DFT Investigations of Aun Nano-Clusters Supported on TiO2 Nanotubes: Structures and Electronic Properties
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
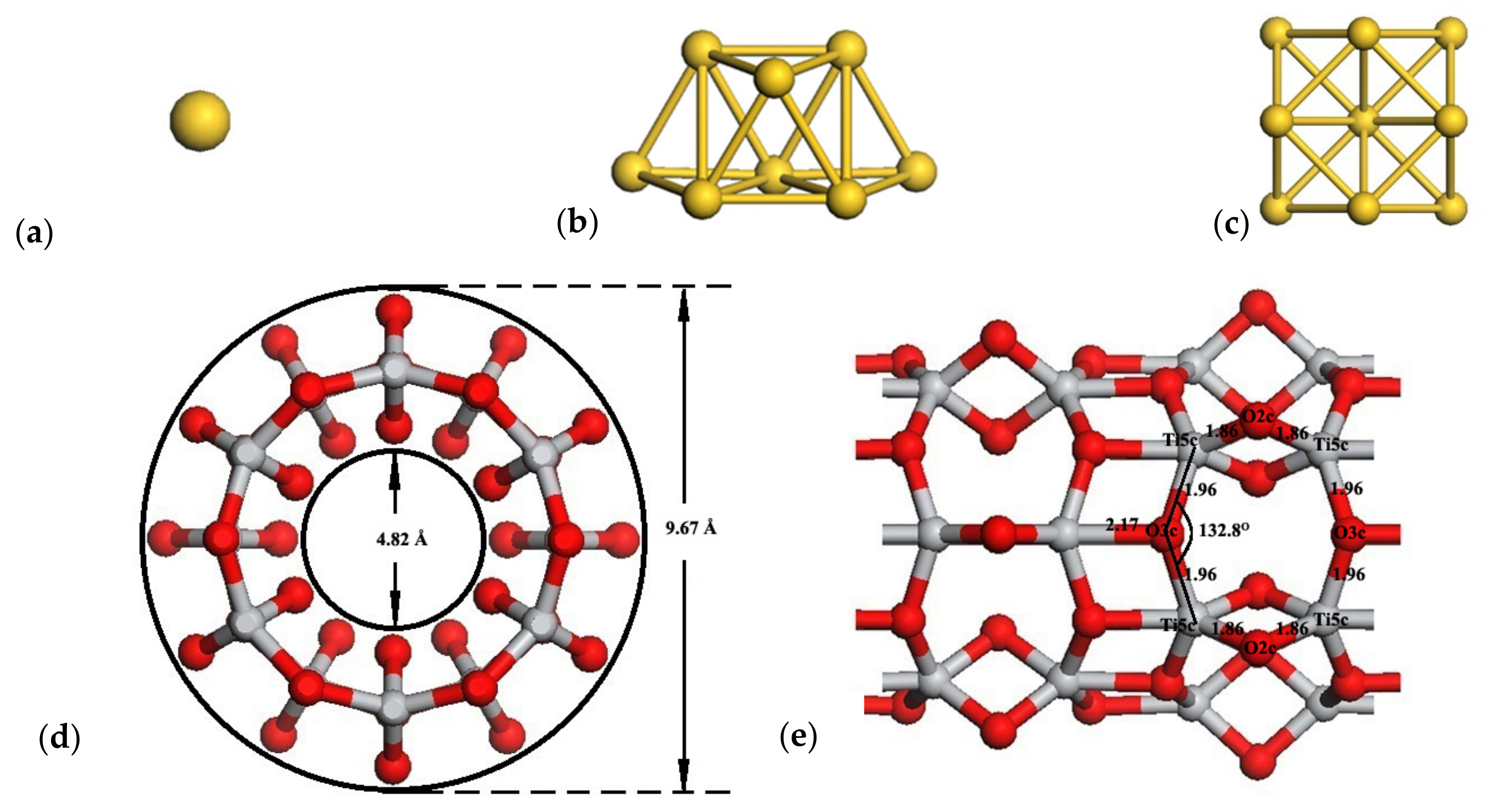
3.1. Structures of Anatase TiO2 Nanotubes
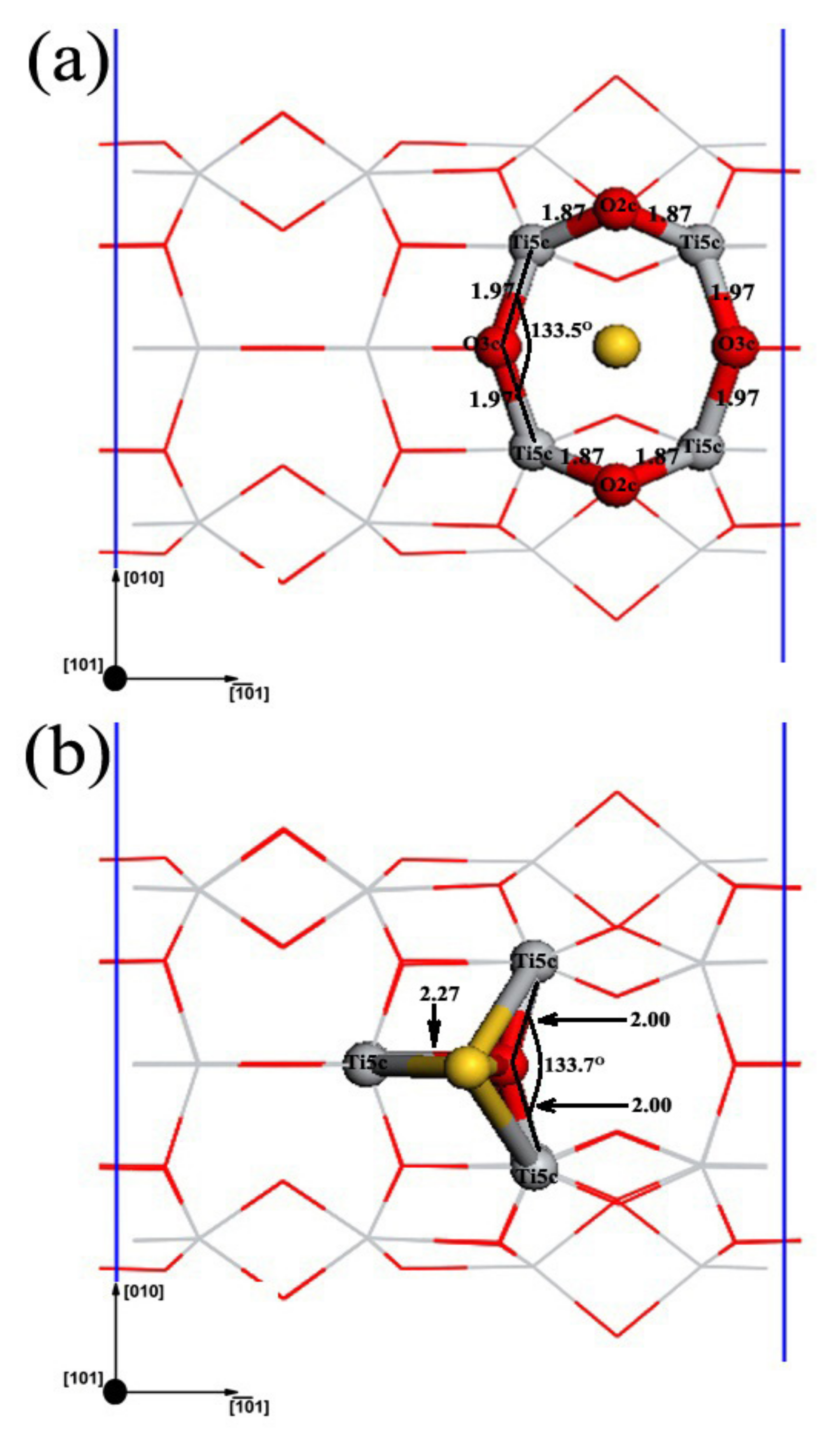
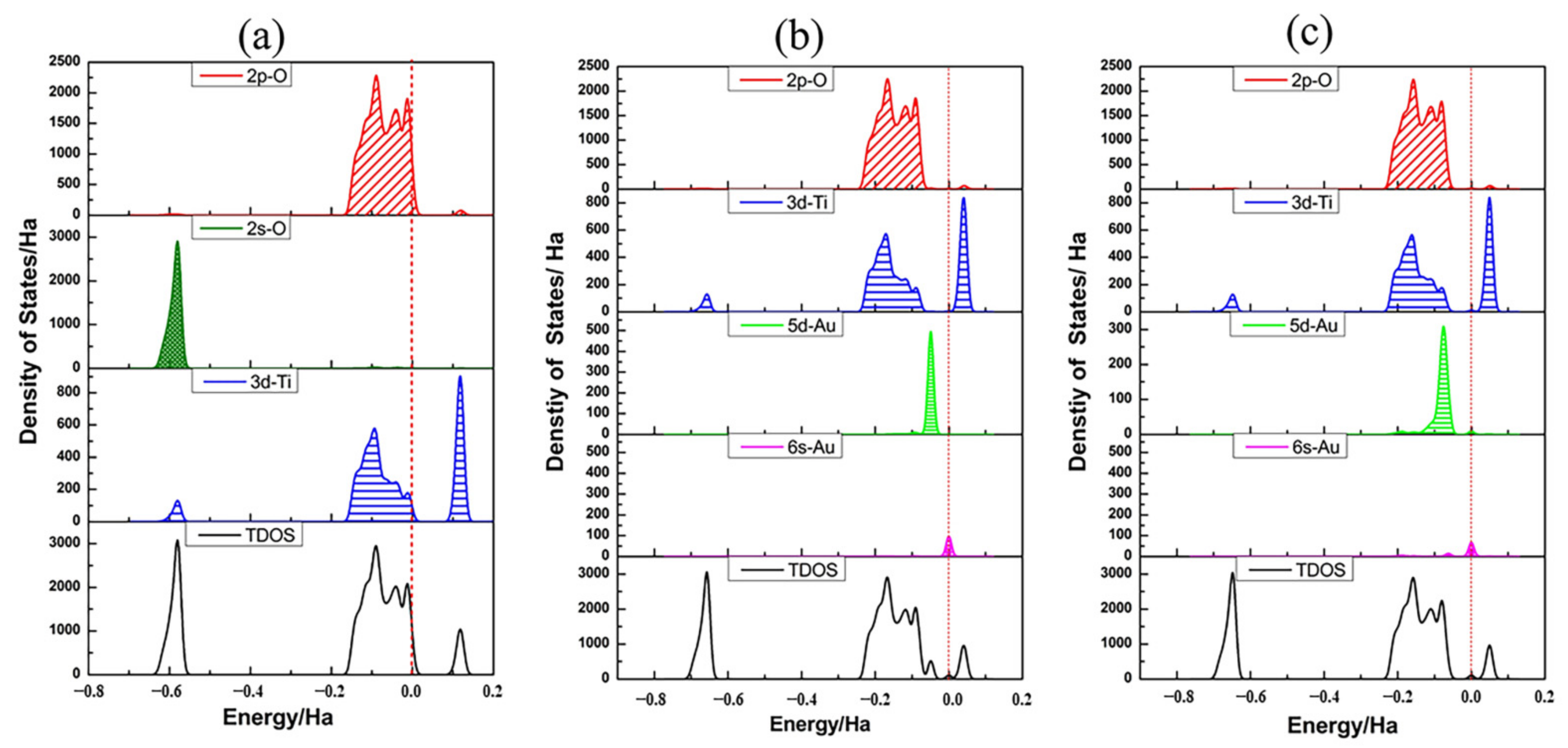
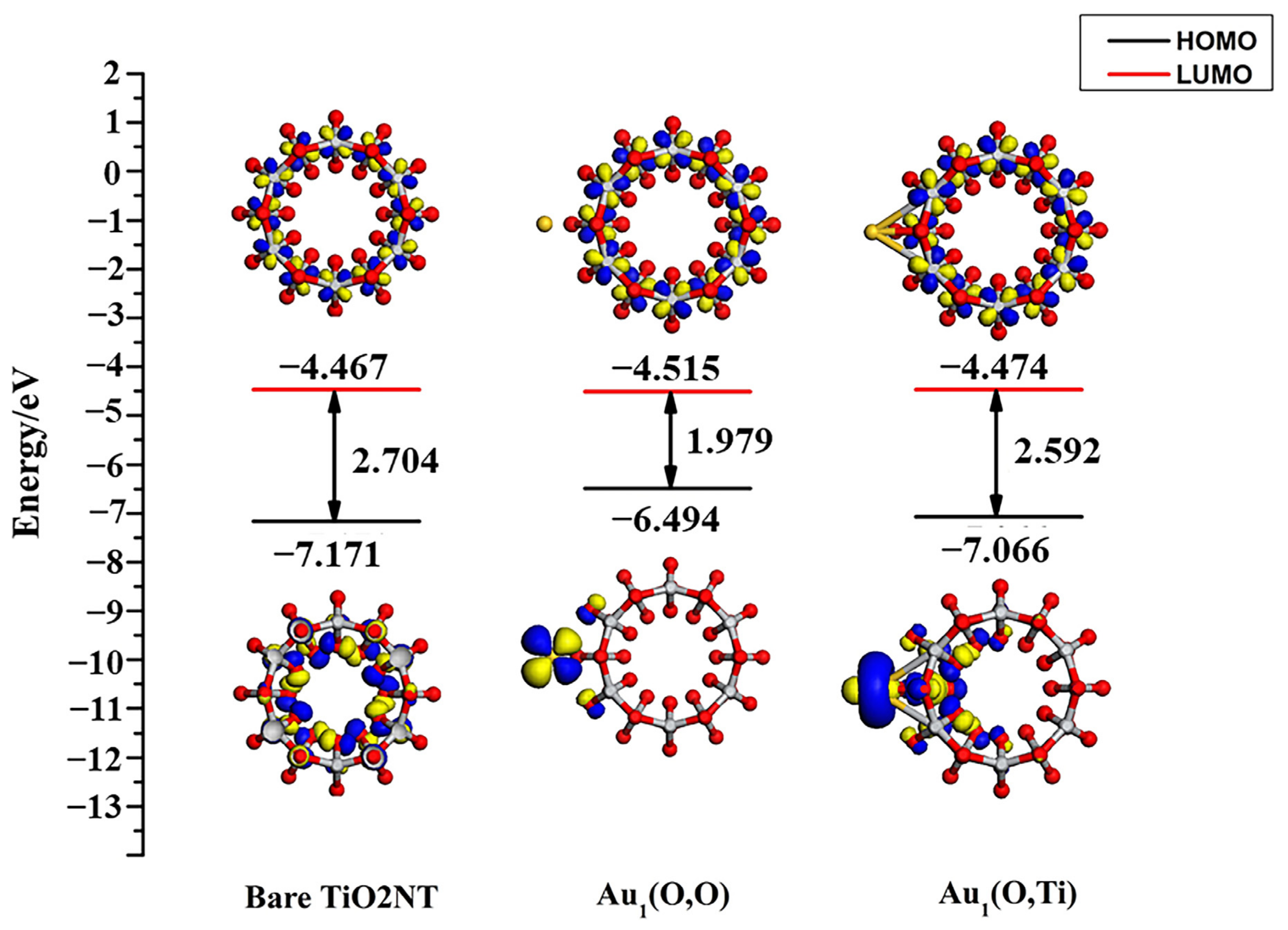
3.2. Structures of Au1/TiO2NTs
3.3. Structures of Au8/TiO2NT
3.4. Structures of Au13/TiO2NT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, X.B. Small Au and Pt Clusters at the Anatase TiO2 (101) Surface: Behavior at Terraces, Steps, and Surface Oxygen Vacancies. J. Am. Chem. Soc. 2008, 130, 370–381. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Ge, Q. Interaction of Pt Clusters with the Anatase TiO2 (101) Surface: A First Principles Study. J. Phys. Chem. B 2006, 110, 7463–7472. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, A.; Selloni, A. Small Gold Clusters on Stoichiometric and Defected TiO2 Anatase (101) and Their Interaction with CO: A Density Functional Study. J. Chem. Phys. 2002, 117, 353–361. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, M.; Chu, J.; Jiang, T.; Yin, H. Preparation, Characterization of Au (or Pt)-Loaded Titania Nanotubes and Their Photocatalytic Activities for Degradation of Methyl Orange. Appl. Surf. Sci. 2009, 255, 3773–3778. [Google Scholar] [CrossRef]
- Goldman, N.; Browning, N.D. Gold Cluster Diffusion Kinetics on Stoichiometric and Reduced Surfaces of Rutile TiO2(110). J. Phys. Chem. C 2011, 115, 11611–11617. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Tang, W.; Neurock, M.; Yates, J.T. Electric Charge of Single Au Atoms Adsorbed on TiO2 (110) and Associated Band Bending. J. Phys. Chem. C 2011, 115, 23848–23853. [Google Scholar] [CrossRef]
- Çakir, D.; Gülseren, O. Adsorption of Pt and Bimetallic PtAu Clusters on the Partially Reduced Rutile (110) TiO2 Surface: A First-Principles Study. J. Phys. Chem. C 2012, 116, 5735–5746. [Google Scholar] [CrossRef]
