterial, and antioxidant activities of natural substances from the wood of Derris reticulata Craib. Nat. Prod. Res. 2021, 35, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Ortmayr, N.; Oostingh, G.J.; Stelzhammer, B. Compounds extracted from larch, birch bark, douglas fir, and alder woods with four different solvents: Effects on five skin-related microbes. Bioresources 2020, 15, 3368–3381. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial activities of beech bark (Fagus sylvatica L.) polyphenolic extract. Environ. Eng. Manag. J. 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Wagner, K.; Roth, C.; Willför, S.; Musso, M.; Petutschnigg, A.; Oostingh, G.; Schnabel, T. Identification of antimicrobial compounds in different hydrophilic larch bark extracts. Bioresources 2019, 14, 5807–5815. [Google Scholar] [CrossRef]
- Laireiter, C.M.; Schnabel, T.; Köck, A.; Stalzer, P.; Petutschnigg, A.; Oostingh, G.J.; Hell, M. Active anti-microbial effects of larch and pine wood on four bacterial strains. Bioresources 2013, 9, 273–281. [Google Scholar] [CrossRef]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J.; Helander, M.; Salminen, J.-P. Phenolic compounds of the inner bark of Betula pendula: Seasonal and genetic variation and induction by wounding. J. Chem. Ecol. 2012, 38, 1410–1418. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 517–532. ISBN 978-981-15-4889-5. [Google Scholar]
