Ameliorative Effect of Ocimum forskolei Benth on Diabetic, Apoptotic, and Adipogenic Biomarkers of Diabetic Rats and 3T3-L1 Fibroblasts Assisted by In Silico Approach
Abstract
:1. Introduction
2. Results
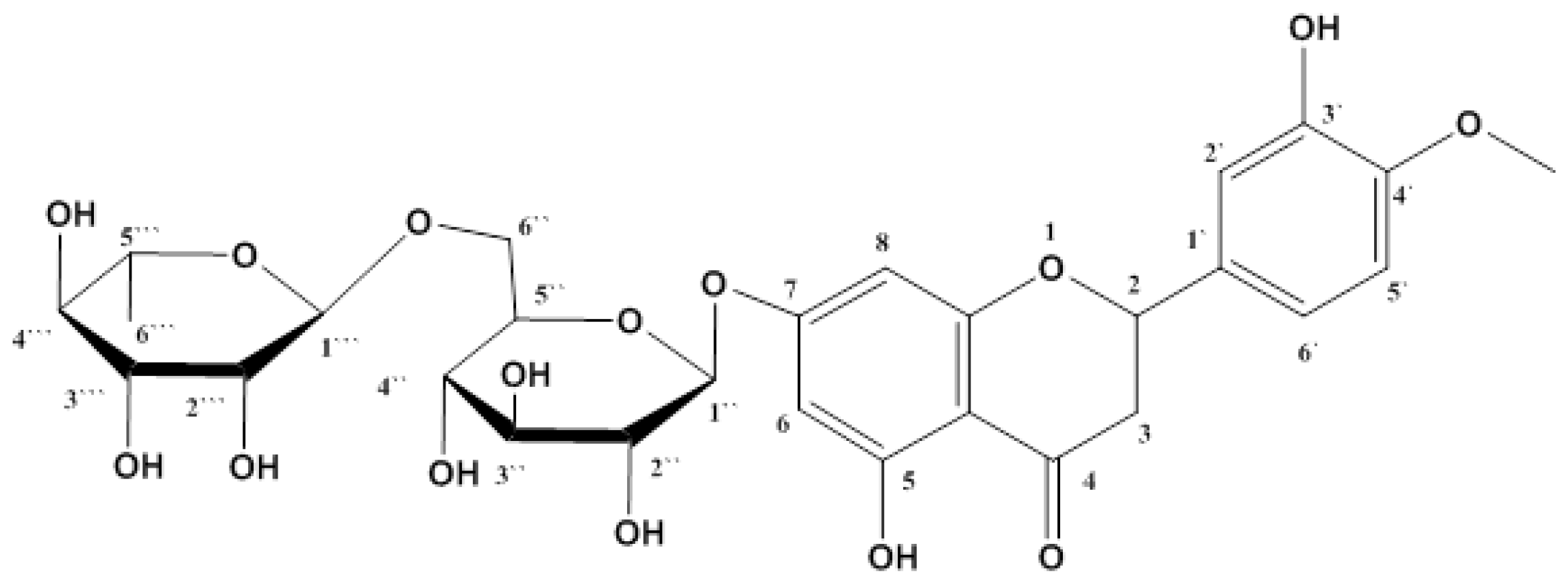
2.1. Isolation and Identification of Hesperidin
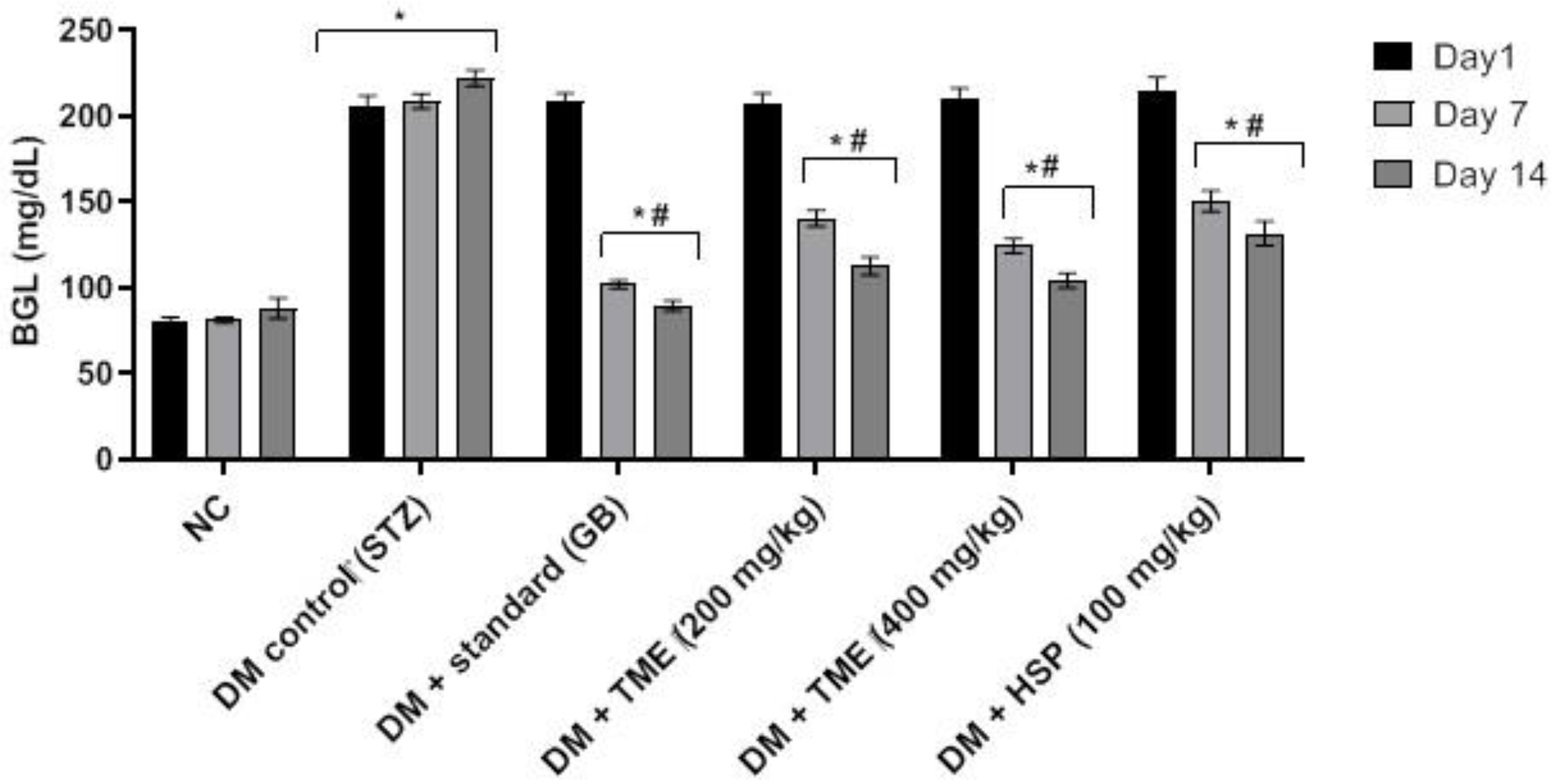
2.2. Effect of TME and HSP on Blood Glucose Level
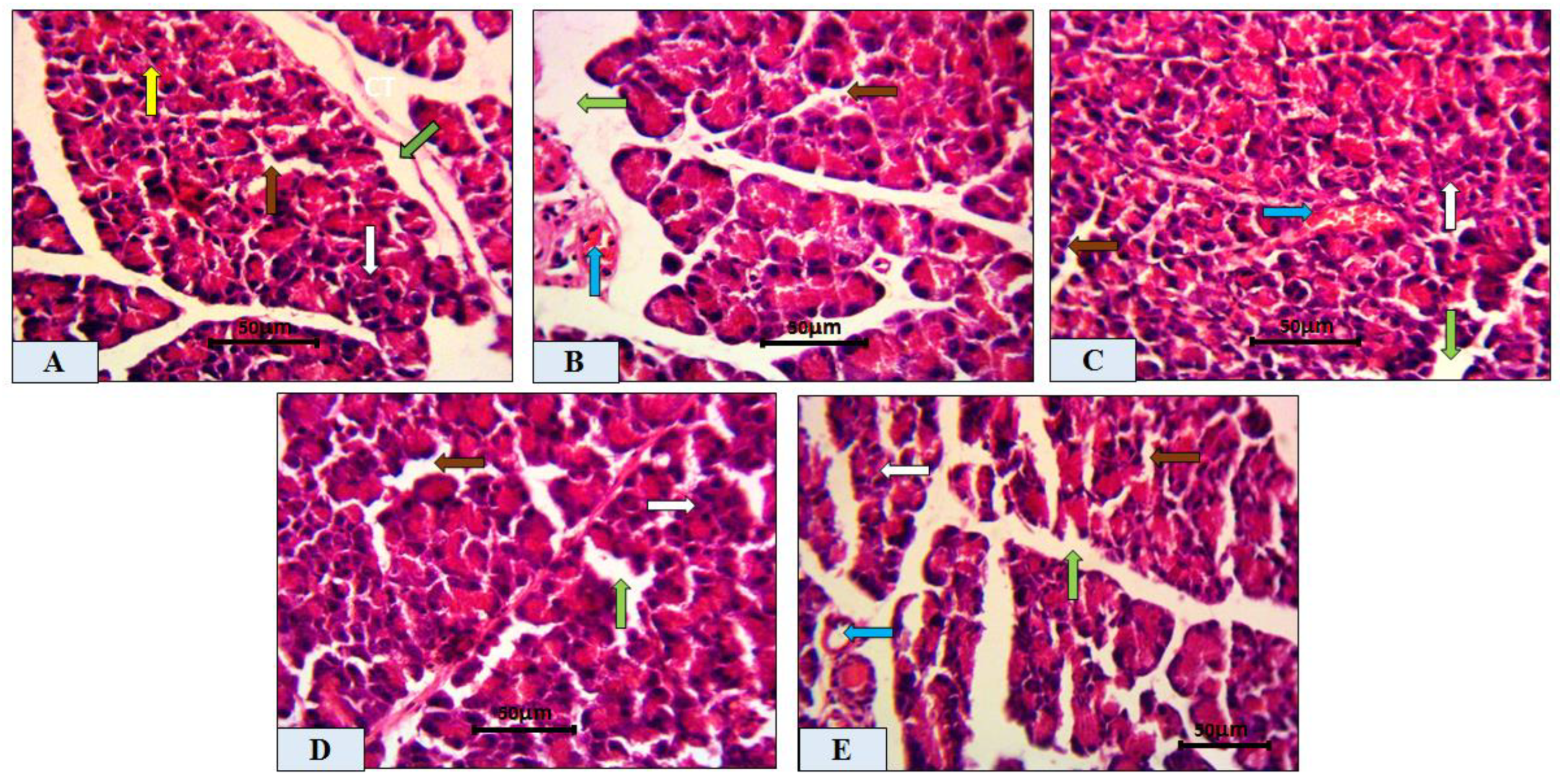
2.3. Histopathological Analysis of Pancreatic Tissue
2.4. Morphometric Analysis of Pancreatic Tissue
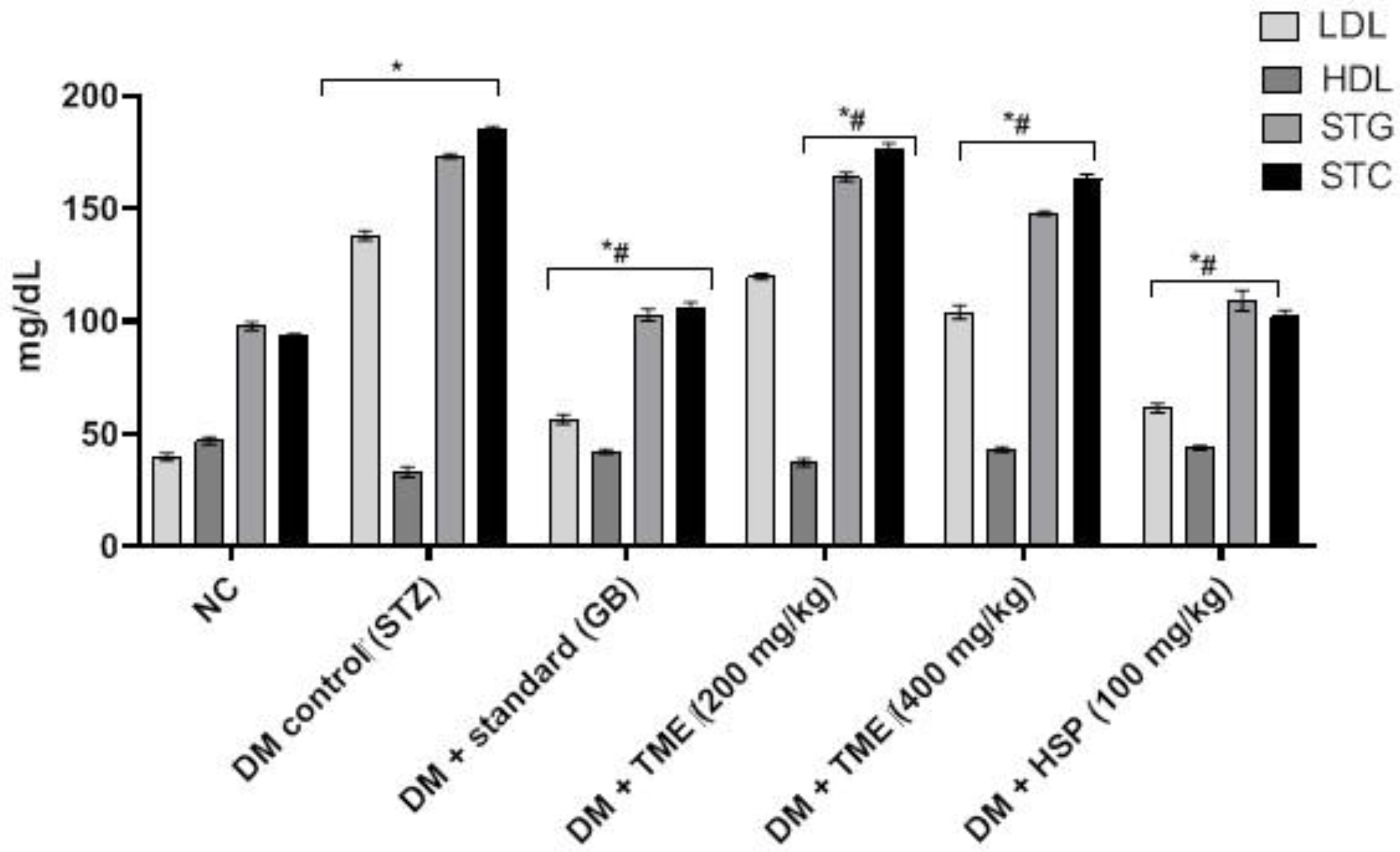
2.5. Effect of TME and HSP on Lipid Profile in HFD-Fed STZ-Induced Diabetic Rats
2.6. Histopathological Analysis of Adipose Tissue
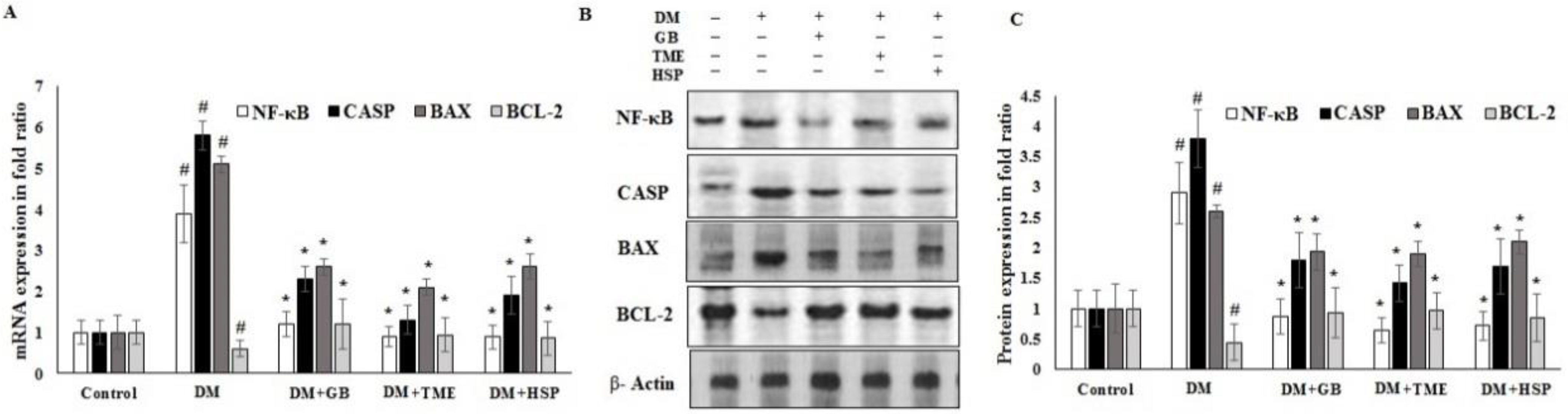
2.7. Effect of TME and HSP on Expression of NF-κB, CASPASE-3, BAX, and BCL2 Markers in Rat Pancreatic Tissue
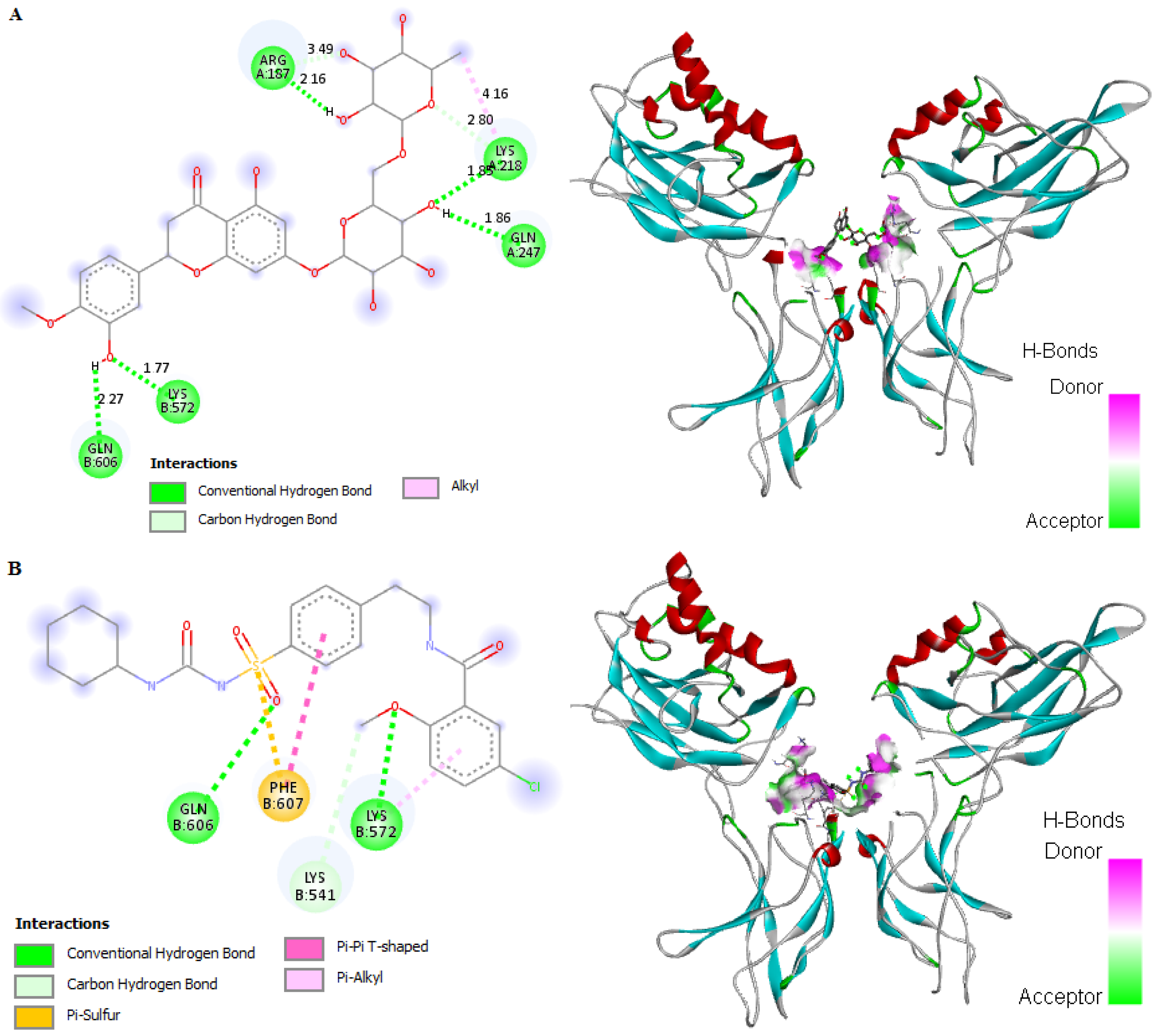
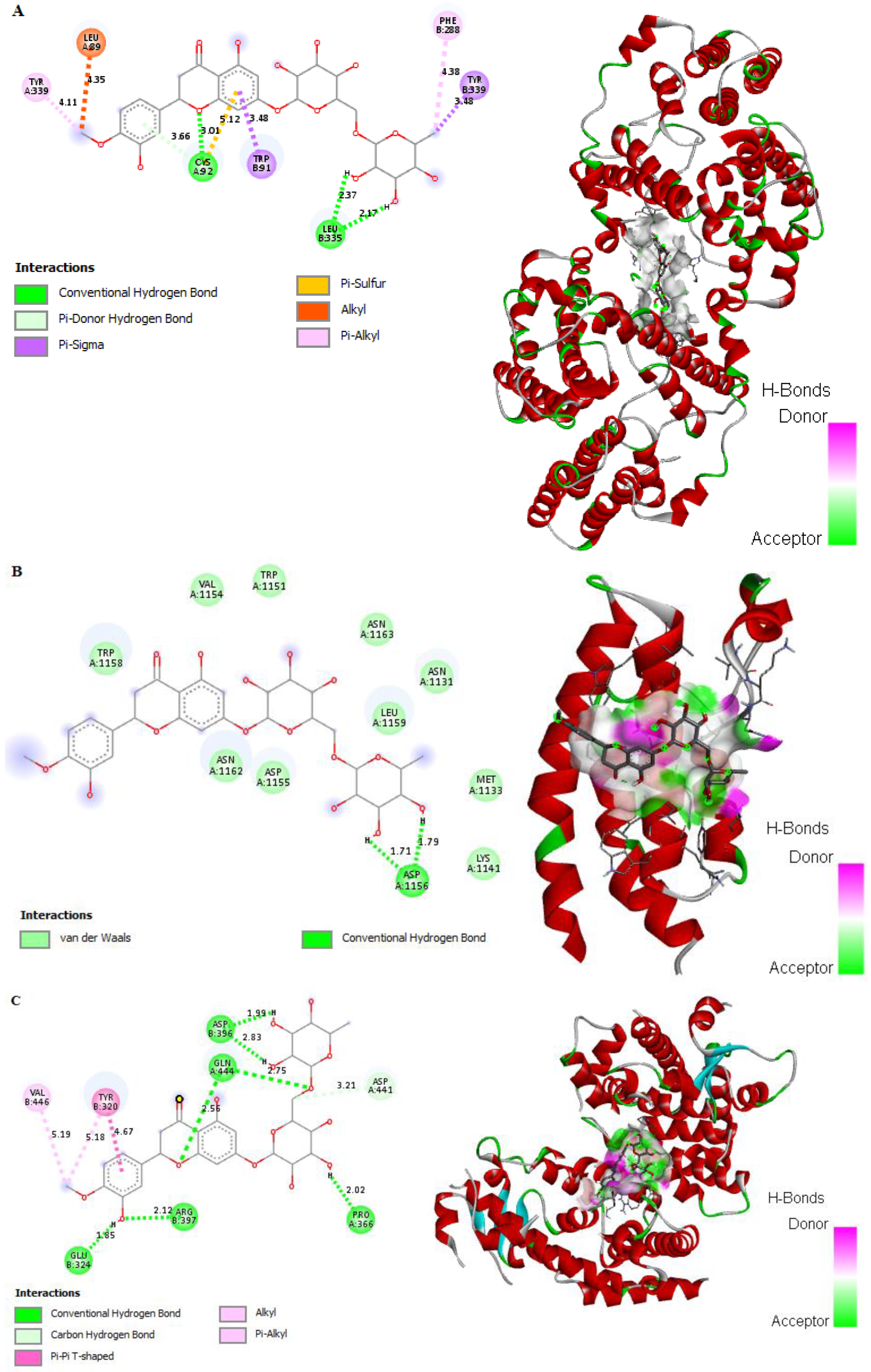
2.8. In Silico Binding of HSP and GB with ABC Transporter SUR1
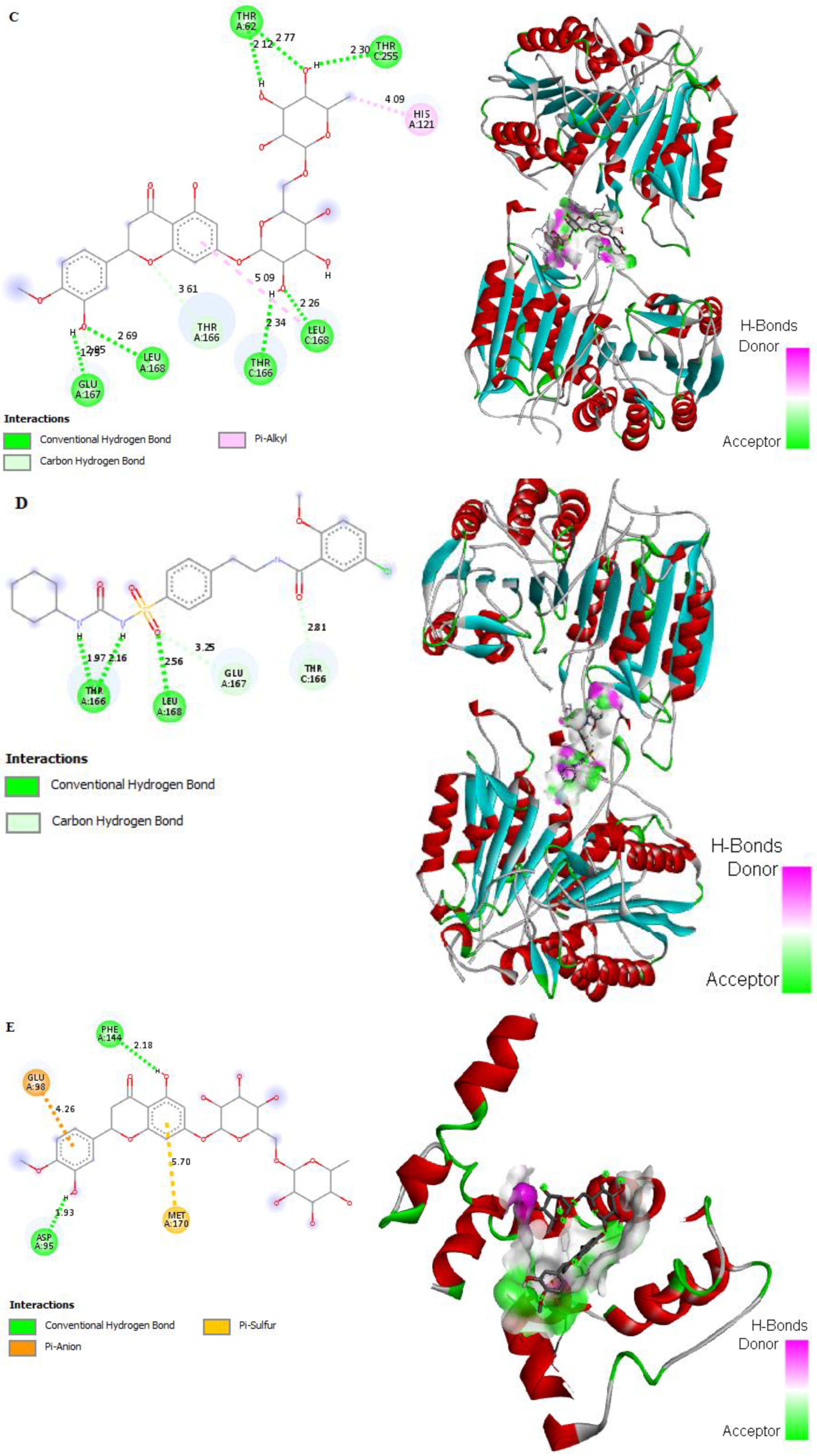
2.9. In Silico Binding of HSP and GB with NF-κB, Cleaved caspase-3, BAX, and BCL2 Markers
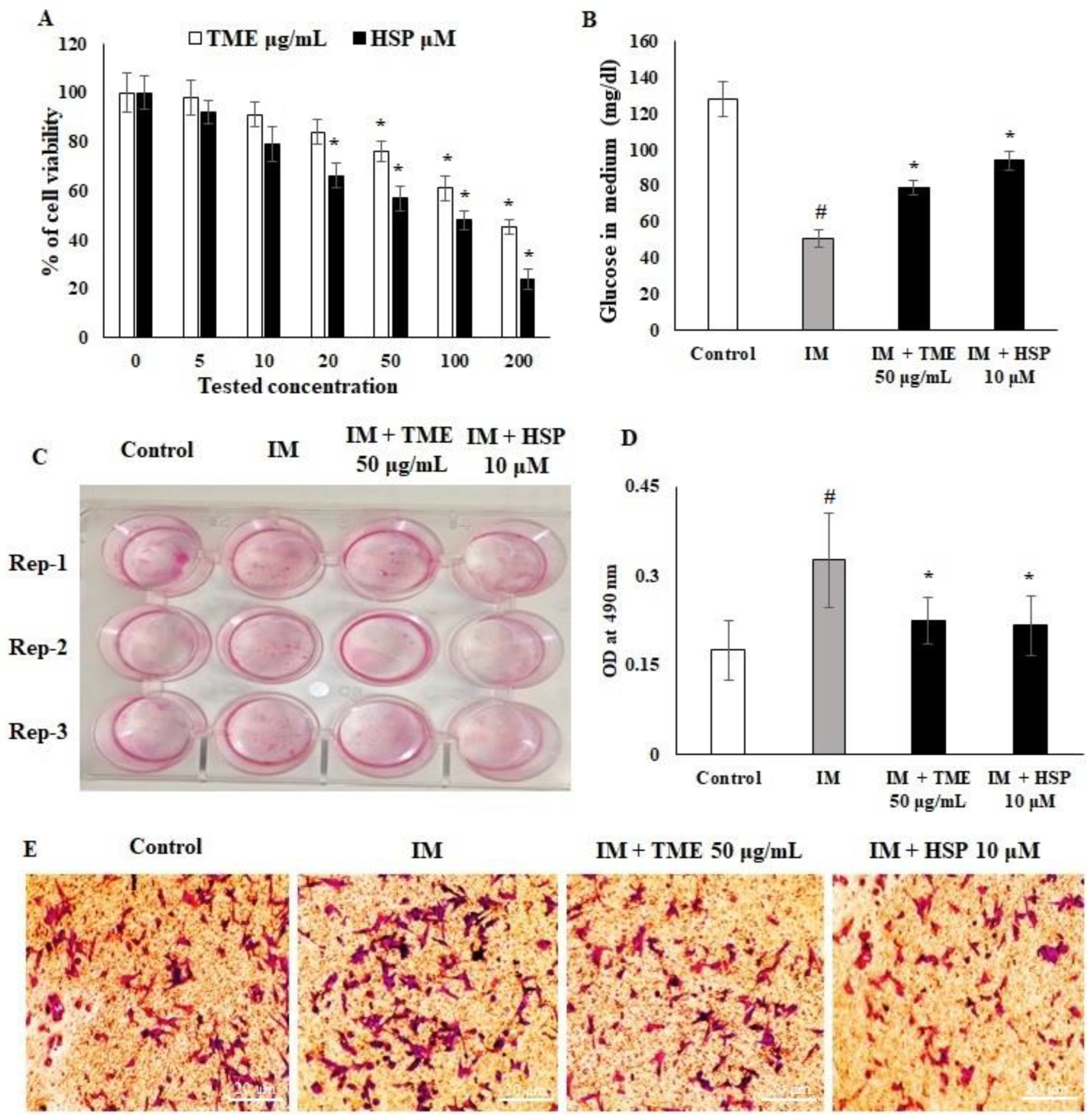
2.10. Effect of HSP on Cell Viability and Glucose Uptake of 3T3-L1 Cell Lines
2.11. HSP Inhibited 3T3-L1 Pre-Adipocyte Differentiation to Adipocytes
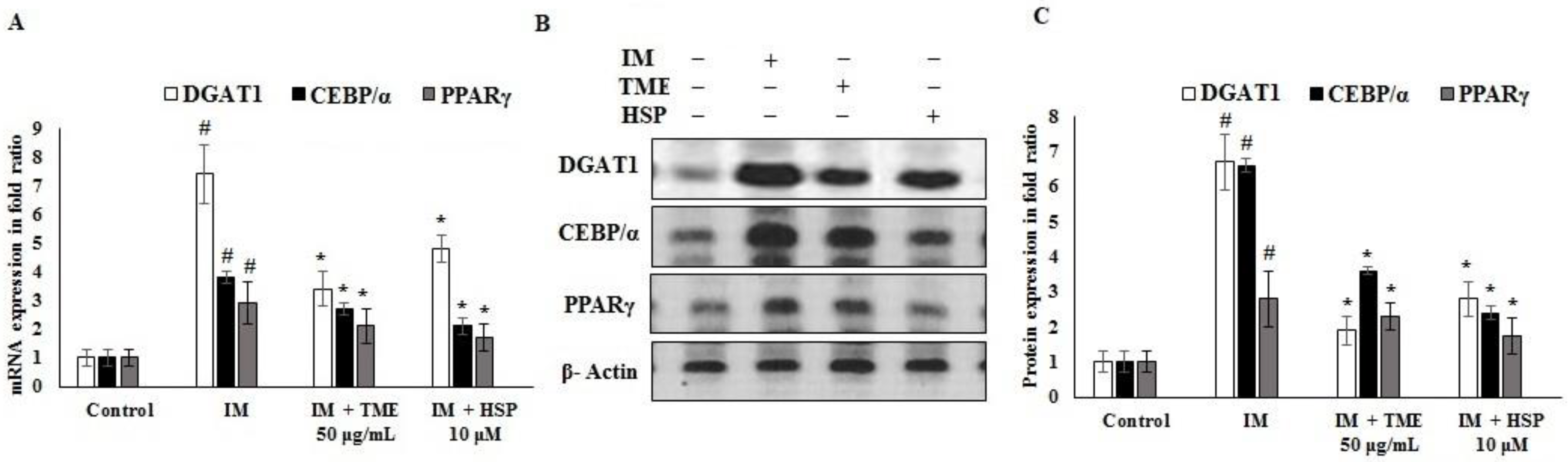
2.12. Effect of TME and HSP on Expression of DGAT1, CEBP/α, and PPARγ in 3T3-L1 Cells
2.13. In Silico Binding of HSP to DGAT1, CEBP/α, and PPARγ Adipogenic Markers in 3T3-L1 Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures and Chemicals
4.2. Plant Material
4.3. Extraction and Isolation of the Major Plant Constituents
4.4. Animals
4.5. Diet
4.6. Design of Experiment
4.7. Tissue Collection
4.8. Morphometric Analysis
4.9. Computational Studies
4.10. Cell Culture
4.11. Measurement of Glucose Content in Medium
4.12. Oil Red O Staining
4.13. mRNA Quantification
4.14. Western Blot Analysis
4.15. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Non-Commun. Dis. 2016, 1, 3–8. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Wilmot, E.; Idris, I. Early onset type 2 diabetes: Risk factors, clinical impact and management. Ther. Adv. Chronic. Dis. 2014, 5, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network pharmacology studies on the bioactive compounds and action mechanisms of natural products for the treatment of diabetes mellitus: A review. Front. Pharmacol. 