A Novel Mechanism for SIRT1 Activators That Does Not Rely on the Chemical Moiety Immediately C-Terminal to the Acetyl-Lysine of the Substrate
Abstract
:1. Introduction
2. Results and Discussion
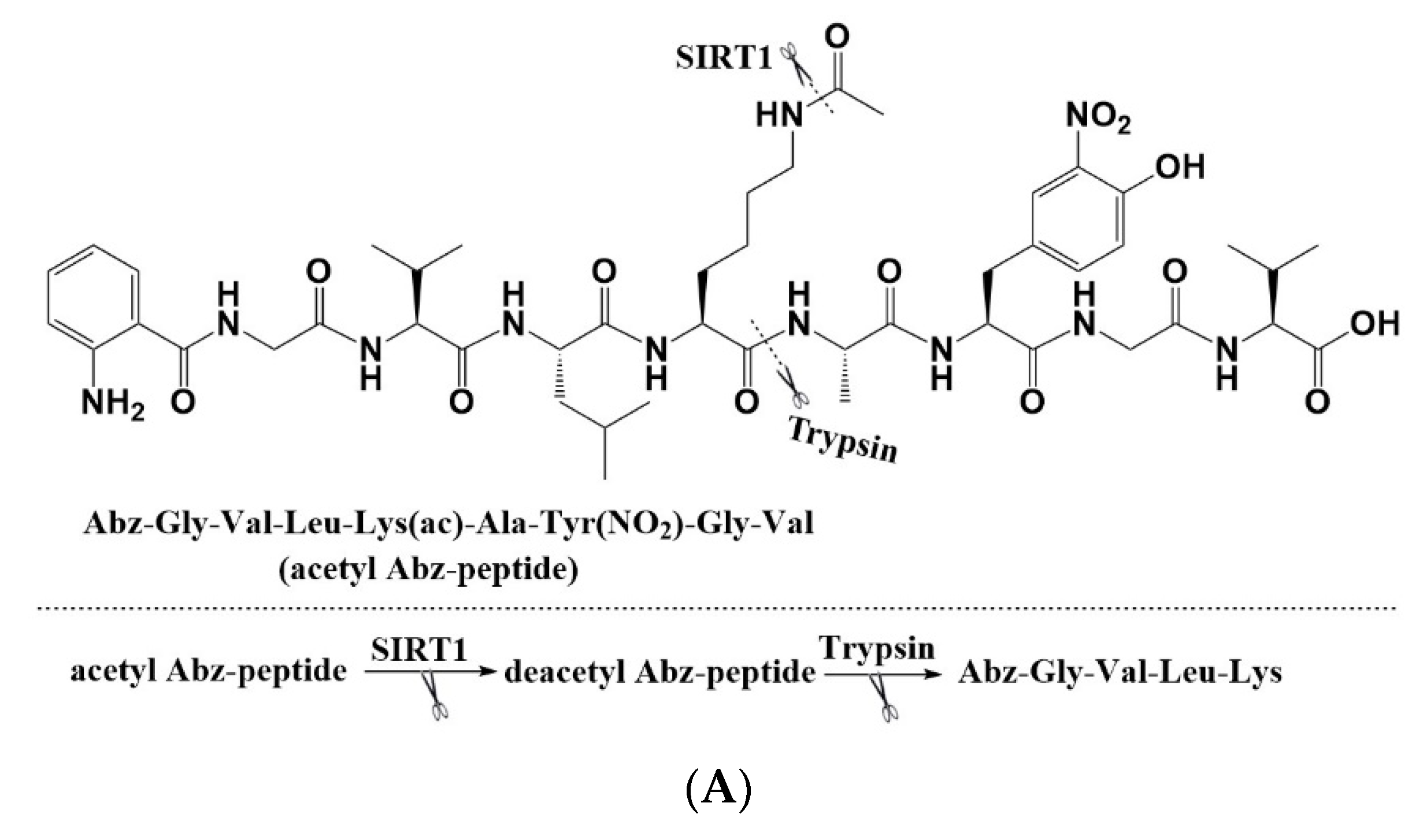
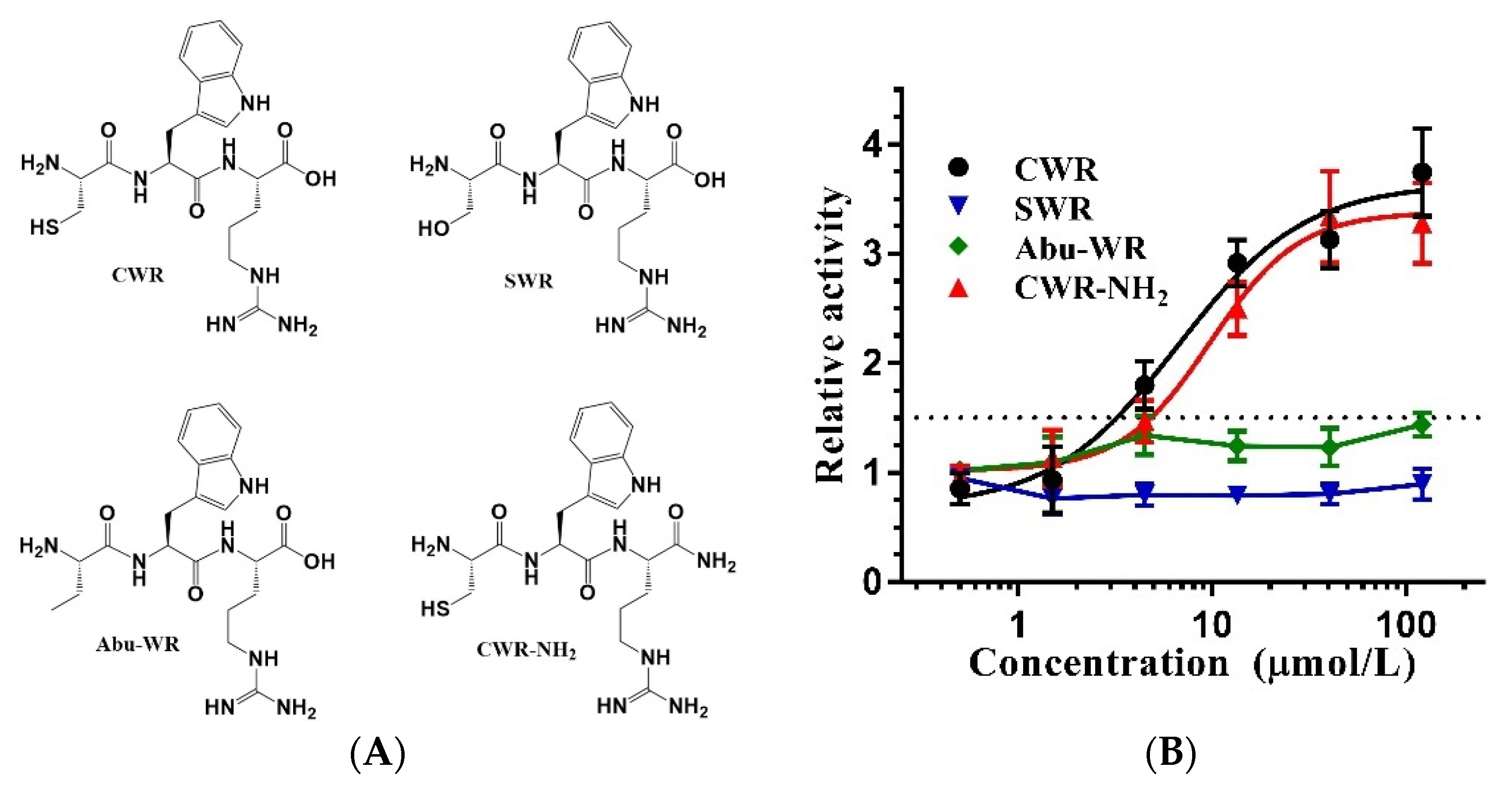
2.1. Amino Acid Sequence Determines the Activity of CWR
2.2. CWR Activity Is Independent of AMC
2.3. Cysteine of CWR Is Critical for Its Activity
2.4. CWR Activity Is Unrelated to Disulfide Bonds
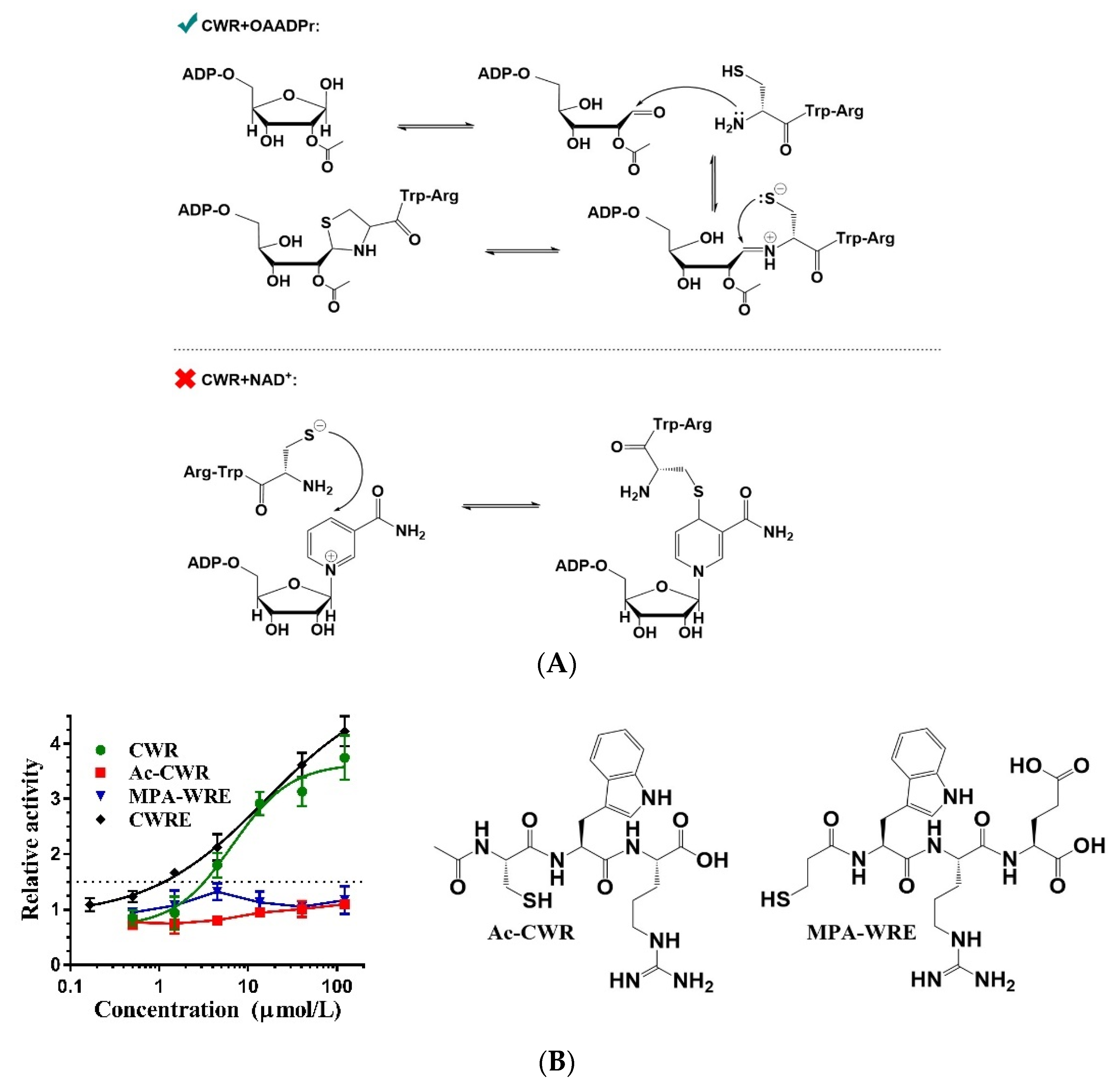
2.5. Covalent Bonding of OAADPr and Cysteines of CWR
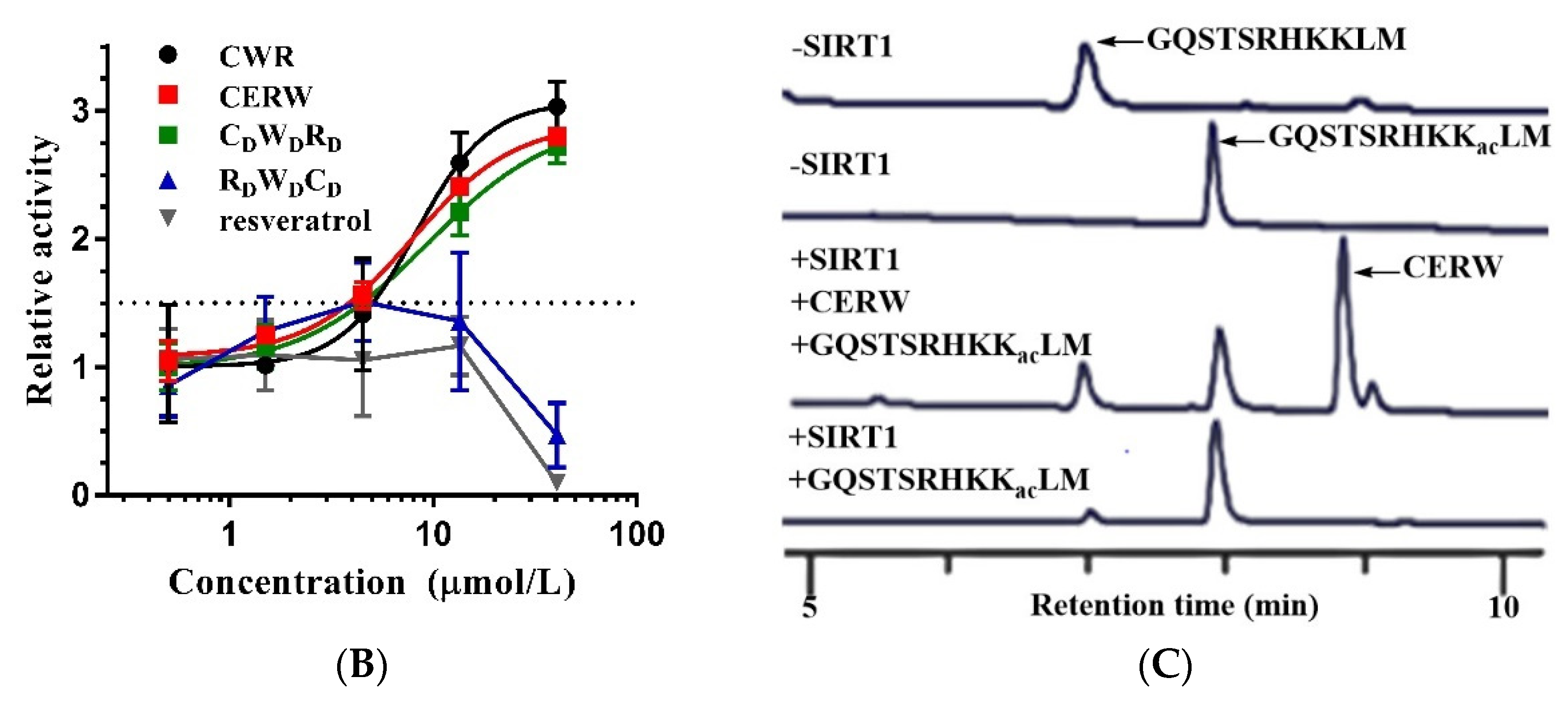
2.6. Mechanism of CWR Selectivity
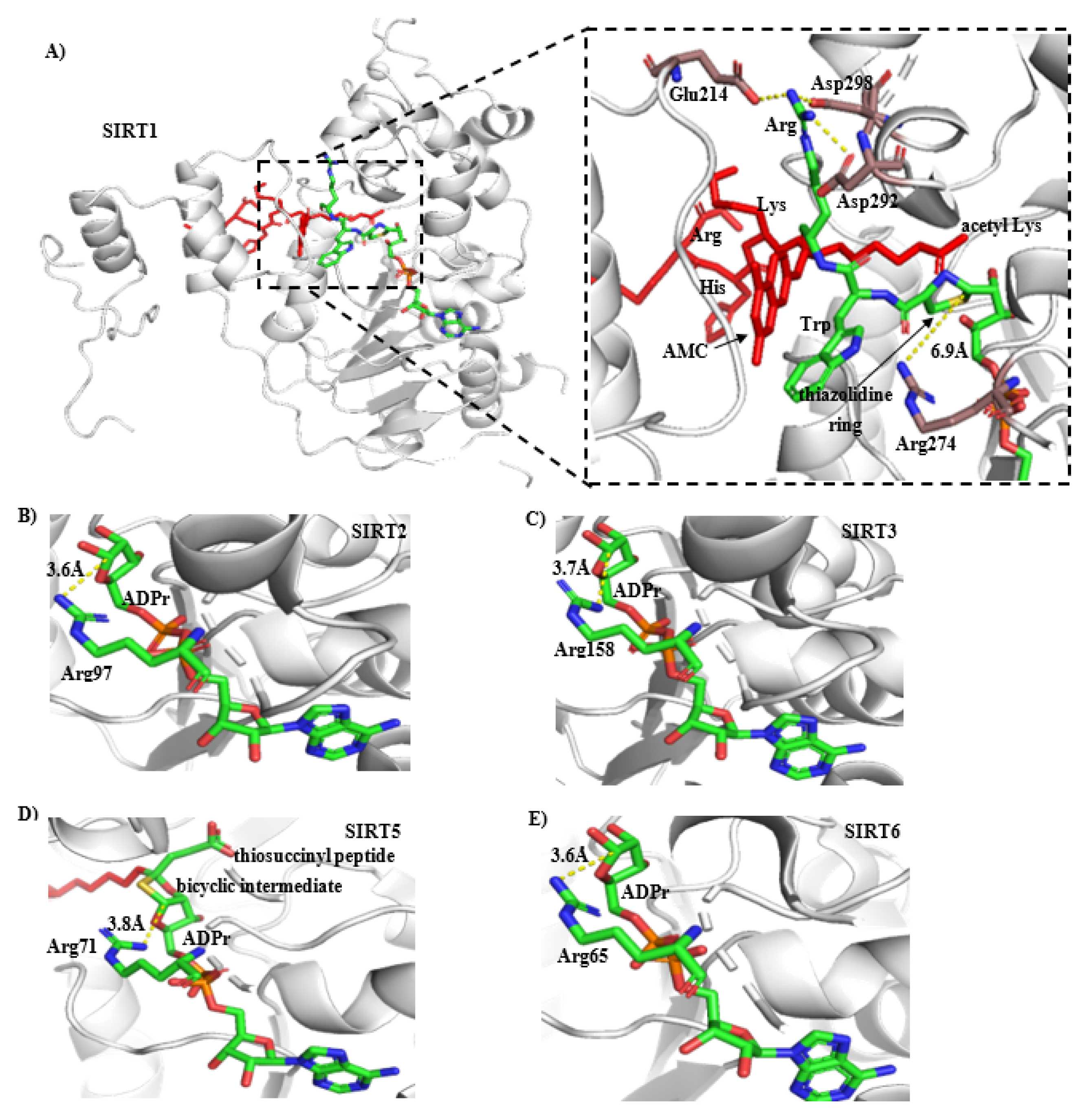
2.7. CWR Activation Mechanism
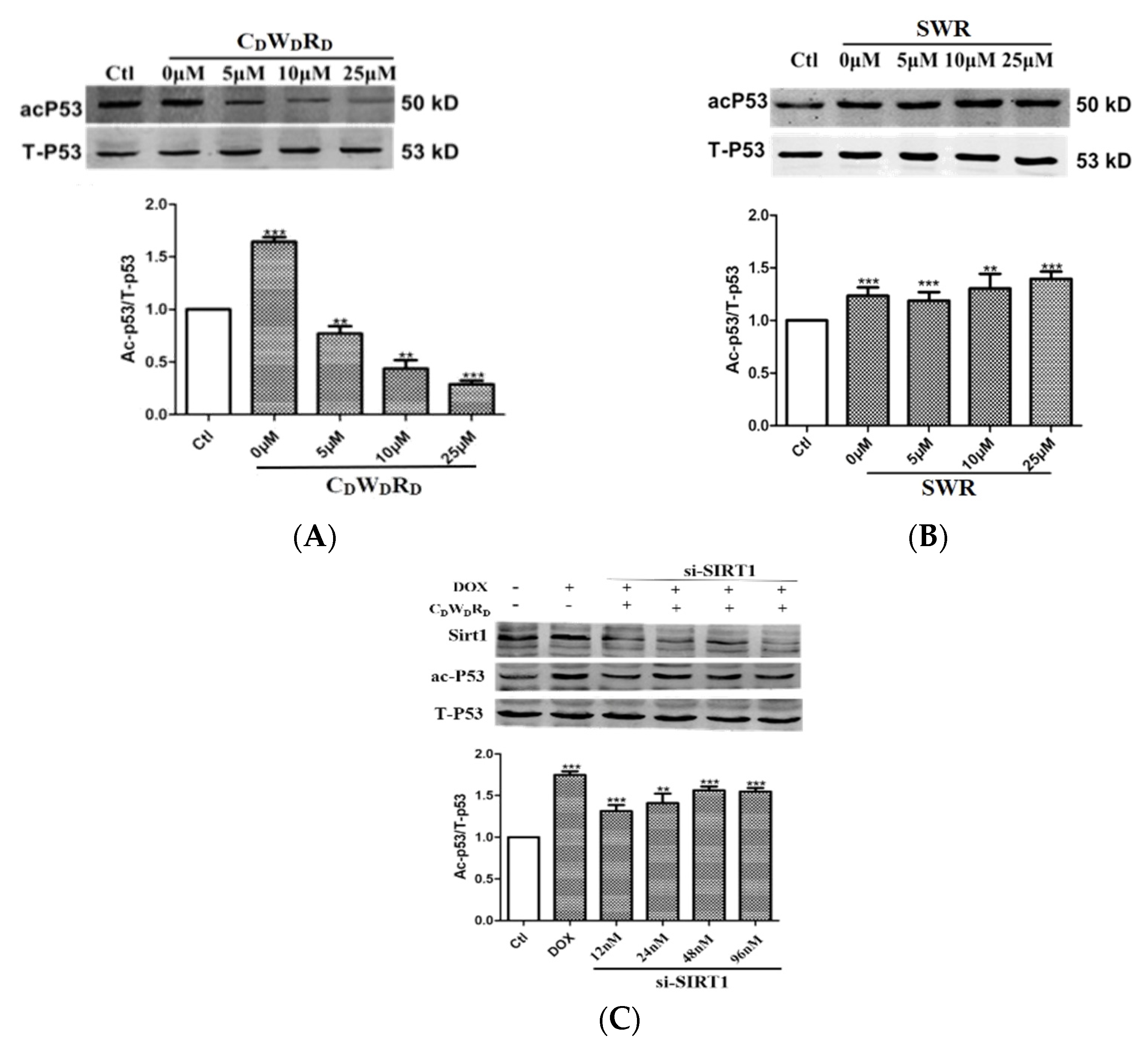
2.8. Tripeptide Promotes p53 Deacetylation in Cells via SIRT1
3. Experimental Section
3.1. Protein Expression and Purification
3.2. Screening of Sirtuin Activators and EC1.5 Determination
3.3. HPLC Assay
3.4. Enzyme Kinetics Assay
3.5. Molecular Docking
3.6. Cell Culture
3.7. Transient Transfection
3.8. Western Blotting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability Statement
References
- Schiedel, M.; Robaa, D.; Rumpf, T.; Sippl, W.; Jung, M. The Current State of NAD(+) -Dependent Histone Deacetylases (Sirtuins) as Novel Therapeutic Targets. Med. Res. Rev. 2018, 38, 147–200. [Google Scholar] [PubMed]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [PubMed] [Green Version]
