Polyoxometalate/Cellulose Nanofibrils Aerogels for Highly Efficient Oxidative Desulfurization
Abstract
:1. Introduction
2. Results
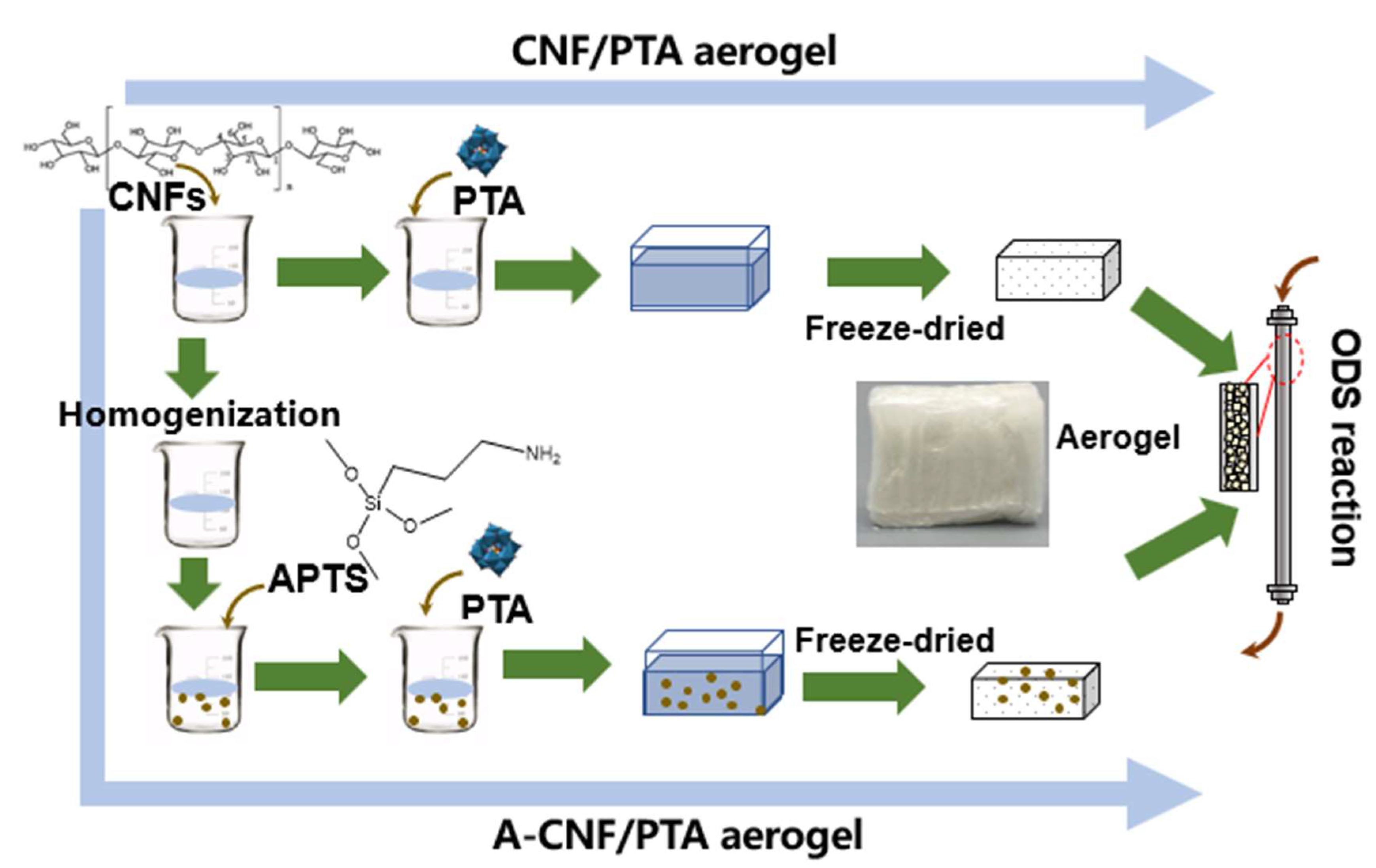
2.1. CNF Aerogel Preparation
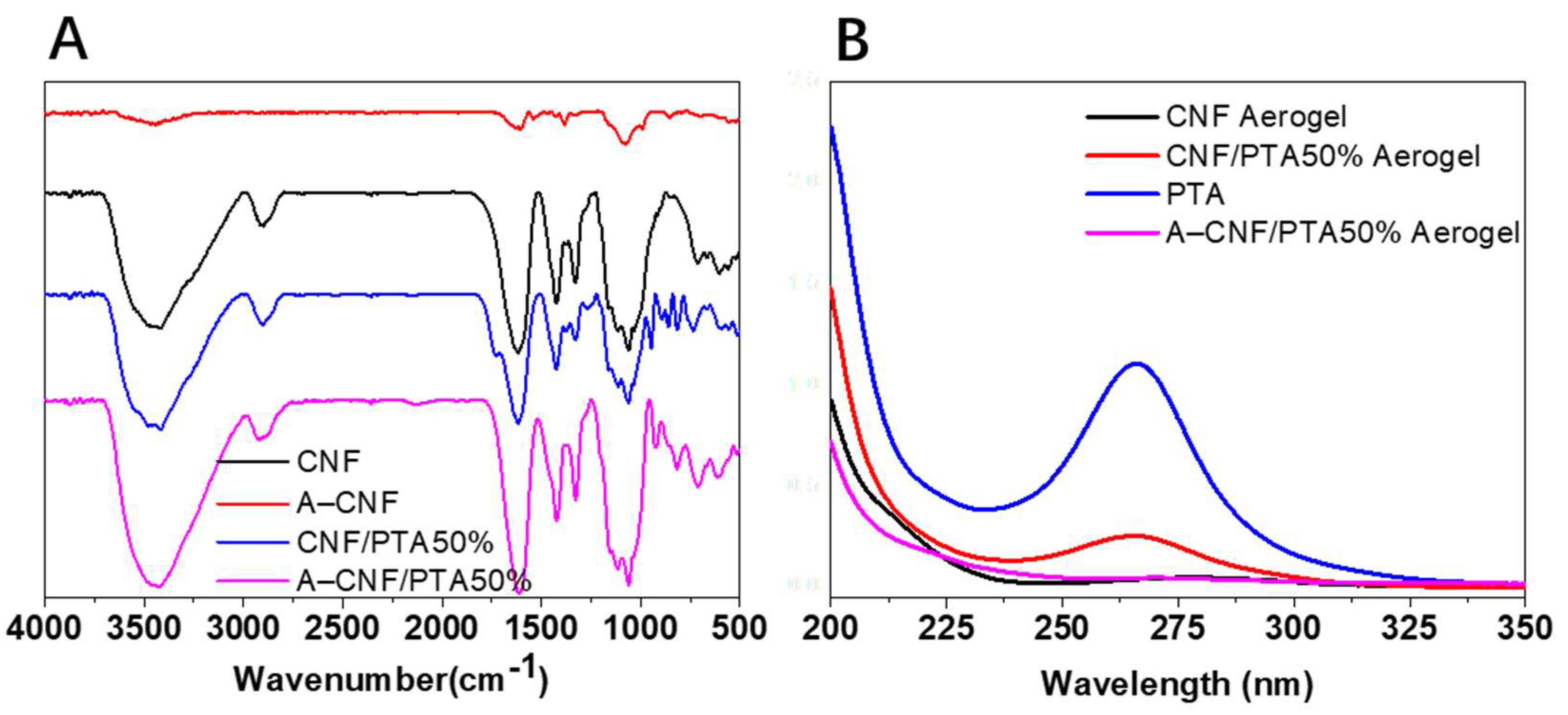
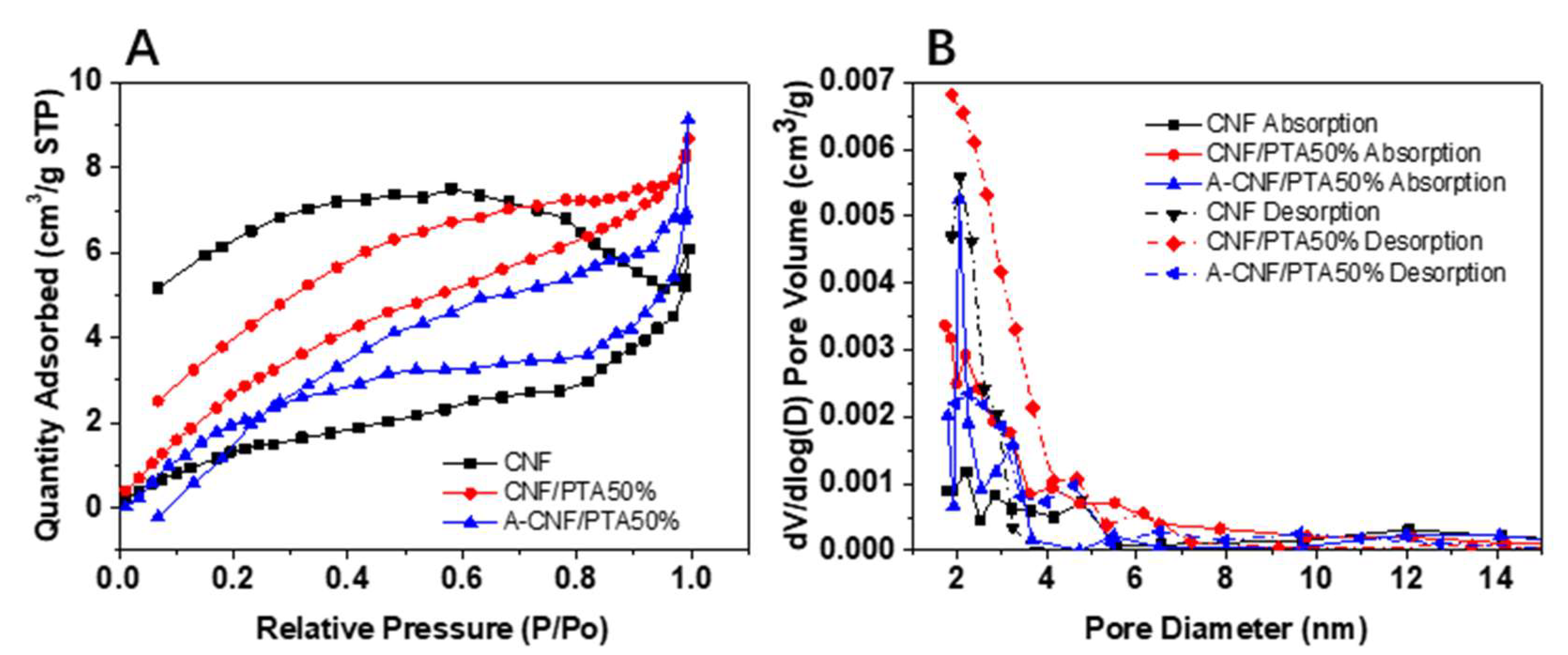
2.2. Morphology and Porosity of CNF Aerogels
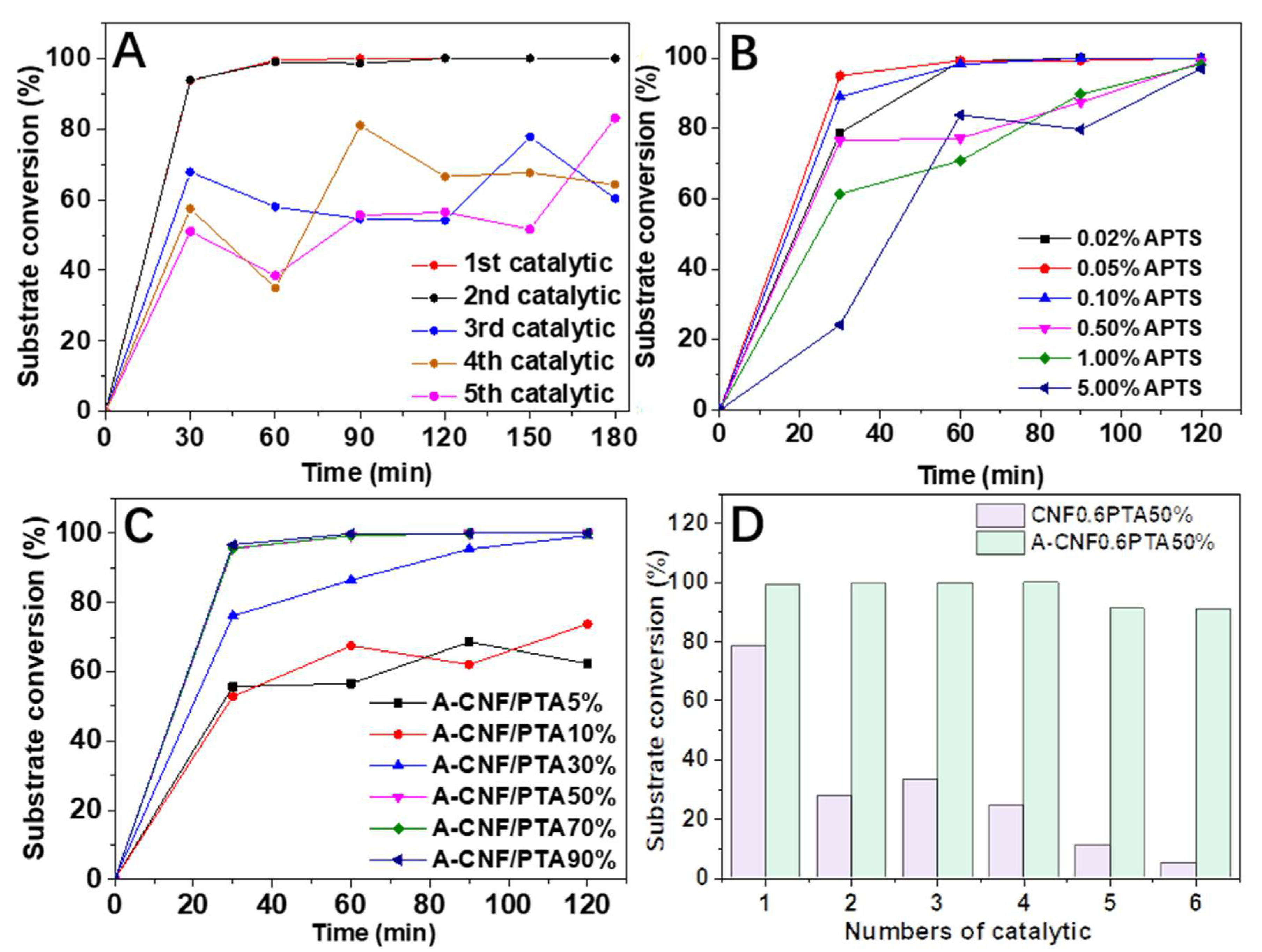
2.3. Static Catalysis
2.4. Dynamic Catalysis
2.5. Changes of CNF Aerogels before and after Catalysis
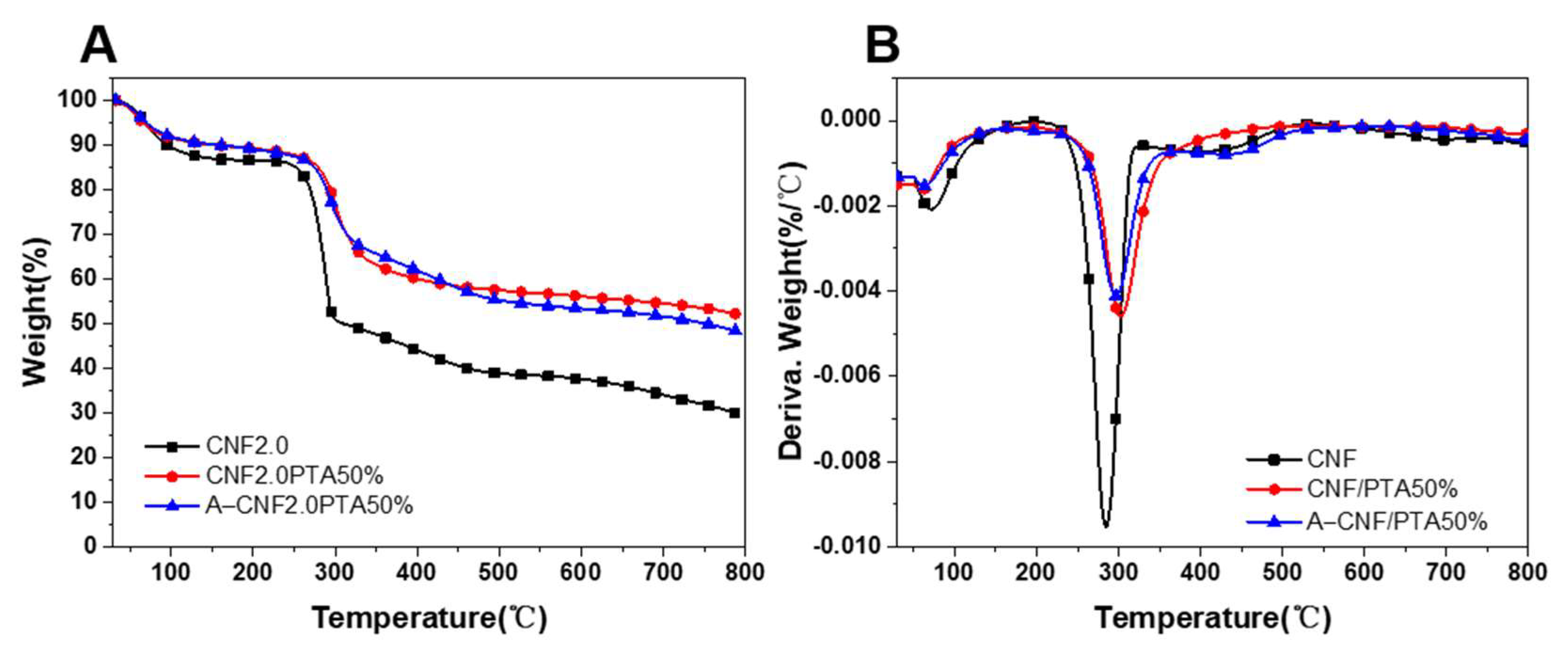
2.6. Thermal Stability of CNF Aerogels
3. Materials and Methods
3.1. Materials
3.2. CNF Aerogel Preparation
3.3. Characterization
3.4. Dissolution Behaviors of the CNF Aerogels
3.5. Static Catalytic Experiments
3.6. Dynamic Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Du, S.; Li, T.; Wang, X.; Zhang, L.; Yang, Z.; Lin, R.; Zhu, T. Molecular simulation on mechanism of thiophene hydrodesulfurization on surface of Ni2P. Energy Explor. Exploit. 2021, 39, 975–992. [Google Scholar] [CrossRef]
- Kochubey, D.I.; Babenko, V.P. Structure of MoS2-based catalysts for hydrodesulfurization prepared via exfoliation. React. Kinet. Catal. Lett. 2002, 77, 237–243. [Google Scholar] [CrossRef]
- Ooi, S.; Zhang, H.B.; Hinode, H. The hydrodesulfurization activity and characterization of cobalt Chevrel phase sulfides. React. Kinet. Catal. Lett. 2004, 82, 89–95. [Google Scholar] [CrossRef]
- Mei, H.; Mei, B.W.; Yen, T.F. A new method for obtaining ultra-low sulfur diesel fuel via ultrasound assisted oxidative desulfurization. Fuel 2003, 82, 405–414. [Google Scholar] [CrossRef]
- Song, C.; Ma, X.L. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B-Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, Y.C.; Li, R.; Zhu, W.S.; Li, H.P.; Zhang, Q.; Wang, M.; Chen, X.; Li, H.M. Magnetic POM-based mesoporous silica for fast oxidation of aromatic sulfur compounds. Fuel 2017, 209, 545–551. [Google Scholar] [CrossRef]
