Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review
Abstract
:1. Introduction
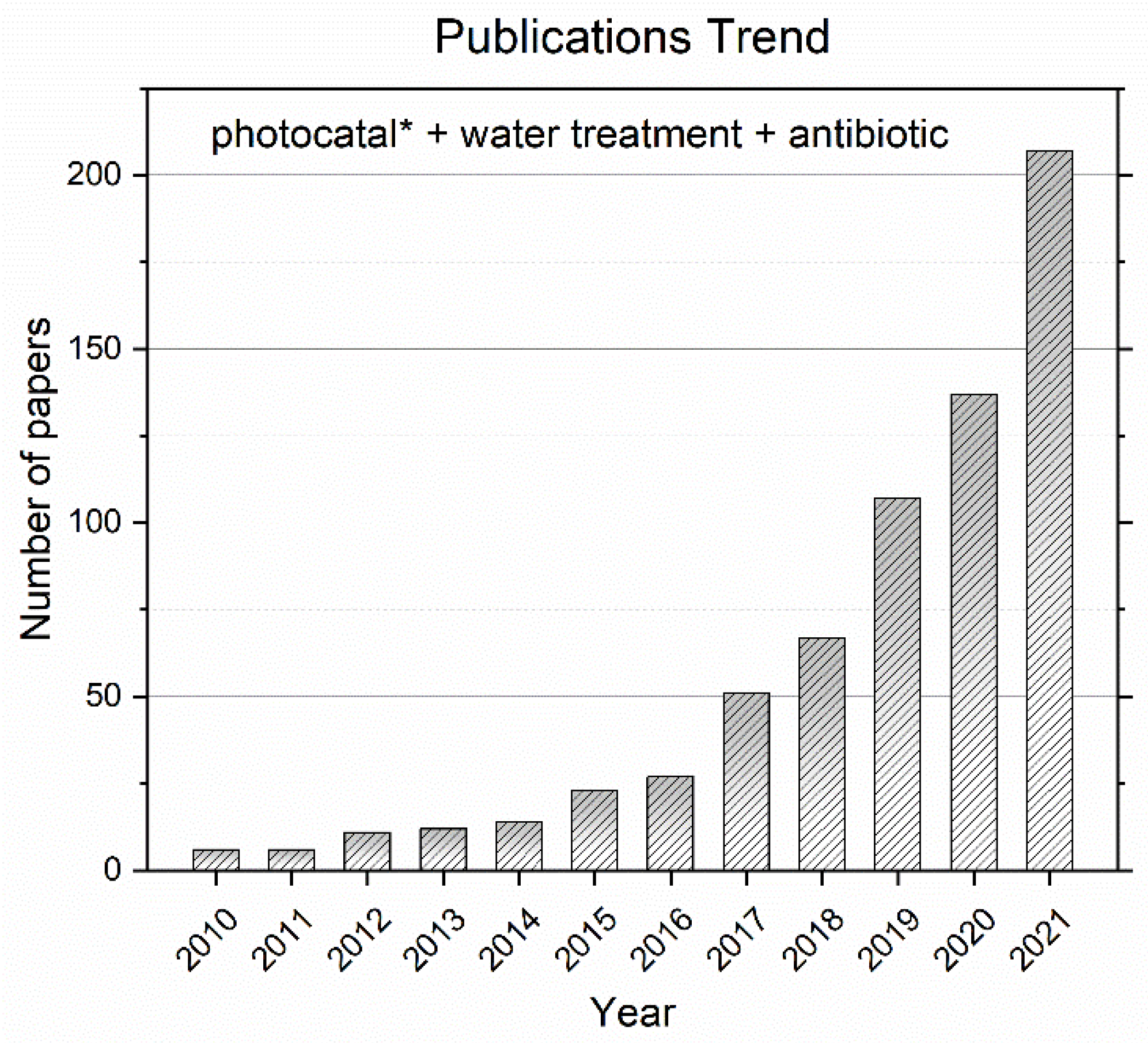
1.1. Method
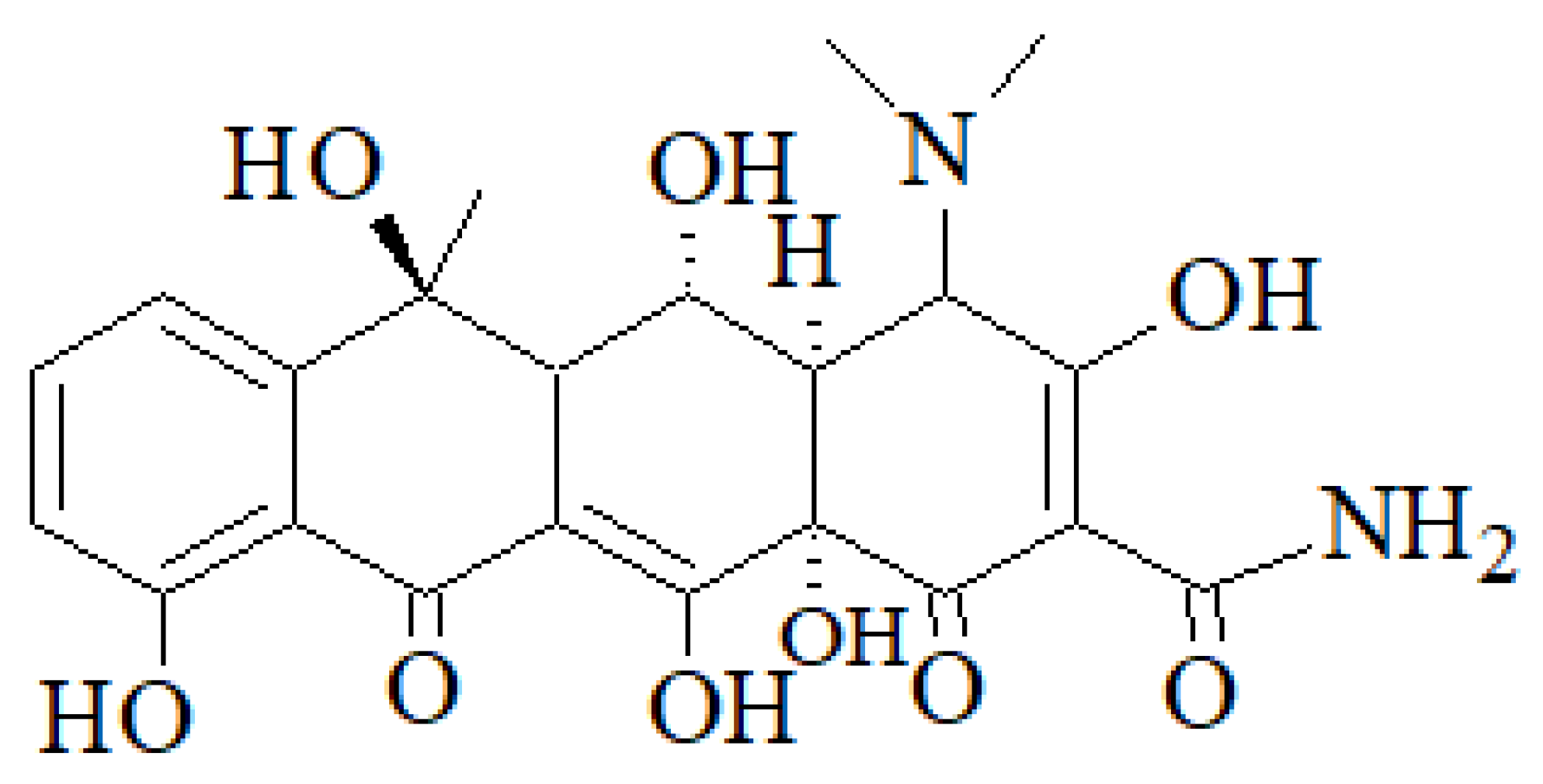
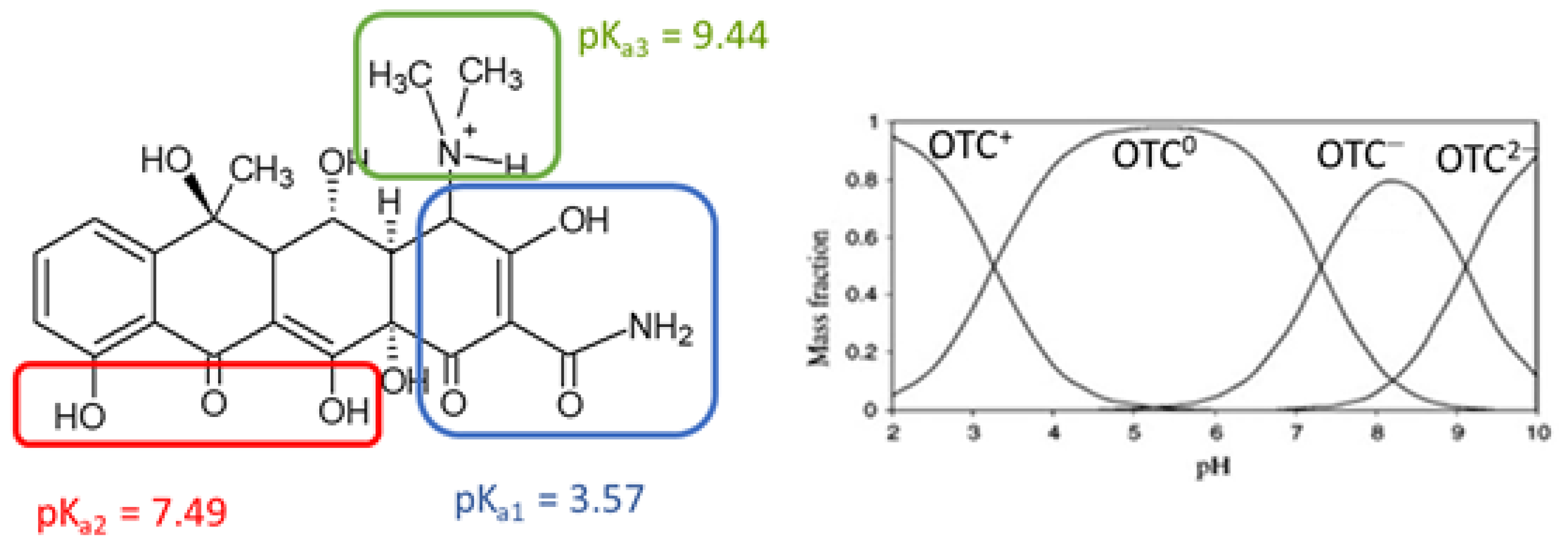
1.2. Occurrence of Oxytetracycline in Wastewater
2. Standard Processes of Removal/Degradation of OTC in Water
2.1. Adsorption
2.2. Bioremediation
2.3. Oxidation
2.4. Photolysis
2.5. Other Processes
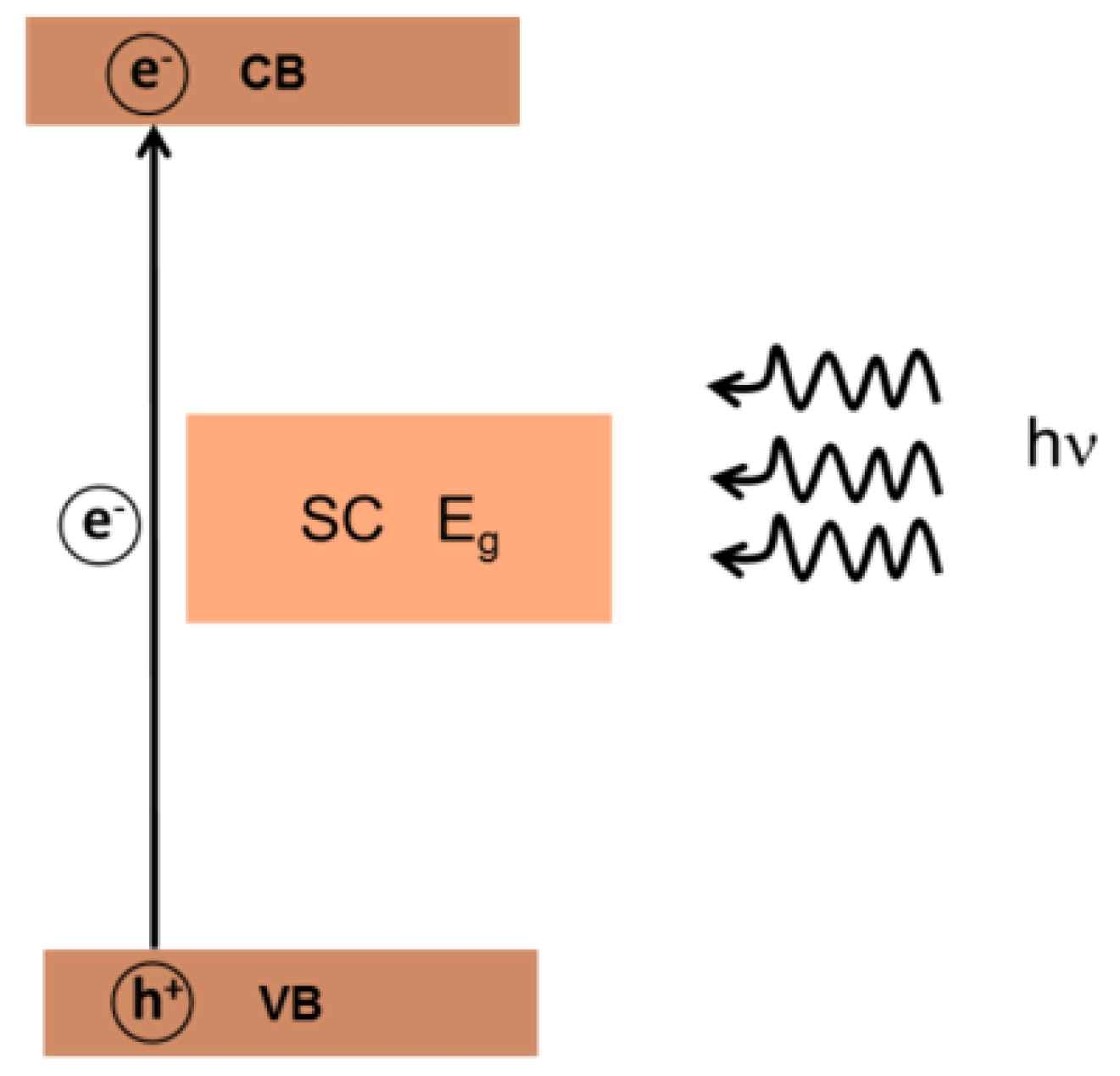
3. Photocatalytic Degradation of Organic Pollutants
3.1. Photocatalytic Oxidation of OTC Using Pure TiO2
3.2. Photocatalytic Oxidation of OTC Using Heterogeneous TiO2-Based Photocatalysts
3.3. Photocatalytic Oxidation of OTC Using Visible-Light Active Semiconductor Oxides
3.3.1. Simple Semiconductor Oxides
3.3.2. Semiconductor-Oxide-Based Heterojunctions
3.4. Photocatalytic Oxidation of OTC Using Graphene-Based Nanocomposites
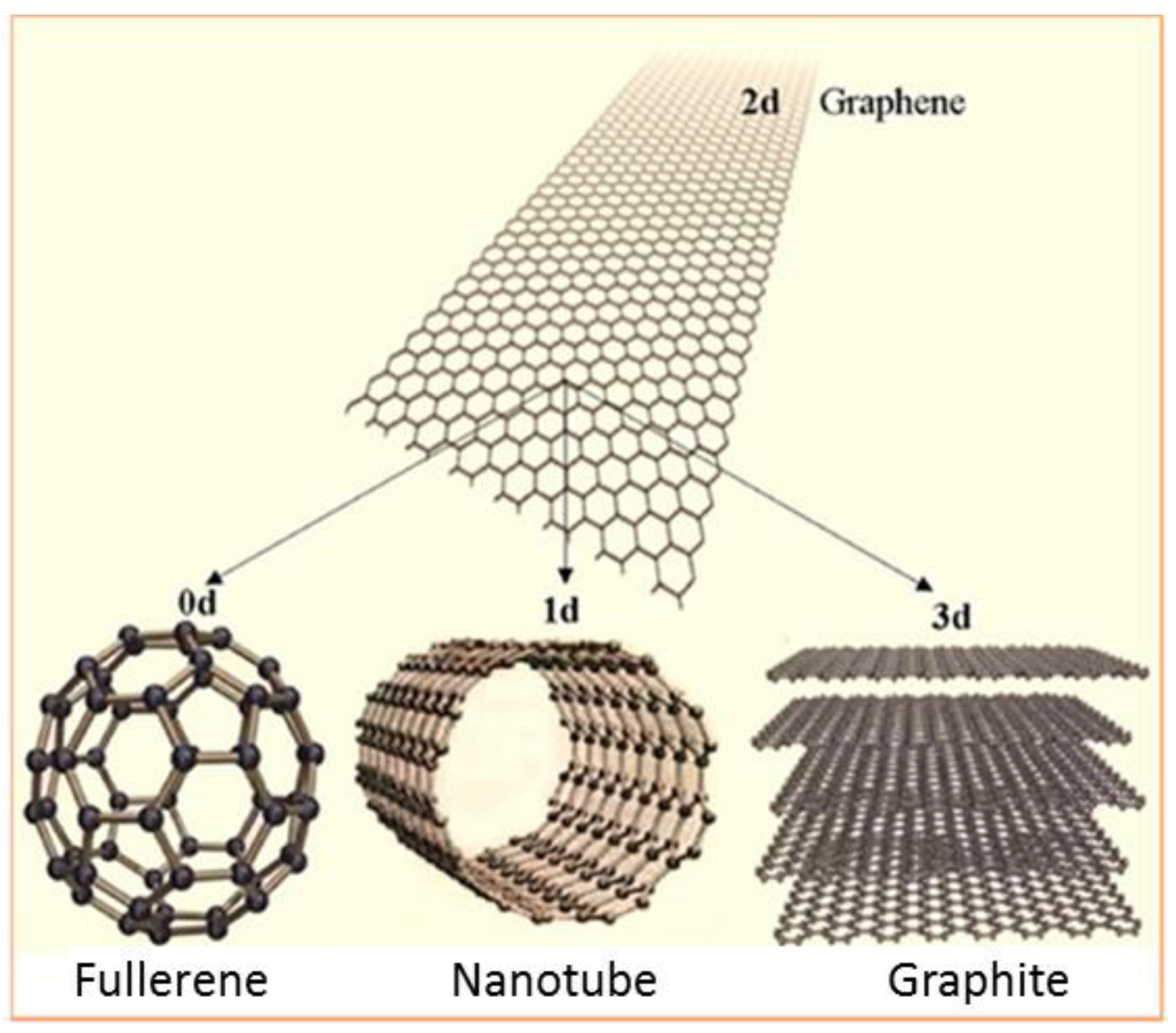
3.4.1. Graphene: Structure and Properties
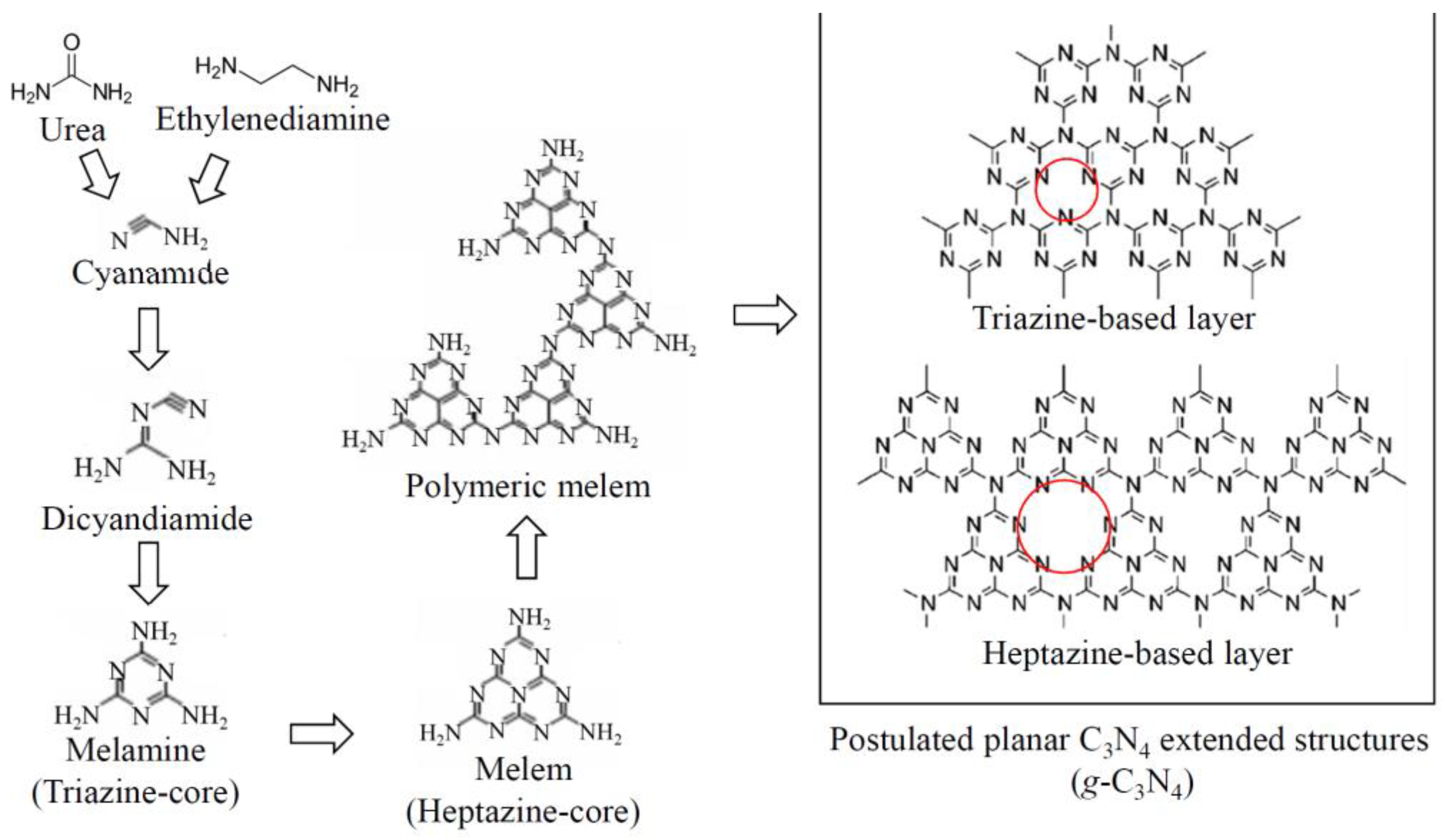
3.4.2. Graphitic Carbon Nitride g-C3N4
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A Review on Emerging Contaminants in Wastewaters and the Environment: Current Knowledge, Understudied Areas and Recommendations for Future Monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xiong, H.; Ma, H.; Li, Z. Effects of Different Fertilizers on Residues of Oxytetracycline and Microbial Activity in Soil. Environ. Sci. Pollut. Res. 2019, 26, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, J.; Zhu, K.; Zhang, H. Influence of Oxytetracycline on the Structure and Activity of Microbial Community in Wheat Rhizosphere Soil. J. Environ. Sci. 2009, 21, 954–959. [Google Scholar] [CrossRef]