- Miranda, M.X.; van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; Stein, S.; Gomes, A.P.; Suri, V.; Ellis, J.L.; et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur. Heart J. 2015, 36, 51–59. [Google Scholar] [PubMed]
- Li, H.; Chou, P.; Du, F.; Sun, L.; Liu, J.; Wang, W. Depleting microRNA-183-3p improves renal tubulointerstitial fibrosis after acute kidney injury via SIRT1/PUMA/FOXO3a deacetylation. Life Sci. 2021, 269, 119017. [Google Scholar] [PubMed]
- Li, M.Z.; Zheng, L.J.; Shen, J.; Li, X.Y.; Zhang, Q.; Bai, X.; Wang, Q.S.; Ji, J.G. SIRT1 facilitates amyloid beta peptide degradation by upregulating lysosome number in primary astrocytes. Neural Regen. Res. 2018, 13, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.A.; Kon, N.; Knight, C.; Matsumoto, M.; Gutiérrez-Juárez, R.; Rossetti, L.; Gu, W.; Accili, D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008, 8, 333–341. [Google Scholar] [PubMed] [Green Version]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. Embo J. 2007, 26, 3169–3179. [Google Scholar]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar]
- Yoshizaki, T.; Milne, J.C.; Imamura, T.; Schenk, S.; Sonoda, N.; Babendure, J.L.; Lu, J.C.; Smith, J.J.; Jirousek, M.R.; Olefsky, J.M. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell Biol. 2009, 29, 1363–1374. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar]