- Zhu, W.S.; Li, H.M.; Jiang, X.; Yan, Y.S.; Lu, J.D.; He, L.N.; Xia, J.X. Commercially available molybdic compound-catalyzed ultra-deep desulfurization of fuels in ionic liquids. Green Chem. 2008, 10, 641–646. [Google Scholar] [CrossRef]
- Fontaine, D.; Eriksen, J.; Sorensen, P. Sulfur from biogas desulfurization: Fate of S during storage in manure and after application to plants. Sci. Total Environ. 2021, 754, 142180. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Polikarpova, P.; Lysenko, S.; Anisimov, A.; Efremenko, E. Formation and use of anaerobic consortia for the biotransformation of sulfur-containing extracts from pre-oxidized crude oil and oil fractions. Bioresour. Technol. 2021, 319, 124248. [Google Scholar] [CrossRef]
- Jiang, X.C.; Nie, Y.; Li, C.X.; Wang, Z. Imidazolium-based alkylphosphate ionic liquids—A potential solvent for extractive desulfurization of fuel. Fuel 2008, 87, 79–84. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Zhou, A.; Yang, C. Desulfurization of model FCC gasoline by extraction with ionic liquid and conventional extraction solvents. Pet. Sci. Technol. 2017, 35, 1699–1705. [Google Scholar] [CrossRef]
- Chen, T.C.; Sapitan, J.F.F.; Ballesteros, F.C.; Lu, M.C. Using activated clay for adsorption of sulfone compounds in diesel. J. Clean. Prod. 2016, 124, 378–382. [Google Scholar] [CrossRef]
- Nanoti, A.; Dasgupta, S.; Goswami, A.N.; Nautiyal, B.R.; Rao, T.V.; Sain, B.; Sharma, Y.K.; Nanoti, S.M.; Garg, M.O.; Gupta, P. Mesoporous silica as selective sorbents for removal of sulfones from oxidized diesel fuel. Microporous Mesoporous Mater. 2009, 124, 94–99. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Coombes, M.J.; Torto, N.; Tshentu, Z.R. The adsorptive extraction of oxidized sulfur-containing compounds from fuels by using molecularly imprinted chitosan materials. React. Funct. Polym. 2014, 81, 61–76. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building Blocks for Functional Nanoscale Systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef]