- Natali Sora, I.; Fumagalli, D. Fast Photocatalytic Degradation of Pharmaceutical Micropollutants and Ecotoxicological Effects. Environ. Sci. Pollut. Res. 2017, 24, 12556–12561. [Google Scholar] [CrossRef]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of Micropollutants in Municipal Wastewater: Ozone or Powdered Activated Carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Quantitative Structure-Activity Relationships (QSARs) for the Transformation of Organic Micropollutants during Oxidative Water Treatment. Water Res. 2012, 46, 6177–6195. [Google Scholar] [CrossRef] [Green Version]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of Granular Activated Carbon to Remove Micropollutants from Municipal Wastewater—A Meta-Analysis of Pilot- and Large-Scale Studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved Removal of Estrogenic and Pharmaceutical Compounds in Sewage Effluent by Full Scale Granular Activated Carbon: Impact on Receiving River Water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef]
- Stalter, D.; Magdeburg, A.; Oehlmann, J. Comparative Toxicity Assessment of Ozone and Activated Carbon Treated Sewage Effluents Using an in Vivo Test Battery. Water Res. 2010, 44, 2610–2620. [Google Scholar] [CrossRef]
- Zimmermann, S.G.; Wittenwiler, M.; Hollender, J.; Krauss, M.; Ort, C.; Siegrist, H.; von Gunten, U. Kinetic Assessment and Modeling of an Ozonation Step for Full-Scale Municipal Wastewater Treatment: Micropollutant Oxidation, by-Product Formation and Disinfection. Water Res. 2011, 45, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Greenbank, M.; Knepper, J. GAC/PAC: Use of Powdered Activated Carbon for Potable Water Treatment in Small Systems. Water Condition & Purification International Magazine. 2002. Available online: https://wcponline.com/2002/11/21/gacpac-use-powdered-activated-carbon-potable-water-treatment-small-systems/ (accessed on 19 April 2022).