2017, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; López Machado, A.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome—Review of classical and new compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Patle, D.; Vyas, M.; Khatik, G.L. A review on natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021, 17, 186–197. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O. Natural products in diabetes research: Quantitative literature analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Thakkar, N.V.; Patel, J.A. Pharmacological evaluation of “Glyoherb”: A polyherbal formulation on streptozotocin-induced diabetic rats. Int. J. Diabetes Dev. Ctries. 2010, 30, 1–7. [Google Scholar]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Etsassala, N.G.; Hussein, A.A.; Nchu, F. Potential application of some lamiaceae species in the management of diabetes. Plants 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant. 2012, 6, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, T.; Mandal, A.; Roy, S.C.; De Sarker, D. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J. Genet. Eng. Biotechnol. 2017, 15, 275–286. [Google Scholar] [CrossRef]
- Parasuraman, S.; Balamurugan, S.; Christapher, P.V.; Petchi, R.R.; Yeng, W.Y.; Sujithra, J.; Vijaya, C. Evaluation of antidiabetic and antihyperlipidemic effects of hydroalcoholic extract of leaves of Ocimum tenuiflorum (Lamiaceae) and prediction of biological activity of its phytoconstituents. Pharmacogn. Res. 2015, 7, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.; Patil, R.; Ahirwar, B.; Ahirwar, D. Isolation and characterization of anti-diabetic component (bioactivity—Guided fractionation) from Ocimum sanctum L. (Lamiaceae) aerial part. Asian Pac. J. Trop. Dis. 2011, 4, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Okoduwa, S.I.; Umar, I.A.; James, D.B.; Inuwa, H.M. Anti-diabetic potential of Ocimum gratissimum leaf fractions in fortified diet-fed streptozotocin treated rat model of type-2 diabetes. Medicines 2017, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, N.; El-Banna, R.; Arafa, M.M.; Hady, M.M. Hypoglycemic efficacy of Rosmarinus officinalis and/or Ocimum basilicum leaves powder as a promising clinico-nutritional management tool for diabetes mellitus in Rottweiler dogs. Vet. World 2020, 13, 73–79. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Kolkeila, A.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Anti-epileptic potential, metabolic profiling and in silico studies of the aqueous fraction from Ocimum menthiifolium benth, family Lamiaceae. Nat. Prod. Res. 2021, 35, 5972–5976. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Chhetri, B.K.; Dosoky, N.S.; Shari, K.; Al-Fahad, A.J.; Wessjohann, L.; Setzer, W.N. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahran, E.M.; Abdelmohsen, U.R.; Shalash, M.M.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Krischke, M.; Mueller, M.; Kamel, M.S. Local anaesthetic potential, metabolic profiling, molecular docking and in silico ADME studies of Ocimum forskolei, family Lamiaceae. Nat. Prod. Res. 2021, 35, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Hussein, A.S.; Salem, M.A.; Khalil, H.E.; Yehia Desoukey, S.; Fouad, M.A.; Kamel, M.S. Antiulcer potential and molecular docking of flavonoids from Ocimum forskolei Benth., family Lamiaceae. Nat. Prod. Res. 2021, 35, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth. (family Lamiaceae). S. Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Zahran, E.M.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. The antiinflammatory activity and LD50 of Ocimum forskolei Benth., family Lamiaceae. J. Adv. Biomed. Pharm. Sci. 2019, 2, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.E.; Alharbi, A.G.A.; Ibrahim, I.M. In vitro antidiabetic assessment of Ocimum forskolei L. growing in Saudi Arabia. J. Pharmacogn. Phytochem. 2019, 8, 355–357. [Google Scholar]
- Adhikari-Devkota, A.; Elbashir, S.M.I.; Watanabe, T.; Devkota, H.P. Chemical constituents from the flowers of Satsuma mandarin and their free radical scavenging and α-glucosidase inhibitory activities. Nat. Prod. Res. 2019, 33, 1670–1673. [Google Scholar] [CrossRef]
- Antony, P.J.; Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Toppo, E.; Sivasankaran, K.; Ignacimuthu, S.; Al-Dhabi, N.A. Myoinositol ameliorates high-fat diet and streptozotocin-induced diabetes in rats through promoting insulin receptor signaling. Biomed. Pharmacother. 2017, 88, 1098–1113. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z. Evaluation of anti-diabetic potential of chromium (III) propionate complex in high-fat diet fed and STZ injected rats. Food Chem. Toxicol. 2011, 49, 3217–3223. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Chen, X.-Q.; Zan, K.; Wen, X.-D.; Chen, H.; Wang, Q.; Lai, M.-X. Antidiabetic and antioxidant effects of extracts from Potentilla discolor Bunge on diabetic rats induced by high fat diet and streptozotocin. J. Ethnopharmacol. 2010, 132, 518–524. [Google Scholar] [CrossRef]
- Ghiasi, R.; Soufi, F.G.; hossein Somi, M.; Mohaddes, G.; Bavil, F.M.; Naderi, R.; Alipour, M.R. Swim training improves HOMA-IR in type 2 diabetes induced by high fat diet and low dose of streptozotocin in male rats. Adv. Pharm. Bull. 2015, 5, 379–384. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Shah, S.; Kumar, R.; Kumar, A.; Shasany, A.K. Comparative temporal metabolomics studies to investigate interspecies variation in three Ocimum species. Sci. Rep. 2020, 10, 5234. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Diversity, phytochemical and medicinal potential of the genus Ocimum L.(Lamiaceae). Phytochem. Rev. 2020, 19, 907–953. [Google Scholar] [CrossRef]
- Antora, R.A.; Salleh, R.M. Antihyperglycemic effect of Ocimum plants: A short review. Asian Pac. J. Trop. Biomed. 2017, 7, 755–759. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Attanayake, A.P.; Kalansuriya, P. Biochemical characterization of high fat diet fed and low dose streptozotocin induced diabetic Wistar rat model. J. Pharmacol. Toxicol. Methods 2022, 113, 107144. [Google Scholar] [CrossRef]
- Radenković, M.; Stojanović, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol. Toxicol. Methods 2016, 78, 13–31. [Google Scholar] [CrossRef]
- Guo, X.-X.; Wang, Y.; Wang, K.; Ji, B.-P.; Zhou, F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B 2018, 19, 559–569. [Google Scholar] [CrossRef]
- Othman, M.S.; Khaled, A.M.; Al-Bagawi, A.H.; Fareid, M.A.; Ghany, R.A.; Habotta, O.A.; Moneim, A.E.A. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother. 2021, 144, 112287. [Google Scholar] [CrossRef]
- Rehman, K.; Munawar, S.M.; Akash, M.S.H.; Buabeid, M.A.; Chohan, T.A.; Tariq, M.; Jabeen, K.; Arafa, E.-S.A. Hesperidin improves insulin resistance via down-regulation of inflammatory responses: Biochemical analysis and in silico validation. PLoS ONE 2020, 15, e0227637. [Google Scholar]