- Yin, P.; Bayaguud, A.; Cheng, P.; Haso, F.; Hu, L.; Wang, J.; Vezenov, D.; Winans, R.E.; Hao, J.; Li, T.; et al. Spontaneous Stepwise Self-Assembly of a Polyoxometalate-Organic Hybrid into Catalytically Active One-Dimensional Anisotropic Structures. Chem.-A Eur. J. 2014, 20, 9589–9595. [Google Scholar] [CrossRef]
- Jiang, Z.-G.; Mao, W.-T.; Huang, D.-P.; Wang, Y.; Wang, X.-J.; Zhan, C.-H. A nonconventional host–guest cubic assembly based on γ-cyclodextrin and a Keggin-type polyoxometalate. Nanoscale 2020, 12, 10166–10171. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef]
- Cheng, L.; Niu, L.; Gong, J.; Dong, S. Electrochemical Growth and Characterization of Polyoxometalate-Containing Monolayers and Multilayers on Alkanethiol Monolayers Self-Assembled on Gold Electrodes. Chem. Mater. 1999, 11, 1465–1475. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Li, H.; Wu, L. Ionic Complexes of Metal Oxide Clusters for Versatile Self-Assemblies. Acc. Chem. Res. 2017, 50, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Li, D.; Liu, T. Solution behaviors and self-assembly of polyoxometalates as models of macroions and amphiphilic polyoxometalate–organic hybrids as novel surfactants. Chem. Soc. Rev. 2012, 41, 7368–7383. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, R.; Auvray, T.; Volatron, F.; Salzemann, C.; Ngo, A.-T.; Albouy, P.-A.; Proust, A.; Petit, C. Binary Superlattices from {Mo132} Polyoxometalates and Maghemite Nanocrystals: Long-Range Ordering and Fine-Tuning of Dipole Interactions. Small 2016, 12, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhang, J.; Wang, Y.; Wu, Y.; Li, Q.; Liang, Y.; Qi, W.; Rao, H.; Su, R.; He, Z. Self-assembly of multifunctional hydrogels with polyoxometalates helical arrays using nematic peptide liquid crystal template. J. Colloid Interface Sci. 2020, 578, 218–228. [Google Scholar] [CrossRef]