- Von Gunten, U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]
- Cates, E.L. Photocatalytic Water Treatment: So Where Are We Going with This? Environ. Sci. Technol. 2017, 51, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Doudrick, K.; Yang, T.; Hristovski, K.; Westerhoff, P. Photocatalytic Nitrate Reduction in Water: Managing the Hole Scavenger and Reaction by-Product Selectivity. Appl. Catal. B Environ. 2013, 136–137, 40–47. [Google Scholar] [CrossRef]
- Testa, J.J.; Grela, M.A.; Litter, M.I. Heterogeneous Photocatalytic Reduction of Chromium(VI) over TiO2 Particles in the Presence of Oxalate: Involvement of Cr(V) Species. Environ. Sci. Technol. 2004, 38, 1589–1594. [Google Scholar] [CrossRef]
- Prairie, M.R.; Evans, L.R.; Stange, B.M.; Martinez, S.L. An Investigation of TiO2 Photocatalysis for the Treatment of Water Contaminated with Metals and Organic Chemicals. Environ. Sci. Technol. 1993, 27, 1776–1782. [Google Scholar] [CrossRef]
- Kim, H.-I.; Choi, Y.; Hu, S.; Choi, W.; Kim, J.-H. Photocatalytic Hydrogen Peroxide Production by Anthraquinone-Augmented Polymeric Carbon Nitride. Appl. Catal. B Environ. 2018, 229, 121–129. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, D. Covalent Organic Frameworks: New Materials Platform for Photocatalytic Degradation of Aqueous Pollutants. Materials 2021, 14, 5600. [Google Scholar] [CrossRef]
- Animal Antibiotics and Antimicrobials Market by Product, Mode of Delivery and Animal—Global Forecast to 2026. Available online: https://www.Businesswire.Com/News/Home/20210324005614/En/Animal-Antibiotics-and-Antimicrobials-Market-by-Product-Mode-of-Delivery-and-Animal---Global-Forecast-to-2026---ResearchAndMarkets.Com (accessed on 2 April 2022).
