Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma
Abstract
:1. Introduction
2. Results
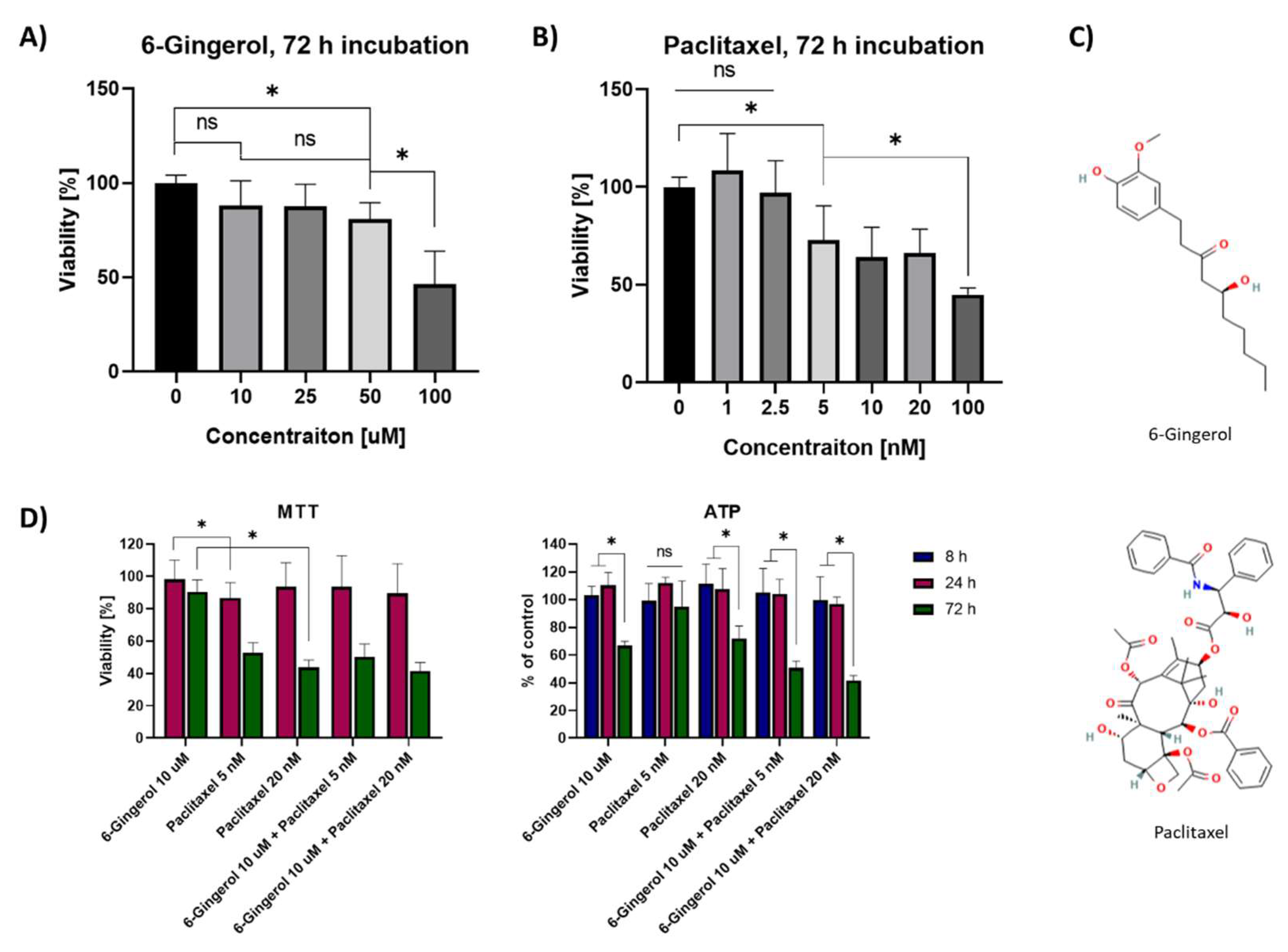
2.1. MTT Viability Assay and ATP Cellular Content
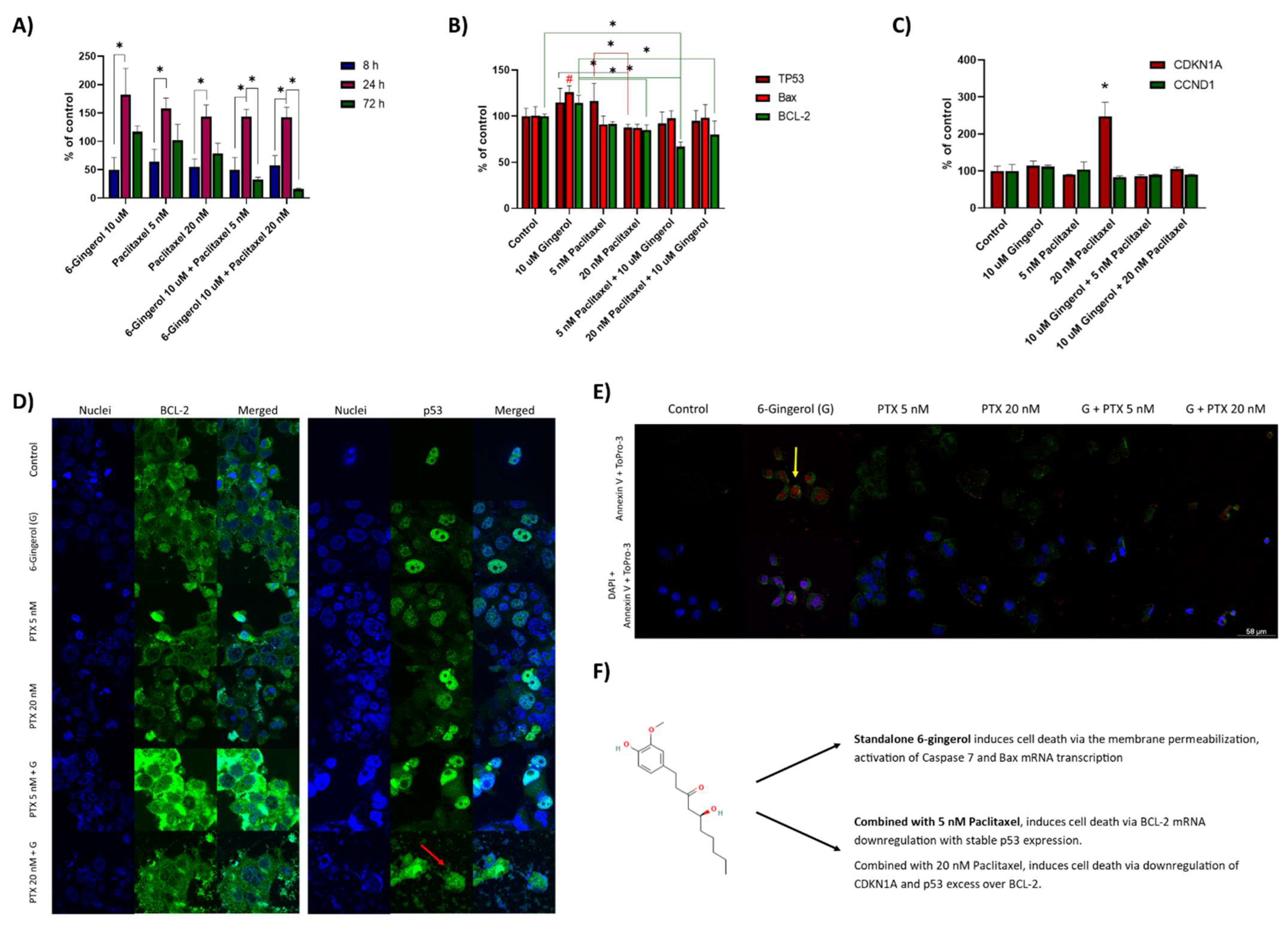
2.2. Caspase-7 Activity Assay, Real Time PCR Study and Confocal Microscopy Studies
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Drug Solutions
4.3. MTT Viability Assay
4.4. Caspase 7 Activity Assay
4.5. CellTiter Glo ATP Assay
4.6. Confocal Microscopy Studies
4.7. Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 17 April 2022).