- Makrygenni, O.; Brouri, D.; Proust, A.; Launay, F.; Villanneau, R. Immobilization of polyoxometalate hybrid catalysts onto mesoporous silica supports using phenylene diisothiocyanate as a cross-linking agent. Microporous Mesoporous Mater. 2019, 278, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Chen, D.; Ma, N.; Hou, Z.; Hu, M.; Wang, C.; Wang, W. Covalent immobilization of a polyoxometalate in a porous polymer matrix: A heterogeneous catalyst towards sustainability. RSC Adv. 2013, 3, 21544–21551. [Google Scholar] [CrossRef]
- Jalkh, C.; Ghazaly, C.; El-Rassy, H. Entrapment vs. immobilization of polyoxometalates into silica and titania aerogels: Application in heterogeneous oxidation catalysis. Mater. Chem. Phys. 2020, 252, 123296. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, J.; Yang, L.; Ma, Z.; Wang, L.; Li, H.; Bai, L.; Wei, D.; Wang, W.; et al. Cellulose nanocrystal shelled with poly(ionic liquid)/polyoxometalate hybrid as efficient catalyst for aerobic oxidative desulfurization. J. Colloid Interface Sci. 2019, 554, 572–579. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Xu, W.; Yun, Z. Dehydration of glycerol to acrolein over Wells-Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem. Eng. J. 2017, 316, 797–806. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, P.; Wang, R.; Liu, Y.; Jiao, W. Covalent immobilization of Dawson polyoxometalates on hairy particles and its catalytic properties for the oxidation desulfurization of tetrahydrothiophene. J. Clean. Prod. 2020, 274, 122774. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, L.; Liu, Z.; Yang, L. Polyoxometalate-based 3DOM ZrO(2)material for deep oxidative desulfurization of DBT with ultrahigh stability. J. Porous Mater. 2020, 28, 109–116. [Google Scholar] [CrossRef]
- Ye, G.; Hu, L.; Gu, Y.; Lancelot, C.; Rives, A.; Lamonier, C.; Nuns, N.; Marinova, M.; Xu, W.; Sun, Y. Synthesis of polyoxometalate encapsulated in UiO-66(Zr) with hierarchical porosity and double active sites for oxidation desulfurization of fuel oil at room temperature. J. Mater. Chem. A 2020, 8, 19396–19404. [Google Scholar] [CrossRef]