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Wu, L.; Huang, Y.; Christie, P. Residues and Potential Ecological Risks of Veterinary Antibiotics in Manures and Composts Associated with Protected Vegetable Farming. Environ. Sci. Pollut. Res. 2015, 22, 5908–5918. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Pailler, J.-Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid Phase Extraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry Analysis of Sulfonamides, Tetracyclines, Analgesics and Hormones in Surface Water and Wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef] [PubMed]
- Birošová, L.; Mackuľak, T.; Bodík, I.; Ryba, J.; Škubák, J.; Grabic, R. Pilot Study of Seasonal Occurrence and Distribution of Antibiotics and Drug Resistant Bacteria in Wastewater Treatment Plants in Slovakia. Sci. Total Environ. 2014, 490, 440–444. [Google Scholar] [CrossRef]
- Sobin, B.A.; Finlay, A.C.; Kane, J.H. Terramycin and Its Production. U.S. Patent US2,516,080, 18 July 1950. [Google Scholar]
- Siedlewicz, G.; Białk-Bielińska, A.; Borecka, M.; Winogradow, A.; Stepnowski, P.; Pazdro, K. Presence, Concentrations and Risk Assessment of Selected Antibiotic Residues in Sediments and near-Bottom Waters Collected from the Polish Coastal Zone in the Southern Baltic Sea—Summary of 3 Years of Studies. Mar. Pollut. Bull. 2018, 129, 787–801. [Google Scholar] [CrossRef]
- Choi, S.; Sim, W.; Jang, D.; Yoon, Y.; Ryu, J.; Oh, J.; Woo, J.-S.; Kim, Y.M.; Lee, Y. Antibiotics in Coastal Aquaculture Waters: Occurrence and Elimination Efficiency in Oxidative Water Treatment Processes. J. Hazard. Mater. 2020, 396, 122585. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and Risk Assessment of Antibiotics in Water and Lettuce in Ghana. Sci. Total Environ. 2018, 622–623, 293–305. [Google Scholar] [CrossRef]
- Loke, M.-L.; Tjørnelund, J.; Halling-Sørensen, B. Determination of the Distribution Coefficient (LogKd) of Oxytetracycline, Tylosin A, Olaquindox and Metronidazole in Manure. Chemosphere 2002, 48, 351–361. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for the Release of Antibiotics in the Environment: A Review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Samuelsen, O.B. Estimates of the Significance of Out-Washing of Oxytetracycline from Sediments under Atlantic Salmon Sea-Cages. Aquaculture 1996, 144, 17–26. [Google Scholar] [CrossRef]
- Li, D.; Yu, T.; Zhang, Y.; Yang, M.; Li, Z.; Liu, M.; Qi, R. Antibiotic Resistance Characteristics of Environmental Bacteria from an Oxytetracycline Production Wastewater Treatment Plant and the Receiving River. Appl. Environ. Microbiol. 2010, 76, 3444–3451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djedouani, D.; Chabani, M.; Amrane, A.; Bensmaili, A. Adsorption Kinetics of Oxytetracycline onto Activated Carbon in a Closed-Loop Fixed Bed Reactor. Int. J. Chem. React. Eng. 2013, 11, 569–576. [Google Scholar] [CrossRef]
- Li, K.; Yediler, A.; Yang, M.; Schulte-Hostede, S.; Wong, M.H. Ozonation of Oxytetracycline and Toxicological Assessment of Its Oxidation By-Products. Chemosphere 2008, 72, 473–478. [Google Scholar] [CrossRef]
- Li, Z.; Qi, W.; Feng, Y.; Liu, Y.; Ebrahim, S.; Long, J. Degradation Mechanisms of Oxytetracycline in the Environment. J. Integr. Agric. 2019, 18, 1953–1960. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Giese, R.F.; Aga, D.S. Investigating the Molecular Interactions of Oxytetracycline in Clay and Organic Matter: Insights on Factors Affecting Its Mobility in Soil. Environ. Sci. Technol. 2004, 38, 4097–4105. [Google Scholar] [CrossRef]
- Jin, X.; Xu, H.; Qiu, S.; Jia, M.; Wang, F.; Zhang, A.; Jiang, X. Direct Photolysis of Oxytetracycline: Influence of Initial Concentration, pH and Temperature. J. Photochem. Photobiol. Chem. 2017, 332, 224–231. [Google Scholar] [CrossRef]
- Migliore, L.; Fiori, M.; Spadoni, A.; Galli, E. Biodegradation of Oxytetracycline by Pleurotus Ostreatus Mycelium: A Mycoremediation Technique. J. Hazard. Mater. 2012, 215–216, 227–232. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Y.; Zheng, J.; Zhao, X.; Zhong, W. The Behavior of Tetracyclines and Their Degradation Products during Swine Manure Composting. Bioresour. Technol. 2011, 102, 5924–5931. [Google Scholar] [CrossRef]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.; Qiao, M. Characteristics of Oxytetracycline Sorption and Potential Bioavailability in Soils with Various Physical–Chemical Properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Y.; Wang, W.; Yue, Q.; Yang, T. Comparative Study on Characterization of Activated Carbons Prepared by Microwave and Conventional Heating Methods and Application in Removal of Oxytetracycline (OTC). Chem. Eng. J. 2011, 171, 1446–1453. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Li, Q.; Huang, L.; Yao, F.; Xu, X. Preparation of Activated Carbon Derived from Cotton Linter Fibers by Fused NaOH Activation and Its Application for Oxytetracycline (OTC) Adsorption. J. Colloid Interface Sci. 2012, 368, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, D.; Mousavi, S.A.; Mehrabi, A. Oxytetracycline Removal from Aqueous Solutions Using Activated Carbon Prepared from Corn Stalks. J. Appl. Res. Water Wastewater 2019, 6. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Qin, T.; Liang, G.; Li, Y.; Xie, X. Efficient Removal of Oxytetracycline from Aqueous Solution by a Novel Magnetic Clay–Biochar Composite Using Natural Attapulgite and Cauliflower Leaves. Environ. Sci. Pollut. Res. 2019, 26, 7463–7475. [Google Scholar] [CrossRef]
- Belaib, F.; Azzedine, M.; Boubeker, B.; Abdeslam-Hassen, M. Experimental Study of Oxytetracycline Retention by Adsorption onto Polyaniline Coated Peanut Shells. Int. J. Hydrogen Energy 2014, 39, 1511–1515. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Yang, S.; Wang, W.; Wang, Y. Adsorption Property and Mechanism of Oxytetracycline onto Willow Residues. Int. J. Environ. Res. Public. Health 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juengchareonpoon, K.; Boonamnuayvitaya, V.; Wanichpongpan, P. Kinetics and Isotherms of Oxytetracycline Adsorption on Β-cyclodextrin/Carboxymethylcellulose Hydrogel Films. Aquac. Res. 2019, 50, 3412–3419. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Studies on Adsorption of Oxytetracycline from Aqueous Solutions onto Hydroxyapatite. Sci. Total Environ. 2018, 628–629, 36–43. [Google Scholar] [CrossRef]
- Martínez-Olivas, A.; Torres-Pérez, J.; Balderas-Hernández, P.; Reyes-López, S.Y. Oxytetracycline Sorption onto Synthetized Materials from Hydroxyapatite and Aluminosilicates. Water Air Soil Pollut. 2020, 231, 264. [Google Scholar] [CrossRef]
- Barbooti, M.M.; AlShuwaiki, N.M.; Su, H. Optimization of Oxytetracycline Sorption onto Iron Modified Montmorillonite. IOP Conf. Ser. Mater. Sci. Eng. 2020, 737, 012207. [Google Scholar] [CrossRef]
- Lye, J.W.P.; Saman, N.; Sharuddin, S.S.N.; Othman, N.S.; Mohtar, S.S.; Md Noor, A.M.; Buhari, J.; Cheu, S.C.; Kong, H.; Mat, H. Removal Performance of Tetracycline and Oxytetracycline From Aqueous Solution Via Natural Zeolites: An Equilibrium and Kinetic Study: Water. CLEAN-Soil Air Water 2017, 45, 1600260. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and Removal of Tetracycline Antibiotics from Aqueous Solution by Graphene Oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Song, Y.-X.; Zhang, Y.; Fan, H.-T. Nanocomposites of Reduced Graphene Oxide with Pure Monoclinic-ZrO2 and Pure Tetragonal-ZrO2 for Selective Adsorptive Removal of Oxytetracycline. Appl. Surf. Sci. 2021, 543, 148810. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Z.; Hu, Y.; Lv, S.; Fan, H.; Zeng, Y.; Hu, J.; Wang, M. Removal of Tetracycline and Oxytetracycline from Water by Magnetic Fe3O4@graphene. Environ. Sci. Pollut. Res. 2017, 24, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Lv, J.; Lou, D. Synthesis of Novel Magnetic Microspheres with Bimetal Oxide Shell for Excellent Adsorption of Oxytetracycline. ACS Sustain. Chem. Eng. 2017, 5, 10298–10306. [Google Scholar] [CrossRef]