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, M.; Anderson, B.O.; Duggan, C.; Adebamowo, C.; Agarwal, G.; Ali, Z.; Bird, P.; Bourque, J.-M.; DeBoer, R.; Gebrim, L.H.; et al. Breast cancer treatment: A phased approach to implementation. Cancer 2020, 126, 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Y.; Cong, W.; Hu, M.; Li, X.; Zhou, C. Experience of women with breast cancer undergoing chemotherapy: A systematic review of qualitative research. Qual. Life Res. 2021, 30, 1249–1265. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Varna, M.; Bousquet, G.; Plassa, L.F.; Bertheau, P.; Janin, A. TP53 status and response to treatment in breast cancers. J. Biomed. Biotechnol. 2011, 2011, 284584. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.U.; Ferdiousi, N.; Sajon, S.R. Anti-cancer agents derived from plant and dietary sources: A review. Int. J. Pharmacogn. 2016, 3, 55–66. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phyther. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Ojo, M.; Afolabi, T.T.; Arowoogun, M.D.; Nwawolor, D.; Farombi, E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017, 270, 15–23. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Kim, C.Y.; Jang, J.H. [6]-Gingerol attenuates β-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2011, 49, 1261–1269. [Google Scholar] [CrossRef]
- De Lima, R.M.T.; dos Reis, A.C.; de Menezes, A.-A.P.M.; de Oliveira Santos, J.V.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phyther. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef] [PubMed]
- Chegu, K.; Mounika, K.; Rajeswari, M.; Vanibala, N.; Sujatha, P.; Sridurga, P.; Reddy, D.R.B. In vitro study of the anticoagulant activity of some plant extracts. World J. Pharm. Pharm. Sci. 2018, 7, 904–913. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological properties of 6-Gingerol: A brief review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef] [Green Version]
- Pashaei-Asl, R.; Pashaei-Asl, F.; Gharabaghi, P.M.; Khodadadi, K.; Ebrahimi, M.; Ebrahimie, E.; Pashaiasl, M. The inhibitory effect of ginger extract on Ovarian cancer cell line; Application of systems biology. Adv. Pharm. Bull. 2017, 7, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Nigam, N.; George, J.; Srivastava, S.; Roy, S.; Bhui, K.; Singh, M.; Shukla, Y. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemother. Pharmacol. 2010, 65, 687–696. [Google Scholar] [CrossRef]
- Kim, E.C.; Min, J.K.; Kim, T.Y.; Lee, S.J.; Yang, H.O.; Han, S.; Kim, Y.M.; Kwon, Y.G. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Lee, J.M.; Bae, S.W.; Jang, K.J. Potential antitumor effects of 6-gingerol in p53-dependent mitochondrial apoptosis and inhibition of tumor sphere formation in breast cancer cells. Int. J. Mol. Sci. 2021, 22, 4660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [Green Version]
- Barbuti, A.M.; Chen, Z.-S. Paclitaxel Through the Ages of Anticancer Therapy: Exploring Its Role in Chemoresistance and Radiation Therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef]
- Walker, F.E. Paclitaxel (TAXOL): Side effects and patient education issues. Semin. Oncol. Nurs. 1993, 9, 6–10. [Google Scholar] [CrossRef]
- Belachew, G.T.; Tekelemariam, B.A.; Hanumanthaiah, P.; Namo, F.M. Therapeutic Value of 6-Gingerol (1-[4′-hydroxy-3′-methoxyphenyl]-5-hydroxy-3-decanone): A Review. J. Pharm. Res. Int. 2021, 33, 63–75. [Google Scholar] [CrossRef]
- Lin, C.B.; Lin, C.C.; Tsay, G.J. 6-gingerol inhibits growth of colon cancer cell LoVo via induction of G2/M arrest. Evid.-Based Complement. Altern. Med. 2012, 2012, 326096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, A.; Murphy, K.; Hoskin, D.W. 10-gingerol inhibits ovarian cancer cell growth by inducing G2 arrest. Adv. Pharm. Bull. 2019, 9, 685–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, V.; Aggarwal, S.; Das, S.N. 6-Gingerol Mediates its Anti Tumor Activities in Human Oral and Cervical Cancer Cell Lines through Apoptosis and Cell Cycle Arrest. Phyther. Res. 2016, 30, 588–595. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.M.T.; dos Reis, A.C.; de Oliveira Santos, J.V.; de Oliveira Ferreira, J.R.; de Oliveira Filho, J.W.G.; Soares Dias, A.C.; de Menezes, A.A.P.M.; da Mata, A.M.O.F.; de Alencar, M.V.O.B.; de Jesus Aguiar dos Santos Andrade, T.; et al. Antitumoral effects of [6]-gingerol [(S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone] in sarcoma 180 cells through cytogenetic mechanisms. Biomed. Pharmacother. 2020, 126, 110004. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.S. The Molecular Activity of Gingerol on Inhibits Proliferation of Breast Cancer Cell Line (MCF7) Through Caspase Activity. Ann. Rom. Soc. Cell Biol. 2021, 25, 11095–11103. [Google Scholar]