- Wang, R.X.; Dang, L.F.; Liu, Y.F.; Jiao, W.Z. Preparation, characterization and photocatalytic activity of Dawson type phosphotungstate/graphene oxide composites. Adv. Powder Technol. 2019, 30, 1400–1408. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Sun, M.; Liu, W.; Huang, J.; Xu, H. Self-assembly of highly-dispersed phosphotungstic acid clusters onto graphitic carbon nitride nanosheets as fascinating molecular-scale Z-scheme heterojunctions for photocatalytic solar-to-fuels conversion. Appl. Catal. B-Environ. 2021, 281, 119473. [Google Scholar] [CrossRef]
- Khudhair, E.M.; Ammar, S.H.; Khadim, H.J. Phosphotungstic acid immobilized onto ZnO coated zerovalent iron (Fe@ZnO/PW) core/shell magnetic nanocomposite for enhanced photocatalytic bacterial inactivation under visible light. J. Photochem. Photobiol. A-Chem. 2021, 404, 112907. [Google Scholar] [CrossRef]
- Bordoloi, A.; Lefebvre, E.; Halligudi, S.B. Selective oxidation of anthracene using inorganic-organic hybrid materials based on molybdovanadophosphoric acids. J. Catal. 2007, 247, 166–175. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.C.; Ferrer, M.L.; del Monte, F. Ice-templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation-induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Chen, D.-M.; Liu, X.-H.; Zhang, N.N.; Liu, C.-S.; Du, M. Immobilization of polyoxometalate in a cage-based metal-organic framework towards enhanced stability and highly effective dye degradation. Polyhedron 2018, 152, 108–113. [Google Scholar] [CrossRef]
- Lauinger, S.M.; Sumliner, J.M.; Yin, Q.; Xu, Z.; Liang, G.; Glass, E.N.; Lian, T.; Hill, C.L. High Stability of Immobilized Polyoxometalates on TiO2 Nanoparticles and Nanoporous Films for Robust, Light-Induced Water Oxidation. Chem. Mater. 2015, 27, 5886–5891. [Google Scholar] [CrossRef]
- Ye, J.-J.; Wu, C.-D. Immobilization of polyoxometalates in crystalline solids for highly efficient heterogeneous catalysis. Dalton Trans. 2016, 45, 10101–10112. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Kuang, X.-F.; Wu, X.-Y.; Lu, C.-Z.; Donahue, J.P. Stabilization and immobilization of polyoxometalates in porous coordination polymers through host-guest interactions. Coord. Chem. Rev. 2009, 253, 2872–2890. [Google Scholar] [CrossRef]
