The Inhibition of Gastric Cancer Cells’ Progression by 23,24-Dihydrocucurbitacin E through Disruption of the Ras/Raf/ERK/MMP9 Signaling Pathway
Abstract
1. Introduction
2. Results
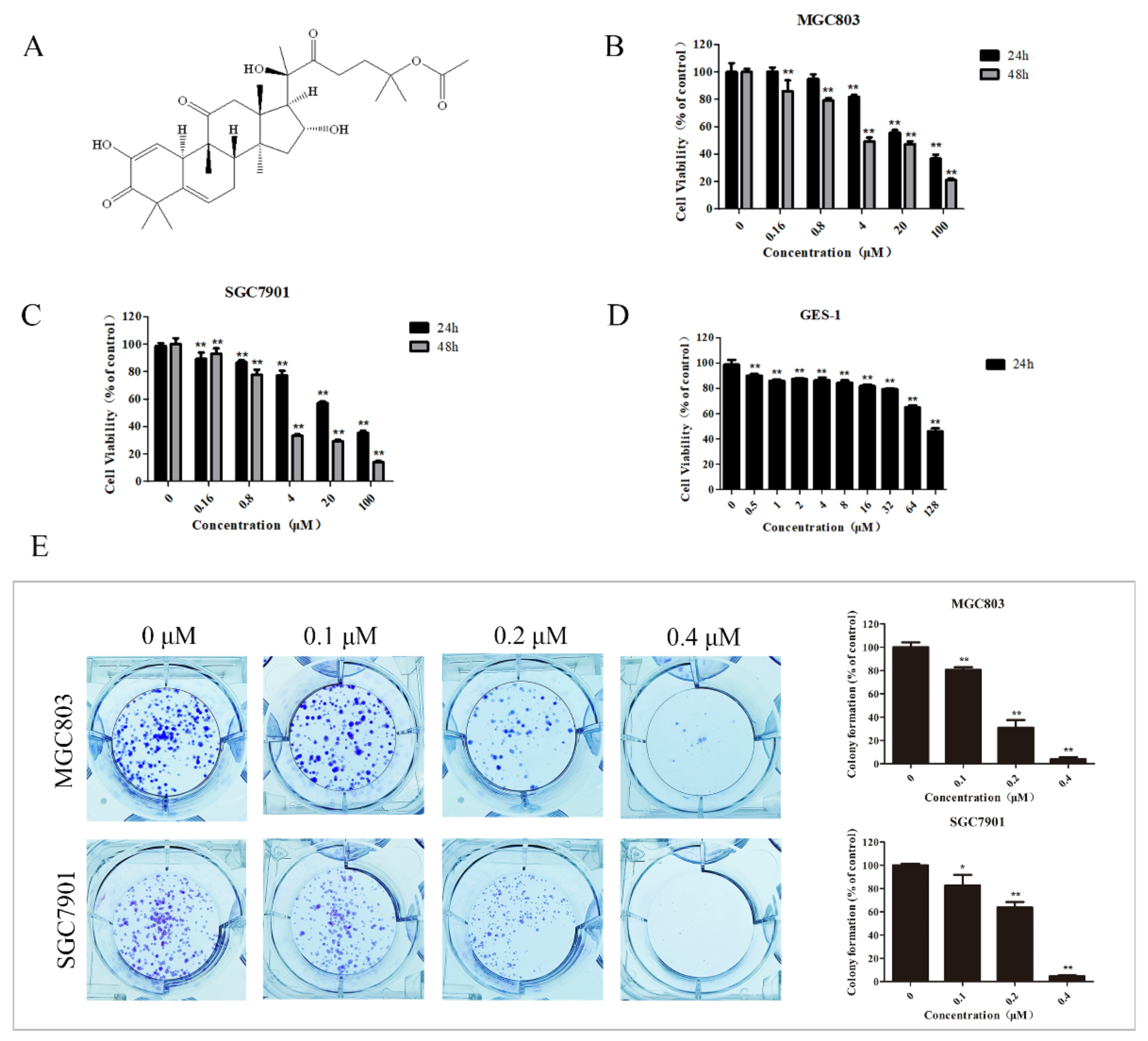
2.1. DHCE Selectively Inhibited the Proliferation of Gastric Cancer Cells
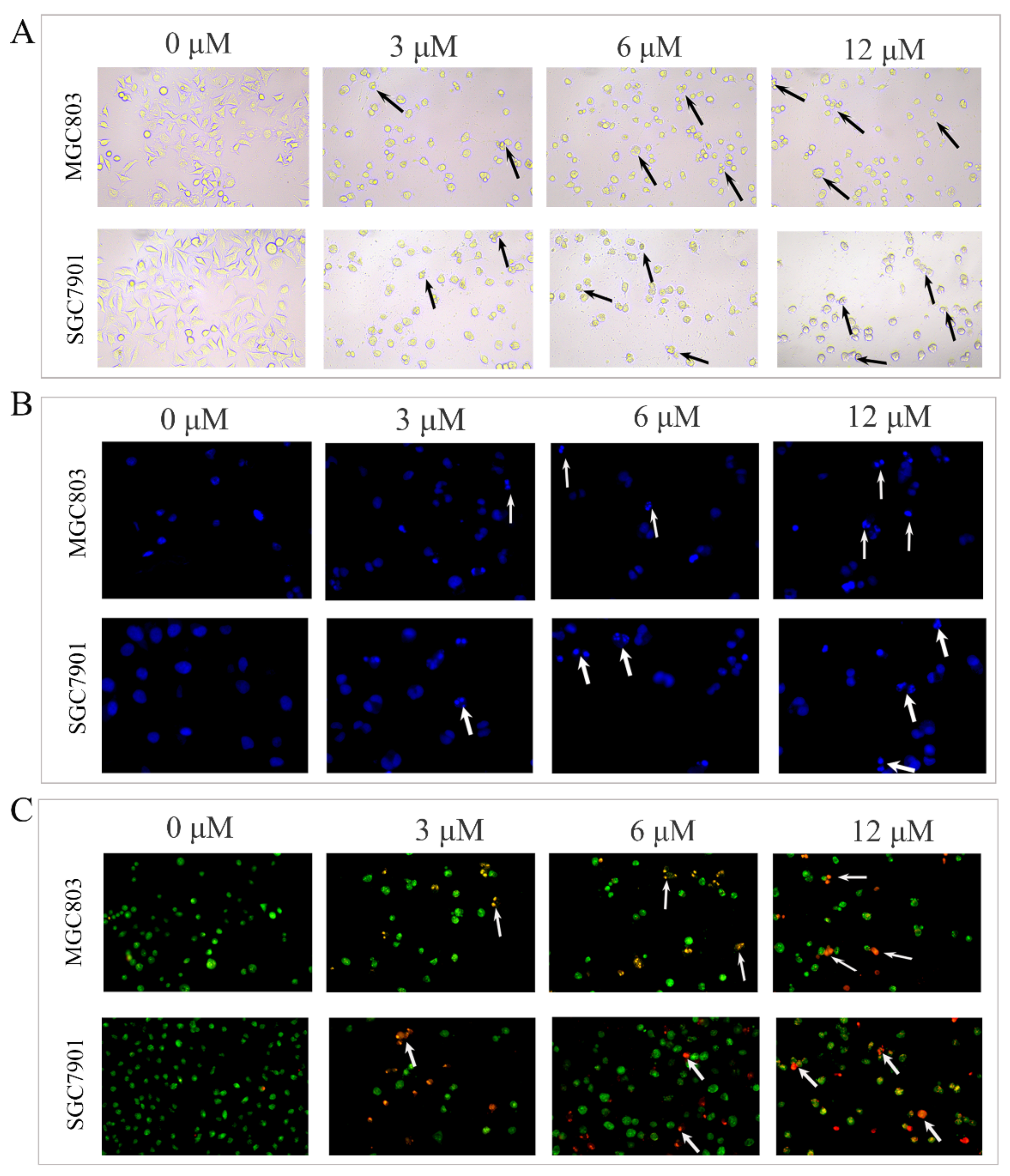
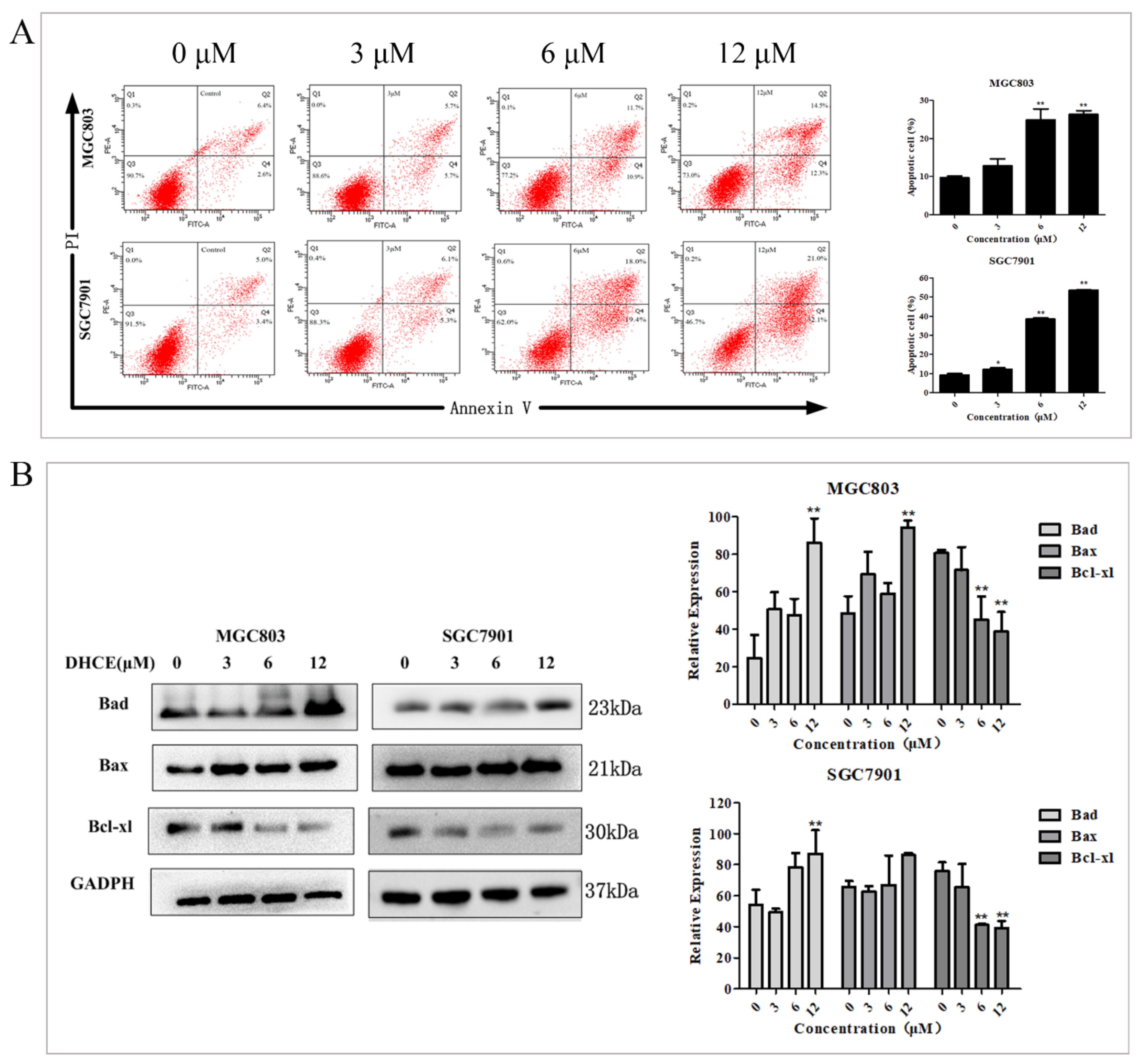
2.2. DHCE Triggered Apoptosis in Gastric Cancer Cells
2.3. DHCE Induces Gastric Cancer Cell G2/M Phase Arrest
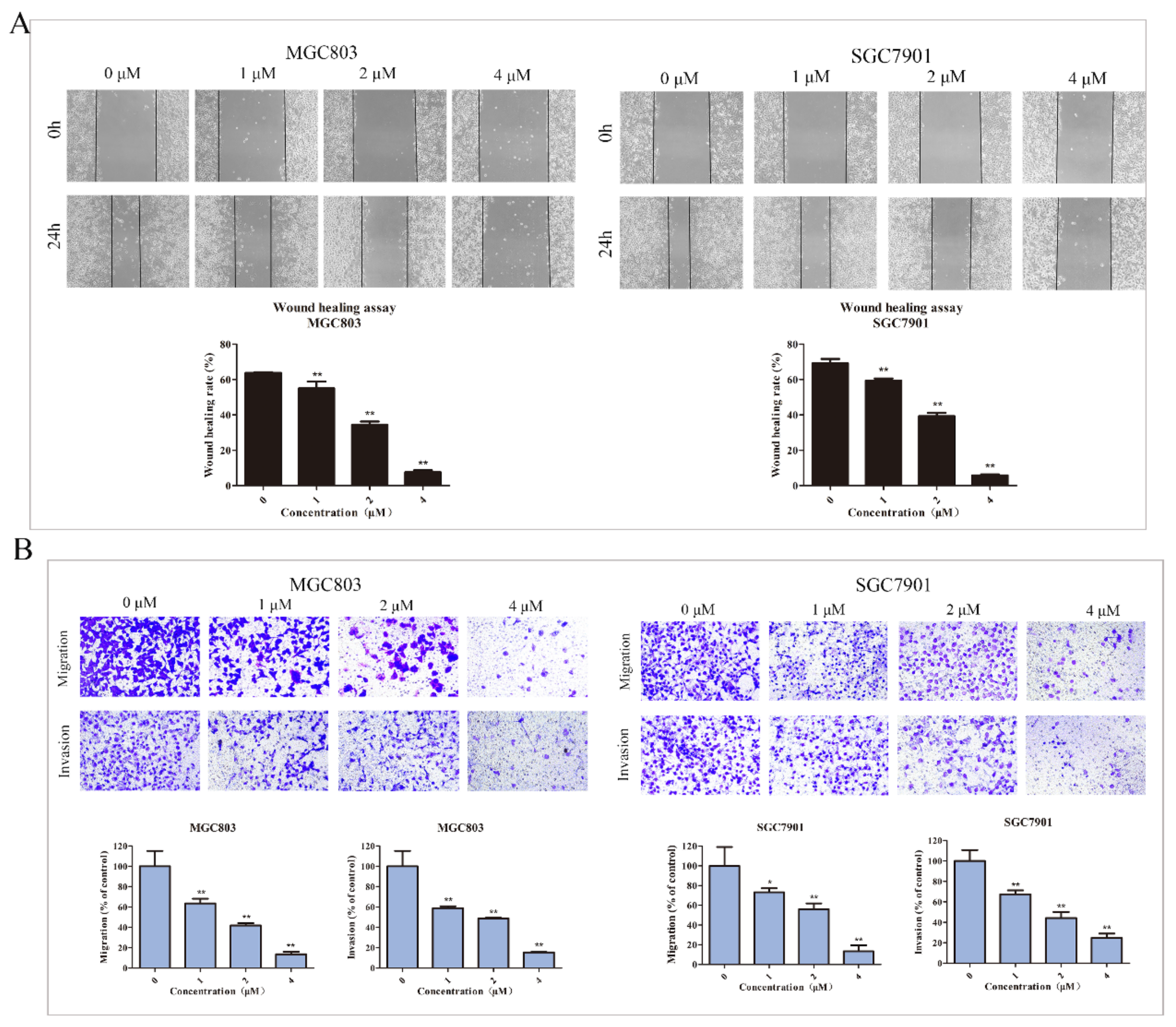
2.4. DHCE Inhibits the Migration and Invasion of Gastric Cancer Cells
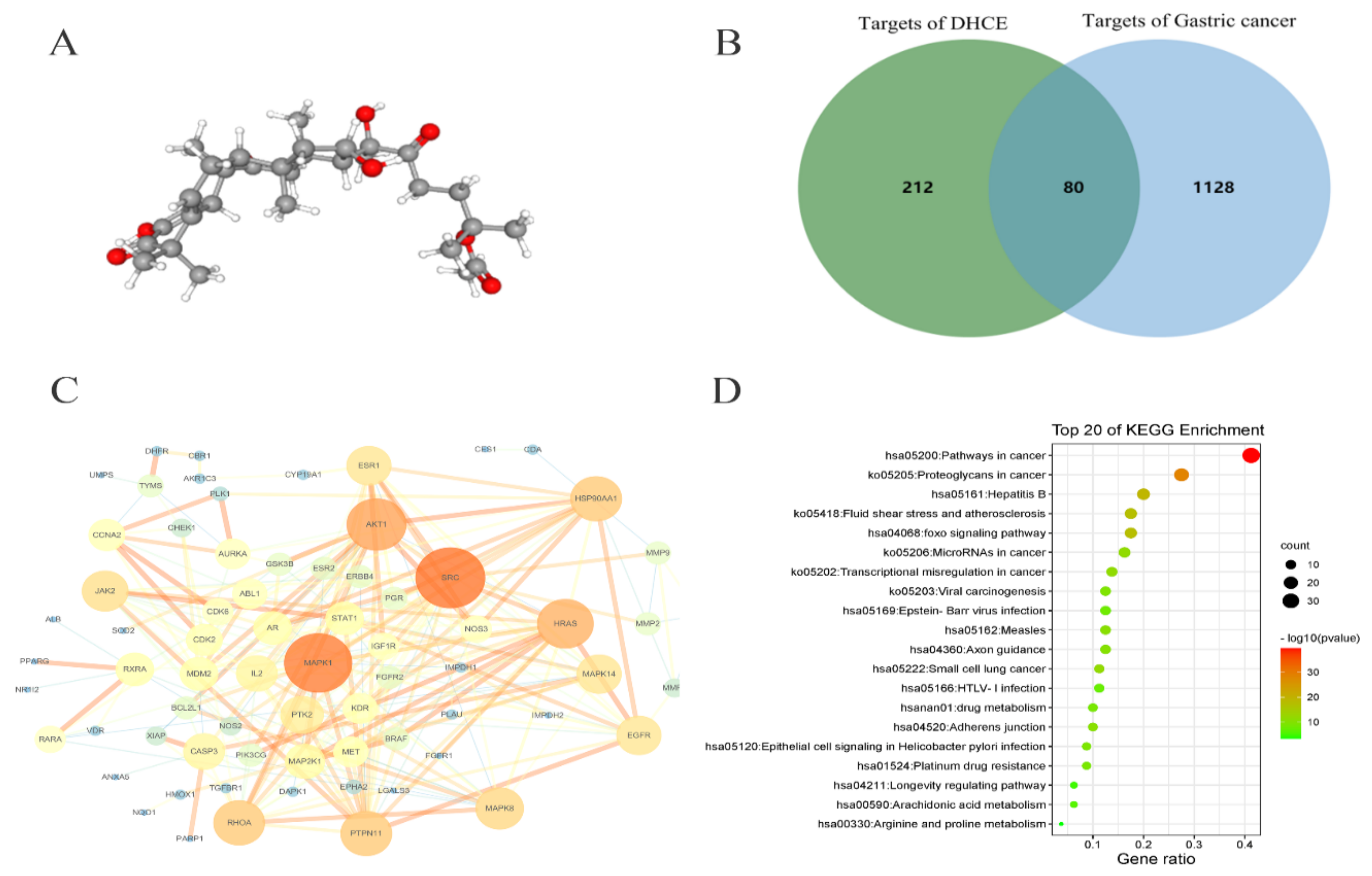
2.5. Network Pharmacology Analysis of DHCE Regulatory Signaling Networks
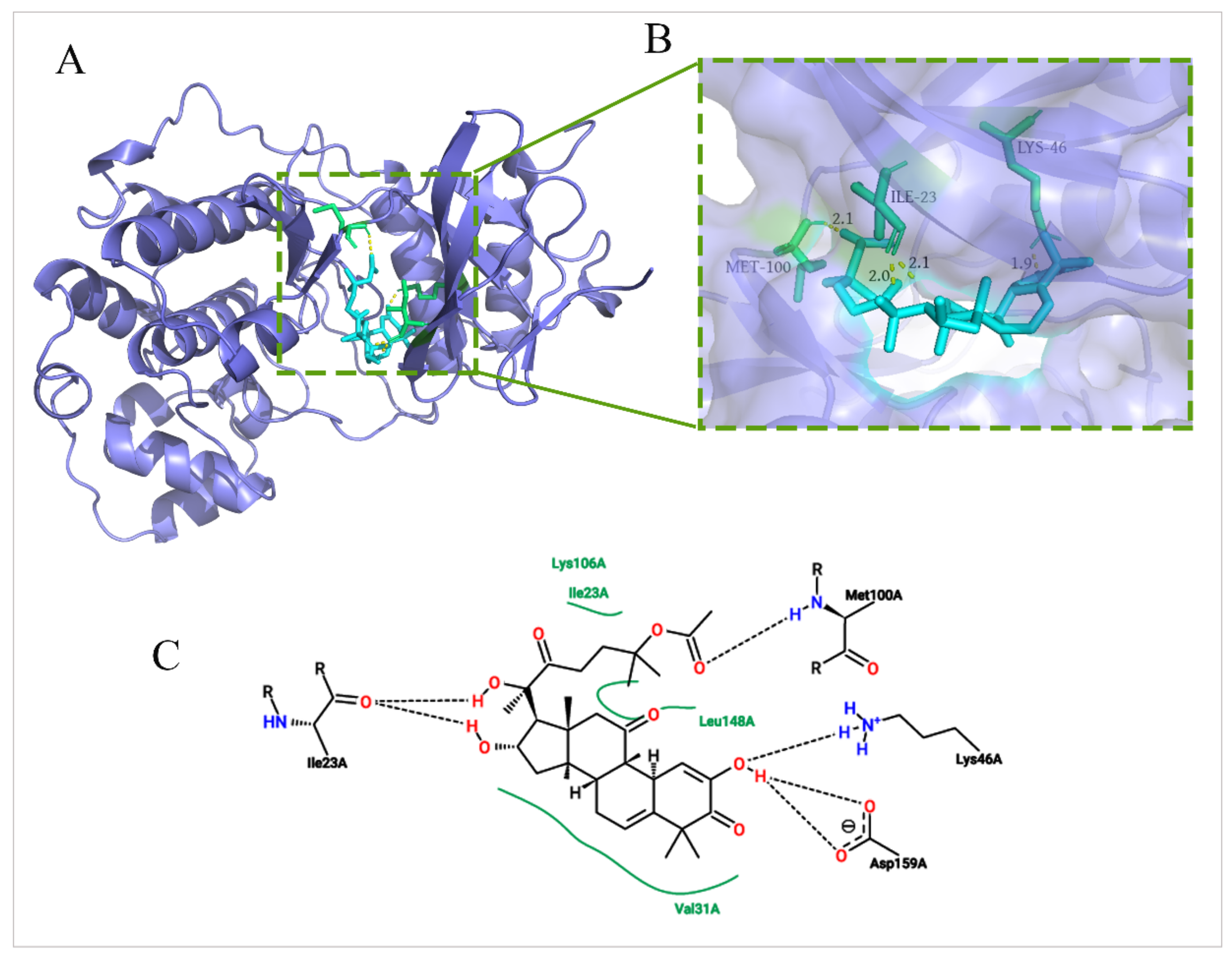
2.6. Molecular Docking between DHCE and ERK2
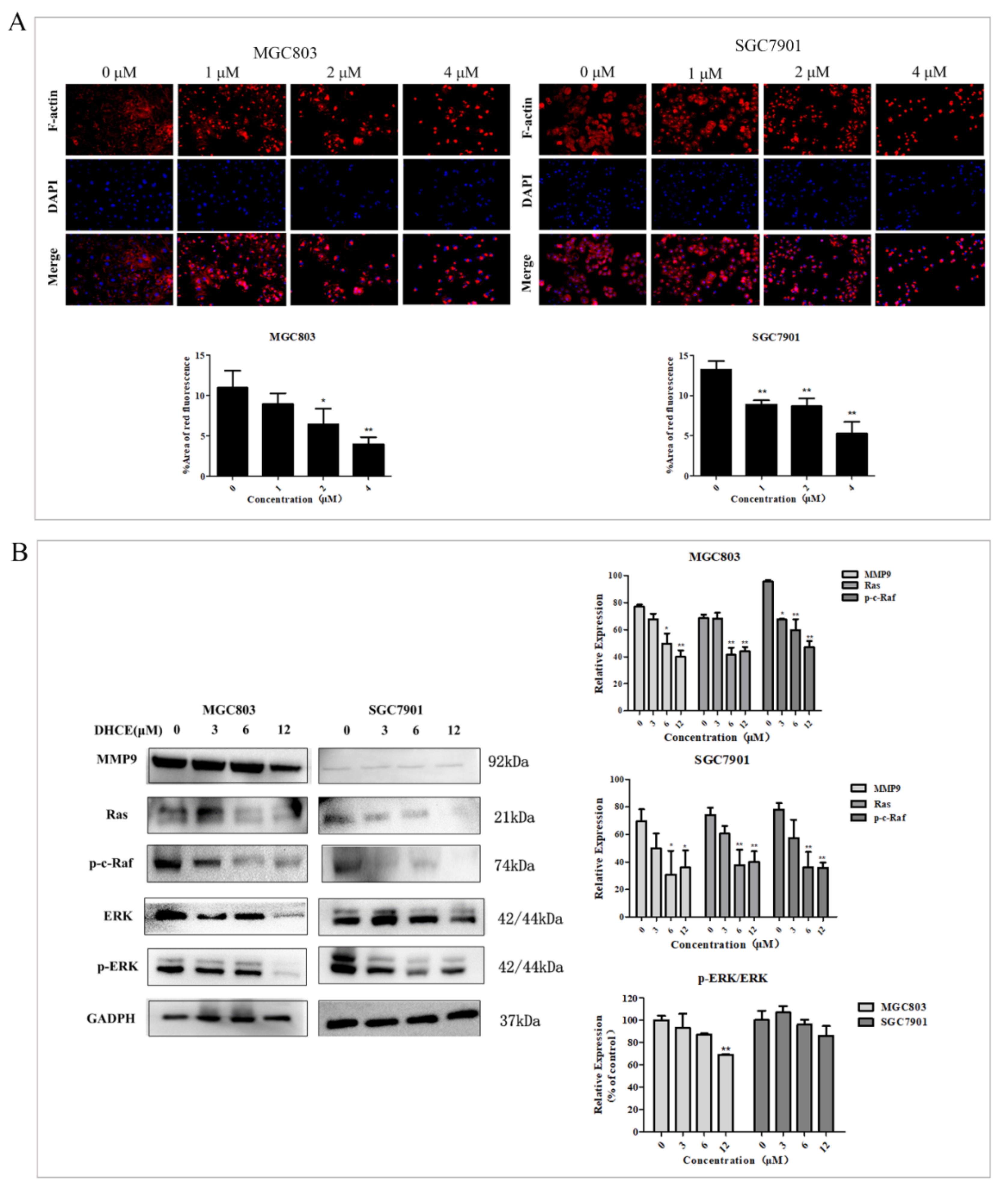
2.7. DHCE Destroys F-actin Microfilament and Attenuates Ras/Raf/ERK/MMP9 Pathways in Gastric Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines and Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Observation of Cell Morphological Changes
4.6. Apoptosis Analysis by Flow Cytometry
4.7. Cell Cycle Analysis by Flow Cytometry
4.8. Wound Healing Assay
4.9. Cell Migration and Invasion Assays
4.10. Potential Target Prediction
4.11. Molecular Docking Calculations
4.12. Cytoskeleton Staining and Observation