- Matthey, D.; Wang, J.G.; Wendt, S.; Matthiesen, J.; Schaub, R.; Lægsgaard, E.; Hammer, B.; Besenbacher, F. Enhanced Bonding of Gold Nanoparticles on Oxidized TiO2 (110). Science 2007, 315, 1692–1696. [Google Scholar] [CrossRef]
- Fu, C.; Li, M.; Li, H.; Li, C.; Wu, X.G.; Yang, B. Fabrication of Au Nanoparticle/TiO2 Hybrid Films for Photoelectrocatalytic Degradation of Methyl Orange. J. Alloys Compd. 2017, 692, 727–733. [Google Scholar] [CrossRef]
- Galeanoa, L.; Valencia, S.; Restrepo, G.; Marin, J.M. Dry-co-Grinding of Doped TiO2 With Nitrogen, Silicon or Selenium for Enhanced Photocatalytic Activity under UV/Visible and Visible Light Irradiation for Environmental Applications. Mater. Sci. Semicond. Process. 2019, 91, 47–57. [Google Scholar] [CrossRef]
- Maolanon, R.; Wongwiriyapan, W.; Pratontep, S. TiO2/Pt/TiO2 Sandwich Nanostructures: Towards Alcohol Sensing and UV Irradiation-Assisted Recovery. J. Chem. 2017, 2017, 8545690. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Gul, T.; Sadiq, M. Efficient Photodegradation of Methyl Violet Dye Using TiO2/Pt and TiO2/Pdphotocatalysts. Appl. Water Sci. 2017, 7, 3841–3848. [Google Scholar] [CrossRef]
- Pan, X.; Chen, X.; Yi, Z. Defective, Porous TiO2 Nanosheets with Pt Decoration as an Efficient Photocatalyst for Ethylene Oxidation Synthesized by a C3N4 Templating Method. ACS Appl. Mater. Interfaces 2016, 8, 10104–10108. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Scott, J.A.; Amal, R. Progress in Heterogeneous Photocatalysis: From Classical Radical Chemistry to Engineering Nanomaterials and Solar Reactors. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef]
- Miyasaka, T. Toward Printable Sensitized Mesoscopic Solar Cells: Light-Harvesting Management with Thin TiO2 Films. J. Phys. Chem. Lett. 2011, 2, 262–269. [Google Scholar] [CrossRef]
- Mora-Seró, I.; Bisquert, J. Breakthroughs in the Development of Semiconductor-Sensitized Solar Cells. J. Phys. Chem. Lett. 2010, 1, 3046–3052. [Google Scholar] [CrossRef]
