Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids
Abstract
:1. Introduction
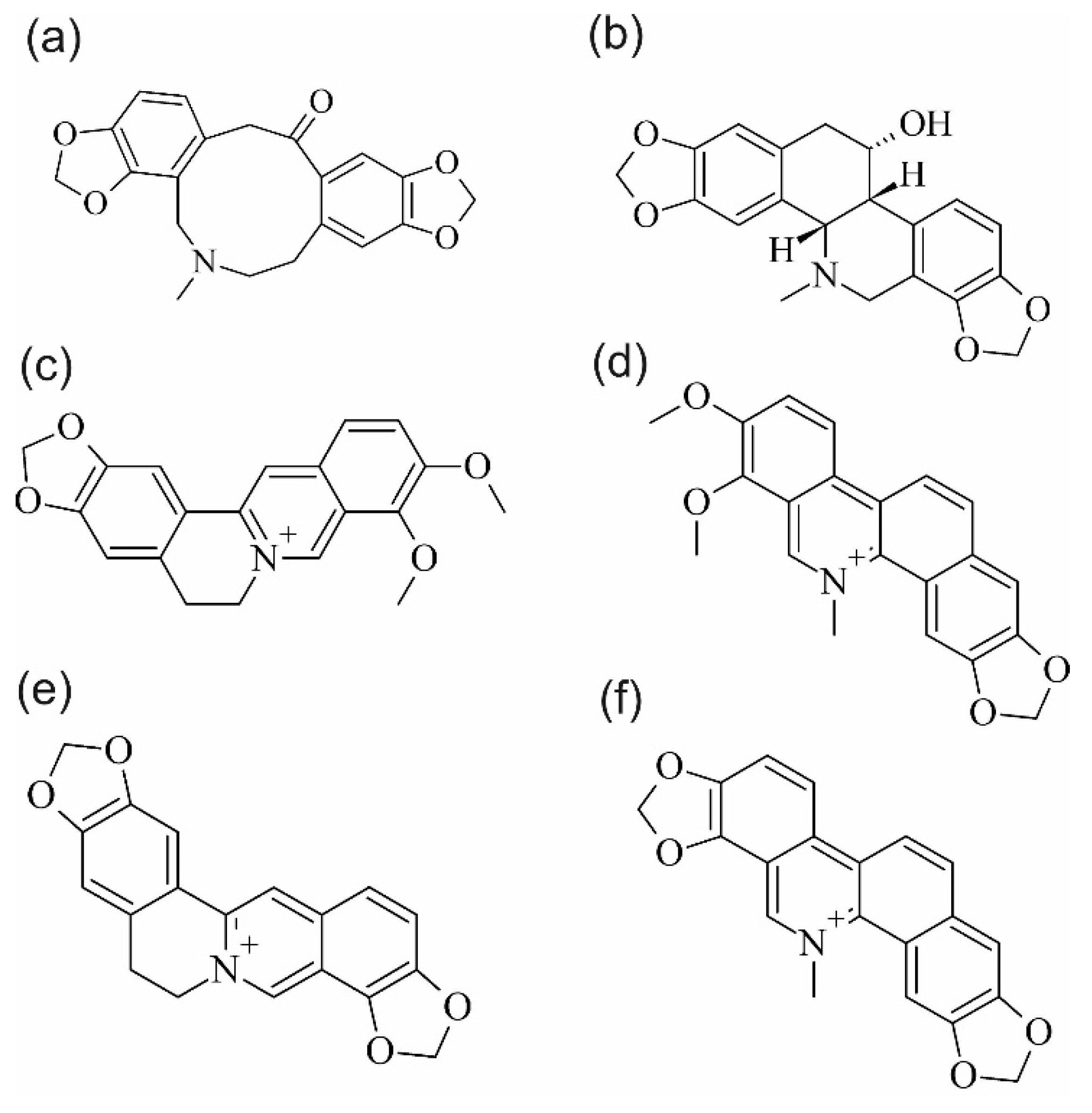
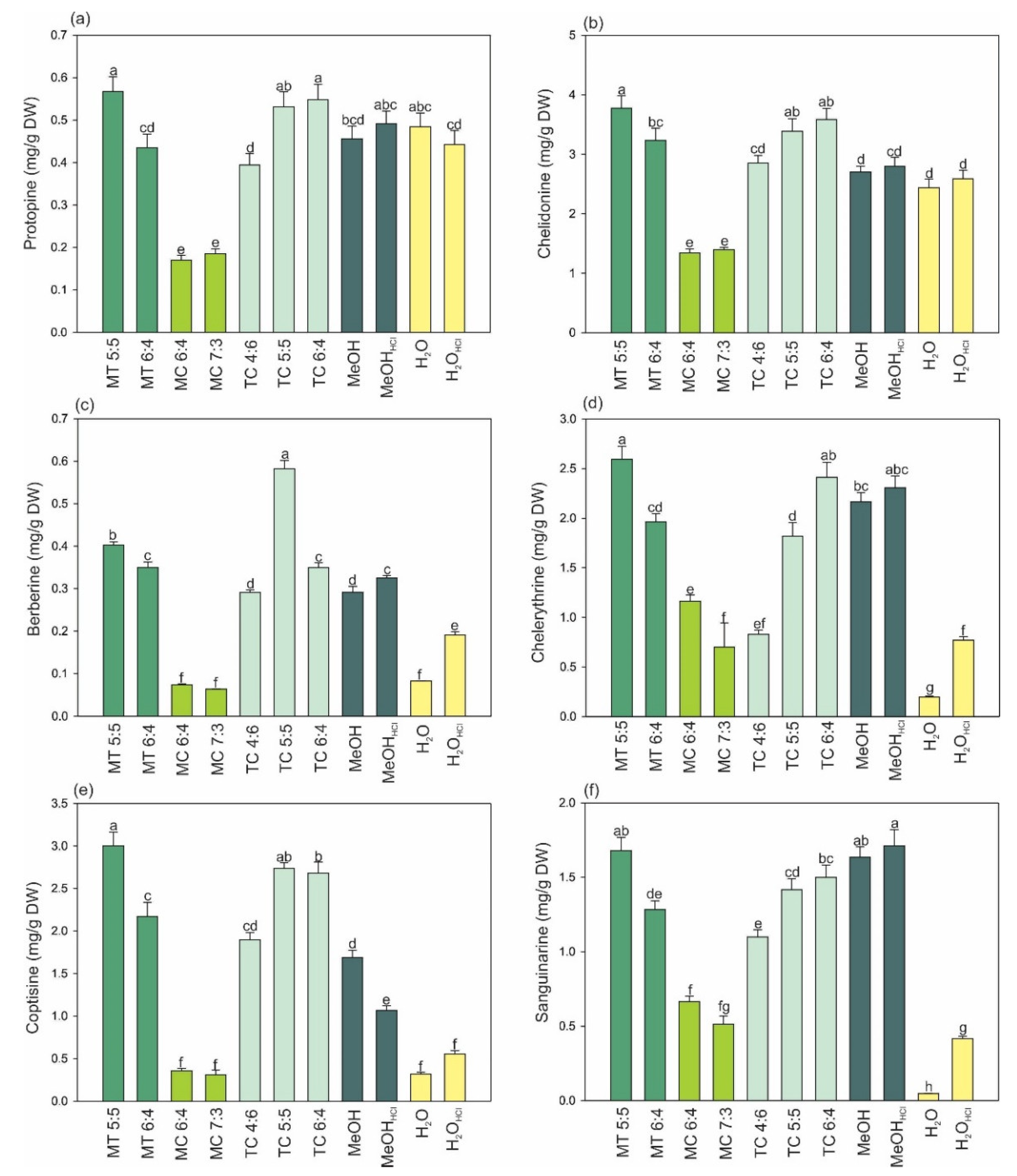
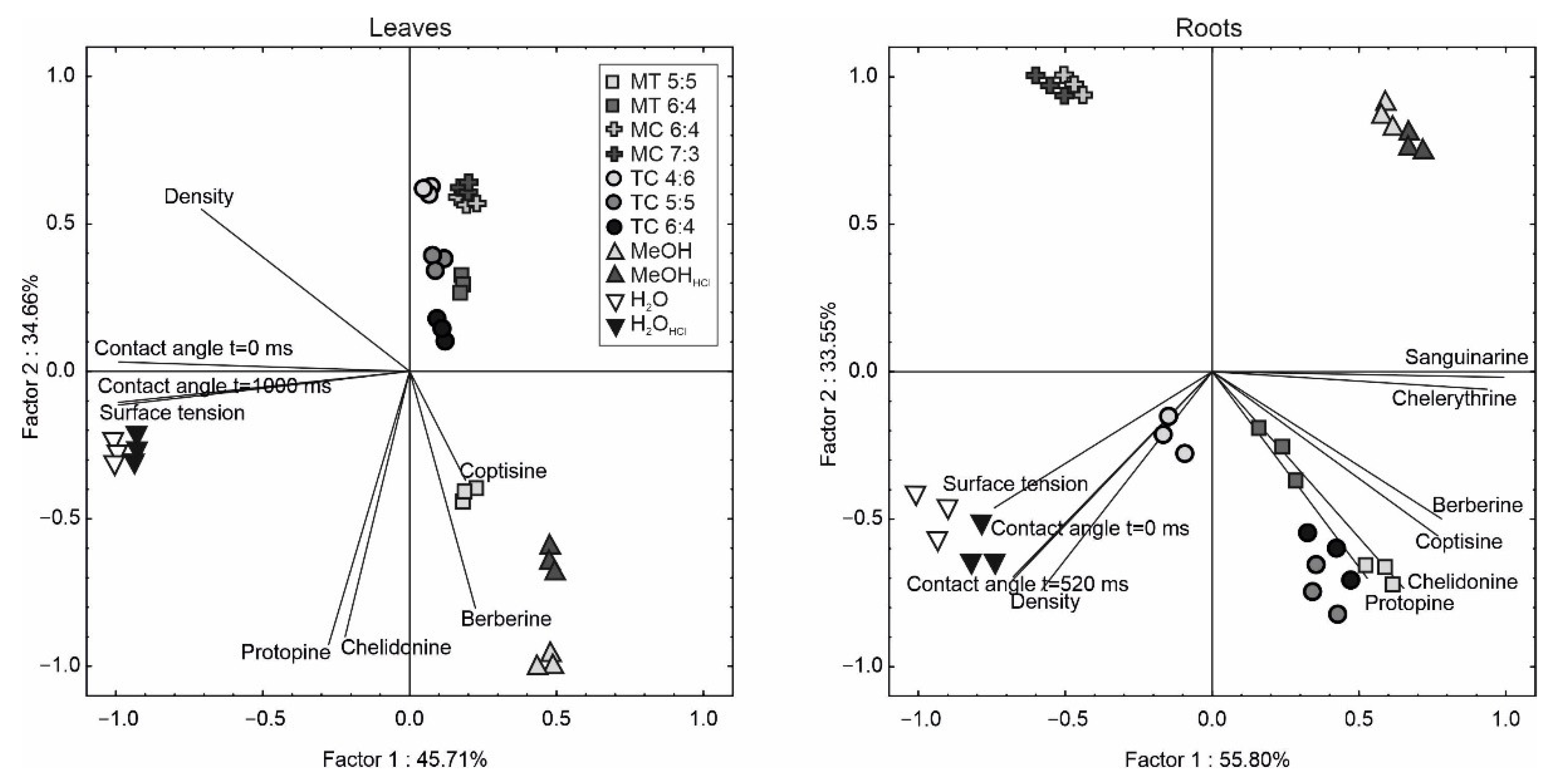
2. Results and Discussion
2.1. Extraction Efficiency
2.2. Physical Properties of Extractants
2.3. Principal Component Analysis
3. Materials and Methods
3.1. Reference Standards and Chemicals
3.2. Preparation of VNADESs and Reference Extractants
3.3. Plant Material and Extraction Procedure
3.4. HPLC Analysis
3.5. Determination of the Physical Properties of the Extractants
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zielinska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater celandine’s ups and downs-21 centuries of medicinal uses of Chelidonium majus from the viewpoint of today’s pharmacology. Front. Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska, S.; Wójciak-Kosior, M.; Płachno, B.J.; Sowa, I.; Włodarczyk, M.; Matkowski, A. Quaternary alkaloids in Chelidonium majus in vitro cultures. Ind. Crops Prod. 2018, 123, 17–24. [Google Scholar] [CrossRef]
- Zielinska, S.; Wójciak-Kosior, M.; Dziagwa-Becker, M.; Glensk, M.; Sowa, I.; Fijalkowski, K.; Ruranska-Smutnicka, D.; Matkowski, A.; Junka, A. The activity of isoquinoline alkaloids and extracts from Chelidonium majus against pathogenic bacteria and Candida sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogucka-Kocka, A.; Zalewski, D. Qualitative and quantitative determination of main alkaloids of Chelidonium majus L.using thin-layer chromatographic-densitometric method. Acta Chromatogr. 2017, 29, 385–397. [Google Scholar] [CrossRef]