- Becker, R.E.N.; Bubeck Wardenburg, J. Staphylococcus aureus and the skin: A longstanding and complex interaction. Skinmed 2015, 13, 111–119. [Google Scholar]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a traditional medicinal plant to a rational drug: Understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef]
- Wardecki, T.; Werner, P.; Thomas, M.; Templin, M.F.; Schmidt, G.; Brandner, J.M.; Merfort, I. Influence of birch bark triterpenes on keratinocytes and fibroblasts from diabetic and nondiabetic donors. J. Nat. Prod. 2016, 79, 1112–1123. [Google Scholar] [CrossRef]
- Tienaho, J.; Reshamwala, D.; Sarjala, T.; Kilpeläinen, P.; Liimatainen, J.; Dou, J.; Viherä-Aarnio, A.; Linnakoski, R.; Marjomäki, V.; Jyske, T. Salix spp. Bark Hot Water Extracts Show Antiviral, Antibacterial, and Antioxidant Activities-The Bioactive Properties of 16 Clones. Front. Bioeng. Biotechnol. 2021, 9, 797939. [Google Scholar] [CrossRef]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Gruber, L.; Seidl, L.; Zanetti, M.; Schnabel, T. Calorific Value and Ash Content of Extracted Birch Bark. Forests 2021, 12, 1480. [Google Scholar] [CrossRef]
- Smailagić, A.; Ristivojević, P.; Dimkić, I.; Pavlović, T.; Dabić Zagorac, D.; Veljović, S.; Fotirić Akšić, M.; Meland, M.; Natić, M. Radical scavenging and antimicrobial properties of polyphenol rich waste wood extracts. Foods 2020, 9, 319. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Zhang, X.; Hänninen, T.; Kyyhkynen, A.; Johansson, L.-S.; Willför, S.; Österberg, M.; Siitonen, A.; Rautkari, L. Antibacterial effects of wood structural components and extractives from Pinus sylvestris and Picea abies on Methicillin-Resistant Staphylococcus aureus and Escherichia coli O157:H7. BioResources 2017, 12, 14. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of Larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial activity and chemical composition of white birch (Betula papyrifera Marshall) bark extracts. Microbiologyopen 2020, 9, e00944. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Kosová, M.; Hrádková, I.; Mátlová, V.; Kadlec, D.; Šmidrkal, J.; Filip, V. Antimicrobial effect of 4-hydroxybenzoic acid ester with glycerol. J. Clin. Pharm. Ther. 2015, 40, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Dai, T.; Hu, P.; Niu, X.; Liu, C.; Chen, J. Binding mechanism and antioxidant capacity of selected phenolic acid-β-casein complexes. Food Res. Int. 2020, 129, 108802. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In vitro human skin penetration, antioxidant and antimicrobial activity of ethanol-water extract of fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Tálos-Nebehaj, E.; Albert, L.; Németh, L. Antioxidant efficiency of Beech (Fagus sylvatica L.) bark polyphenols assessed by chemometric methods. Ind. Crops Prod. 2017, 108, 26–35. [Google Scholar] [CrossRef]