- Li, Q.; Hou, Y.-H.; Dong, L.-Y.; Huang, M.-X.; Weng, W.-Z.; Xia, W.-S.; Wan, H.-L. Catalytic Behaviors and Stability of Aerogel Silica-Supported Ni Catalysts for the Partial Oxidation of Methane into Synthesis Gas. Acta Phys.-Chim. Sin. 2013, 29, 2245–2254. [Google Scholar] [CrossRef]
- Li, H.; Pan, J.; Gao, C.; Ma, M.; Lu, L.; Xiong, Y.; Dong, F. Mercapto-Functionalized Porous Organosilica Monoliths Loaded with Gold Nanoparticles for Catalytic Application. Molecules 2019, 24, 4366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, D.-Y.; Qin, J.-S.; Li, S.-L.; Su, Z.-M.; Lan, Y.-Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [Green Version]
- Chi, M.; Su, T.; Sun, L.; Zhu, Z.; Liao, W.; Ren, W.; Zhao, Y.; Lu, H. Biomimetic oxygen activation and electron transfer mechanism for oxidative desulfurization. Appl. Catal. B-Environ. 2020, 275, 119134. [Google Scholar] [CrossRef]
- Li, C.; Jiang, Z.X.; Gao, J.B.; Yang, Y.X.; Wang, S.J.; Tian, F.P.; Sun, F.X.; Sun, X.P.; Ying, P.L.; Han, C.R. Ultra-deep desulfurization of diesel: Oxidation with a recoverable catalyst assembled in emulsion. Chem.-A Eur. J. 2004, 10, 2277–2280. [Google Scholar] [CrossRef]
- Azimzadeh, H.; Akbari, A.; Omidkhah, M.R. Catalytic oxidative desulfurization performance of immobilized NMP center dot FeCl3 ionic liquid on gamma-Al2O3 support. Chem. Eng. J. 2017, 320, 189–200. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Wu, F.; Cao, R. Deep oxidative desulfurization of fuels based on C(4)mimCl CoCl2 ionic liquid oxone solutions at room temperature. Fuel 2017, 208, 508–513. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, S. Sulfoxide and Sulfone Synthesis via Electrochemical Oxidation of Sulfides. J. Org. Chem. 2021, 86, 13790–13799. [Google Scholar] [CrossRef]