4.13. Western Blot Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.M.; Beckman, M. Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med. Sci. Monit. 2019, 25, 3537–3541. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liao, Y.; Li, L.; Xu, X.; Cao, L. Zeylenone Induces Mitochondrial Apoptosis and Inhibits Migration and Invasion in Gastric Cancer. Molecules 2018, 23, 2149. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Patel, T.H.; Cecchini, M. Targeted Therapies in Advanced Gastric Cancer. Curr. Treat. Options Oncol. 2020, 21, 70. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Werner, D. Recent advances and future trends in the targeted therapy of metastatic gastric cancer. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 555–569. [Google Scholar] [CrossRef]
- Moulick, K.; Ahn, J.H.; Zong, H.; Rodina, A.; Cerchietti, L.; Gomes DaGama, E.M.; Caldas-Lopes, E.; Beebe, K.; Perna, F.; Hatzi, K.; et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat. Chem. Biol. 2011, 7, 818–826. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, J.; Song, P. Correlation of ERK/MAPK signaling pathway with proliferation and apoptosis of colon cancer cells. Oncol. Lett. 2019, 17, 2266–2270. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.T.; Feng, Y.M.; Ke, Z.H.; Qiu, M.J.; He, X.X.; Wang, M.M.; Li, Y.N.; Xu, J.; Shi, L.L.; Xiong, Z.F. KCNN4 promotes invasion and metastasis through the MAPK/ERK pathway in hepatocellular carcinoma. J. Investig. Med. 2020, 68, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Yang, L.; Wu, S.; Zhang, Q.; Liu, F.; Wu, P. 23,24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria-dependent apoptosis in human breast cancer cells (Bcap37). Cancer Lett. 2007, 256, 267–278. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, X.; He, C.; Li, P.; Xiao, F.; Wang, Y.; Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chin. Med. 2015, 43, 1331–1350. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Guo, Q.; Liu, T.; Jiang, Y.; Zhao, M.; Zeng, K.; Tu, P. Cucurbitacin E Inhibits Huh7 Hepatoma Carcinoma Cell Proliferation and Metastasis via Suppressing MAPKs and JAK/STAT3 Pathways. Molecules 2020, 25, 560. [Google Scholar] [CrossRef]
- Si, W.; Lyu, J.; Liu, Z.; Wang, C.; Huang, J.; Jiang, L.; Ma, T. Cucurbitacin E inhibits cellular proliferation and enhances the chemo-response in gastric cancer by suppressing AKt activation. J. Cancer 2019, 10, 5843–5851. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.Y.; Jin, M.L.; Park, G.; Son, H.J. Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 473–480. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, K.; Ku, J.M.; Choi, Y.J.; Mok, K.; Kim, D.; Cheon, C.; Ko, S.G. Cucurbitacin D Induces G2/M Phase Arrest and Apoptosis via the ROS/p38 Pathway in Capan-1 Pancreatic Cancer Cell Line. Evid. Based Complement. Alternat. Med. 2020, 2020, 6571674. [Google Scholar] [CrossRef]
- Chen, X.; Bao, J.; Guo, J.; Ding, Q.; Lu, J.; Huang, M.; Wang, Y. Biological activities and potential molecular targets of cucurbitacins: A focus on cancer. Anticancer Drugs 2012, 23, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, W.; Cao, J.; Li, W.; Zhao, Y. Bioassay-guided isolation and identification of cytotoxic compounds from Bolbostemma paniculatum. J. Ethnopharmacol. 2015, 169, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Z.; Tan, R.; Jiao, R.H.; Deng, X.Z.; Tan, R.X. Herpecaudin from Herpetospermum caudigerum, a Xanthine Oxidase Inhibitor with a Novel Isoprenoid Scaffold. Planta Med. 2016, 82, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-L.; Lin, F.-W.; Wu, T.-S.; Kuoh, C.-S.; Lee, K.-H.; Leed, S.-J. Cytotoxic and Anti-HIV Principles from the Rhizomes of Begonia nantoensis. Chem. Pharm. Bull. 2004, 52, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Galustian, C.; Dye, J.; Leach, L.; Clark, P.; Firth, J.A. Actin cytoskeletal isoforms in human endothelial cells in vitro: Alteration with cell passage. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 796–802. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Hartogh, D.J.D.; Azizi, K.; Tsiani, E. Anticancer Properties of Carnosol: A Summary of in Vitro and In Vivo Evidence. Antioxidants 2020, 9, 961. [Google Scholar] [CrossRef]
- Pai, J.T.; Hsu, M.W.; Leu, Y.L.; Chang, K.T.; Weng, M.S. Induction of G2/M Cell Cycle Arrest via p38/p21(Waf1/Cip1)-Dependent Signaling Pathway Activation by Bavachinin in Non-Small-Cell Lung Cancer Cells. Molecules 2021, 26, 5161. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging Albany NY 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Ma, W.; Xiang, Y.; Yang, R.; Zhang, T.; Xu, J.; Wu, Y.; Liu, X.; Xiang, K.; Zhao, H.; Liu, Y.; et al. Cucurbitacin B induces inhibitory effects via the CIP2A/PP2A/C-KIT signaling axis in t(8;21) acute myeloid leukemia. J. Pharmacol. Sci. 2019, 139, 304–310. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, H.; Sun, C.; Shan, X.; Yang, X.; Li-Ling, J.; Deng, Y. Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother. Pharmacol. 2009, 63, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.H.; Gao, F.C.; Tian, J.; Zhang, W.B. Hsa_circ_101882 promotes migration and invasion of gastric cancer cells by regulating EMT. J. Clin. Lab. Anal. 2019, 33, e23002. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, J.; Huang, Z.; Yao, Q.; Chen, F.; Liu, H.; Zhang, Z.; Lin, J. ADMA mediates gastric cancer cell migration and invasion via Wnt/beta-catenin signaling pathway. Clin. Transl. Oncol. 2021, 23, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.; Si, J.; Li, G.; Cao, L. Umbelliprenin isolated from Ferula sinkiangensis inhibits tumor growth and migration through the disturbance of Wnt signaling pathway in gastric cancer. PLoS ONE 2019, 14, e0207169. [Google Scholar] [CrossRef]
- Li, X.M.; Li, M.T.; Jiang, N.; Si, Y.C.; Zhu, M.M.; Wu, Q.Y.; Shi, D.C.; Shi, H.; Luo, Q.; Yu, B. Network Pharmacology-Based Approach to Investigate the Molecular Targets of Sinomenine for Treating Breast Cancer. Cancer Manag. Res. 2021, 13, 1189–1204. [Google Scholar] [CrossRef]
- Yang, B.; Wang, N.; Wang, S.; Li, X.; Zheng, Y.; Li, M.; Song, J.; Zhang, F.; Mei, W.; Lin, Y.; et al. Network-pharmacology-based identi fi cation of caveolin-1 as a key target of Oldenlandia diffusa to suppress breast cancer metastasis. Biomed. Pharmacother. 2019, 112, 108607. [Google Scholar] [CrossRef]
- Guo, N.; Liu, Z.; Zhao, W.; Wang, E.; Wang, J. Small Molecule APY606 Displays Extensive Antitumor Activity in Pancreatic Cancer via Impairing Ras-MAPK Signaling. PLoS ONE 2016, 11, e0155874. [Google Scholar] [CrossRef]