- Chen, W.-R.; Huang, C.-H. Adsorption and Transformation of Tetracycline Antibiotics with Aluminum Oxide. Chemosphere 2010, 79, 779–785. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, H.; Ma, J.; Zhu, R.; Sun, W.; Lin, X.; Zhang, J.; Zheng, H.; Zhang, X. Degradation of Tetracycline Antibiotics by Arthrobacter Nicotianae OTC-16. J. Hazard. Mater. 2021, 403, 123996. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Degradation of Oxytetracycline (OTC) and Nitrogen Conversion Characteristics Using a Novel Strain. Chem. Eng. J. 2018, 354, 758–766. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Torres, E.; Mera, R.; Abalde, J. Bioremediation of Oxytetracycline in Seawater by Living and Dead Biomass of the Microalga Phaeodactylum Tricornutum. J. Hazard. Mater. 2016, 320, 315–325. [Google Scholar] [CrossRef]
- Liu, M.; Cao, J.; Wang, C. Bioremediation by Earthworms on Soil Microbial Diversity and Partial Nitrification Processes in Oxytetracycline-Contaminated Soil. Ecotoxicol. Environ. Saf. 2020, 189, 109996. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of Tetracycline by Immobilized Laccase and the Proposed Transformation Pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Jia, Y.; Li, J. Degradation of Tetracycline and Oxytetracycline by Crude Lignin Peroxidase Prepared from Phanerochaete Chrysosporium—A White Rot Fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, Z.R.; Blaney, L. A Novel Approach to Modeling the Reaction Kinetics of Tetracycline Antibiotics with Aqueous Ozone. Sci. Total Environ. 2014, 468–469, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Uslu, M.Ö.; Balcıoğlu, I.A. Comparison of the Ozonation and Fenton Process Performances for the Treatment of Antibiotic Containing Manure. Sci. Total Environ. 2009, 407, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Xie, G.; Dai, R.; Kuang, C.; Xu, Y.; Pan, Z.; Zheng, L.; Yu, L.; Ye, S.; Chen, Z.; et al. Kinetics and Mechanisms of Oxytetracycline Degradation in an Electro-Fenton System with a Modified Graphite Felt Cathode. J. Environ. Manag. 2020, 257, 109968. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-Activated Persulfate Oxidation System for Tetracycline Antibiotics Degradation in Aqueous Solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Bilgin Oncu, N.; Mercan, N.; Akmehmet Balcioglu, I. The Impact of Ferrous Iron/Heat-Activated Persulfate Treatment on Waste Sewage Sludge Constituents and Sorbed Antimicrobial Micropollutants. Chem. Eng. J. 2015, 259, 972–980. [Google Scholar] [CrossRef]
- Liu, D.; Li, M.; Li, X.; Ren, F.; Sun, P.; Zhou, L. Core-Shell Zn/Co MOFs Derived Co3O4/CNTs as an Efficient Magnetic Heterogeneous Catalyst for Persulfate Activation and Oxytetracycline Degradation. Chem. Eng. J. 2020, 387, 124008. [Google Scholar] [CrossRef]
- Pouliquen, H.; Delépée, R.; Larhantec-Verdier, M.; Morvan, M.-L.; Le Bris, H. Comparative Hydrolysis and Photolysis of Four Antibacterial Agents (Oxytetracycline Oxolinic Acid, Flumequine and Florfenicol) in Deionised Water, Freshwater and Seawater under Abiotic Conditions. Aquaculture 2007, 262, 23–28. [Google Scholar] [CrossRef]
- Xuan, R.; Arisi, L.; Wang, Q.; Yates, S.R.; Biswas, K.C. Hydrolysis and Photolysis of Oxytetracycline in Aqueous Solution. J. Environ. Sci. Health Part B 2009, 45, 73–81. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The Influence of Light Exposure, Water Quality and Vegetation on the Removal of Sulfonamides and Tetracyclines: A Laboratory-Scale Study. Chemosphere 2013, 90, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Stankov, V.; Stankov, M.N.; Cvetnić, M.; Sigurnjak Bureš, M.; Ukić, Š.; Kučić Grgić, D.; Lončarić Božić, A.; Kušić, H.; Bolanča, T. Environmental Aspects of UV-C-Based Processes for the Treatment of Oxytetracycline in Water. Environ. Pollut. 2021, 277, 116797. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ali, J.; Wang, Z.; Oladoja, N.A.; Cheng, R.; Zhang, C.; Mailhot, G.; Pan, G. Oxygen Nanobubbles Enhanced Photodegradation of Oxytetracycline under Visible Light: Synergistic Effect and Mechanism. Chem. Eng. J. 2020, 388, 124227. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, D. Membrane (RO-UF) Filtration for Antibiotic Wastewater Treatment and Recovery of Antibiotics. Sep. Purif. Technol. 2004, 34, 109–114. [Google Scholar] [CrossRef]
- Ma, W.; Yao, B.; Zhang, W.; He, Y.; Yu, Y.; Niu, J. Fabrication of PVDF-Based Piezocatalytic Active Membrane with Enhanced Oxytetracycline Degradation Efficiency through Embedding Few-Layer E-MoS2 Nanosheets. Chem. Eng. J. 2021, 415, 129000. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, D.; Rao, Y.; Zhang, J.; Qu, Y.; Gu, J. Evaluation of Antibiotic Oxytetracycline Removal in Water Using a Gas Phase Dielectric Barrier Discharge Plasma. J. Environ. Manag. 2018, 226, 22–29. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Selli, E.; Bianchi, C.L.; Pirola, C.; Cappelletti, G.; Ragaini, V. Efficiency of 1,4-Dichlorobenzene Degradation in Water under Photolysis, Photocatalysis on TiO2 and Sonolysis. J. Hazard. Mater. 2008, 153, 1136–1141. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical Properties of TiO2 Photocatalyst and Its Applications for Environmental Purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Vilar, V.J.P.; Borges, M.T.; González, O.; Esplugas, S.; Boaventura, R.A.R. Photocatalytic Degradation of Oxytetracycline Using TiO2 under Natural and Simulated Solar Radiation. Sol. Energy 2011, 85, 2732–2740. [Google Scholar] [CrossRef]
- Bennemla, M.; Chabani, M.; Amrane, A. Photocatalytic Degradation of Oxytetracycline in Aqueous Solutions with TiO2 in Suspension and Prediction by Artificial Neural Networks. Int. J. Chem. Kinet. 2016, 48, 464–473. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic Membrane Reactor Performance towards Oxytetracycline Removal from Synthetic and Real Matrices: Suspended vs Immobilized TiO2-P25. Chem. Eng. J. 2019, 378. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Reis, A.C.; Queirós, D.; Nunes, O.C.; Borges, M.T.; Vilar, V.P.; Boaventura, R.A.R. Insights into Solar TiO2-Assisted Photocatalytic Oxidation of Two Antibiotics Employed in Aquatic Animal Production, Oxolinic Acid and Oxytetracycline. Sci. Total Environ. 2013, 463–464, 274–283. [Google Scholar] [CrossRef]
- Singh, J.; Soni, R.K. Fabrication of Hydroxyl Group-Enriched Mixed-Phase TiO2 Nanoflowers Consisting of Nanoflakes for Efficient Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2020, 31, 12546–12560. [Google Scholar] [CrossRef]
- Singh, J.; Palsaniya, S.; Soni, R.K. Mesoporous Dark Brown TiO2 Spheres for Pollutant Removal and Energy Storage Applications. Appl. Surf. Sci. 2020, 527, 146796. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, Challenge and Perspective of Heterogeneous Photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Wei, M.; Song, N.; Li, F.; Qi, Z.-N.; Yao, M.-M. Efficient Photodegradation of Organic Pollutants with Co–B Codoped TiO2/SiO2 Composite Films under Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 2017, 28, 6320–6327. [Google Scholar] [CrossRef]
- Han, C.; Likodimos, V.; Khan, J.A.; Nadagouda, M.N.; Andersen, J.; Falaras, P.; Rosales-Lombardi, P.; Dionysiou, D.D. UV–Visible Light-Activated Ag-Decorated, Monodisperse TiO2 Aggregates for Treatment of the Pharmaceutical Oxytetracycline. Environ. Sci. Pollut. Res. 2014, 21, 11781–11793. [Google Scholar] [CrossRef]
- Shao, N.; Wang, J.; Wang, D.; Corvini, P. Preparation of Three-Dimensional Ag3PO4/TiO2@MoS2 for Enhanced Visible-Light Photocatalytic Activity and Anti-Photocorrosion. Appl. Catal. B Environ. 2017, 203, 964–978. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, S.; Xin, Y. Synthesis of Au-CuS-TiO2 Nanobelts Photocatalyst for Efficient Photocatalytic Degradation of Antibiotic Oxytetracycline. Chem. Eng. J. 2016, 302, 377–387. [Google Scholar] [CrossRef]
- Bao, S.; Liang, H.; Li, C.; Bai, J. A Heterostructure BiOCl Nanosheets/TiO2 Hollow-Tubes Composite for Visible Light-Driven Efficient Photodegradation Antibiotic. J. Photochem. Photobiol. Chem. 2020, 397, 112590. [Google Scholar] [CrossRef]
- Wang, W.; Han, Q.; Zhu, Z.; Zhang, L.; Zhong, S.; Liu, B. Enhanced Photocatalytic Degradation Performance of Organic Contaminants by Heterojunction Photocatalyst BiVO4/TiO2/RGO and Its Compatibility on Four Different Tetracycline Antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. [Google Scholar] [CrossRef]