- Jesionek, W.; Fornal, E.; Majer-Dziedzic, B.; Móricz, Á.M.; Nowicky, W.; Choma, I.M. Investigation of the composition and antibacterial activity of UkrainTM drug using liquid chromatography techniques. J. Chromatogr. A 2016, 1429, 340–347. [Google Scholar] [CrossRef]
- Migas, P.; Heyka, M.; Pobłocka-Olech, L.; Krauze-Baranowska, M. BMD-TLC—The useful technique for quantitative analysis of chelidonine, chelerithrine and berberine in herbal drugs. J. Planar Chromatogr. -Mod. TLC 2012, 25, 439–444. [Google Scholar] [CrossRef]
- Sárközi, Á.; Móricz, Á.M.; Ott, P.G.; Tyihák, E.; Kéry, Á. Investigation of Chelidonium alkaloids by use of a complex bioautographic system. J. Planar Chromatogr. -Mod. TLC 2006, 19, 267–272. [Google Scholar] [CrossRef]
- Sárközi, Á.; Janicsák, G.; Kursinszki, L.; Kéry, Á. Alkaloid composition of Chelidonium majus L. studied by different chromatographic techniques. Chromatographia 2006, 63, S81–S86. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- de los Ángeles Fernández, M.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef] [Green Version]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ahlgren, S.; Korthout, H.A.A.J.; Salomé-Abarca, L.F.; Bayona, L.M.; Verpoorte, R.; Choi, Y.H. Broad range