- Park, S.J.; Ahmad, F.; Um, J.H.; Brown, A.L.; Xu, X.; Kang, H.; Ke, H.; Feng, X.; Ryall, J.; Philp, A.; et al. Specific Sirt1 Activator-mediated Improvement in Glucose Homeostasis Requires Sirt1-Independent Activation of AMPK. EBioMedicine 2017, 18, 128–138. [Google Scholar]
- McCallum, S.; Wald, J.H.; Jayaramachandran, E.S.; Bhatt, K.; Englehart, S.; Hernandez, C.; Shukla, R.; Sueda, K.; Hussaini, A.; Ellis, J.L. Modified Release Formulations Do Not Enhance the PK of the Novel SIRT1 Activator SRT2104 (GSK2245840B). In Proceedings of the American Association of Pharmaceutical Scientists—2014 Annual Meeting and Exposition, San Diego, CA, USA, 2–6 November 2014. [Google Scholar]
- Mai, A.; Valente, S.; Meade, S.; Carafa, V.; Tardugno, M.; Nebbioso, A.; Galmozzi, A.; Mitro, N.; De Fabiani, E.; Altucci, L.; et al. Study of 1,4-dihydropyridine structural scaffold: Discovery of novel sirtuin activators and inhibitors. J. Med. Chem. 2009, 52, 5496–5504. [Google Scholar]
- Bemis, J.E.; Vu, C.B.; Xie, R.; Nunes, J.J.; Ng, P.Y.; Disch, J.S.; Milne, J.C.; Carney, D.P.; Lynch, A.V.; Jin, L.; et al. Discovery of oxazolo[4,5-b]pyridines and related heterocyclic analogs as novel SIRT1 activators. Bioorg. Med. Chem. Lett. 2009, 19, 2350–2353. [Google Scholar]
- Yao, Z.Q.; Zhang, X.; Zhen, Y.; He, X.Y.; Zhao, S.; Li, X.F.; Yang, B.; Gao, F.; Guo, F.Y.; Fu, L.; et al. A novel small-molecule activator of Sirtuin-1 induces autophagic cell death/mitophagy as a potential therapeutic strategy in glioblastoma. Cell Death Dis. 2018, 9, 767. [Google Scholar]