- Braga, A.; Giménez, S.; Concina, I.; Vomiero, A.; Mora-Seró, I. Panchromatic Sensitized Solar Cells Based on Metal Sulfide Quantum Dots Grown Directly on Nanostructured TiO2 Electrodes. J. Phys. Chem. Lett. 2011, 2, 454–460. [Google Scholar] [CrossRef]
- Mowbray, D.J.; Martínez, J.I.; Calle-Vallejo, F.; Rossmeisl, J.; Thygesen, K.S.; Jacobsen, K.W.; Nørskov, J.K. Trends in Metal Oxide Stability for Nanorods, Nanotubes, and Surfaces. J. Phys. Chem. C 2011, 115, 2244–2252. [Google Scholar] [CrossRef]
- Yu, J.G.; Hai, Y.; Cheng, B. Enhanced Photocatalytic H2-Production Activity of TiO2 by Ni(OH)2 Cluster Modification. J. Phys. Chem. C 2011, 115, 4953–4958. [Google Scholar] [CrossRef]
- Petrik, N.G.; Kimmel, G.A. Electron-and Hole-Mediated Reactions in UV-Irradiated O2 Adsorbed on Reduced Rutile TiO2 (110). J. Phys. Chem. C 2011, 115, 152–164. [Google Scholar] [CrossRef]
- Chrétien, S.; Metiu, H. Electronic Structure of Partially Reduced Rutile TiO2 (110) Surface: Where Are the Unpaired Electrons Located? J. Phys. Chem. C 2011, 115, 4696–4705. [Google Scholar] [CrossRef]
- Deskins, N.A.; Rousseau, R.; Dupuis, M. Defining the Role of Excess Electrons in the Surface Chemistry of TiO2. J. Phys. Chem. C 2010, 114, 5891–5897. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and Optical Properties of Anatase TiO2. Phys. Rev. B 2000, 6, 7459–7465. [Google Scholar] [CrossRef]
- Tang, H.; Berger, H.; Schmid, P.E.; Lévy, F. Photoluminescence in TiO2 Anatase Single Crystals. Solid State Commun. 1993, 87, 847–850. [Google Scholar] [CrossRef]
- Pu, Y.C.; Wang, G.; Chang, K.D.; Ling, Y.; Lin, Y.K.; Fitzmorris, B.C.; Liu, C.M.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity across Entire UV-visible Region for Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis. Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous Nanomaterials for the Enhancement of TiO2 Photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Gao, L.D.; Le, Y.; Wang, J.X.; Chen, J.F. Preparation and Characterization of Titanium Nanotubes with Mesostructured Walls. Mater. Lett. 2006, 60, 3882–3886. [Google Scholar] [CrossRef]