- Akiyama, S.; Katsumata, S.-I.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2009, 46, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic potency, antioxidant effects, and mode of actions of Citrus reticulata fruit peel hydroethanolic extract, hesperidin, and quercetin in nicotinamide/streptozotocin-induced Wistar diabetic rats. Oxid. Med. Cell. Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Jin, J.; Zou, G.; Sui, Y.; Han, Y.; Zhao, D.; Liu, L. Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway. Exp. Ther. Med. 2021, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Ezeani, C.; Ezenyi, I.; Okoye, T.; Okoli, C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. J. Intercult. Ethnopharmacol. 2017, 6, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.K.; Verma, P.R.; Itankar, P.R.; Prasad, S.K.; Nakhate, K.T. Evaluation of pancreatic regeneration activity of Tephrosia purpurea leaves in rats with streptozotocin-induced diabetes. J. Tradit. Complement. Med. 2021, 11, 435–445. [Google Scholar] [CrossRef]
- Almalki, D.A. Renoprotective effect of Ocimum Basilicum (basil) against diabetes-induced renal affection in albino rats. Mater. Socio. Med. 2019, 31, 236–240. [Google Scholar] [CrossRef]
- You, S.; Jang, M.; Kim, G.-H. Mori cortex radicis attenuates high fat diet-induced cognitive impairment via an IRS/Akt signaling pathway. Nutrients 2020, 12, 1851. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative stress, NF-κb-mediated inflammation and apoptosis in the testes of streptozotocin–induced diabetic rats: Combined protective effects of malaysian propolis and metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Gu, Q.; Xiang, L.; Dong, X.; Li, H.; Ni, J.; Wan, L.; Cai, G.; Chen, G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017, 13, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Xie, Z.; Zhang, Q.; Ma, Y.; Bi, X.; Yang, X.; Li, B.; Chen, J. Hyperoside ameliorates diabetic retinopathy via anti-oxidation, inhibiting cell damage and apoptosis induced by high glucose. Front. Pharmacol. 2020, 11, 797. [Google Scholar] [CrossRef]