- Kumar, D.; Landry, C.C. Immobilization of a Mo,V-polyoxometalate on cationically modified mesoporous silica: Synthesis and characterization studies. Microporous Mesoporous Mater. 2007, 98, 309–316. [Google Scholar] [CrossRef]
- Duan, Y.P.; Wei, W.; Xiao, F.; Xi, Y.R.; Chen, S.L.; Wang, J.L.; Xu, Y.Q.; Hu, C.W. High-valent cationic metal-organic macrocycles as novel supports for immobilization and enhancement of activity of polyoxometalate catalysts. Catal. Sci. Technol. 2016, 6, 8540–8547. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.C.; Lu, M. Synthesis of novel magnetic silica supported hybrid ionic liquid combining TEMPO and polyoxometalate and its application for selective oxidation of alcohols. RSC Adv. 2012, 2, 8265–8268. [Google Scholar] [CrossRef]
- Leng, Y.; Liu, J.; Jiang, P.P.; Wang, J. Heteropolyanion-based polymeric hybrids: Highly efficient and recyclable catalysts for oxidation of alcohols with H2O2. Rsc Adv. 2012, 2, 11653–11656. [Google Scholar] [CrossRef]
- Kasai, J.; Nakagawa, Y.; Uchida, S.; Yamaguchi, K.; Mizuno, N. gamma-1,2-H2SiV2W10O40 immobilized on surface-modified SiO2 as a heterogeneous catalyst for liquid-phase oxidation with H2O2. Chem.-A Eur. J. 2006, 12, 4176–4184. [Google Scholar] [CrossRef]
- Dianat, S.; Tangestaninejad, S.; Yadollahi, B.; Bordbar, A.-K.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I. Stability investigation of some heteropolyoxotungstate and heteropolyoxomolybdate salts in buffer solutions. J. Mol. Liq. 2012, 174, 76–79. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Z.; Dong, L.; Miao, G.; Liao, N.; Li, Z.; Xiao, J. Dynamic catalytic adsorptive desulfurization of real diesel over ultra-stable and low-cost silica gel-supported TiO2. AlChE J. 2018, 64, 2146–2159. [Google Scholar] [CrossRef]
- Brander, S.; Tokin, R.; Ipsen, J.O.; Jensen, P.E.; Hernandez-Rollan, C.; Norholm, M.H.H.; Lo Leggio, L.; Dupree, P.; Johansen, K.S. Scission of Glucosidic Bonds by a Lentinus similis Lytic Polysaccharide Monooxygenases Is Strictly Dependent on H2O2 while the Oxidation of Saccharide Products Depends on O2. ACS Catal. 2021, 11, 13848–13859. [Google Scholar] [CrossRef]