- Wang, W.; Fang, G.; Rudolph, J. Ras inhibition via direct Ras binding—Is there a path forward? Bioorg. Med. Chem. Lett. 2012, 22, 5766–5776. [Google Scholar] [CrossRef]
- Wang, Z.L.; Fan, Z.Q.; Jiang, H.D.; Qu, J.M. Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal transition in human lung cancer cells via activating MEK-ERK signaling. Carcinogenesis 2013, 34, 638–646. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, J.; Zhao, S.; Luo, Q.; Tang, Q.; Yang, L.; Li, L.; Wu, W.; Hann, S.S. Baicalein increases the expression and reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKalpha and MEK/ERK1/2 signaling pathways in human non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2015, 34, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Kim, Y.S.; Oh, H.-W.; Kim, K.; Oh, S.-S.; Kim, J.-T.; Kim, B.Y.; Lee, S.-J.; Choe, Y.-K.; Kim, D.H.; et al. Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget 2014, 5, 519–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Q.; Yang, P.; Tu, K.; Zhang, H.; Zheng, X.; Yao, Y.; Liu, Q. TPX2 knockdown suppressed hepatocellular carcinoma cell invasion via inactivating AKT signaling and inhibiting MMP2 and MMP9 expression. Chin. J. Cancer Res. 2014, 26, 410–417. [Google Scholar] [PubMed]