- Kumar, R.; Nigam, L.; Singh, A.P.; Singh, K.; Subbarao, N.; Dey, S. Design, synthesis of allosteric peptide activator for human SIRT1 and its biological evaluation in cellular model of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 909–916. [Google Scholar]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.M. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar]
- Beher, D.; Wu, J.; Cumine, S.; Kim, K.W.; Lu, S.C.; Atangan, L.; Wang, M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 2009, 74, 619–624. [Google Scholar]
- Lakshminarasimhan, M.; Rauh, D.; Schutkowski, M.; Steegborn, C. Sirt1 activation by resveratrol is substrate sequence-selective. Aging 2013, 5, 151–154. [Google Scholar]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [PubMed] [Green Version]
- Roessler, C.; Tüting, C.; Meleshin, M.; Steegborn, C.; Schutkowski, M. A Novel Continuous Assay for the Deacylase Sirtuin 5 and Other Deacetylases. J. Med. Chem. 2015, 58, 7217–7223. [Google Scholar]
- De Cesco, S.; Kurian, J.; Dufresne, C.; Mittermaier, A.K.; Moitessier, N. Covalent inhibitors design and discovery. Eur. J. Med. Chem. 2017, 138, 96–114. [Google Scholar] [PubMed]
- Jin, L.; Wei, W.; Jiang, Y.; Peng, H.; Cai, J.; Mao, C.; Dai, H.; Choy, W.; Bemis, J.E.; Jirousek, M.R.; et al. Crystal structures of human SIRT3 displaying substrate-induced conformational changes. J. Biol. Chem. 2009, 284, 24394–24405. [Google Scholar] [PubMed] [Green Version]