chemical profiling of natural deep eutectic solvent extracts using a high performance thin layer chromatography–based method. J. Chromatogr. A 2018, 1532, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Mulia, K.; Krisanti, E.; Terahadi, F.; Putri, S. Selected natural deep eutectic solvents for the extraction of α-mangostin from mangosteen (Garcinia mangostana L.) pericarp. Int. J. Technol. 2015, 6, 1211–1220. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Ping-Kou; Jiang, Y.W.; Wang, L.T.; Niu, L.J.; Liu, Z.M.; Fu, Y.J. Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef]
- Koel, M.; Kuhtinskaja, M.; Vaher, M. Extraction of bioactive compounds from Catharanthus roseus and Vinca minor. Sep. Purif. Technol. 2020, 252, 117438. [Google Scholar] [CrossRef]
- Rachmaniah, O.; Wilson, E.G.; Choi, Y.H.; Witkamp, G.-J.; Verpoorte, R. Pressurized natural deep eutectic solvent extraction of galanthamine and related alkaloids from Narcissus pseudonarcissus. Planta Med. 2022, in press . [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Morais, E.S.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Extraction of high value triterpenic acids from Eucalyptus globulus biomass using hydrophobic deep eutectic solvents. Molecules 2020, 25, 210. [Google Scholar] [CrossRef] [Green Version]
- Bugatti, C.; Colombo, M.L.; Tomè, F. High-performance liquid chromatographic separation of quaternary alkaloids of Chelidonium majus L. roots. J. Chromatogr. A 1987, 393, 312–316. [Google Scholar] [CrossRef]