- Nunzi, F.; Angelis, F.D. DFT Investigations of Formic Acid Adsorption on Single-Wall TiO2 Nanotubes: Effect of the Surface Curvature. J. Phys. Chem. C 2011, 115, 2179–2186. [Google Scholar] [CrossRef]
- Shankar, K.; Bandara, J.; Paulose, M.; Wietasch, H.; Varghese, O.K.; Mor, G.K.; LaTempa, T.J.; Thelakkat, M.; Grimes, C.A. Highly Efficient Solar Cells using TiO2 Nanotube Arrays Sensitized with a Donor-Antenna Dye. Nano Lett. 2008, 8, 1654–1659. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Nano Micro Small 2007, 3, 300–304. [Google Scholar] [CrossRef]
- Kuang, D.; Brillet, J.R.M.; Chen, P.; Takata, M.; Uchida, S.; Miura, H.; Sumioka, K.; Zakeeruddin, S.M.; Grätzel, M. Application of Highly Ordered TiO2 Nanotube Arrays in Flexible Dye-Sensitized Solar Cells. ACS Nano 2008, 2, 1113–1116. [Google Scholar] [CrossRef]
- Hossain, F.M.; Evteev, A.V.; Belova, I.V.; Nowotny, J.; Murch, G.E. Electronic and Optical Properties of Anatase TiO2 Nanotubes. Comput. Mater. Sci. 2010, 48, 854–858. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Chang, Y.C.; Tsai, C.W.; Liu, R.S.; Hu, S.F.; Chang, W.S.; Chen, K.H. Quantum Dot Monolayer Sensitized ZnO Nanowire-Array Photoelectrodes: True Efficiency for Water Splitting. Angew. Chem. Int. Ed. 2010, 49, 5966–5969. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chi, C.F.; Liau, S.Y. CdS/CdSe Co-Sensitized TiO2 Photoelectrode for Efficient Hydrogen Generation in a Photoelectrochemical Cell. Chem. Mater. 2010, 22, 922–927. [Google Scholar] [CrossRef]
- Gao, Z.D.; Liu, H.F.; Li, C.Y.; Song, Y.Y. Biotemplated Synthesis of Au Nanoparticles–TiO2 Nanotube Junctions for Enhanced Direct Electrochemistry of Heme Proteins. Chem. Commun. 2013, 49, 774–776. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, X.; Ren, S.; Tian, Z.; Fan, S.; Sun, S.; Liu, S.; Wang, Q. Improved Visible Solar Absorber Based on TiO2 Nanotube Film by Surface-Loading of Plasmonic Au Nanoparticles. J. Appl. Phys. 2013, 114, 063510. [Google Scholar] [CrossRef]