- Adeneye, A.A.; Adeyemi, O.O.; Agbaje, E.O. Anti-obesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidaemia. J. Ethnopharmacol. 2010, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Gómez, C.; Salgado, L.M.; González-Gallardo, A.; Ramos-Gómez, M.; Loarca-Piña, G.; Reynoso-Camacho, R. Consumption of Ocimum sanctum L. and Citrus paradisi infusions modulates lipid metabolism and insulin resistance in obese rats. Food Funct. 2014, 5, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.; Locher, K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007, 581, 935–938. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Bryan, L.; Nichols, C.G.; Wechsler, S.W.; Clement, J.P., IV; Boyd, A., III; González, G.; Herrera-Sosa, H.; Nguy, K.; Bryan, J.; Nelson, D.A. Cloning of the β cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science 1995, 268, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.K.; Chen, J.; MacKinnon, R. Molecular structure of human KATP in complex with ATP and ADP. eLife 2017, 6, e32481. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Tinahones, F.J. Impaired adipose tissue expandability and lipogenic capacities as ones of the main causes of metabolic disorders. J. Diabetes Res. 2015, 2015, 970375. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-orchestrated metabolic homeostasis in the adipose tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, X.; Yan, Z.; Wang, X.; Gun, S. Isoimperatorin enhances 3T3-L1 preadipocyte differentiation by regulating PPARγ and C/EBPα through the Akt signaling pathway. Exp. Ther. Med. 2019, 18, 2160–2166. [Google Scholar] [CrossRef] [Green Version]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Mirghani, S.J.; Peeri, M.; Yekani, O.Y.; Zamani, M.; Feizolahi, F.; Nikbin, S.; Derakhshideh, A.; Mousavi, N.; Khojasteh, Z.; Nasrollahi, Z. Role or synergistic interaction of adenosine and vitamin D3 alongside high-intensity interval training and isocaloric moderate intensity training on metabolic parameters: Protocol for an Experimental Study. JMIR Res. Protoc. 2019, 8, e10753. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Zhu, C.; Yu, W.; Jiang, X.; Zhang, F. High-fat diet/low-dose streptozotocin-induced type 2 diabetes in rats impacts osteogenesis and Wnt signaling in bone marrow stromal cells. PLoS ONE 2015, 10, e0136390. [Google Scholar] [CrossRef]
- Abdelwahab, S.A.; Elsebay, S.A.G.; Ibrahim, M.F.G.; Hafez, S.M.N.A. Cerebral and cerebellar histological changes in the rat animal model of rotenone induced parkinsonism can be ameliorated by bone marrow derived stem cell conditioned media. J. Chem. Neuroanat. 2021, 111, 101892. [Google Scholar] [CrossRef]