- Du, Y.-B.; Zhang, L.; Ruan, M.; Niu, C.-G.; Wen, X.-J.; Liang, C.; Zhang, X.-G.; Zeng, G.-M. Template-Free Synthesis of Three-Dimensional Porous CdS/TiO2 with High Stability and Excellent Visible Photocatalytic Activity. Mater. Chem. Phys. 2018, 212, 69–77. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Guan, L.-X.; Yao, M.-M. Nanocrystalline Co2+/F− Codoped TiO2-SiO2 Composite Films for Environmental Applications. Chem. Eng. J. 2014, 252, 1–10. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt Promoted TiO2/GO for the Photocatalytic Degradation of Oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Gaeta, M.; Sanfilippo, G.; Fraix, A.; Sortino, G.; Barcellona, M.; Conti, G.O.; Fragalà, M.E.; Ferrante, M.; Purrello, R.; D’urso, A. Photodegradation of Antibiotics by Noncovalent Porphyrin-Functionalized TiO2 in Water for the Bacterial Antibiotic Resistance Risk Management. Int. J. Mol. Sci. 2020, 21, 3775. [Google Scholar] [CrossRef]
- Li, R.; Jia, Y.; Wu, J.; Zhen, Q. Photocatalytic Degradation and Pathway of Oxytetracycline in Aqueous Solution by Fe2O3-TiO2 Nanopowder. RSC Adv. 2015, 5, 40764–40771. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, J.; Ji, B.; Ma, L.; Yang, Z. Novel Formation of Large Area N-TiO2/Graphene Layered Materials and Enhanced Photocatalytic Degradation of Antibiotics. J. Environ. Chem. Eng. 2020, 8, 102206. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.; Gu, P.; Zhang, T.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Conjugate Polymer-Clothed TiO2@V2O5 Nanobelts and Their Enhanced Visible Light Photocatalytic Performance in Water Remediation. J. Colloid Interface Sci. 2020, 578, 402–411. [Google Scholar] [CrossRef]
- Huo, P.; Lu, Z.; Liu, X.; Liu, X.; Gao, X.; Pan, J.; Wu, D.; Ying, J.; Li, H.; Yan, Y. Preparation Molecular/Ions Imprinted Photocatalysts of La3+@POPD/TiO2/Fly-Ash Cenospheres: Preferential Photodegradation of TCs Antibiotics. Chem. Eng. J. 2012, 198–199, 73–80. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; He, X.; Du, T.; Wang, Y.; Li, Y.; Hao, T. Facile Prepared Ball-like TiO2 at GO Composites for Oxytetracycline Removal under Solar and Visible Lights. Water Res. 2019, 160, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, Y.; Ridder, D.J.D.; Zhai, J.; Wei, Y.; Deng, H. Advantages of TiO2/5A Composite Catalyst for Photocatalytic Degradation of Antibiotic Oxytetracycline in Aqueous Solution: Comparison between TiO2 and TiO2/5A Composite System. Chem. Eng. J. 2014, 248, 280–289. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of Oxytetracycline in Aqueous by 5A and 13X Loaded with TiO2 under UV Irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Rishikesh; Manna, A.K.; Soni, R.K. Fabrication of ZnO–TiO2 Nanohybrids for Rapid Sunlight Driven Photodegradation of Textile Dyes and Antibiotic Residue Molecules. Opt. Mater. 2020, 107, 110138. [Google Scholar] [CrossRef]
- Changanaqui, K.; Brillas, E.; Alarcón, H.; Sirés, I. ZnO/TiO2/Ag2Se Nanostructures as Photoelectrocatalysts for the Degradation of Oxytetracycline in Water. Electrochim. Acta 2020, 331, 135194. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.M.; Burrell, A.K.; Officer, D.L.; Jolley, K.W. Porphyrins as Light Harvesters in the Dye-Sensitised TiO2 Solar Cell. Coord. Chem. Rev. 2004, 248, 1363–1379. [Google Scholar] [CrossRef]
- Silva, E.F.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simões, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of Singlet-Oxygen and Superoxide-Ion Generation by Porphyrins and Bacteriochlorins and Their Implications in Photodynamic Therapy. Chem.-Eur. J. 2010, 16, 9273–9286. [Google Scholar] [CrossRef]
- Raizada, P.; Kumari, J.; Shandilya, P.; Dhiman, R.; Pratap Singh, V.; Singh, P. Magnetically Retrievable Bi2WO6/Fe3O4 Immobilized on Graphene Sand Composite for Investigation of Photocatalytic Mineralization of Oxytetracycline and Ampicillin. Process Saf. Environ. Prot. 2017, 106, 104–116. [Google Scholar] [CrossRef]
- Xu, K.; Yang, X.; Ruan, L.; Qi, S.; Liu, J.; Liu, K.; Pan, S.; Feng, G.; Dai, Z.; Yang, X.; et al. Superior Adsorption and Photocatalytic Degradation Capability of Mesoporous LaFeO3/g-C3N4 for Removal of Oxytetracycline. Catalysts 2020, 10, 301. [Google Scholar] [CrossRef] [Green Version]
- Raizad, P.; Kumari, J.; Shandilya, P.; Singh, P. Kinetics of Photocatalytic Mineralization of Oxytetracycline and Ampicillin Using Activated Carbon Supported ZnO/ZnWO4 Nanocomposite in Simulated Wastewater. Desalin. Water Treat. 2017, 79, 204–213. [Google Scholar] [CrossRef]
- Cheng, L.; Tian, Y.; Zhang, J. Construction of P-n Heterojunction Film of Cu2O/α-Fe2O3 for Efficiently Photoelectrocatalytic Degradation of Oxytetracycline. J. Colloid Interface Sci. 2018, 526, 470–479. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, H.; Su, J.; Xia, Y.; Fu, X.; Kang, L.; Wang, L.; Wu, M. Polyacrylamide Gel Synthesis and Photocatalytic Performance of CuCo2O4 Nanoparticles. Mater. Lett. 2021, 288, 129375. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Yan, Y.; Yang, Z.; Wang, Y.; Liu, G.; Ni, T. Tunable and Sustainable Photocatalytic Activity of Photochromic Y-WO3 under Visible Light Irradiation. RSC Adv. 2021, 11, 1147–1152. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, X.; Xu, Y.; Lai, W.; Yan, K.; Huang, L.; Ling, J.; Zheng, L. Photocatalytic Performance of Multi-Walled Carbon Nanotube/BiVO4 Synthesized by Electro-Spinning Process and Its Degradation Mechanisms on Oxytetracycline. Chem. Eng. J. 2019, 373, 880–890. [Google Scholar] [CrossRef]
- Xu, J.; Bian, Z.; Xin, X.; Chen, A.; Wang, H. Size Dependence of Nanosheet BiVO4 with Oxygen Vacancies and Exposed {001} Facets on the Photodegradation of Oxytetracycline. Chem. Eng. J. 2018, 337, 684–696. [Google Scholar] [CrossRef]
- Du, Z.; Feng, L.; Guo, Z.; Yan, T.; Hu, Q.; Lin, J.; Huang, Y.; Tang, C.; Fang, Y. Ultrathin H-BN/Bi2MoO6 Heterojunction with Synergetic Effect for Visible-Light Photocatalytic Tetracycline Degradation. J. Colloid Interface Sci. 2021, 589, 545–555. [Google Scholar] [CrossRef]
- Hernández-Arellano, D.L.; Durán-Álvarez, J.C.; Zanella, R.; López-Juárez, R. Effect of Heat Treatment on the Structure and Photocatalytic Properties of BiYO3 and BiY0.995Ni0.005O3 Ceramic Powders. Ceram. Int. 2020, 46, 20291–20298. [Google Scholar] [CrossRef]
- Gautam, S.; Shandilya, P.; Singh, V.P.; Raizada, P.; Singh, P. Solar Photocatalytic Mineralization of Antibiotics Using Magnetically Separable NiFe2O4 Supported onto Graphene Sand Composite and Bentonite. J. Water Process Eng. 2016, 14, 86–100. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Singh, P. Magnetically Recoverable Graphitic Carbon Nitride and NiFe2O4 Based Magnetic Photocatalyst for Degradation of Oxytetracycline Antibiotic in Simulated Wastewater under Solar Light. J. Environ. Chem. Eng. 2018, 6, 3874–3883. [Google Scholar] [CrossRef]
- Gautam, S.; Shandilya, P.; Priya, B.; Singh, V.P.; Raizada, P.; Rai, R.; Valente, M.A.; Singh, P. Superparamagnetic MnFe2O4 Dispersed over Graphitic Carbon Sand Composite and Bentonite as Magnetically Recoverable Photocatalyst for Antibiotic Mineralization. Sep. Purif. Technol. 2017, 172, 498–511. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, M.; Dai, K.; Zhang, X.; Chen, H. Controllable Synthesis of Bi2MoO6 Nanosheets and Their Facet-Dependent Visible-Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2018, 430, 505–514. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Kong, J.; Yuan, J.; Sun, C.; Xian, Q.; Yang, S.; He, H. High Photocatalytic Degradation Efficiency of Oxytetracycline Hydrochloride over Ag/AgCl/BiVO4 Plasmonic Photocatalyst. Solid State Sci. 2019, 96, 105946. [Google Scholar] [CrossRef]