- Wen, J.; Yin, Y.; Peng, X.; Zhang, S. Using H2O2 to selectively oxidize recyclable cellulose yarn with high carboxyl content. Cellulose 2019, 26, 2699–2713. [Google Scholar] [CrossRef]
- Qi, Z.; Huang, Z.; Wang, H.; Li, L.; Ye, C.; Qiu, T. In situ bridging encapsulation of a carboxyl-functionalized phosphotungstic acid ionic liquid in UiO-66: A remarkable catalyst for oxidative desulfurization. Chem. Eng. Sci. 2020, 225, 115818. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Yu, Y.-Z.; Hong, Y.-L.; Li, N.-F.; Han, Y.-M.; Cao, J.-P.; Sun, Q.; Mei, H.; Xu, Y. Polyoxometalate-Based Metal-Organic Frameworks with Unique High-Nuclearity Water Clusters. ACS Appl. Mater. Interfaces 2020, 12, 57174–57181. [Google Scholar] [CrossRef] [PubMed]

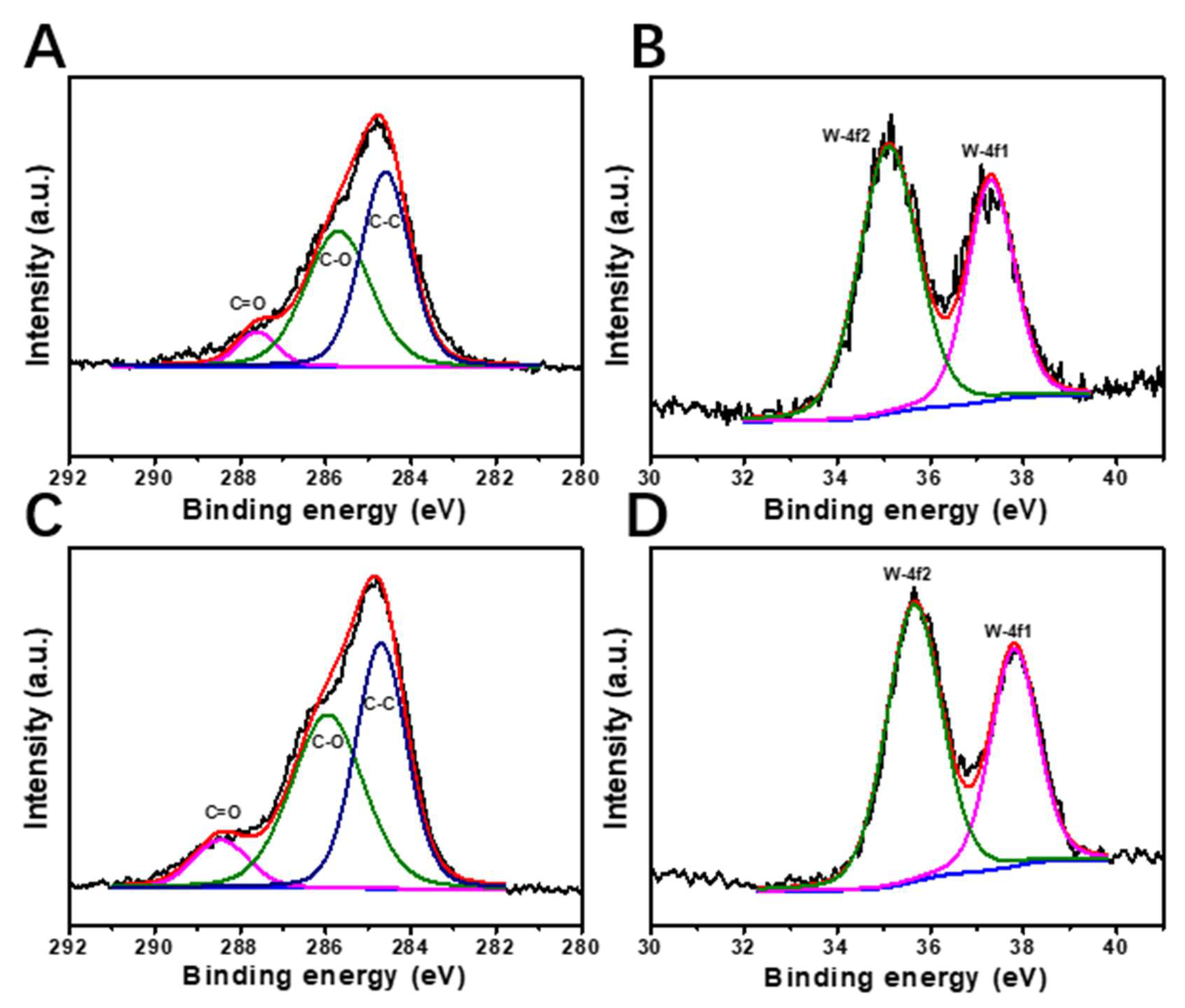
| Sample | Elemental Quantitative Analysis by XPS (%) | ||||
|---|---|---|---|---|---|
| C-C | C-O | C=O | W-4f1 | W-4f2 | |
| Before | 48.37 | 44.65 | 6.98 | 39.27 | 60.73 |
| After | 45.18 | 46.46 | 8.36 | 38.50 | 61.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Zhang, X.; Wang, H.; Liu, C. Polyoxometalate/Cellulose Nanofibrils Aerogels for Highly Efficient Oxidative Desulfurization. Molecules 2022, 27, 2782. https://doi.org/10.3390/molecules27092782
Song R, Zhang X, Wang H, Liu C. Polyoxometalate/Cellulose Nanofibrils Aerogels for Highly Efficient Oxidative Desulfurization. Molecules. 2022; 27(9):2782. https://doi.org/10.3390/molecules27092782
Chicago/Turabian StyleSong, Rui, Xueqin Zhang, Huihui Wang, and Chuanfu Liu. 2022. "Polyoxometalate/Cellulose Nanofibrils Aerogels for Highly Efficient Oxidative Desulfurization" Molecules 27, no. 9: 2782. https://doi.org/10.3390/molecules27092782
APA StyleSong, R., Zhang, X., Wang, H., & Liu, C. (2022). Polyoxometalate/Cellulose Nanofibrils Aerogels for Highly Efficient Oxidative Desulfurization. Molecules, 27(9), 2782. https://doi.org/10.3390/molecules27092782