- Chen, S.; Malig, M.; Tian, M.; Chen, A. Electrocatalytic Activity of PtAu Nanoparticles Deposited on TiO2 Nanotubes. J. Phys. Chem. C 2012, 116, 3298–3304. [Google Scholar] [CrossRef]
- Bao, H.H. Microwave Synthesis and Charaterization of Ag, Au, Pt nanoparticles Supported on TiO2 Nanotubes. Chin. J. Inorg. Chem. 2005, 21, 374–378. [Google Scholar]
- Xiao, F. An Efficient Layer-by-Layer Self-Assembly of Metal-TiO2 Nanoring/Nanotube Heterostructures, M/T-NRNT (M = Au, Ag, Pt), for Versatile Catalytic Applications. Chem. Commun. 2012, 48, 6538–6540. [Google Scholar] [CrossRef]
- Zhu, B.L.; Guo, Q.; Wang, S.; Zheng, X.; Zhang, S.; Wu, S.; Huang, W. Synthesis of MetalDdoped TiO2 Nanotubes and Their Catalytic Performance for Low-Temperature CO Oxidation. React. Kinet. Catal. Lett. 2006, 88, 301–308. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Tang, L.; Wang, J.; Pan, H.; Du, M. Electrochemical Sensor for Detection of Hydrazine Based on Au@Pd Core–Shell Nanoparticles Supported on Amino-Functionalized TiO2 Nanotubes. Mater. Sci. Eng. C 2014, 34, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, Characterization and Visible-Light-Driven Photocatalytic Activity of Fe-Doped Titaniananorods and First-Principles Study for Electronic Structures. App. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Diebold, U.; Lehman, J.; Mahmoud, T.; Kuhn, M.; Leonardelli, G.; Hebenstreit, W.; Schmid, M.; Varga, P. Intrinsic Defects on a TiO2 (110)(1×1) Surface and Their Reaction with Oxygen: A Scanning Tunneling Microscopy Study. Surf. Sci. 1998, 411, 137–153. [Google Scholar] [CrossRef]
- Hengerer, R.; Bolliger, B.; Erbudak, M.; Gratzel, M. Structure and Stability of the Anatase TiO2 (101) and (001) Surfaces. Surf. Sci. 2000, 460, 162–169. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and Energetics of Stoichiometric TiO2 Anatase Surfaces. Phys. Rev. B 2001, 63, 155409. [Google Scholar] [CrossRef]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef]
- De Sekhar, H.; Krishnamurty, S.; Sourav, P. Understanding the Reactivity Properties of Aun (6 ≤ n ≤ 13) Clusters Using Density Functional Theory Based Reactivity Descriptors. J. Phys. Chem. C 2010, 114, 6690–6703. [Google Scholar] [CrossRef]