- Mohan, H.; Selvaraj, D.; Kuppusamy, S.; Venkatachalam, J.; Park, Y.; Seralathan, K.; Oh, B. E-Waste Based V2O5/RGO/Pt Nanocomposite for Photocatalytic Degradation of Oxytetracycline. Environ. Prog. Sustain. Energy 2019, 38, 13123. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Yu, C.; Wei, L.; Ji, H. Fabrication and Characterization of I Doped Bi2MoO6 Microspheres with Distinct Performance for Removing Antibiotics and Cr(VI) under Visible Light Illumination. Sep. Purif. Technol. 2020, 247, 116951. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, N.; Wang, T.; Chen, G.; Qiu, X.; Ouyang, S.; Mei, Z.; Liu, X.; Ma, R. Ag1.69Sb2.27O6.25 Coupled Carbon Nitride Photocatalyst with High Redox Potential for Efficient Multifunctional Environmental Applications. Appl. Surf. Sci. 2019, 487, 82–90. [Google Scholar] [CrossRef]
- Li, R.; Sun, C.; Liu, J.; Zhen, Q. Sulfur-Doped CoFe2O4 Nanopowders for Enhanced Visible-Light Photocatalytic Activity and Magnetic Properties. RSC Adv. 2017, 7, 50546–50554. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Guo, F.; Tang, Y.; Lu, C. Construction of CuBi2O4/Bi2MoO6 p-n Heterojunction with Nanosheets-on-Microrods Structure for Improved Photocatalytic Activity towards Broad-Spectrum Antibiotics Degradation. Chem. Eng. J. 2020, 394, 125009. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Zhang, L. In-Situ Fabrication of 3D Hierarchical Flower-like β-Bi2O3@CoO Z-Scheme Heterojunction for Visible-Driven Simultaneous Degradation of Multi-Pollutants. J. Hazard. Mater. 2021, 403, 123566. [Google Scholar] [CrossRef]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically Structured Ternary Heterojunctions Based on Ce3+/Ce4+ Modified Fe3O4 Nanoparticles Anchored onto Graphene Oxide Sheets as Magnetic Visible-Light-Active Photocatalysts for Decontamination of Oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Huo, P.; Zhou, M.; Tang, Y.; Liu, X.; Ma, C.; Yu, L.; Yan, Y. Incorporation of N–ZnO/CdS/Graphene Oxide Composite Photocatalyst for Enhanced Photocatalytic Activity under Visible Light. J. Alloys Compd. 2016, 670, 198–209. [Google Scholar] [CrossRef]
- Ma, D.-K.; Guan, M.-L.; Liu, S.-S.; Zhang, Y.-Q.; Zhang, C.-W.; He, Y.-X.; Huang, S.-M. Controlled Synthesis of Olive-Shaped Bi2S3/BiVO4 Microspheres through a Limited Chemical Conversion Route and Enhanced Visible-Light-Responding Photocatalytic Activity. Dalton Trans. 2012, 41, 5581–5586. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Zeng, G.-M. Fabrication of SnO2 Nanopaticles/BiOI n–p Heterostructure for Wider Spectrum Visible-Light Photocatalytic Degradation of Antibiotic Oxytetracycline Hydrochloride. ACS Sustain. Chem. Eng. 2017, 5, 5134–5147. [Google Scholar] [CrossRef]
- Priya, B.; Raizada, P.; Singh, N.; Thakur, P.; Singh, P. Adsorptional Photocatalytic Mineralization of Oxytetracycline and Ampicillin Antibiotics Using Bi2O3/BiOCl Supported on Graphene Sand Composite and Chitosan. J. Colloid Interface Sci. 2016, 479, 271–283. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. An in Depth Mechanism Insight of the Degradation of Multiple Refractory Pollutants via a Novel SrTiO3/BiOI Heterojunction Photocatalysts. J. Catal. 2017, 356, 283–299. [Google Scholar] [CrossRef]
- Wei, Z.; Benlin, D.; Fengxia, Z.; Xinyue, T.; Jiming, X.; Lili, Z.; Shiyin, L.; Leung, D.Y.C.; Sun, C. A Novel 3D Plasmonic P-n Heterojunction Photocatalyst: Ag Nanoparticles on Flower-like p-Ag2S/n-BiVO4 and Its Excellent Photocatalytic Reduction and Oxidation Activities. Appl. Catal. B Environ. 2018, 229, 171–180. [Google Scholar] [CrossRef]
- Mohan, H.; Lim, J.; Lee, S.; Cho, M.; Park, Y.; Seralathan, K.; Oh, B. V2O5/RGO/Pt Nanocomposite on Oxytetracycline Degradation and Pharmaceutical Effluent Detoxification. J. Chem. Technol. Biotechnol. 2020, 95, 297–307. [Google Scholar] [CrossRef]
- Yang, S.-F.; Niu, C.-G.; Huang, D.-W.; Zhang, H.; Zeng, G.-M. Ag/AgCl Nanoparticles-Modified CdSnO3·3H2O Nanocubes Photocatalyst for the Degradation of Methyl Orange and Antibiotics under Visible Light Irradiation. J. Colloid Interface Sci. 2017, 505, 96–104. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, F.; Gu, W.; Sun, L.; Shi, W.; Hua, Y. Construction of Nitrogen-Doped Graphene Quantum Dots-BiVO4/g-C3N4 Z-Scheme Photocatalyst and Enhanced Photocatalytic Degradation of Antibiotics under Visible Light. RSC Adv. 2016, 6, 61162–61174. [Google Scholar] [CrossRef]
- Guan, D.-L.; Niu, C.-G.; Wen, X.-J.; Guo, H.; Deng, C.-H.; Zeng, G.-M. Enhanced Escherichia Coli Inactivation and Oxytetracycline Hydrochloride Degradation by a Z-Scheme Silver Iodide Decorated Bismuth Vanadate Nanocomposite under Visible Light Irradiation. J. Colloid Interface Sci. 2018, 512, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-L.; Liu, C.-M.; Huang, F.-Q.; Zheng, C.; Wang, W.-D. Study of the Electronic Structure and Photocatalytic Activity of the BiOCl Photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Prakruthi, K.; Ujwal, M.P.; Yashas, S.R.; Mahesh, B.; Kumara Swamy, N.; Shivaraju, H.P. Recent Advances in Photocatalytic Remediation of Emerging Organic Pollutants Using Semiconducting Metal Oxides: An Overview. Environ. Sci. Pollut. Res. 2022, 29, 4930–4957. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Abdel Hamid, Z. Graphene-Based Nanocomposites: Synthesis, Mechanical Properties, and Characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, D.; Sun, Z.; Zheng, M.; Li, J.; Zhang, Y.; Zhang, G.; Zhao, H.; Liu, X.; Xie, Z. Three Colors Emission from S,N Co-Doped Graphene Quantum Dots for Visible Light H2 Production and Bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zhang, M.; Liu, S.; Yi, H.; Liu, X.; An, N.; Zhou, X.; Li, L.; Fu, Y.; et al. N, S-GQDs and Au Nanoparticles Co-Modified Ultrathin Bi2MoO6 Nanosheet with Enhanced Charge Transport Dynamics for Full-Spectrum-Light-Driven Molecular Oxygen Activation. Chem. Eng. J. 2021, 409, 128281. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, D.; Zhou, C.; Wang, W.; Xiong, W.; Song, B.; Yi, H.; et al. In Situ Grown Single-Atom Cobalt on Polymeric Carbon Nitride with Bidentate Ligand for Efficient Photocatalytic Degradation of Refractory Antibiotics. Small 2020, 16, 2001634. [Google Scholar] [CrossRef]
- Guo, H.; Niu, C.-G.; Feng, C.-Y.; Liang, C.; Zhang, L.; Wen, X.-J.; Yang, Y.; Liu, H.-Y.; Li, L.; Lin, L.-S. Steering Exciton Dissociation and Charge Migration in Green Synthetic Oxygen-Substituted Ultrathin Porous Graphitic Carbon Nitride for Boosted Photocatalytic Reactive Oxygen Species Generation. Chem. Eng. J. 2020, 385, 123919. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, F.; Wen, K.; Lu, J. Enhanced Photocatalytic Activity for Tetracyclines Degradation with Ag Modified g-C3N4 Composite under Visible Light. J. Photochem. Photobiol. Chem. 2020, 389, 112217. [Google Scholar] [CrossRef]
- Viet, N.M.; Trung, D.Q.; Giang, B.L.; Tri, N.L.M.; Thao, P.; Pham, T.H.; Kamand, F.Z.; Al Tahtamouni, T.M. Noble Metal -Doped Graphitic Carbon Nitride Photocatalyst for Enhancement Photocatalytic Decomposition of Antibiotic Pollutant in Wastewater under Visible Light. J. Water Process Eng. 2019, 32, 100954. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Chen, F.; Shi, H. Metallic NiSe Cocatalyst Decorated g-C3N4 with Enhanced Photocatalytic Activity. Chem. Eng. J. 2021, 413, 127474. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, D.K.; Selvaraj, R.; Kim, Y. Facile Synthesis of Br-Doped g-C3N4 Nanosheets via One-Step Exfoliation Using Ammonium Bromide for Photodegradation of Oxytetracycline Antibiotics. J. Ind. Eng. Chem. 2019, 79, 473–481. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Yu, H.; Zou, Y.; Dong, X. Enhanced Photocatalytic Performance of Boron and Phosphorous Co-Doped Graphitic Carbon Nitride Nanosheets for Removal of Organic Pollutants. Sep. Purif. Technol. 2019, 226, 128–137. [Google Scholar] [CrossRef]
- Yu, C.; Tan, L.; Shen, S.; Fang, M.; Yang, L.; Fu, X.; Dong, S.; Sun, J. In Situ Preparation of g-C3N4/Polyaniline Hybrid Composites with Enhanced Visible-Light Photocatalytic Performance. J. Environ. Sci. 2021, 104, 317–325. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, D.; Zhou, C.; Wang, W.; Xiong, W.; Li, X.; Li, B.; et al. Molecular Engineering of Polymeric Carbon Nitride for Highly Efficient Photocatalytic Oxytetracycline Degradation and H2O2 Production. Appl. Catal. B Environ. 2020, 272, 118970. [Google Scholar] [CrossRef]
