DFT Calculations of 31P NMR Chemical Shifts in Palladium Complexes
Abstract
1. Introduction
2. Results and Discussion
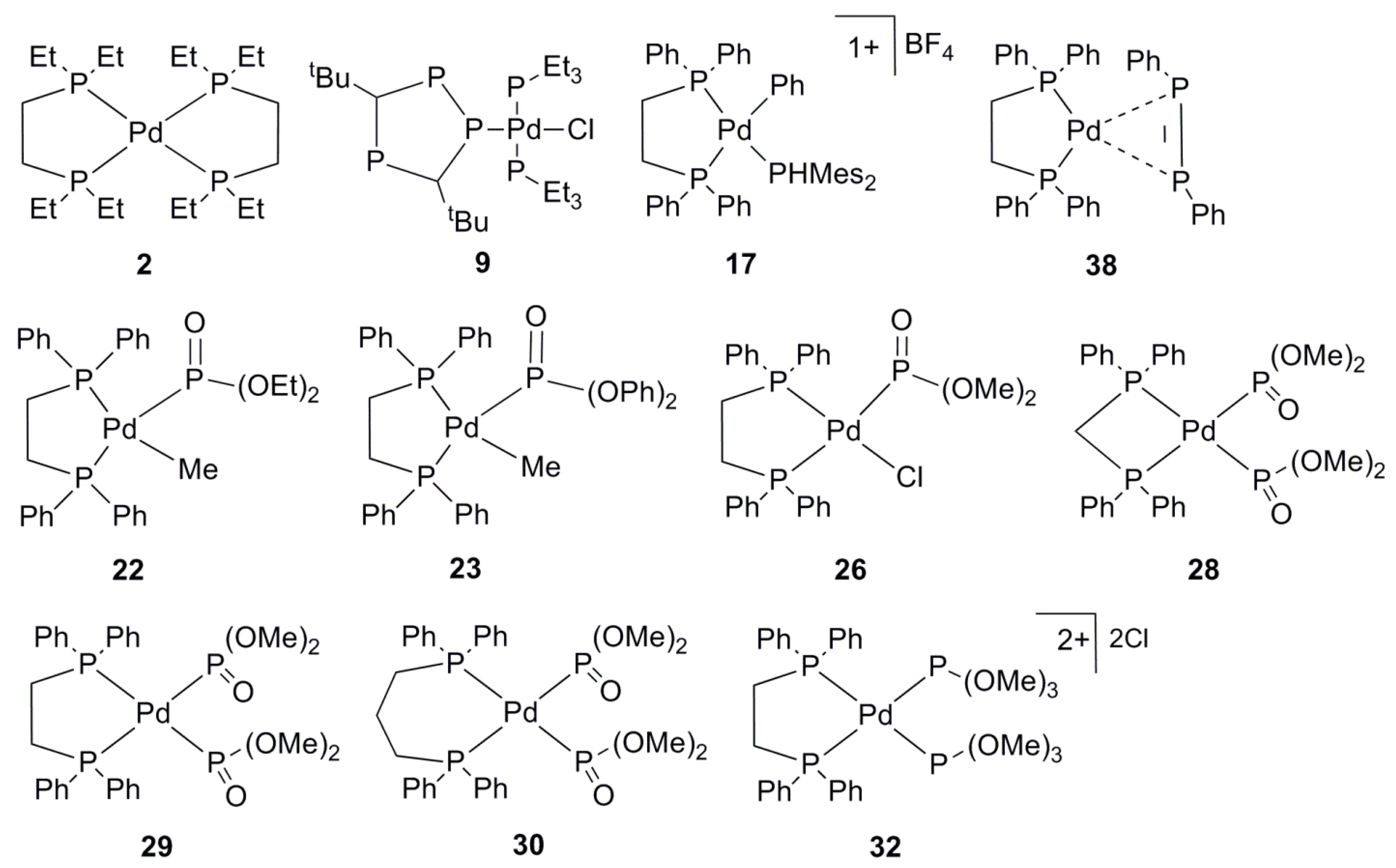
2.1. General Overview
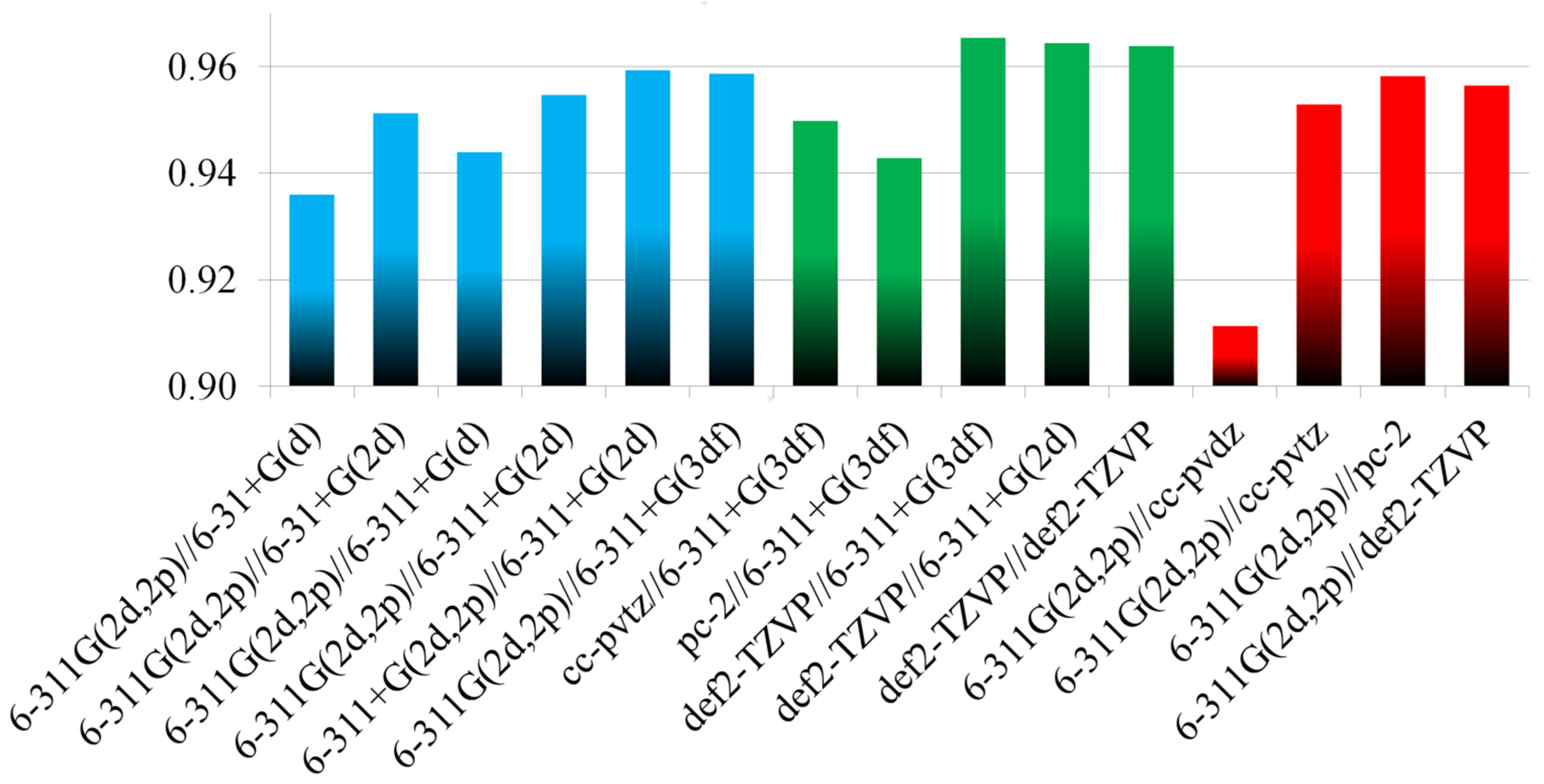
2.2. Optimization of the Computational Approach
2.3. Practical Aspects, Quality of Calculations, and Computational Costs
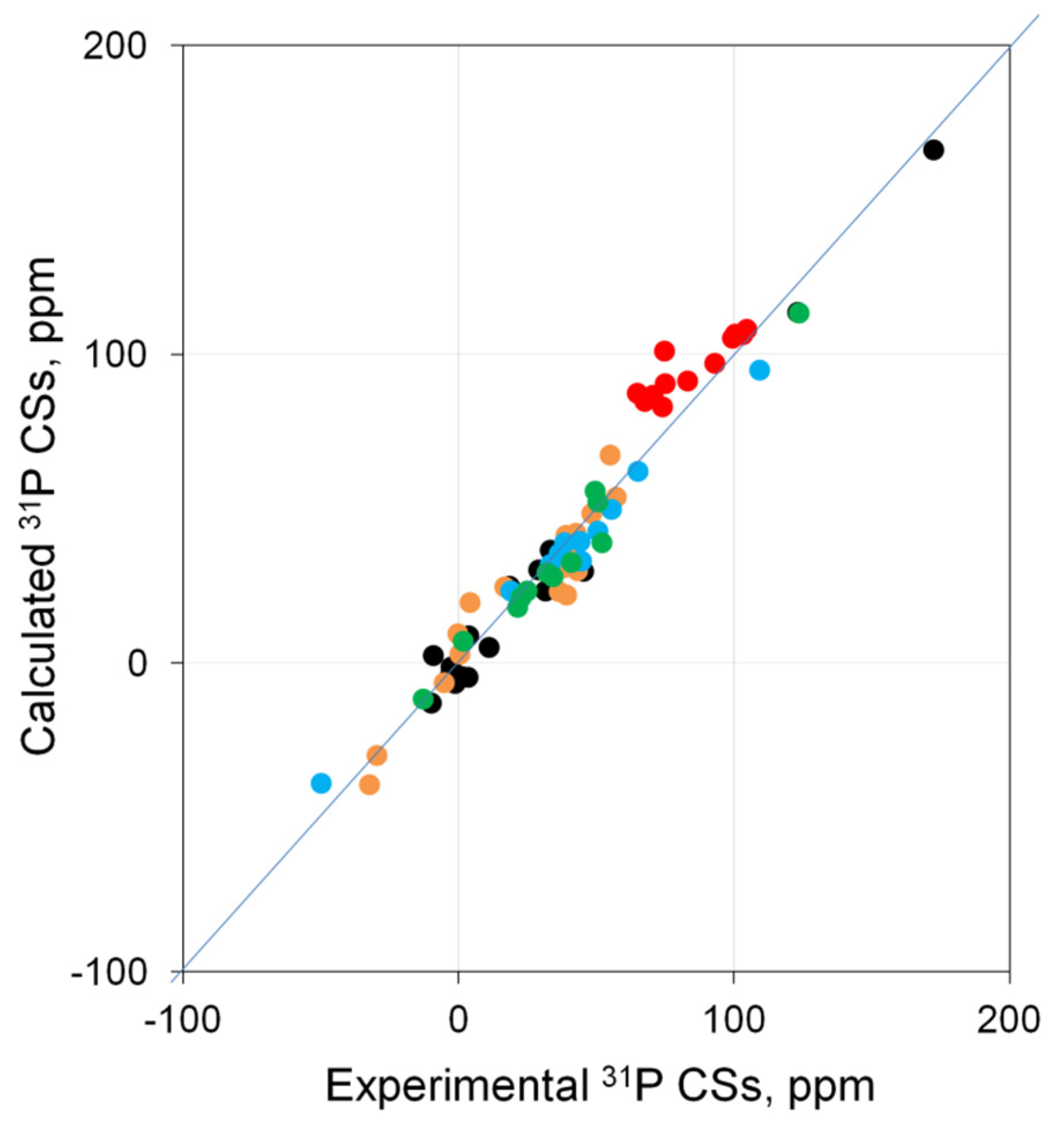
2.4. Checking the Leading Combinations on the Entire Test Set of Pd Complexes
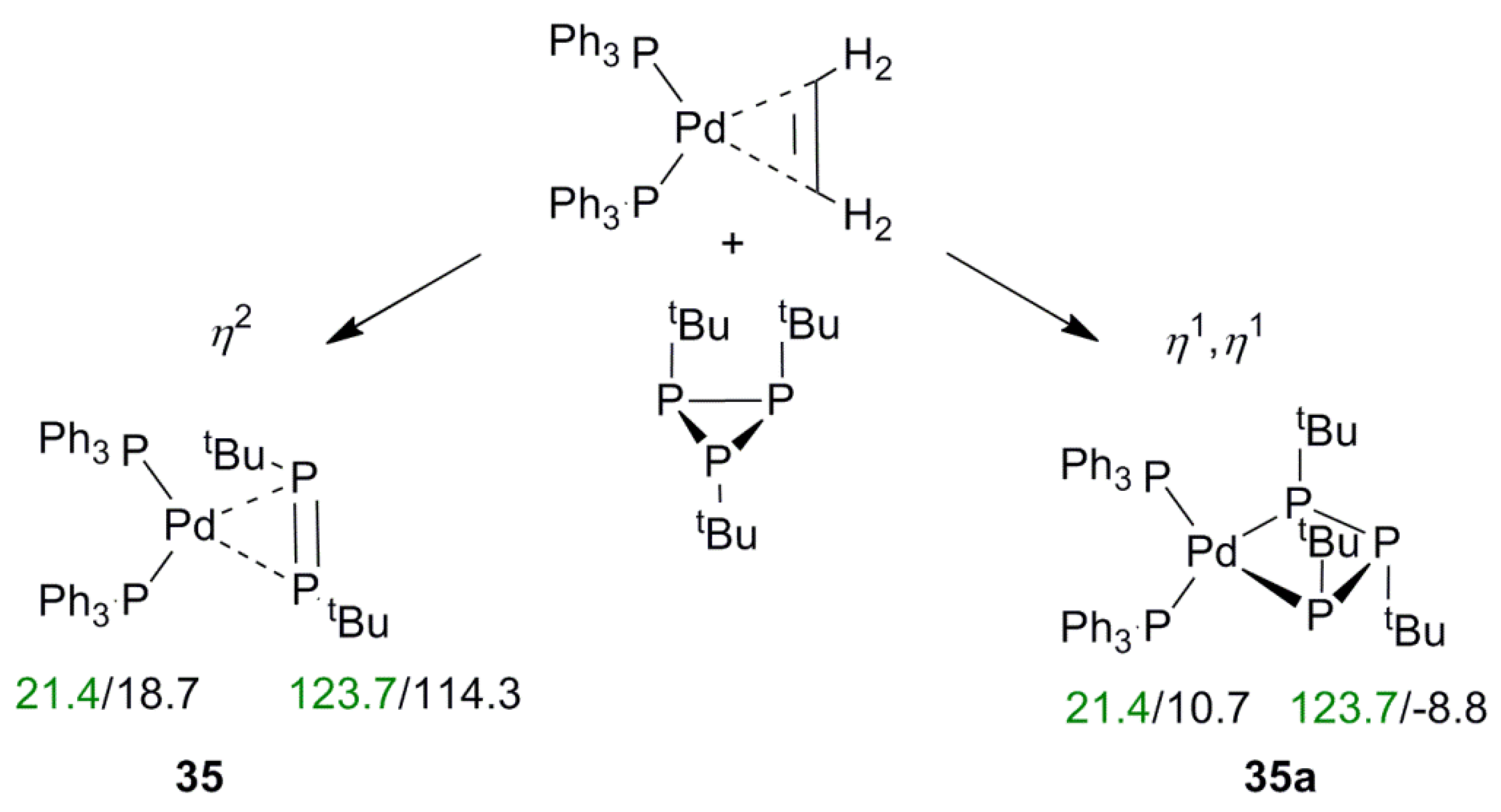
2.5. Impact of Coordination Type on 31P NMR Shifts
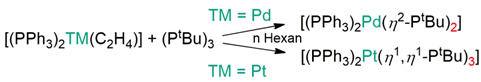
3. Materials and Methods
Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Korch, K.M.; Watson, D.A. Cross-coupling of heteroatomic electrophiles. Chem. Rev. 2019, 119, 8192–8228. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.-I. Magical power of transition metals: Past, present, and future. Angew. Chem. Int. Ed. 2011, 50, 6738–6764. [Google Scholar] [CrossRef] [PubMed]
- Kinzel, N.W.; Werlé, C.; Leitner, W. Transition metal complexes as catalysts for the electroconversion of CO2: An organometallic perspective. Angew. Chem. Int. Ed. 2021, 60, 11628–11686. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J. Catalysis using transition metal complexes featuring main group metal and metalloid compounds as supporting ligands. Chem. Sci. 2021, 12, 1964–1981. [Google Scholar] [CrossRef]
- Bühl, M.; Kaupp, M.; Malkina, O.L.; Malkin, V.G. The DFT route to NMR chemical shifts. J. Comput. Chem. 1999, 20, 91–105. [Google Scholar] [CrossRef]
- Autschbach, J.; Ziegler, T. Double perturbation theory: A powerful tool in computational coordination chemistry. Coord. Chem. Rev. 2003, 238–239, 83–126. [Google Scholar] [CrossRef]
- Vıcha, J.; Novotný, J.; Straka, M.; Repisky, M.; Ruud, K.; Komorovsky, S.; Marek, R. Structure, solvent, and relativistic effects on the NMR chemical shifts in square-planar transition-metal complexes: Assessment of DFT approaches1. Phys. Chem. Chem. Phys. 2015, 17, 24944–24955. [Google Scholar] [CrossRef]