- Van Eys, J.; Kaplan, N.O. The addition of sulfhydryl compounds to diphosphopyridine nucleotide and its analogues. J. Biol. Chem. 1957, 228, 305–314. [Google Scholar] [PubMed]
- McDonald, L.J.; Wainschel, L.A.; Oppenheimer, N.J.; Moss, J. Amino acid-specific ADP-ribosylation: Structural characterization and chemical differentiation of ADP-ribose-cysteine adducts formed nonenzymatically and in a pertussis toxin-catalyzed reaction. Biochemistry 1992, 31, 11881–11887. [Google Scholar] [PubMed]
- Ogata, K.; Yajima, Y.; Nakamura, S.; Kaneko, R.; Goto, M.; Ohshima, T.; Yoshimune, K. Inhibition of homoserine dehydrogenase by formation of a cysteine-NAD covalent complex. Sci. Rep. 2018, 8, 5749. [Google Scholar]
- Thompson, P.S.; Amidon, K.M.; Mohni, K.N.; Cortez, D.; Eichman, B.F. Protection of abasic sites during DNA replication by a stable thiazolidine protein-DNA cross-link. Nat. Struct. Mol. Biol. 2019, 26, 613–618. [Google Scholar]
- Avalos, J.L.; Boeke, J.D.; Wolberger, C. Structural basis for the mechanism and regulation of Sir2 enzymes. Mol. Cell 2004, 13, 639–648. [Google Scholar]
- Sauve, A.A.; Celic, I.; Avalos, J.; Deng, H.; Boeke, J.D.; Schramm, V.L. Chemistry of gene silencing: The mechanism of NAD+-dependent deacetylation reactions. Biochemistry 2001, 40, 15456–15463. [Google Scholar]