- Majumdar, A.; Ghosh, U.; Pal, A. Novel 2D/2D g-C3N4/Bi4NbO8Cl Nano-Composite for Enhanced Photocatalytic Degradation of Oxytetracycline under Visible LED Light Irradiation. J. Colloid Interface Sci. 2021, 584, 320–331. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D Porous Tubular g-C3N4 Capture Black Phosphorus Quantum Dots as 1D/0D Metal-Free Photocatalysts for Oxytetracycline Hydrochloride Degradation and Hexavalent Chromium Reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, Q.; Sun, C.; Xia, D.; Huang, H.; Yang, G.; Wang, G.; Leung, D.Y.C. A Novel Au/g-C3N4 Nanosheets/CeO2 Hollow Nanospheres Plasmonic Heterojunction Photocatalysts for the Photocatalytic Reduction of Hexavalent Chromium and Oxidation of Oxytetracycline Hydrochloride. Chem. Eng. J. 2021, 409, 128185. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Wang, S.; Yao, F.; Sun, J.; Wang, Y.; Zhang, C.; Li, X.; Niu, C.; Wang, D.; et al. Graphene Oxide and Carbon Nitride Nanosheets Co-Modified Silver Chromate Nanoparticles with Enhanced Visible-Light Photoactivity and Anti-Photocorrosion Properties towards Multiple Refractory Pollutants Degradation. Appl. Catal. B Environ. 2017, 209, 493–505. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
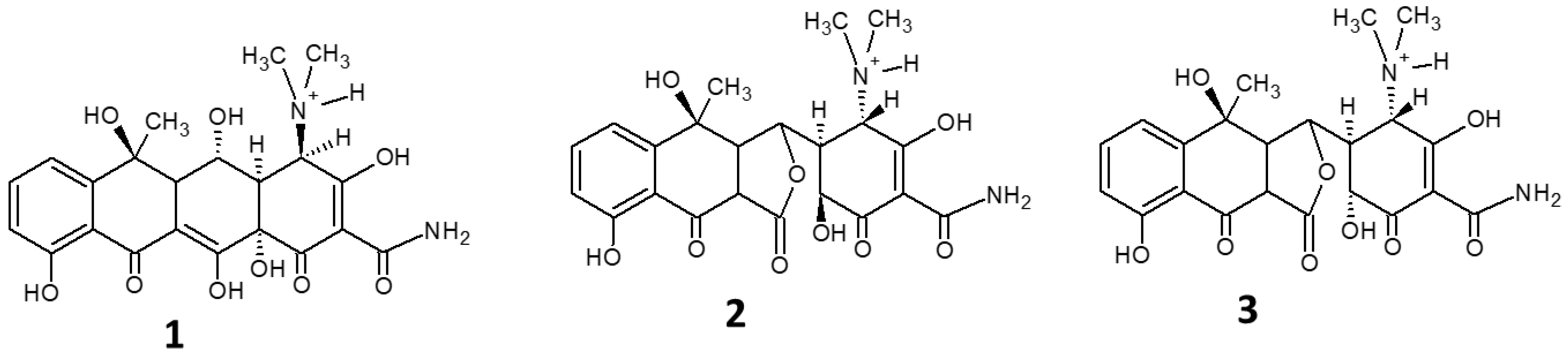
| Photocatalyst | Light Source | (OTC) (mg L−1) | Catalyst (g L−1) | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| TiO2 P25 Degussa powder | Xe 1000 W | 20 | 0.5 | 95 | 35 | [84] |
| TiO2 P25 Degussa powder | Hg 24 W | 5 | 1 | 100 | 180 | [85] |
| TiO2 P25 Degussa powder | UVA 6 W | 5 | 0.4 | 90 | 30 | [86] |
| TiO2 powder | Solar UV | 20 | 0.5 | 100 | n.a. | [87] |
| TiO2 nanoflowers | Sunlight | 0.5 | 1 | 80 | 60 | [88] |
| Brown TiO2 spheres | Sunlight | 5 | 5 | ≈50 | 80 | [89] |
| Photocatalyst | Light Source | (OTC) (mg L−1) | Catalyst (g L−1) | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Co-B co-doped TiO2/SiO2 | Vis. light | 5 | film | 37 | 100 | [91] |
| Ag-decorated TiO2 | 30 W | 0.5 | 0.5 | 100 | 60 (UV) 180 (Vis) | [92] |
| Ag3PO4/TiO2@MoS2 | Xe 800 W | 5 | 1 | 90 | 24 | [93] |
| Au/CuS/TiO2 | Xe 35 W | 5 | sheet | 96 | 60 | [94] |
| BiOCl/TiO2 hollow tubes | Xe 300 W | 20 | 0.5 | 51 | 150 | [95] |
| BiVO4/TiO2/RGO * | Xe 1000 W | 10 | n.a. | ≈99 | 120 | [96] |
| CdS/TiO2 | Xe 300 W | 40 | 1 | 81 | 50 | [97] |
| Co2+/F− co-doped TiO2-SiO2 | W lamp | 100 | film | 42 | 40 | [98] |
| Co3O4/TiO2 | Xe 300 W | 10 | 0.25 | 76 | 90 | [99] |
| Co3O4/TiO2/GO * | Xe 300 W | 10 | 0.25 | 91 | 90 | [99] |
| Cu-porphyrin-TiO2 | UV 300 W | 14 | 0.02 | ≈65 | 40 | [100] |
| Fe2O3/TiO2 | W 300 W | 60 | 1 | 95 | 300 | [101] |
| N-TiO2/graphene | Hg 250 W | 30 | film | ≈62 | 160 | [102] |
| Polypyrrole TiO2@V2O5 | Xe 300 W | 50 | 0.6 | 85 | 120 | [103] |
| POPD/TiO2/fly ash | W 300 W | 10 | 0.1 | 73 | 30 | [104] |
| TiO2@GO * | Xe 500 W | 20 | 0.6 | 99.4 | 240 | [105] |
| TiO2/5A zeolite | UV 32 W | 50 | 1 | 100 | 150 | [106] |
| TiO2/5A zeolite | UV 32 W | 50 | 0.5 | 100 | 210 | [107] |
| ZnO/TiO2 | Solar light | 60 | 1 | 90.3 | 8 | [108] |
| ZnO/TiO2/Ag2Se | Blue LED 36 W | 5 | film | ≈55 | 360 | [109] |
| Photocatalyst | Light Source | (OTC) (mg L−1) | Catalyst (g L−1) | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Fe2O3/TiO2 | W-Halide 300 W | 60 | 1 | ≈50 | 300 | [101] |
| Fe3O4 | Sunlight 1 | 46 | 0.5 | 42 | 120 | [113] |
| Bi2WO6 | Sunlight 1 | 46 | 0.5 | 40 | 120 | [113] |
| LaFeO3 | LED 40 W | 40 | 0.5 | ≈50 | 120 | [114] |
| ZnO | Sunlight 1 | 46 | 0.5 | 41 | 120 | [115] |
| ZnWO4 | Sunlight 1 | 46 | 0.5 | 46 | 120 | [115] |
| CuCo2O4 | Xe 500 W | 20 | 1.0 | 91.5 | 180 | [117] |
| yellow-WO3 | Au-Halide 500 W | 20 | 1.0 | 87.9 | 60 | [118] |
| BiVO4 | Xe 500 W | 10 | 0.25 | 47.4 | 60 | [119] |
| BiVO4 nanosheets | Xe 500 W | 20 | 1 | 95.8 | 120 | [120] |
| Bi2MoO6 | Xe 300 W | 20 | 0.5 | ≈80 | 140 | [121] |
| BiYO3 | Xe 25 W | 30 | 0.25 | 59 | 300 | [122] |
| BiY0.995Ni0.005O3 | Xe 25 W | 30 | 0.25 | 97 | 300 | [122] |
| NiFe2O4 | Sunlight 1 | 46 | 0.50 | <30 | 120 | [123] |
| GSC/NiFe2O4 * | Sunlight 1 | 46 | 0.50 | 80 | 120 | [123] |
| BT/NiFe2O4 * | Sunlight 1 | 46 | 0.50 | 69 | 120 | [123] |
| NiFe2O4 | Sunlight 1 | 46 | 0.50 | 65 | 60 | [124] |
| MnFe2O4 | Sunlight 1 | 46 | 0.50 | <50 | 120 | [125] |
| GSC/MnFe2O4 * | Sunlight 1 | 46 | 0.50 | 99 | 120 | [125] |
| BT/MnFe2O4 * | Sunlight 1 | 46 | 0.50 | 90 | 120 | [125] |
| Bi2MoO6 nanosheets | Xe 300 W | 4.6 | 1 | ≈50 | 120 | [126] |
| BiVO4 | Xe 1000 W | 20 | 1 | 61.1 | 120 | [127] |
| V2O5 | Xe 150 W | 50 | 0.5 | 58 | 60 | [128] |
| Bi2MoO6 | Xe 350 W | 10 | 0.6 | 57.1 | 300 | [129] |
| I-Bi2MoO6 | Xe 350 W | 10 | 0.6 | 89.6 | 300 | [129] |
| Ag1.69Sb2.27O6.25 | Xe 300 W | 16 | 0.5 | 63 | 120 | [130] |
| S-CoFe2O4 | W-Iodine 300 W | 80 | 1 | 83 | 300 | [131] |
| Photocatalyst | Light Source | (OTC) (mg L−1) | Catalyst (g L−1) | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4/Bi2WO6 | Sunlight 1 | 46 | 0.5 | 71 | 120 | [113] |
| GSC/Fe3O4/Bi2WO6 * | Sunlight 1 | 46 | 0.5 | 94 | 120 | [113] |
| g-C3N4/LaFeO3 (2%) * | LED 40 W | 40 | 0.5 | 90 | 120 | [114] |
| ZnO/ZnWO4 | Sunlight 1 | 46 | 0.5 | 70 | 120 | [115] |
| AC/ZnO/ZnWO4 * | Sunlight 1 | 46 | 0.5 | 96 | 120 | [115] |
| Cu2O/α-Fe2O3 2 | Xe 300 W | 10 | n.a.2 | 73.3 | 60 | [116] |
| MWCN/BiVO4 * | Xe 500 W | 10 | 0.25 | 88.7 | 60 | [119] |
| hBN/Bi2MoO6 * | Xe 300 W | 20 | 0.5 | 95.3 | 140 | [121] |
| g-C3N4/NiFe2O4 * | Sunlight 1 | 46 | 10 | 97 | 60 | [124] |
| Ag/AgCl/BiVO4 | Xe 1000 W | 20 | 1 | 97.6 | 120 | [127] |
| AgCl/BiVO4 | Xe 1000 W | 20 | 1 | 76.5 | 120 | [127] |
| 20%RGO/V2O5 * | Xe 150 W | 50 | 0.5 | 85 | 60 | [128] |
| pCN/Ag1.69Sb2.27O6.25 * | Xe 300 W | 16 | 0.5 | 94 | 120 | [130] |
| CuBi2O4/Bi2MoO6 | Xe 300 W | 20 | 0.30 | 74 | 60 | [132] |
| β-Bi2O3/CoO | Xe 300 W | 10 | 0.40 | 86 | 120 | [133] |
| GO/CeO2/Fe3O4 * | Xe 220 W | 30 | 0.8 | 60 | 120 | [134] |
| GO/Fe3-xCexO4 * | Xe 220 W | 30 | 0.8 | 88 | 120 | [134] |
| N-ZnO/CdS/GO * | Xe 300 W | 20 | 0.5 | 50 | 60 | [135] |
| Bi2S3/BiVO4 | Xe 500 W | 200 | 1 | 67 | 960 | [136] |
| 30% SnO2/BiOI | Xe 300 W | 10 | 1 | 94 | 90 | [137] |
| GSC/Bi2O3/BiOCl * | Sunlight 1 | 46 | 0.5 | 86 | 120 | [138] |
| CT/Bi2O3/BiOCl * | Sunlight 1 | 46 | 0.5 | 90 | 120 | [138] |
| 22% SrTiO3/BiOI | Xe 300 W | 20 | 1 | 85.3 | 90 | [139] |
| Ag/Ag2S/BiVO4 | Xe 500 W | 20 | 0.4 | 99.8 | 150 | [140] |
| 20%RGO/V2O5/Pt(1%) * | Xe 150 W | 50 | 0.5 | 99 | 40 | [141] |
| Ag/AgCl/CdSnO3 | Xe 300 W | 10 | 1 | 90 | 60 | [142] |
| Photocatalyst | Light source | (OTC) (mg L−1) | Catalyst (g L−1) | Removal (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| N-ZnO/CdS/GO * | Xe 300 W | 20 | 0.5 | 50 | 60 | [135] |
| N,S-GQDs/BMO * | Xe 300 W | 10 | 0.1 | 81 | 60 | [150] |
| Co(1.28%)–pCN * | Xe 300 W | 20 | 0.3 | 18.3 | 40 | [151] |
| OCN * | Xe 300 W | 20 | 1 | 85.7 | 120 | [152] |
| Ag(8%)/g-C3N4 * | Xe 300 W | 20 | 1 | 81 | 120 | [153] |
| Ag(7%)/g-C3N4 * | Xe 300 W | 30 | 0.2 | 98.7 | 120 | [154] |
| NiSe(3%)/g-C3N4 * | Xe 300 W | 20 | 1 | 98.7 | 60 | [155] |
| Br(15%)/g-C3N4 * | LED 38.5 W | 10 | 1 | 75 | 120 | [156] |
| BPCNNS * | Xe 300 W | 15 | 1 | 71 | 120 | [157] |
| PANI(5%)/g-C3N4 * | Xe 350 W | 5 | 0.5 | 88 | 100 | [158] |
| ACN * | Xe 300 W | 20 | 0.3 | 79.3 | 60 | [159] |
| B4NbO8Cl/g-C3N4 | LED 18 W | 20 | 1 | 87 | 60 | [160] |
| BPQDs/g-C3N4 * | Xe 300 W | 10 | 0.6 | 81 | 60 | [161] |
| Au(6 wt%)/g-C3N4/CeO2 | Xe 500 W | 15 | 0.4 | 88 | 150 | [162] |
| GO/Ag2CrO4/g-C3N4 * | Xe 300 W | 10 | 0.2 | 94.2 | 90 | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743. https://doi.org/10.3390/molecules27092743
Pelosato R, Bolognino I, Fontana F, Sora IN. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules. 2022; 27(9):2743. https://doi.org/10.3390/molecules27092743
Chicago/Turabian StylePelosato, Renato, Isabella Bolognino, Francesca Fontana, and Isabella Natali Sora. 2022. "Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review" Molecules 27, no. 9: 2743. https://doi.org/10.3390/molecules27092743
APA StylePelosato, R., Bolognino, I., Fontana, F., & Sora, I. N. (2022). Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules, 27(9), 2743. https://doi.org/10.3390/molecules27092743