- Sanchez, A.; Abbet, S.; Heiz, U.; Schneider, W.D.; Häkkinen, H.; Barnett, R.N.; Landman, U. When Gold Is Not Noble: Nanoscale Gold Catalysts. J. Phys. Chem. A 1999, 103, 9573–9578. [Google Scholar] [CrossRef]
- Häkkinen, H.; Abbet, S.; Sanchez, A.; Heiz, U.; Landman, U. Structural, Electronic, and Impurity-Doping Effects in Nanoscale Chemistry: Supported Gold Nanoclusters. Angew. Chem. Int. Ed. 2003, 42, 1297–1300. [Google Scholar] [CrossRef]
- Yoon, B.; Häkkinen, H.; Landman, U.; Wörz, A.S.; Antonietti, J.M.; Abbet, S.; Judai, K.; Heiz, U. Charging Effects on Bonding and Catalyzed Oxidation of CO on Au8 Clusters on MgO. Science 2005, 307, 403–407. [Google Scholar] [CrossRef]
- Fernández, E.M.; Soler, J.M.; Garzón, I.L.; Balbás, L.C. Trends in the Structure and Bonding of Noble Metal Clusters. Phys. Rev. B 2004, 70, 165403. [Google Scholar] [CrossRef]
- Oviedo, J.; Palmer, R.E. Amorphous Structures of Cu, Ag, and Au Nanoclusters from First Principles Calculations. J. Chem. Phys. 2002, 117, 9548–9551. [Google Scholar] [CrossRef]
- Chang, C.M.; Chou, M.Y. Alternative Low-Symmetry Structure for 13-Atom Metal Clusters. Phys. Rev. Lett. 2004, 93, 133401–133404. [Google Scholar] [CrossRef]
- Shafai, G.; Hong, S.; Bertino, M.; Rahman, T.S. Effect of Ligands on the Geometric and Electronic Structure of Au13 Clusters. J. Phys. Chem. C 2009, 113, 12072–12078. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Delley, B. An Allelectron Numerical Method for Solving the Local Density Functional Forpolyatomic Molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From Molecules to Solids with the DMol3 Approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Dong, C.; Li, X.; Qi, J. First-Principles Investigation on Electronic Properties of Quantum Dot-Sensitized Solar Cells Based on Anatase TiO2 Nanotubes. J. Phys. Chem. C 2011, 115, 20307–20315. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Burdett, J.K.; Hughbanks, T.; Miller, G.J.; Richardson, J.W., Jr.; Smith, V. Structural-Electronic Relationships in Inorganic Solids: Powder Neutron Diffraction Studies of the Rutile and Anatase Polymorphs of Titanium Dioxide at 15 and 295 K. J. Am. Chem. Soc. 1987, 109, 3639–3646. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Zhukovskii, Y.F.; Bandura, A.V.; Piskunov, S. Symmetry and Models of Single-Walled TiO2 Nanotubes with Rectangular Morphology. Cent. Eur. J. Phys. 2011, 9, 492–501. [Google Scholar] [CrossRef]
- Bandura, A.V.; Evarestov, R.A. From Anatase (101) Surface to TiO2 Nanotubes: Rolling Procedure and First Principles LCAO Calculations. Surf. Sci. 2009, 603, 117–120. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, C.; Moon, H.; Choi, Y.; Park, S.; Lee, C.-K.; Han, S. Electronic Structure Tailoring and Selective Adsorption Mechanism of Metal-coated Nanotubes. Nano Lett. 2008, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Springborg, M. Global Structure Optimization Study on Au2–20. Eur. Phys. J. D 2007, 43, 15–18. [Google Scholar] [CrossRef]
- Hong, S.; Rahman, T.S. Rationale for the Higher Reactivity of Interfacial Sites in Methanol Decomposition on Au13/TiO2(110). J. Am. Chem. Soc. 2013, 135, 7629–7635. [Google Scholar] [CrossRef]
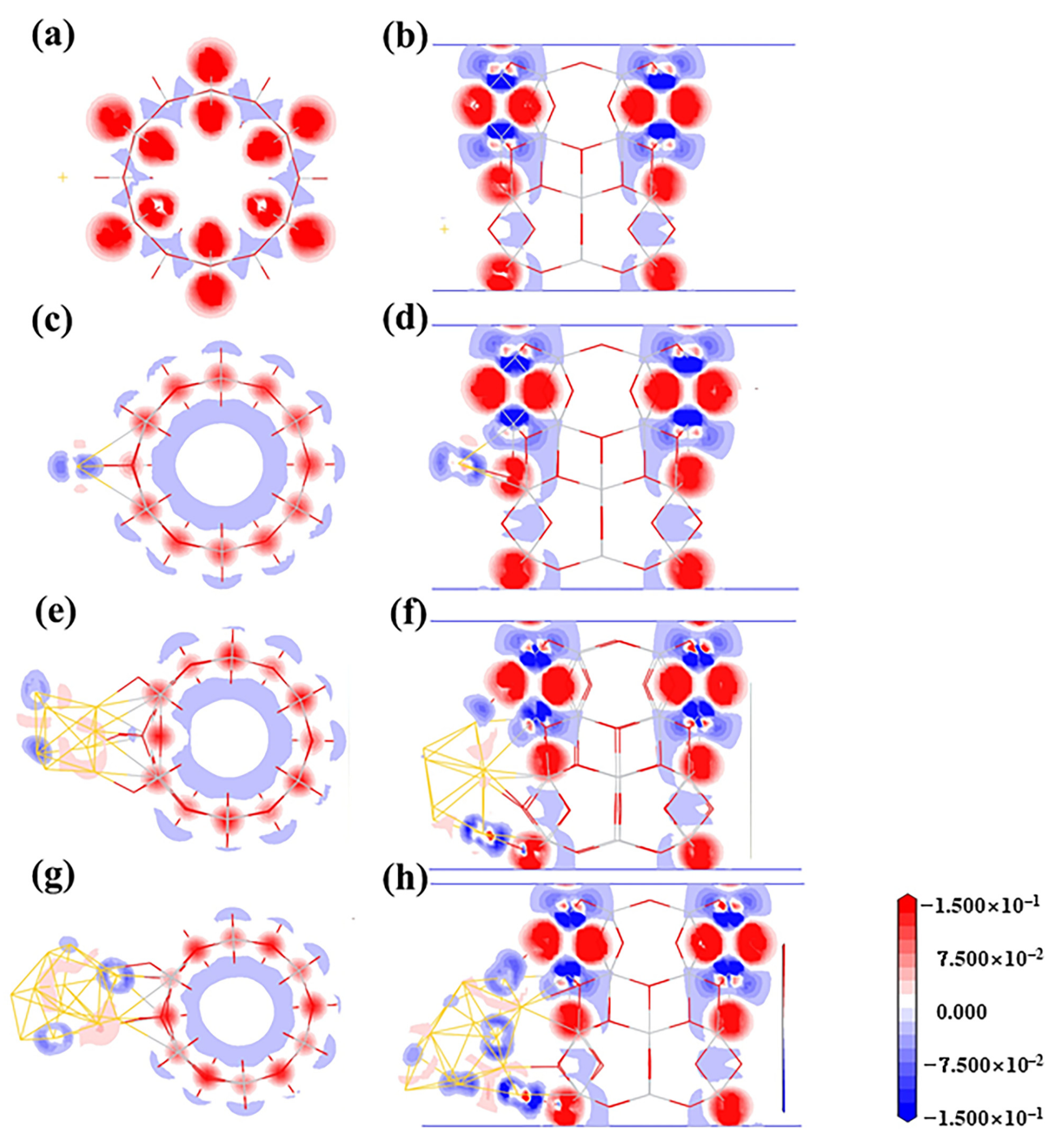
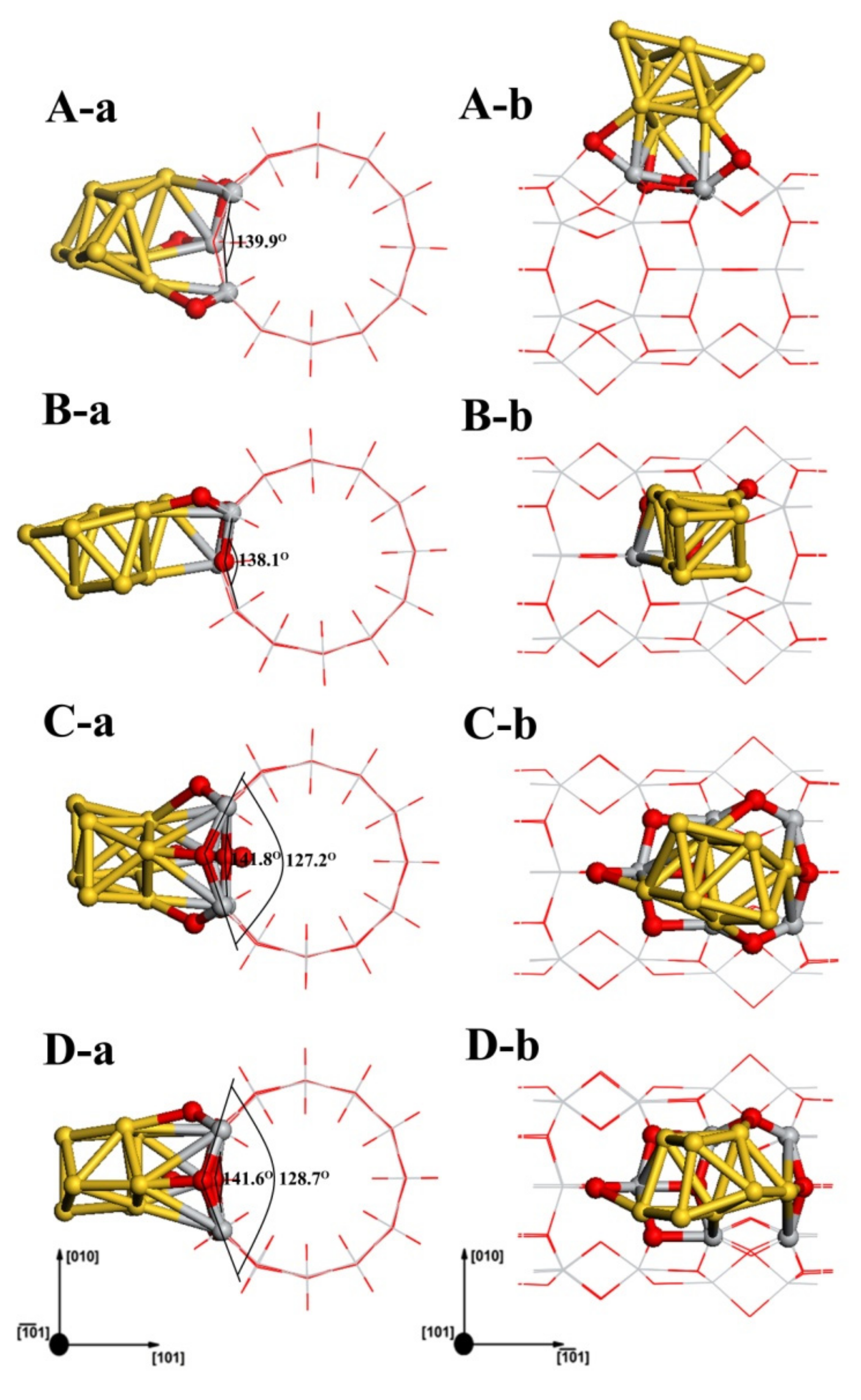

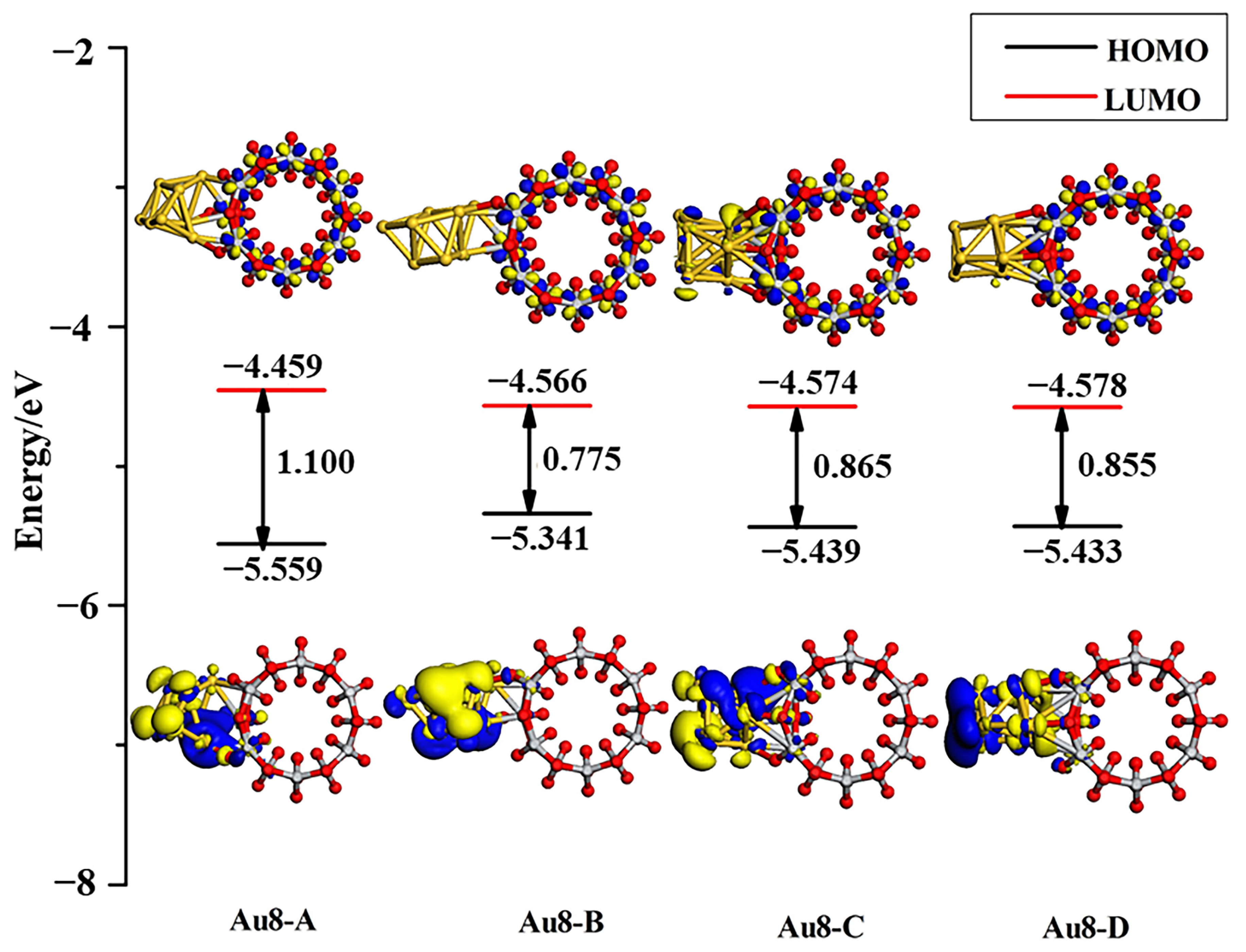
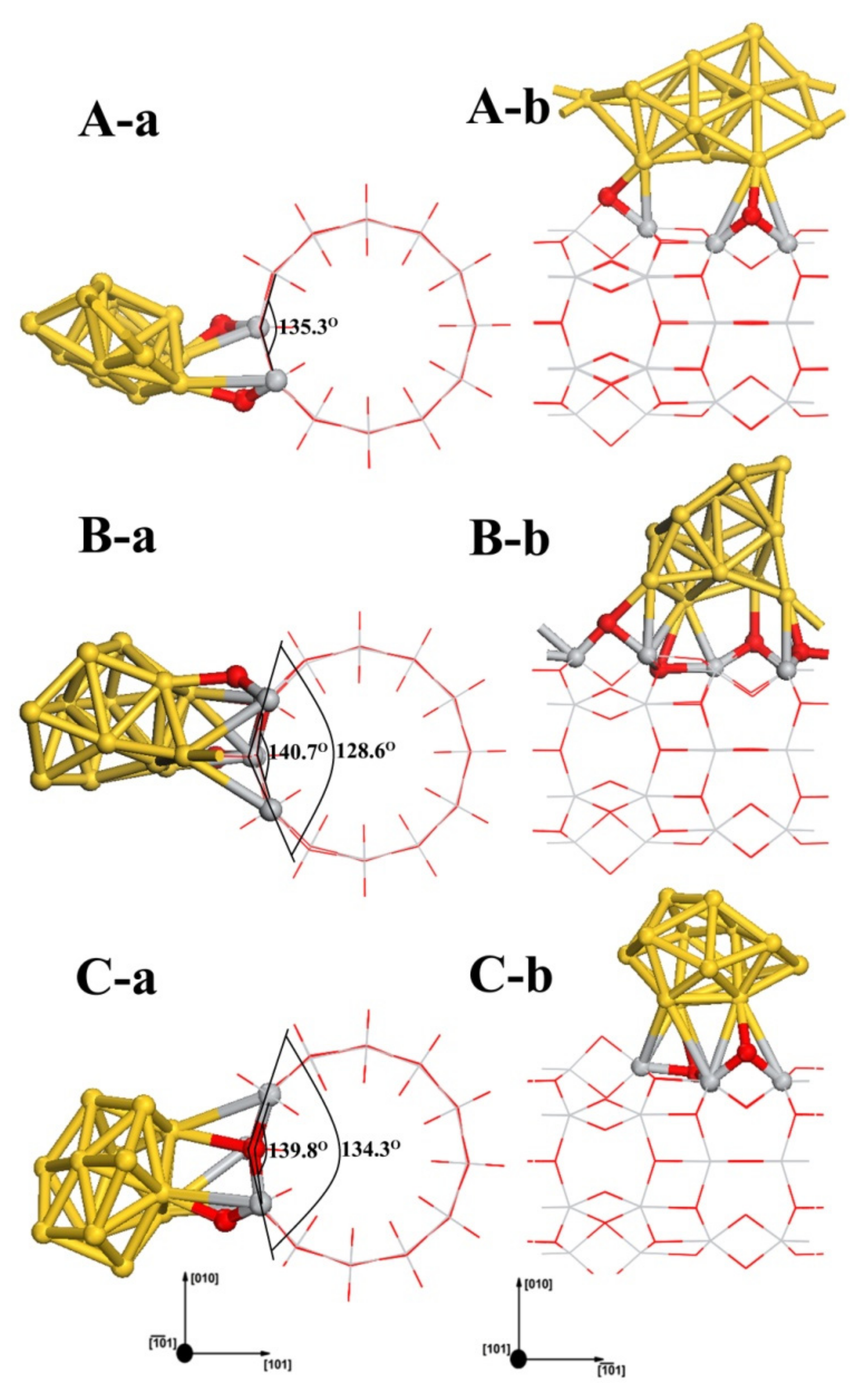
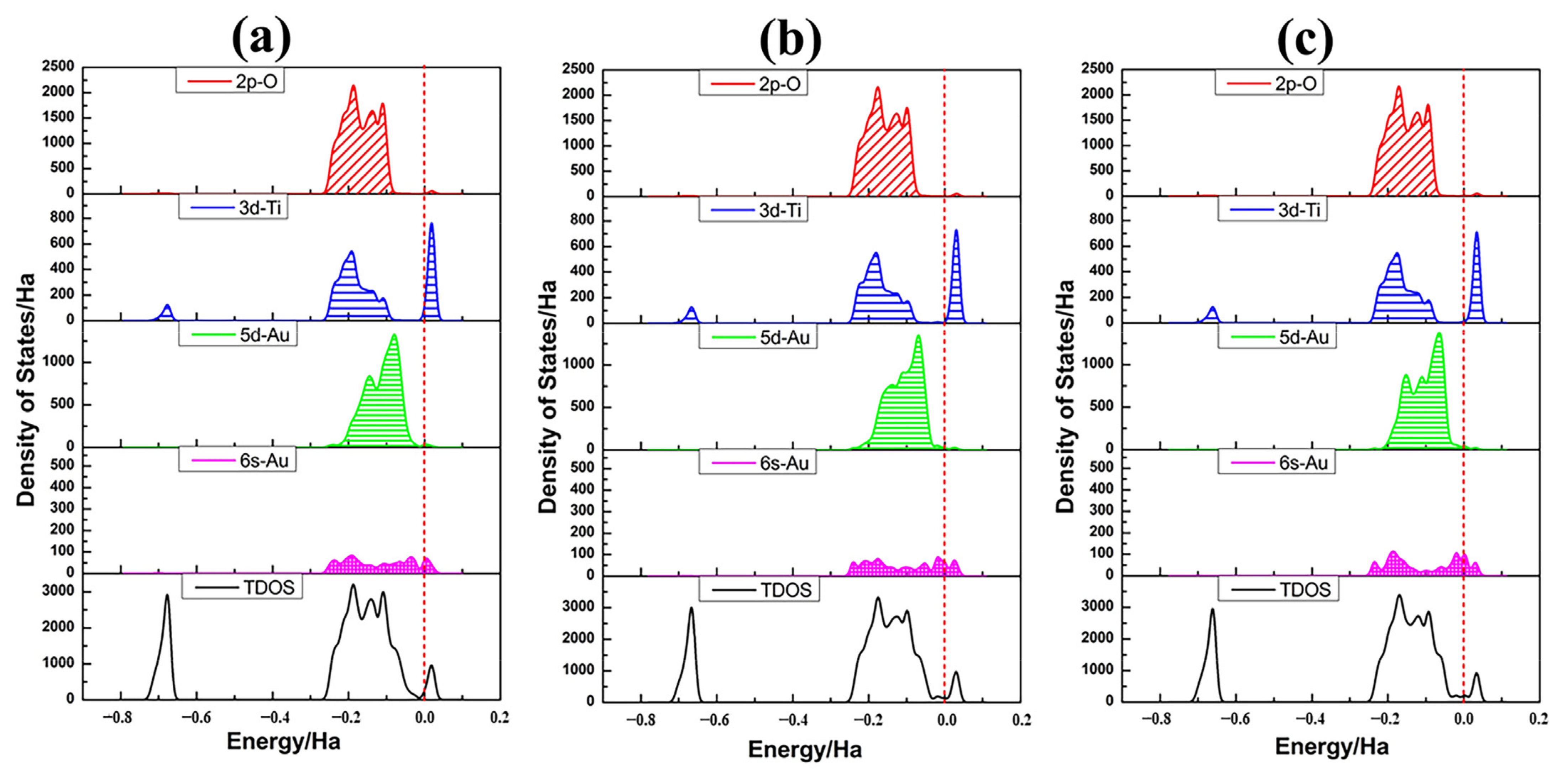
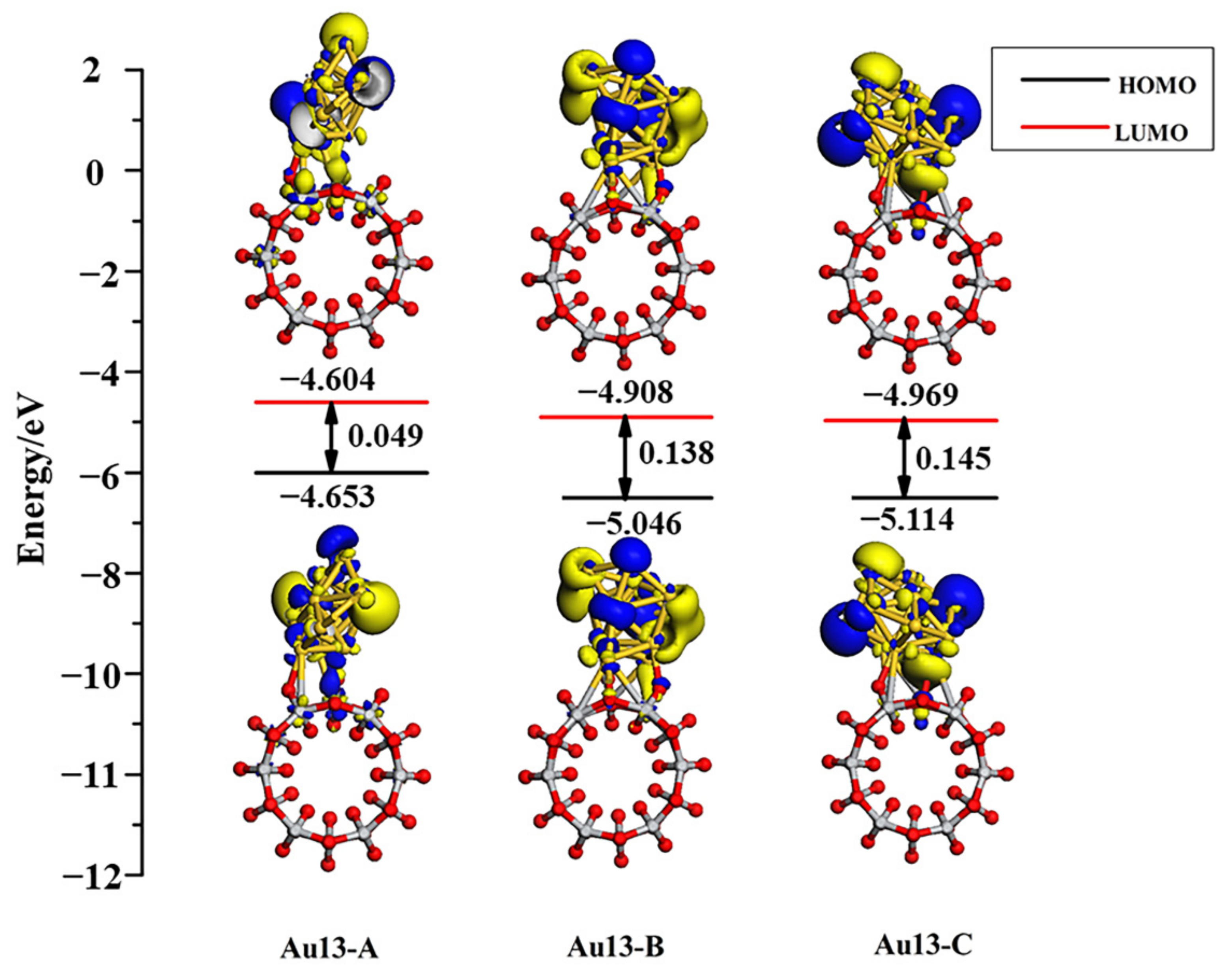
| Configuration | Energy | Mulliken Charge (a.u.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Au | 3cO 1 | 2cO 1 | 3cO 2 | 2cO 2 | 5cTi 1 | 5cTi 2 | 5cTi 3 | ||
| Bare TiO2NT | −0.966 | −0.779 | −0.965 | −0.779 | 1.714 | 1.714 | 1.713 | ||
| Au1(O,O) | 0.20 | −0.060 | −0.958 | −0.770 | −0.958 | −0.770 | |||
| Au1(O,Ti) | 0.49 | −0.075 | −0.940 | 1.732 | 1.731 | 1.676 | |||
| Configuration | Energy | Mulliken Charge (a.u.) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Au8 | 3cO 1 | 2cO 1 | 2cO 2 | 3cO 2 | 2cO 3 | 5cTi 1 | 5cTi 2 | 5cTi 3 | 5cTi 4 | 5cTi 5 | |||