- Peck, B.; Chen, C.Y.; Ho, K.K.; Di Fruscia, P.; Myatt, S.S.; Coombes, R.C.; Fuchter, M.J.; Hsiao, C.D.; Lam, E.W. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol. Cancer Ther. 2010, 9, 844–855. [Google Scholar]
- Wu, J.; Zhang, D.; Chen, L.; Li, J.; Wang, J.; Ning, C.; Yu, N.; Zhao, F.; Chen, D.; Chen, X.; et al. Discovery and mechanism study of SIRT1 activators that promote the deacetylation of fluorophore-labeled substrate. J. Med. Chem. 2013, 56, 761–780. [Google Scholar]
- Hang, T.; Chen, W.; Wu, M.; Zhan, L.; Wang, C.; Jia, N.; Zhang, X.; Zang, J. Structural insights into the molecular mechanism underlying Sirt5-catalyzed desuccinylation of histone peptides. Biochem. J. 2019, 476, 211–223. [Google Scholar]
- Li, C.; Hu, S.S.; Yang, L.; Wang, M.; Long, J.D.; Wang, B.; Han, H.; Zhu, H.; Zhao, S.; Liu, J.G.; et al. Discovery of 5-Benzylidene-2-phenyl-1,3-dioxane-4,6-diones as Highly Potent and Selective SIRT1 Inhibitors. ACS Med. Chem. Lett. 2021, 12, 397–403. [Google Scholar]
- Pirola, L.; Fröjdö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar]
- Huber, J.L.; McBurney, M.W.; Distefano, P.S.; McDonagh, T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med. Chem. 2010, 2, 1751–1759. [Google Scholar]
- Nguyen, G.T.; Schaefer, S.; Gertz, M.; Weyand, M.; Steegborn, C. Structures of human sirtuin 3 complexes with ADP-ribose and with carba-NAD+ and SRT1720: Binding details and inhibition mechanism. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1423–1432. [Google Scholar]