- Bagno, A.; Saielli, G. Relativistic DFT calculations of the NMR properties and reactivity of transition metal methane σ-complexes: Insights on C–H bond activation. Phys. Chem. Chem. Phys. 2011, 13, 4285–4291. [Google Scholar] [CrossRef]
- Vícha, J.; Novotny, J.; Komorovsky, S.; Straka, M.; Kaupp, M.; Marek, R. Relativistic heavy-neighbor-atom effects on NMR shifts: Concepts and trends across the periodic table. Chem. Rev. 2020, 120, 7065–7103. [Google Scholar] [CrossRef]
- Kondrashova, S.A.; Polyancev, F.M.; Ganushevich, Y.S.; Latypov, S.K. DFT approach for predicting 13C NMR shifts of atoms directly coordinated to nickel. Organometallics 2021, 40, 1614–1625. [Google Scholar] [CrossRef]
- Latypov, S.K.; Kondrashova, S.A.; Polyancev, F.M.; Sinyashin, O.G. Quantum chemical calculations of 31P NMR chemical shifts in nickel complexes: Scope and limitations. Organometallics 2020, 39, 1413–1422. [Google Scholar] [CrossRef]
- Payard, P.A.; Perego, L.A.; Grimaud, L.; Ciofini, I. A DFT protocol for the prediction of 31P NMR chemical shifts of phosphine ligands in first-row transition-metal complexes. Organometallics 2020, 39, 3121–3130. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Jadhav, B.D.; Pardeshi, S.K. Synthesis and catalytic application of Pd complex catalysts: Atom-efficient cross-coupling of triarylbismuthines with haloarenes and acid chlorides under mild conditions. Appl. Organomet. Chem. 2017, 31, e3591. [Google Scholar] [CrossRef]
- Zhu, J.; Lindsay, V.N. Benzimidazolyl palladium complexes as highly active and general bifunctional catalysts in sustainable cross-coupling reactions. ACS Catal. 2019, 9, 6993–6998. [Google Scholar] [CrossRef]
- Schroeter, F.; Soellner, J.; Strassner, T. Cross-coupling catalysis by an anionic palladium complex. ACS Catal. 2017, 7, 3004–3009. [Google Scholar] [CrossRef]