- Gu, Y.; Qian, D.; Duan, J.A.; Wang, Z.; Guo, J.; Tang, Y.; Guo, S. Simultaneous determination of seven main alkaloids of Chelidonium majus L. by ultra-performance LC with photodiode-array detection. J. Sep. Sci. 2010, 33, 1004–1009. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Arbid, M.S.; Asaad, G.F. Chelidonium majus leaves methanol extract and its chelidonine alkaloid ingredient reduce cadmium-induced nephrotoxicity in rats. J. Nat. Med. 2013, 67, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.; Yahyazadeh, M.; Hänsel, S.; Kleinwächter, M.; Ibrom, K.; Selmar, D. 13,14-Dihydrocoptisine—The genuine alkaloid from Chelidonium majus. Phytochemistry 2015, 111, 149–153. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiao, S.; Wu, J.; Fang, H. Influence of multiple factors on the wettability and surface free energy of leaf surface. Appl. Sci. 2019, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Wójciak-Kosior, M.; Sowa, I.; Dresler, S.; Kováčik, J.; Staniak, M.; Sawicki, J.; Zielińska, S.; Świeboda, R.; Strzemski, M.; Kocjan, R. Polyaniline based material as a new SPE sorbent for pre-treatment of Chelidonium majus extracts before chromatographic analysis of alkaloids. Talanta 2019, 194, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Dang-Vu, T.; Jha, R.; Wu, S.Y.; Tannant, D.D.; Masliyah, J.; Xu, Z. Wettability determination of solids isolated from oil sands. Colloids Surf. A Physicochem. Eng. Asp. 2009, 337, 80–90. [Google Scholar] [CrossRef]