- Tanase, C.; Mocan, A.; Coșarcă, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.-M.; Vodnar, D.C.; Muntean, D.-L.; Crișan, O. Biological and Chemical Insights of Beech (Fagus sylvatica L.) Bark: A Source of Bioactive Compounds with Functional Properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef]
- Vek, V.; Oven, P.; Poljanšek, I.; Ters, T. Contribution to Understanding the Occurrence of Ex-tractives in Red Heart of Beech. BioResources 2014, 10, 970–985. [Google Scholar] [CrossRef][Green Version]
- Missiakas, D.M.; Schneewind, O. Growth and laboratory maintenance of Staphylococcus aureus. Curr. Protoc. Microbiol. 2013, 28, 9C-1. [Google Scholar] [CrossRef]
- Oliveira, F.; França, A.; Cerca, N. Staphylococcus epidermidis is largely dependent on iron availability to form biofilms. Int. J. Med. Microbiol. 2017, 307, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Eady, E.A.; Layton, A.M.; Cove, J.H. A honey trap for the treatment of acne: Manipulating the follicular microenvironment to control Propionibacterium acnes. Biomed. Res. Int. 2013, 2013, 679680. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care (New Rochelle) 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Hubert, J.; Angelis, A.; Aligiannis, N.; Rosalia, M.; Abedini, A.; Bakiri, A.; Reynaud, R.