- Liu, Z.; Ding, Y.; Ye, N.; Wild, C.; Chen, H.; Zhou, J. Direct Activation of Bax Protein for Cancer Therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.G.; Qu, J.; Zhang, Q.; Prasad, C.; Wei, Z.J. Assessment of anti-cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells. Food Chem. Toxicol. 2017, 109, 910–922. [Google Scholar] [CrossRef]
- Radhakrishnan, E.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.T.; Soniya, E.V.; Anto, R.J. [6]-Gingerol Induces Caspase-Dependent Apoptosis and Prevents PMA-Induced Proliferation in Colon Cancer Cells by Inhibiting MAPK/AP-1 Signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef]
- Nafees, S.; Zafaryab, M.; Mehdi, S.H.; Zia, B.; Rizvi, M.A.; Khan, M.A. Anti-Cancer Effect of Gingerol in Cancer Prevention and Treatment. Anticancer. Agents Med. Chem. 2020, 21, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Xing-Yu, Z.; Mo, Z.; Zhi-Hua, Z.; Jian-Cheng, H.; Zhang Wei, L.B. Effect and mechanism of 6-gingerol on invasion and migration of HPV-positive and negative cervical cancer cells. Med. J. Chin. People’s Lib. Army 2020, 45, 691–696. [Google Scholar] [CrossRef]
- Liebmann, J.E.; Cook, J.A.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J.B. Cytotoxic studies of pacfitaxel (Taxol®) in human tumour cell lines. Br. J. Cancer 1993, 68, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-W.; Oh, D.-H.; Koh, J.-T.; Lim, Y.-C. Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells. Int. J. Oral Biol. 2015, 40, 223–228. [Google Scholar] [CrossRef]
- Yusof, K.M.; Makpol, S.; Jamal, R.; Harun, R.; Mokhtar, N.; Ngah, W.Z.W. γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells. Molecules 2015, 20, 10280–10297. [Google Scholar] [CrossRef] [Green Version]
- Al-Abbasi, F.A.; Alghamdi, E.A.; Baghdadi, M.A.; Alamoudi, A.J.; El-Halawany, A.M.; El-Bassossy, H.M.; Aseeri, A.H.; Al-Abd, A.M. Gingerol Synergizes the Cytotoxic Effects of Doxorubicin against Liver Cancer Cells and Protects from Its Vascular Toxicity. Molecules 2016, 21, 886. [Google Scholar] [CrossRef]
- Rahman, A.A.; Makpol, S.; Jamal, R.; Harun, R.; Mokhtar, N.; Ngah, W.Z.W. Tocotrienol-Rich Fraction, [6]-Gingerol and Epigallocatechin Gallate Inhibit Proliferation and Induce Apoptosis of Glioma Cancer Cells. Molecules 2014, 19, 14528–14541. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, N.; Duggal, S.; Singh, S.K.; Porwal, K.; Srivastava, V.K.; Maurya, R.; Bhatt, M.L.B.; Mishra, D.P. Proteasome inhibition mediates p53 reactivation and anti-cancer activity of 6-Gingerol in cervical cancer cells. Oncotarget 2015, 6, 43310. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Kim, D.W.; Jung, C.H.; Lee, Y.J.; Park, D. Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells. Toxicol. Appl. Pharmacol. 2014, 279, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wei, Q.; Yang, Q.; Cao, X.; Li, Q.; Shi, F.; Tong, S.S.; Feng, C.; Yu, Q.; Yu, J.; et al. A novel formulation of [6]-gingerol: Proliposomes with enhanced oral bioavailability and antitumor effect. Int. J. Pharm. 2018, 535, 308–315. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wala, K.; Szlasa, W.; Sauer, N.; Kasperkiewicz-Wasilewska, P.; Szewczyk, A.; Saczko, J.; Rembiałkowska, N.; Kulbacka, J.; Baczyńska, D. Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma. Molecules 2022, 27, 2693. https://doi.org/10.3390/molecules27092693
Wala K, Szlasa W, Sauer N, Kasperkiewicz-Wasilewska P, Szewczyk A, Saczko J, Rembiałkowska N, Kulbacka J, Baczyńska D. Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma. Molecules. 2022; 27(9):2693. https://doi.org/10.3390/molecules27092693
Chicago/Turabian StyleWala, Kamila, Wojciech Szlasa, Natalia Sauer, Paulina Kasperkiewicz-Wasilewska, Anna Szewczyk, Jolanta Saczko, Nina Rembiałkowska, Julita Kulbacka, and Dagmara Baczyńska. 2022. "Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma" Molecules 27, no. 9: 2693. https://doi.org/10.3390/molecules27092693
APA StyleWala, K., Szlasa, W., Sauer, N., Kasperkiewicz-Wasilewska, P., Szewczyk, A., Saczko, J., Rembiałkowska, N., Kulbacka, J., & Baczyńska, D. (2022). Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma. Molecules, 27(9), 2693. https://doi.org/10.3390/molecules27092693