- Cao, Q.; Bailie, D.S.; Fu, R.; Muldoon, M.J. Cationic palladium (II) complexes as catalysts for the oxidation of terminal olefins to methyl ketones using hydrogen peroxide. Green Chem. 2015, 17, 2750–2757. [Google Scholar] [CrossRef]
- Scattolin, T.; Voloshkin, V.A.; Martynova, E.; Broeck, S.M.V.; Beliš, M.; Cazin, C.S.; Nolan, S.P. Synthesis and catalytic activity of palladium complexes bearing N-heterocyclic carbenes (NHCs) and 1,4,7-triaza-9-phosphatricyclo[5.3.2.1]tridecane (CAP) ligands. Dalton Trans. 2021, 50, 9491–9499. [Google Scholar] [CrossRef]
- Zhou, T.; Szostak, M. Palladium-catalyzed cross-couplings by C–O bond activation. Catal. Sci. Technol. 2020, 10, 5702–5739. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Labinger, J.A. Platinum-catalyzed C–H functionalization. Chem. Rev. 2017, 117, 8483–8496. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Davies, P.W. Catalytic carbophilic activation: Catalysis by platinum and gold π acids. Angew. Chem. Int. Ed. 2007, 46, 3410–3449. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum group metals: A review of resources, production and usage with a focus on catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chem. Acc. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Hansen, A.E.; Bouman, T.D. Localized orbital/local origin method for calculation and analysis of NMR shieldings. Applications to 13C shielding tensors. J. Chem. Phys. 1985, 82, 5035–5047. [Google Scholar] [CrossRef]
- Pierens, G.K. 1H and 13C NMR scaling factors for the calculation of chemical shifts in commonly used solvents using density functional theory. J. Comput. Chem. 2014, 35, 1388–1394. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, I.A.; Broadbelt, L.J. Regression formulas for density functional theory calculated 1H and 13C NMR chemical shifts in toluene-d8. J. Phys. Chem. A 2011, 115, 12364–12372. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Noro, T.; Sekiya, M.; Koga, T.; Saito, S.L. Relativistic contracted Gaussian-type basis functions for atoms K through Xe. Chem. Phys. Lett. 2009, 481, 229–233. [Google Scholar] [CrossRef]