- Abdel Hafez, S.M.N.; Allam, F.A.F.A.; Elbassuoni, E. Sex differences impact the pancreatic response to chronic immobilization stress in rats. Cell Stress Chaperones 2021, 26, 199–215. [Google Scholar] [CrossRef]
- Elbassuoni, E.A.; Abdel Hafez, S.M. Impact of chronic exercise on counteracting chronic stress-induced functional and morphological pancreatic changes in male albino rats. Cell Stress Chaperones 2019, 24, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambantham, K.; Muralidaran, S.; Mandal, A.K.A. Molecular cloning, computational and expression analysis of anthocyanidin reductase in tea (Camellia sinensis). Appl. Biochem. Biotechnol. 2014, 174, 130–145. [Google Scholar] [CrossRef]
- Elhady, S.S.; Youssef, F.S.; Alahdal, A.M.; Almasri, D.M.; Ashour, M.L. Anti-Hyperglycaemic Evaluation of Buddleia indica Leaves Using In Vitro, In Vivo and In Silico Studies and Its Correlation with the Major Phytoconstituents. Plants 2021, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Kwon, Y.I.; Lee, C.; Apostolidis, E.; Kim, Y.C. Antidiabetic effect of chitosan oligosaccharide (GO2KA1) is mediated via inhibition of intestinal alpha-glucosidase and glucose transporters and PPARγ expression. BioFactors 2017, 43, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, H.E.; Ibrahim, H.-I.M.; Darrag, H.M.; Matsunami, K. Insight into Analysis of Essential Oil from Anisosciadium lanatum Boiss.—Chemical Composition, Molecular Docking, and Mitigation of Hepg2 Cancer Cells through Apoptotic Markers. Plants 2022, 11, 66. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.-I.M.; Ahmed, E.A.; Emeka, P.M.; Alhaider, I.A. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals 2022, 15, 154. [Google Scholar] [CrossRef]
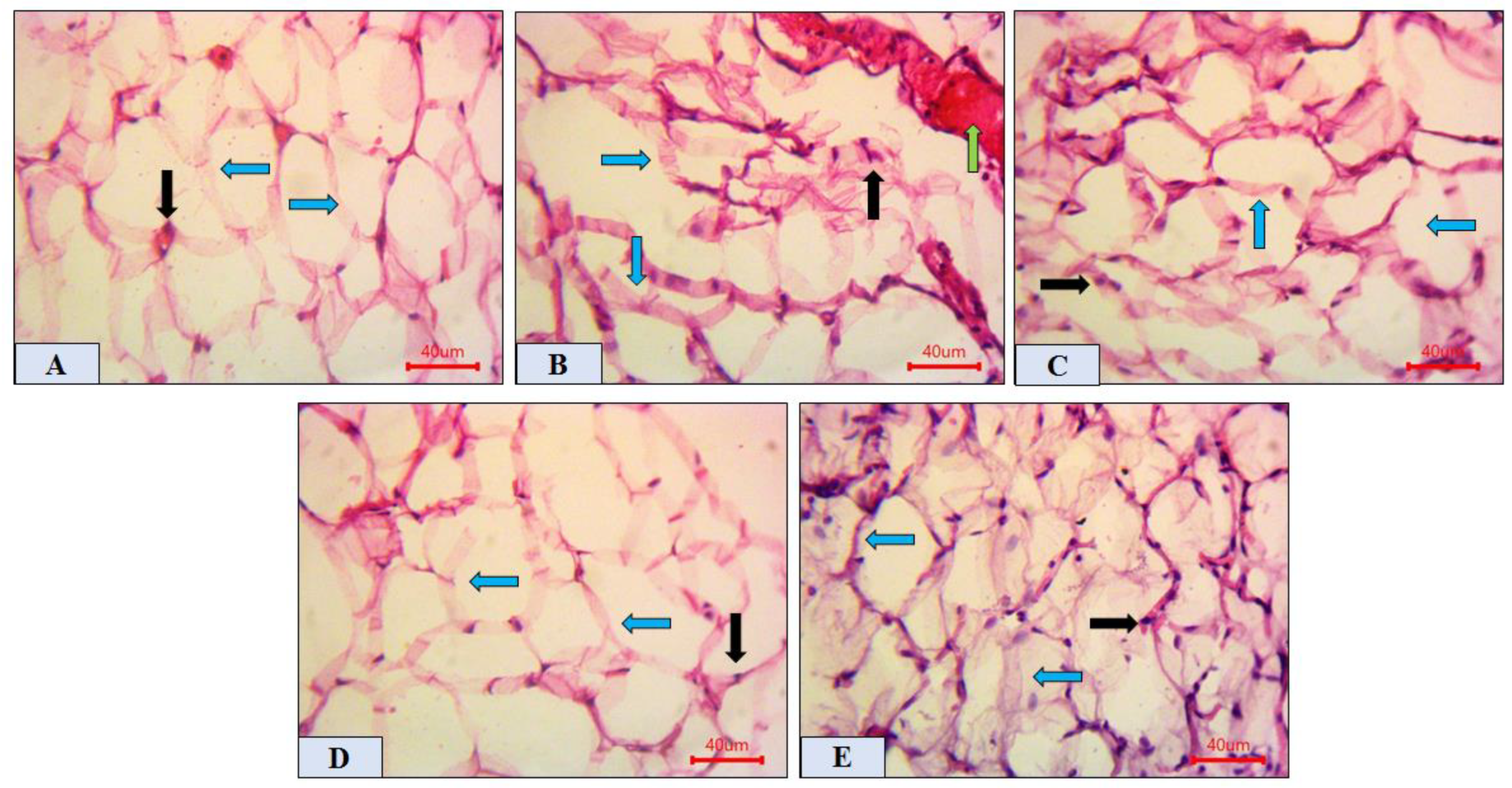


| Groups | NC | DM | DM + GB | DM + TME | DM + HSP |
|---|---|---|---|---|---|
| Degeneration and necrosis | 0.14 ± 0.11 | 6.43 ± 0.37 ac | 4.43 ± 0.30 ab | 2.86 ± 0.26 abc | 4.71 ± 0.42 ab |
| Mononuclear cellular infiltration | 0.63 ± 0.26 | 23.14 ± 0.74 ac | 14.00 ± 1.02 ab | 9.29 ± 0.97 a | 14.57 ± 1.04 ab |
| Hemorrhage | 1.13 ± 0.25 | 4.00 ± 0.27 ac | 2.75 ± 0.16 ab | 1.63 ± 0.26 abc | 3.00 ± 0.27 ab |