| Bare TiO2NT | −0.966 | −0.779 | −0.779 | −0.966 | −0.779 | 1.717 | 1.714 | 1.718 | 1.714 | 1.713 | |||
| Au8-A(2cO, 3cO, 2cO) | 1.11 | 2.12 | −0.321 | −0.836 | −0.965 | −0.809 | 1.720 | 1.834 | 1.766 | 1.892 | |||
| Au8-B(2cO,5cTi) | 0.86 | 2.09 | −0.293 | −0.851 | −0.960 | 1.815 | 1.803 | ||||||
| Au8-C(2cO,2cO,3cO) | 0.81 | 2.09 | −0.424 | −0.993 | −0.826 | −0.758 | −0.843 | 1.926 | 1.874 | 1.716 | 1.783 | 1.905 | |
| Au8-D(2cO,2cO,3cO) | 0.72 | 2.07 | −0.343 | −0.987 | −0.818 | −0.817 | 1.733 | 1.769 | 1.751 | 1.783 | 1.870 | ||
| Configuration | Energy | Mulliken Charge (a.u.) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Au13 | 3cO 1 | 2cO 1 | 3cO 2 | 2cO 2 | 3cO 3 | 2cO 3 | 5cTi 1 | 5cTi 2 | 5cTi 3 | 5cTi 4 | 5cTi 5 | 5cTi 6 | |||
| Bare TiO2NT | −0.966 | −0.779 | −0.965 | −0.779 | −0.966 | −0.779 | 1.717 | 1.714 | 1.718 | 1.714 | 1.713 | 1.717 | |||
| Au13-A(2cO,2cO) | 3.12 | 2.28 | −0.225 | −0.870 | −0.833 | 1.788 | 1.794 | 1.819 | |||||||
| Au13-B(2cO,3cO,2cO,3cO) | 2.23 | 2.21 | −0.322 | −0.996 | −0.809 | −0.929 | −0.830 | 1.736 | 1.761 | 1.805 | 1.848 | 1.727 | |||
| Au13-C(3cO,2cO) | 1.6 | 2.19 | −0.171 | −0.854 | −0.934 | 1.762 | 1.791 | 1.737 | 1.778 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, G. DFT Investigations of Aun Nano-Clusters Supported on TiO2 Nanotubes: Structures and Electronic Properties. Molecules 2022, 27, 2756. https://doi.org/10.3390/molecules27092756
Wang Y, Zhou G. DFT Investigations of Aun Nano-Clusters Supported on TiO2 Nanotubes: Structures and Electronic Properties. Molecules. 2022; 27(9):2756. https://doi.org/10.3390/molecules27092756
Chicago/Turabian StyleWang, Ying, and Ge Zhou. 2022. "DFT Investigations of Aun Nano-Clusters Supported on TiO2 Nanotubes: Structures and Electronic Properties" Molecules 27, no. 9: 2756. https://doi.org/10.3390/molecules27092756
APA StyleWang, Y., & Zhou, G. (2022). DFT Investigations of Aun Nano-Clusters Supported on TiO2 Nanotubes: Structures and Electronic Properties. Molecules, 27(9), 2756. https://doi.org/10.3390/molecules27092756