- Rabideau, A.E.; Pentelute, B.L. A d-Amino Acid at the N-Terminus of a Protein Abrogates Its Degradation by the N-End Rule Pathway. ACS Cent. Sci. 2015, 1, 423–430. [Google Scholar]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta Biochim. Biophys. Sin. 2017, 49, 916–925. [Google Scholar]
- Carmona, G.; Rodriguez, A.; Juarez, D.; Corzo, G.; Villegas, E. Improved protease stability of the antimicrobial peptide Pin2 substituted with D-amino acids. Protein J. 2013, 32, 456–466. [Google Scholar]
- Feng, Z.; Xu, B. Inspiration from the mirror: D-amino acid containing peptides in biomedical approaches. Biomol. Concepts 2016, 7, 179–187. [Google Scholar]
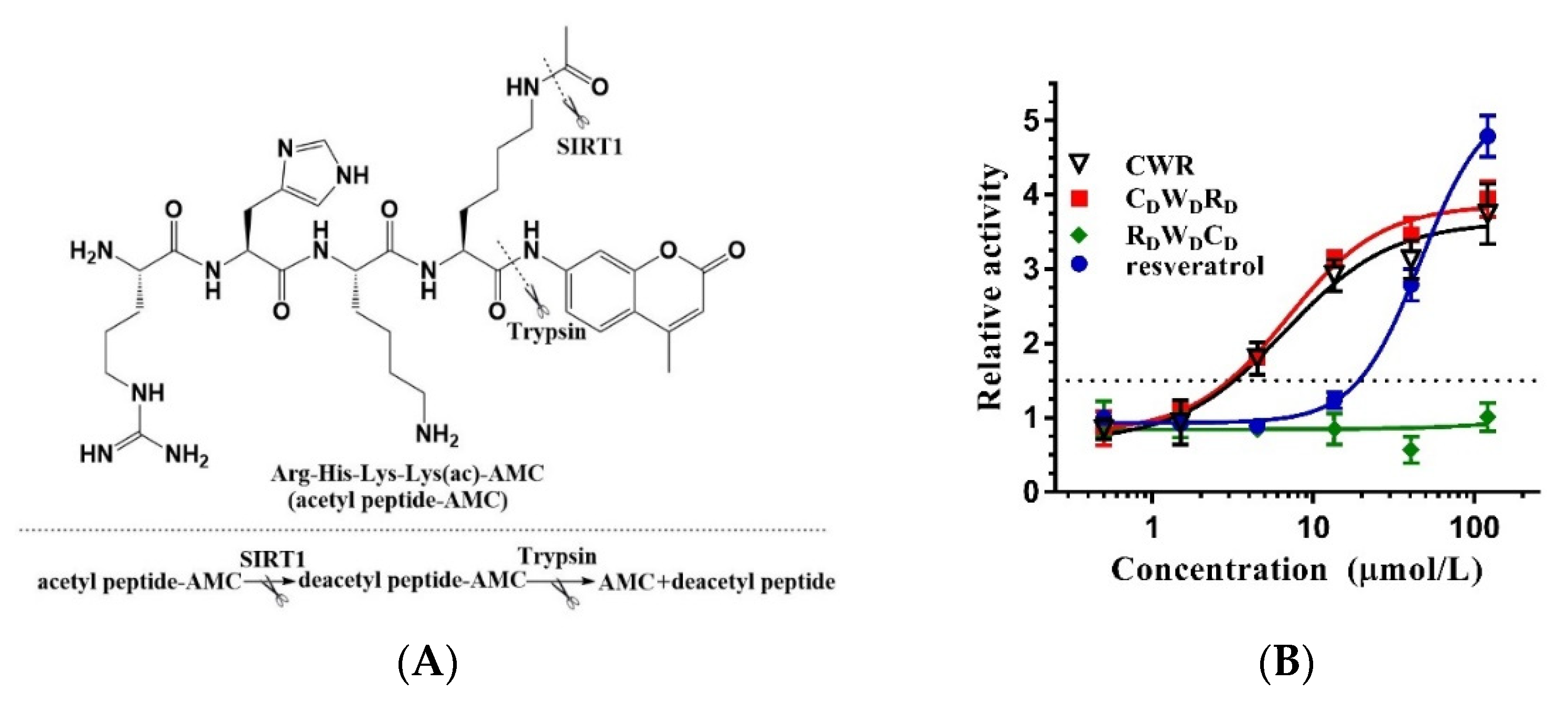



| Compounds | EC1.5 (μmol/L) a | Max A (%) b |
|---|---|---|
| Resveratrol | 19.2 ± 1.60 | 528 ± 26.0 |
| CWR | 3.16 ± 0.35 | 364 ± 20.5 |
| CDWDRD | 2.99 ± 0.38 | 386 ± 12.5 |
| CDWR | 3.18 ± 0.21 | 295 ± 32.9 |
| CWDR | 5.17 ± 0.71 | 275 ± 32.1 |
| CWRD | 3.11 ± 0.32 | 374 ± 15.1 |
| CDWDR | 3.02 ± 0.20 | 333 ± 20.3 |
| CDWRD | 3.71 ± 0.17 | 320 ± 18.8 |
| CWDRD | 4.57 ± 0.27 | 344 ± 23.4 |
| RDWDCD | NA c | NA |
| CWN | 10.0 ± 1.23 | 210 ± 11.4 |
| SWR | NA | NA |
| SDWDRD | NA | NA |
| CERW | 1.98 ± 0.10 | 306 ± 20.3 |
| CWRE | 1.06 ± 0.07 | 523 ± 44.5 |
| CWR dimer | NA | NA |
| SIRT1 + Pep | RHKKac-AMC | NAD+ | ||||
|---|---|---|---|---|---|---|
| Kcat | Km | Kcat/Km | Kcat | Km | Kcat/Km | |
| (S−1) | (μmol/L) | (S−1 mmol−1L) | (S−1) | (μmol/L) | (S−1 mmol−1L) | |