- Osanai, Y.O.U.; Sekiya, M.; Noro, T.; Koga, T. Valence and correlating basis sets for the second transition-metal atoms from Y to Cd. Mol. Phys. 2003, 101, 65–71. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Jensen, F. Polarization consistent basis sets: Principles. J. Chem. Phys. 2001, 115, 9113–9125. [Google Scholar] [CrossRef]
- Jensen, F. Polarization consistent basis sets. II. Estimating the Kohn-Sham basis set limit. J. Chem. Phys. 2002, 116, 7372–7379. [Google Scholar] [CrossRef]
- Jensen, F.; Helgaker, T. Polarization consistent basis sets. V. The elements Si-Cl. J. Chem. Phys. 2004, 121, 3463–3470. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian-basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schaefer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian-basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Fenske, D.; Schottmueller, H. New Research of Reaction Behaviour of Triorganylcyclotriphosphines. The Crystal Structures of [(PPh3)2Pt(PtBu)3], [(PPh3)2Pd(PtBu)2, [(CO)4Cr{(PiPr)3}2], [RhCl(PPh3)(PtBu)3], [(NiCO)6(μ2-CO)3{(PtBu)2}2], and [(CpFeCO)2(μ-CO)(μ-PHtBu)]+∙[FeCl3(thf)]–. Z. Anorg. Allg. Chem. 1998, 624, 443–451. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision B.04; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Spitznagel, G.W.; Clark, T.; von Ragué Schleyer, P.; Hehre, W.J. An evaluation of the performance of diffuse function-augmented basis sets for second row elements, Na-Cl. J. Comput. Chem. 1987, 8, 1109–1116. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gausian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. A New Basis Set Exchange: An Open, Up-to-date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Portnoy, M.; Milstein, D. Chelate effect on the structure and reactivity of electron-rich palladium complexes and its relevance to catalysis. Organometallics 1993, 12, 1655–1664. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Miedaner, A.; Curtis, C.J.; Miller, S.M.; Anderson, O.P.; DuBois, D.L. Using ligand bite angles to control the hydricity of palladium diphosphine complexes. J. Am. Chem. Soc. 2004, 126, 5502–5514. [Google Scholar] [CrossRef]
- Nixon, J.F.; Sillett, G.J. 31P and 195Pt NMR studies on fluxional η1-ligated 1,2,4-triphosphacyclopentadienyl palladium(II) and platinum(II) complexes. J. Organomet. Chem. 1993, 461, 237–245. [Google Scholar] [CrossRef]
- Kane, J.C.; Wong, E.H.; Yap, G.P.; Rheingold, A.L. Synthesis and structural studies of molybdenum and palladium complexes of 1,5-diaza-3,7-diphosphacyclooctane ligands. Polyhedron 1999, 18, 1183–1188. [Google Scholar] [CrossRef]
- Gouygou, M.; Tissot, O.; Daran, J.C.; Balavoine, G.G. Biphosphole: A C2 Symmetry Chiral Bidentate Ligand. Synthesis and Characterization Of Nickel, Palladium, Platinum, and Rhodium Complexes. Organometallics 1997, 16, 1008–1015. [Google Scholar] [CrossRef]
- Wächtler, E.; Priver, S.H.; Wagler, J.; Heine, T.; Zhechkov, L.; Bennett, M.A.; Bhargava, S.K. Metallophilic contacts in 2-C6F4PPh2 bridged heterobinuclear complexes: A crystallographic and computational study. Inorg. Chem. 2015, 54, 6947–6957. [Google Scholar] [CrossRef]
- David, M.A.; Wicht, D.K.; Glueck, D.S.; Yap, G.P.; Liable-Sands, L.M.; Rheingold, A.L. Palladium-catalyzed phosphaketene decarbonylation: Diphosphaureylene intermediates in diphosphene formation. Organometallics 1997, 16, 4768–4770. [Google Scholar] [CrossRef]
- Sevillano, P.; Habtemariam, A.; Parsons, S.; Castiñeiras, A.; García, M.E.; Sadler, P.J. Gold (I)-induced chelate ring-opening of palladium (II) and platinum (II) triphos complexes. J. Chem. Soc. Dalton Trans. 1999, 16, 2861–2870. [Google Scholar] [CrossRef]