| Primer Name | Forward | Reverse | PCR Product Size in Base Pair (bp) |
|---|---|---|---|
| PPARγ | GAAAGACAACGGACAAATCACC | GGGGGTGATATGTTTGAACTTG, | 169 |
| CEBP/α | TTGTTTGGCTTTATCTCGGC, | CCAAGAAGTCGGTGGACAAG | |
| DGAT1 | CTACAGGTCATCTCAGTGCT | GAAGTAGAGCACAGCGATGA | 121 |
| BAX | TGGCAGCTGACATGTTTTCTGAC | TCACCCAACCACCCTGGTCTT- | 195 |
| BCL2 | TCGCCCTGTGGATGACTGA | CAGAGACAGCCAGGAGAAATCA | 134 |
| CASPASE-3 | GTGGAACTGACGATGATATGGC | CGCAAAGTGACTGGATGAACC | 211 |
| NF-κB | CATGAAGAGAAGACACTGACC ATGGAAA | TGGATAGAGGCTAAGTGTAGA CACG | 310 |
| GAPDH | CGTCCCGTAGACAAAATGGT, | TTGATGGCAACAATCTCCAC | 212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.E.; Abdelwahab, M.F.; Emeka, P.M.; Badger-Emeka, L.I.; Thirugnanasambantham, K.; Ibrahim, H.-I.M.; Naguib, S.M.; Matsunami, K.; Abdel-Wahab, N.M. Ameliorative Effect of Ocimum forskolei Benth on Diabetic, Apoptotic, and Adipogenic Biomarkers of Diabetic Rats and 3T3-L1 Fibroblasts Assisted by In Silico Approach. Molecules 2022, 27, 2800. https://doi.org/10.3390/molecules27092800
Khalil HE, Abdelwahab MF, Emeka PM, Badger-Emeka LI, Thirugnanasambantham K, Ibrahim H-IM, Naguib SM, Matsunami K, Abdel-Wahab NM. Ameliorative Effect of Ocimum forskolei Benth on Diabetic, Apoptotic, and Adipogenic Biomarkers of Diabetic Rats and 3T3-L1 Fibroblasts Assisted by In Silico Approach. Molecules. 2022; 27(9):2800. https://doi.org/10.3390/molecules27092800
Chicago/Turabian StyleKhalil, Hany Ezzat, Miada F. Abdelwahab, Promise Madu Emeka, Lorina I. Badger-Emeka, Krishnaraj Thirugnanasambantham, Hairul-Islam Mohamed Ibrahim, Sara Mohamed Naguib, Katsuyoshi Matsunami, and Nada M. Abdel-Wahab. 2022. "Ameliorative Effect of Ocimum forskolei Benth on Diabetic, Apoptotic, and Adipogenic Biomarkers of Diabetic Rats and 3T3-L1 Fibroblasts Assisted by In Silico Approach" Molecules 27, no. 9: 2800. https://doi.org/10.3390/molecules27092800
APA StyleKhalil, H. E., Abdelwahab, M. F., Emeka, P. M., Badger-Emeka, L. I., Thirugnanasambantham, K., Ibrahim, H.-I. M., Naguib, S. M., Matsunami, K., & Abdel-Wahab, N. M. (2022). Ameliorative Effect of Ocimum forskolei Benth on Diabetic, Apoptotic, and Adipogenic Biomarkers of Diabetic Rats and 3T3-L1 Fibroblasts Assisted by In Silico Approach. Molecules, 27(9), 2800. https://doi.org/10.3390/molecules27092800