- Blake, J.F.; Burkard, M.; Chan, J.; Chen, H.; Chou, K.J.; Diaz, D.; Dudley, D.A.; Gaudino, J.J.; Gould, S.E.; Grina, J.; et al. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) Inhibitor in Early Clinical Development. J. Med. Chem. 2016, 59, 5650–5660. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, H.; Dong, A.; Huo, X.; Wang, H.; Wang, J.; Si, J. The Inhibition of Gastric Cancer Cells’ Progression by 23,24-Dihydrocucurbitacin E through Disruption of the Ras/Raf/ERK/MMP9 Signaling Pathway. Molecules 2022, 27, 2697. https://doi.org/10.3390/molecules27092697
Liu H, Wang H, Dong A, Huo X, Wang H, Wang J, Si J. The Inhibition of Gastric Cancer Cells’ Progression by 23,24-Dihydrocucurbitacin E through Disruption of the Ras/Raf/ERK/MMP9 Signaling Pathway. Molecules. 2022; 27(9):2697. https://doi.org/10.3390/molecules27092697
Chicago/Turabian StyleLiu, Huiping, Huijuan Wang, Aijun Dong, Xiaoshuang Huo, Huaxiang Wang, Junchi Wang, and Jianyong Si. 2022. "The Inhibition of Gastric Cancer Cells’ Progression by 23,24-Dihydrocucurbitacin E through Disruption of the Ras/Raf/ERK/MMP9 Signaling Pathway" Molecules 27, no. 9: 2697. https://doi.org/10.3390/molecules27092697
APA StyleLiu, H., Wang, H., Dong, A., Huo, X., Wang, H., Wang, J., & Si, J. (2022). The Inhibition of Gastric Cancer Cells’ Progression by 23,24-Dihydrocucurbitacin E through Disruption of the Ras/Raf/ERK/MMP9 Signaling Pathway. Molecules, 27(9), 2697. https://doi.org/10.3390/molecules27092697