| TC | MT | MC | MeOH | H2O | |
|---|---|---|---|---|---|
| Density (g/cm3) | 0.95 | 0.93 | 0.91 | 0.79 | 0.99 |
| Surface tension (J/m2) | 32 | 30 | 29 | 24 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzemski, M.; Dresler, S.; Podkościelna, B.; Skic, K.; Sowa, I.; Załuski, D.; Verpoorte, R.; Zielińska, S.; Krawczyk, P.; Wójciak, M. Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids. Molecules 2022, 27, 2815. https://doi.org/10.3390/molecules27092815
Strzemski M, Dresler S, Podkościelna B, Skic K, Sowa I, Załuski D, Verpoorte R, Zielińska S, Krawczyk P, Wójciak M. Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids. Molecules. 2022; 27(9):2815. https://doi.org/10.3390/molecules27092815
Chicago/Turabian StyleStrzemski, Maciej, Sławomir Dresler, Beata Podkościelna, Kamil Skic, Ireneusz Sowa, Daniel Załuski, Rob Verpoorte, Sylwia Zielińska, Paweł Krawczyk, and Magdalena Wójciak. 2022. "Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids" Molecules 27, no. 9: 2815. https://doi.org/10.3390/molecules27092815
APA StyleStrzemski, M., Dresler, S., Podkościelna, B., Skic, K., Sowa, I., Załuski, D., Verpoorte, R., Zielińska, S., Krawczyk, P., & Wójciak, M. (2022). Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids. Molecules, 27(9), 2815. https://doi.org/10.3390/molecules27092815