- Zhuravel, M.A.; Grewal, N.S.; Glueck, D.S.; Lam, K.C.; Rheingold, A.L. Cyclometalation of Dimesitylphosphine in Cationic Palladium (II) and Platinum (II) Complexes: P–H vs. C–H Activation. Organometallics 2000, 19, 2882–2890. [Google Scholar] [CrossRef]
- Mizuta, T.; Okano, A.; Sasaki, T.; Nakazawa, H.; Miyoshi, K. Palladium (II) and platinum (II) complexes of a tetraphosphamacrocycle. X-ray crystal structures of phosphorus analogs of a (tetramethylcyclam) metal complex. Inorg. Chem. 1997, 36, 200–203. [Google Scholar] [CrossRef]
- Mason, L.J.; Perrault, E.M.; Miller, S.M.; Helm, M.L. Group 10 metal complexes of a cyclic diphosphine: The crystal structures of bis (cis-P,P′-diphenyl-1,4-diphospha-cyclohexane)M(II)chloride, M= palladium, platinum. Inorg. Chem. Com. 2006, 9, 946–948. [Google Scholar] [CrossRef]
- Melaimi, M.; Thoumazet, C.; Ricard, L.; Le Floch, P. Syntheses of a 2,6-bis-(methylphospholyl) pyridine ligand and its cationic Pd(II) and Ni(II) complexes—Application in the palladium-catalyzed synthesis of arylboronic esters. J. Organomet. Chem. 2004, 689, 2988–2994. [Google Scholar] [CrossRef]
- Kohler, M.C.; Grimes, T.V.; Wang, X.; Cundari, T.R.; Stockland, R.A., Jr. Arylpalladium phosphonate complexes as reactive intermediates in phosphorus—Carbon bond forming reactions. Organometallics 2009, 28, 1193–1201. [Google Scholar] [CrossRef][Green Version]
- Stockland, R.A.; Levine, A.M.; Giovine, M.T.; Guzei, I.A.; Cannistra, J.C. Reductive elimination from metal phosphonate complexes: Circumvention of competing protonolysis reactions. Organometallics 2004, 23, 647–656. [Google Scholar] [CrossRef]
- Stockland, R.A., Jr.; Maher, D.L.; Anderson, G.K.; Rath, N.P. Demethylation of trimethylphosphite promoted by dichlorodiphosphineplatinum and palladium complexes. Structures of the metallophosphonate complexes [Pt(P∧P){P(O)(OMe)2}2](P∧P= dppe, dppp). Polyhedron 1999, 18, 1067–1075. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Hey-Hawkins, E. Oxidative cleavage of tetraaryltetraphosphane-1, 4-diides by nickel (ii) and palladium (ii): Formation of unusual Ni0 and Pd0 diaryldiphosphene complexes. Dalton Trans. 2007, 48, 5678–5683. [Google Scholar] [CrossRef]