| SIRT1 | 0.61 ± 0.03 | 223.1 ± 38.22 | 2.740 ± 0.78 | 0.65 ± 0.05 | 203.2 ± 39.74 | 3.20 ± 1.25 |
| SIRT1 + CWR | 0.79 ± 0.05 | 72.70 ± 20.39 | 10.90 ± 2.45 | 1.34 ± 0.05 | 202.8 ± 24.45 | 6.63 ± 2.04 |
| SIRT1 + SWR | 0.61 ± 0.05 | 225.9 ± 32.79 | 2.690 ± 1.52 | 0.66 ± 0.04 | 202.3 ± 37.89 | 3.26 ± 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.-D.; Wang, B.; Li, X.-Z.; Han, H.-Z.; Liu, D. A Novel Mechanism for SIRT1 Activators That Does Not Rely on the Chemical Moiety Immediately C-Terminal to the Acetyl-Lysine of the Substrate. Molecules 2022, 27, 2714. https://doi.org/10.3390/molecules27092714
Yu N-D, Wang B, Li X-Z, Han H-Z, Liu D. A Novel Mechanism for SIRT1 Activators That Does Not Rely on the Chemical Moiety Immediately C-Terminal to the Acetyl-Lysine of the Substrate. Molecules. 2022; 27(9):2714. https://doi.org/10.3390/molecules27092714
Chicago/Turabian StyleYu, Nian-Da, Bing Wang, Xin-Zhu Li, Hao-Zhen Han, and Dongxiang Liu. 2022. "A Novel Mechanism for SIRT1 Activators That Does Not Rely on the Chemical Moiety Immediately C-Terminal to the Acetyl-Lysine of the Substrate" Molecules 27, no. 9: 2714. https://doi.org/10.3390/molecules27092714
APA StyleYu, N.-D., Wang, B., Li, X.-Z., Han, H.-Z., & Liu, D. (2022). A Novel Mechanism for SIRT1 Activators That Does Not Rely on the Chemical Moiety Immediately C-Terminal to the Acetyl-Lysine of the Substrate. Molecules, 27(9), 2714. https://doi.org/10.3390/molecules27092714