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; et al. In Vitro Dermo-Cosmetic Evaluation of Bark Extracts from Common Temperate Trees. Planta Med. 2016, 82, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Schwieger-Briel, A.; Ott, H.; Kiritsi, D.; Laszczyk-Lauer, M.; Bodemer, C. Mechanism of Oleo-gel-S10: A triterpene preparation for the treatment of epidermolysis bullosa. Dermatol. Ther. 2019, 32, e12983. [Google Scholar] [CrossRef] [PubMed]
- Pásztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Devel. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
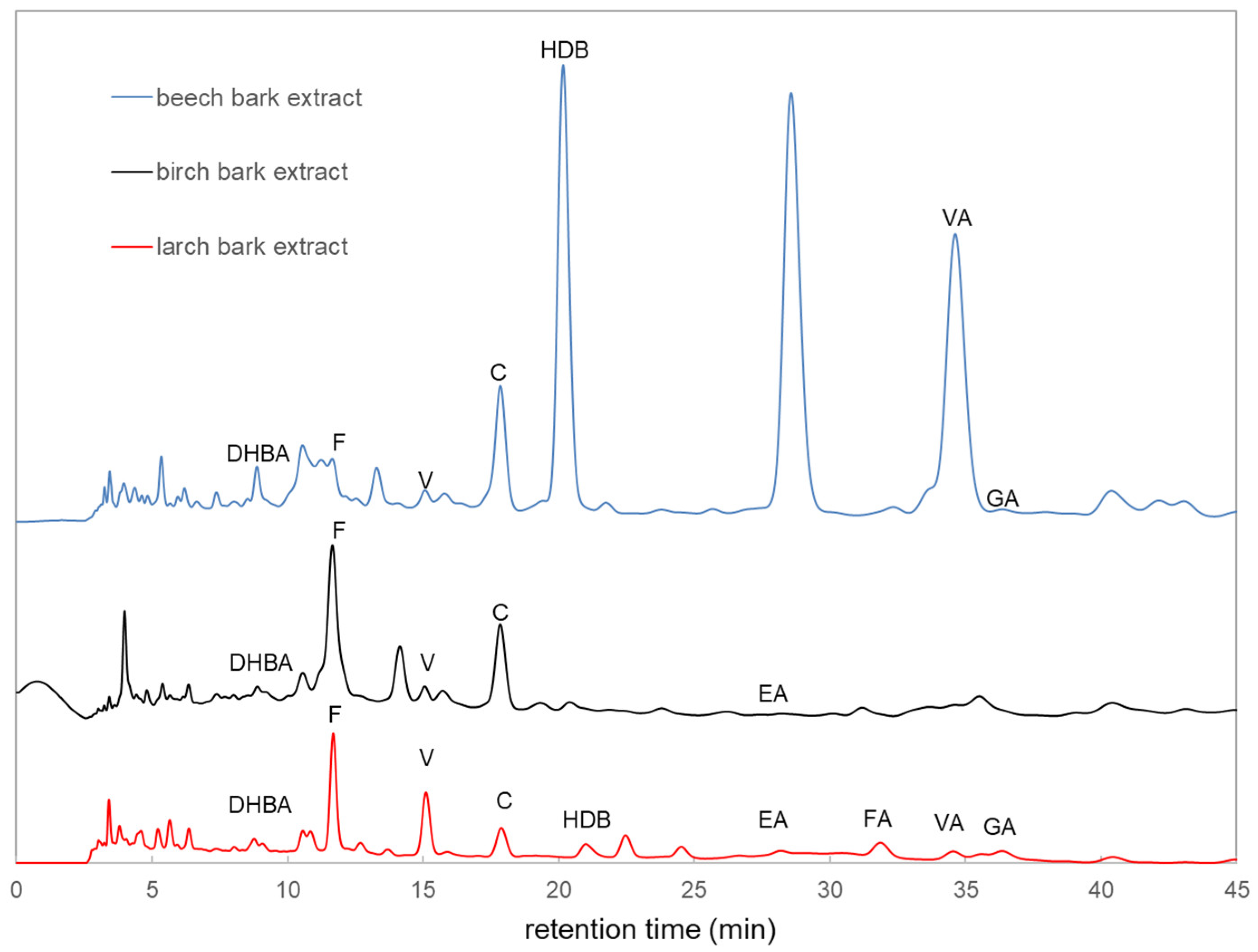

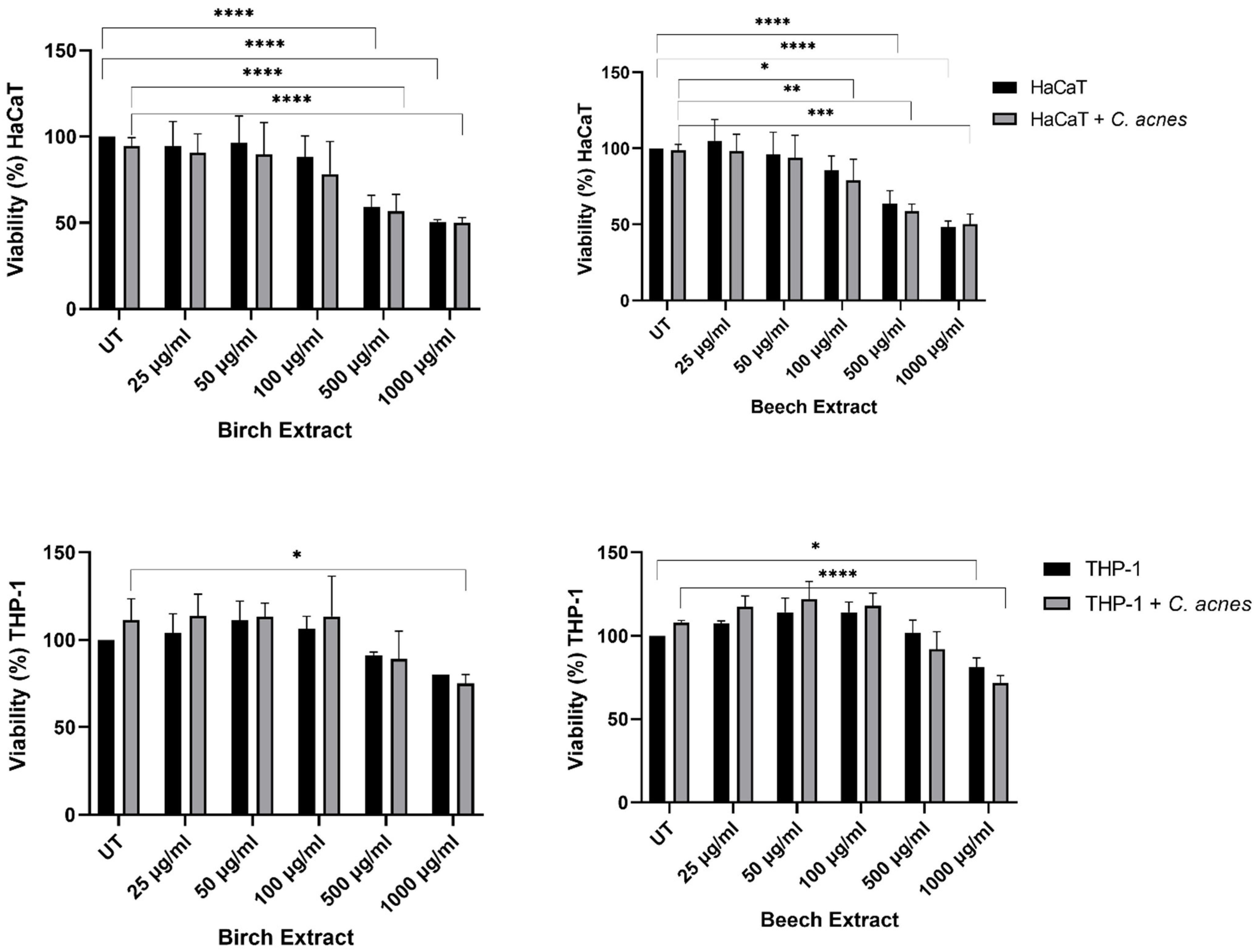
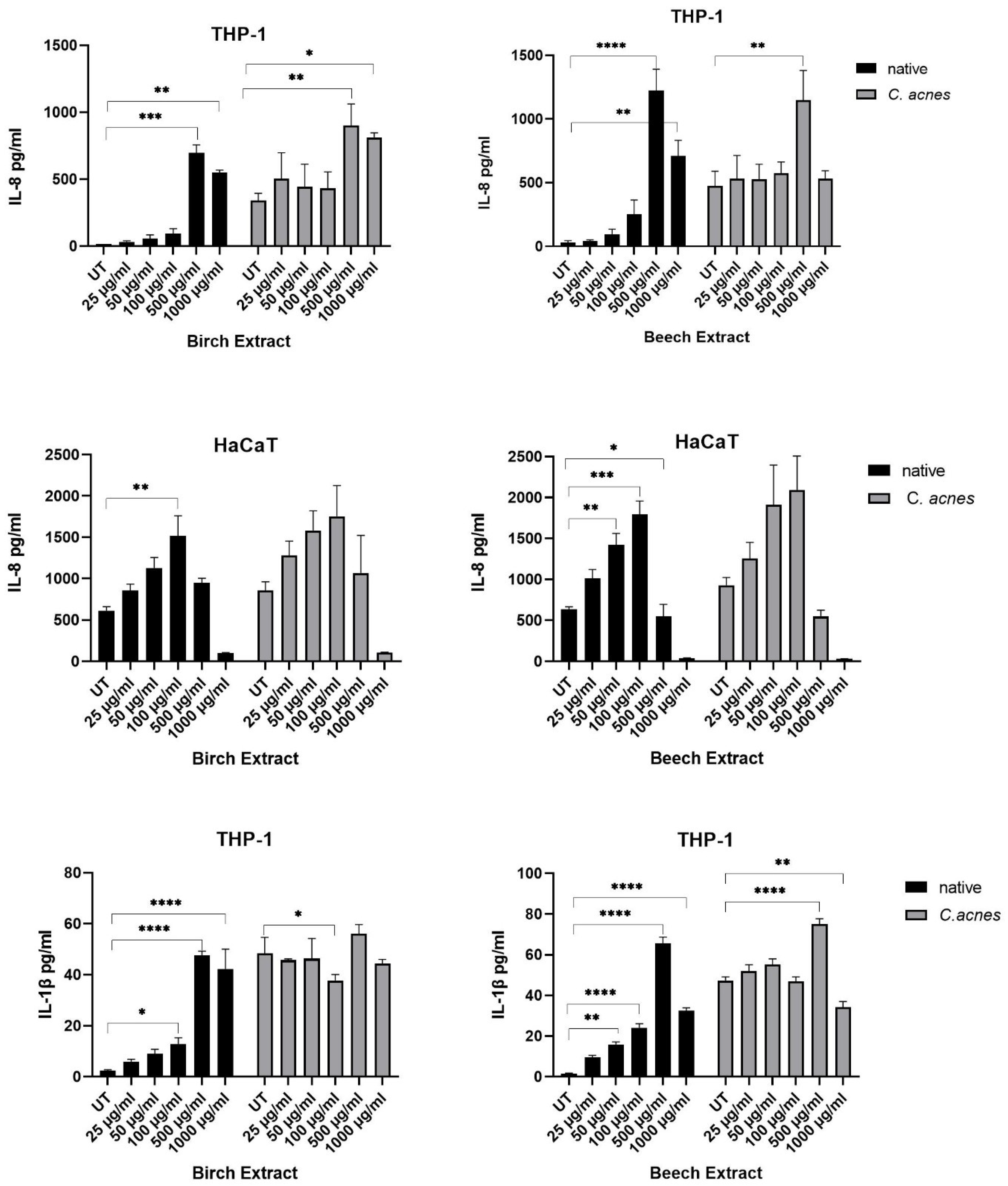
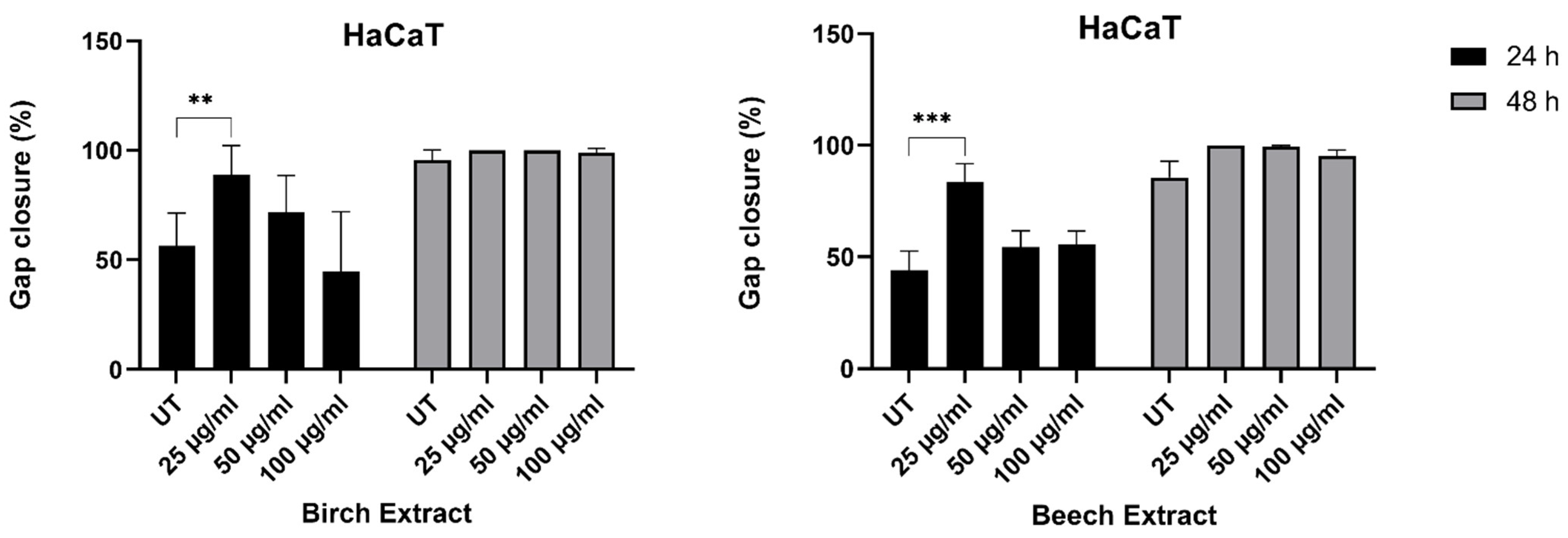
| Total Phenolic Content (mg GAE/g) | Antioxidant Activity (%) | |
|---|---|---|
| Birch | 440.74 | 81.94 |
| Beech | 297.86 | 50.68 |
| Larch | 277.62 | 48.35 |
| Birch µg/mL | Beech µg/mL | Larch µg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CFU/mL | 200 | 400 | 600 | 200 | 400 | 600 | 200 | 400 | 600 | |
| MRSA | 100,000 | ns | **** | **** | ns | **** | **** | **** | **** | **** |
| 10,000 | ns | **** | **** | ** | **** | **** | **** | **** | **** | |
| 1000 | ** | **** | **** | * | **** | **** | **** | **** | **** | |
| E. coli | 100,000 | *** | ** | ns | ns | ns | ns | ns | ns | ns |
| 10,000 | * | ns | ns | ns | ns | ns | ns | ns | ns | |
| 1000 | **** | ns | ns | * | ns | ns | ns | ns | ns | |