- Phillips, I.G.; Ball, R.G.; Cavell, R.G. Reactions of perfluoromethyl-substituted cyclopolyphosphines with zerovalent group 10 metal complexes. Crystal and molecular structure of a complex with a coordinated diphosphene, [Pd(η2-CF3P=PCF3)(PPh3)2]. Inorg. Chem. 1992, 31, 1633–1641. [Google Scholar] [CrossRef]
- Chatt, J.; Hitchcock, P.B.; Pidcock, A.; Warrens, C.P.; Dixon, K.R. The nature of the co-ordinate link. Part 11. Synthesis and phosphorus-31 nuclear magnetic resonance spectroscopy of platinum and palladium complexes containing side-bonded (E)-diphenyldiphosphene. X-Ray crystal and molecular structures of [Pd{(E)-PhP=PPh}(Ph2PCH2CH2PPh2)] and [Pd{[(E)-PhP=PPh][W(CO)5]2}(Ph2PCH2CH2PPh2)]. J. Chem. Soc. Dalton Trans. 1984, 10, 2237–2244. [Google Scholar]
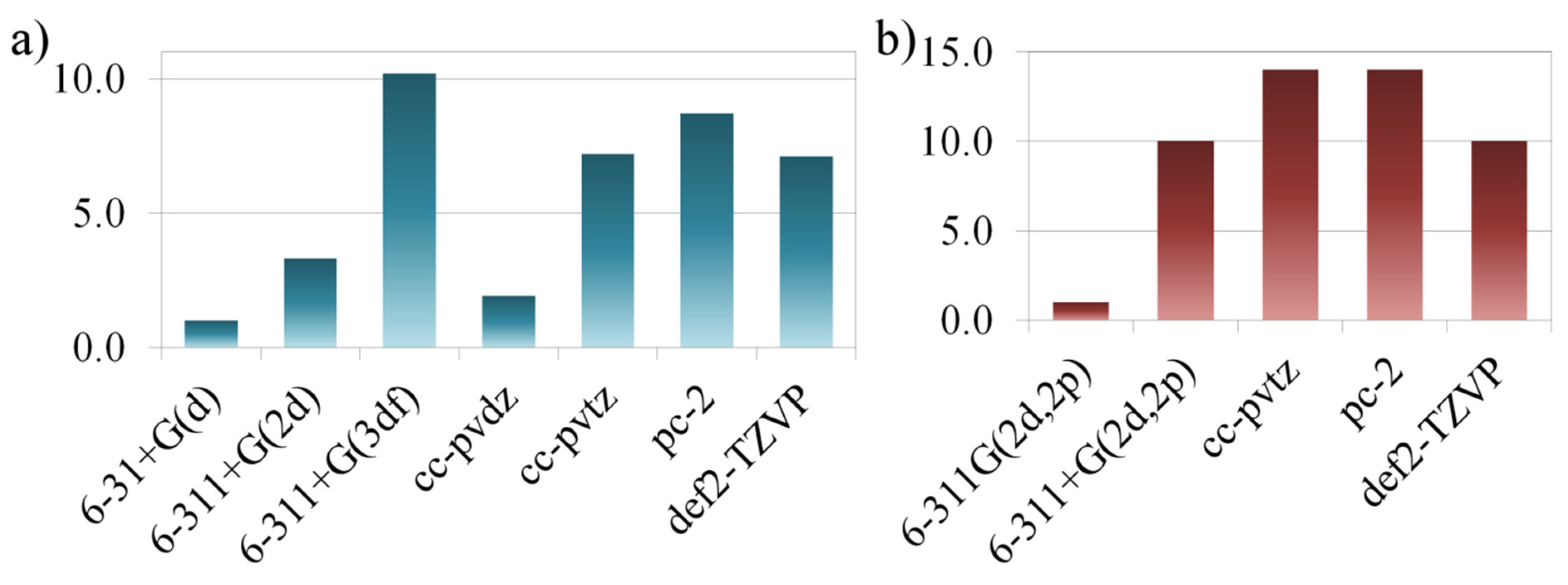
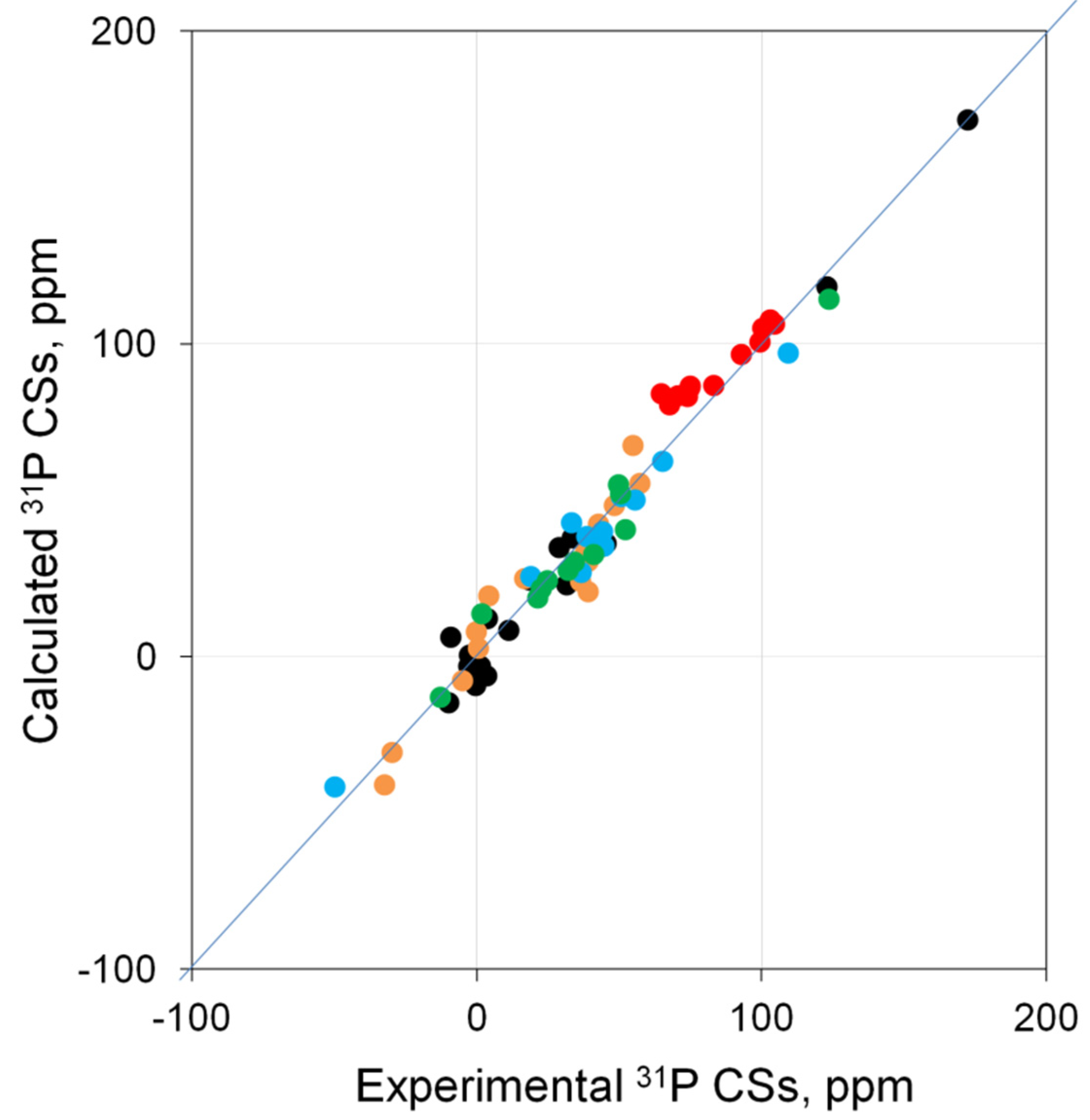
| Entry | Combination | Basis Set a | R2 | Δδ b, ppm | |
|---|---|---|---|---|---|
| Elements | Pd | ||||
| 1 | comb_1 | 6-311G(2d,2p)//6-31+G(d) | SDD//SDD | 0.936 | 24 |
| 2 | comb_2 | 6-311G(2d,2p)//6-31+G(2d) | SDD//SDD | 0.951 | 19 |
| 3 | comb_3 | 6-311G(2d,2p)//6-311+G(d) | SDD//SDD | 0.944 | 22 |
| 4 | comb_4 | 6-311G(2d,2p)//6-311+G(2d) | SDD//SDD | 0.955 | 18 |
| 5 | comb_5 | 6-311+G(2d,2p)//6-311+G(2d) | SDD//SDD | 0.959 | 18 |
| 6 | comb_6 | 6-311G(2d,2p)//6-311+G(3df) | SDD//SDD | 0.959 | 14 |
| 7 | comb_7 | 6-311G(2d,2p)//6-311+G(2d) | def2-TZVPD//def2-TZVPD | 0.953 | 19 |
| 8 | comb_8 | 6-311G(2d,2p)//6-311+G(2d) | SDD//DKH3-DZP | 0.904 | 20 |
| 9 | comb_9 | 6-311G(2d,2p)//6-311+G(2d) | DKH3-DZP//DKH3-DZP | 0.829 | 28 (65) c |
| 10 | comb_10 | cc-pVTZ//6-311+G(3df) | SDD//SDD | 0.950 | 15 |
| 11 | comb_11 | pc-2//6-311+G(3df) | SDD//SDD | 0.943 | 19 |
| 12 | comb_12 | def2-TZVP//6-311+G(3df) | SDD//SDD | 0.965 | 9 |
| 13 | comb_13 | def2-TZVP//6-311+G(2d) | SDD//SDD | 0.964 | 13 |
| 14 | comb_14 | TZV//6-311+G(3df) | SDD//SDD | 0.833 | 68 |
| 15 | comb_15 | def2-TZVP//def2-TZVP | SDD//SDD | 0.964 | 11 |
| 16 | comb_16 | 6-311G(2d,2p)//cc-pVDZ | SDD//SDD | 0.911 | 36 |
| 17 | comb_17 | 6-311G(2d,2p)//cc-pVTZ | SDD//SDD | 0.953 | 17 |
| 18 | comb_18 | 6-311G(2d,2p)//pc-2 | SDD//SDD | 0.958 | 11 |
| 19 | comb_19 | 6-311G(2d,2p)//def2-TZVP | SDD//SDD | 0.956 | 15 |
| 20 | comb_20 | TZV//TZV | SDD//SDD | 0.689 | 118 (101) c |
| 21 | comb_21 | cc-pVTZ//6-31+G(d) | SDD//SDD | 0.927 | 22 |
| 22 | comb_22 | cc-pVTZ//cc-pVDZ | SDD//SDD | 0.901 | 37 |
| Level of Theory | R2 | Slope | Intercept | RMSE |
|---|---|---|---|---|
| PBE0/6-311G(2d,2p)//PBE0/6-31+G(d) | 0.954 | 1.1766 | −12.297 | 8.9 |
| PBE0/6-311G(2d,2p)//PBE0/6-311+G(2d) | 0.963 | 1.1346 | −13.8962 | 8.0 |
| PBE0/def2-TZVP//PBE0/6-311+G(2d) | 0.972 | 0.9297 | 1.1235 | 6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondrashova, S.A.; Polyancev, F.M.; Latypov, S.K. DFT Calculations of 31P NMR Chemical Shifts in Palladium Complexes. Molecules 2022, 27, 2668. https://doi.org/10.3390/molecules27092668
Kondrashova SA, Polyancev FM, Latypov SK. DFT Calculations of 31P NMR Chemical Shifts in Palladium Complexes. Molecules. 2022; 27(9):2668. https://doi.org/10.3390/molecules27092668
Chicago/Turabian StyleKondrashova, Svetlana A., Fedor M. Polyancev, and Shamil K. Latypov. 2022. "DFT Calculations of 31P NMR Chemical Shifts in Palladium Complexes" Molecules 27, no. 9: 2668. https://doi.org/10.3390/molecules27092668
APA StyleKondrashova, S. A., Polyancev, F. M., & Latypov, S. K. (2022). DFT Calculations of 31P NMR Chemical Shifts in Palladium Complexes. Molecules, 27(9), 2668. https://doi.org/10.3390/molecules27092668