| CFU/mL | 25 | 50 | 100 | 25 | 50 | 100 | 25 | 50 | 100 | |
| C. acnes | 100,000 | ns | **** | **** | *** | **** | **** | ns | * | * |
| 10,000 | **** | **** | **** | **** | **** | **** | ns | *** | **** | |
| 1000 | **** | **** | **** | **** | **** | **** | ns | ns | ns | |
| CFU/mL | 100 | 150 | 300 | 100 | 150 | 300 | 100 | 150 | 300 | |
| S. epi1 | 100,000 | ns | * | **** | ns | **** | **** | * | ** | * |
| 10,000 | ns | **** | **** | **** | **** | **** | * | ** | * | |
| 1000 | **** | **** | **** | **** | **** | **** | ns | ns | **** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emrich, S.; Schuster, A.; Schnabel, T.; Oostingh, G.J. Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts. Molecules 2022, 27, 2817. https://doi.org/10.3390/molecules27092817
Emrich S, Schuster A, Schnabel T, Oostingh GJ. Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts. Molecules. 2022; 27(9):2817. https://doi.org/10.3390/molecules27092817
Chicago/Turabian StyleEmrich, Stefanie, Anja Schuster, Thomas Schnabel, and Gertie Janneke Oostingh. 2022. "Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts" Molecules 27, no. 9: 2817. https://doi.org/10.3390/molecules27092817
APA StyleEmrich, S., Schuster, A., Schnabel, T., & Oostingh, G. J. (2022). Antimicrobial Activity and Wound-Healing Capacity of Birch, Beech and Larch Bark Extracts. Molecules, 27(9), 2817. https://doi.org/10.3390/molecules27092817







