Docking Analysis of Some Bioactive Compounds from Traditional Plants against SARS-CoV-2 Target Proteins
Abstract
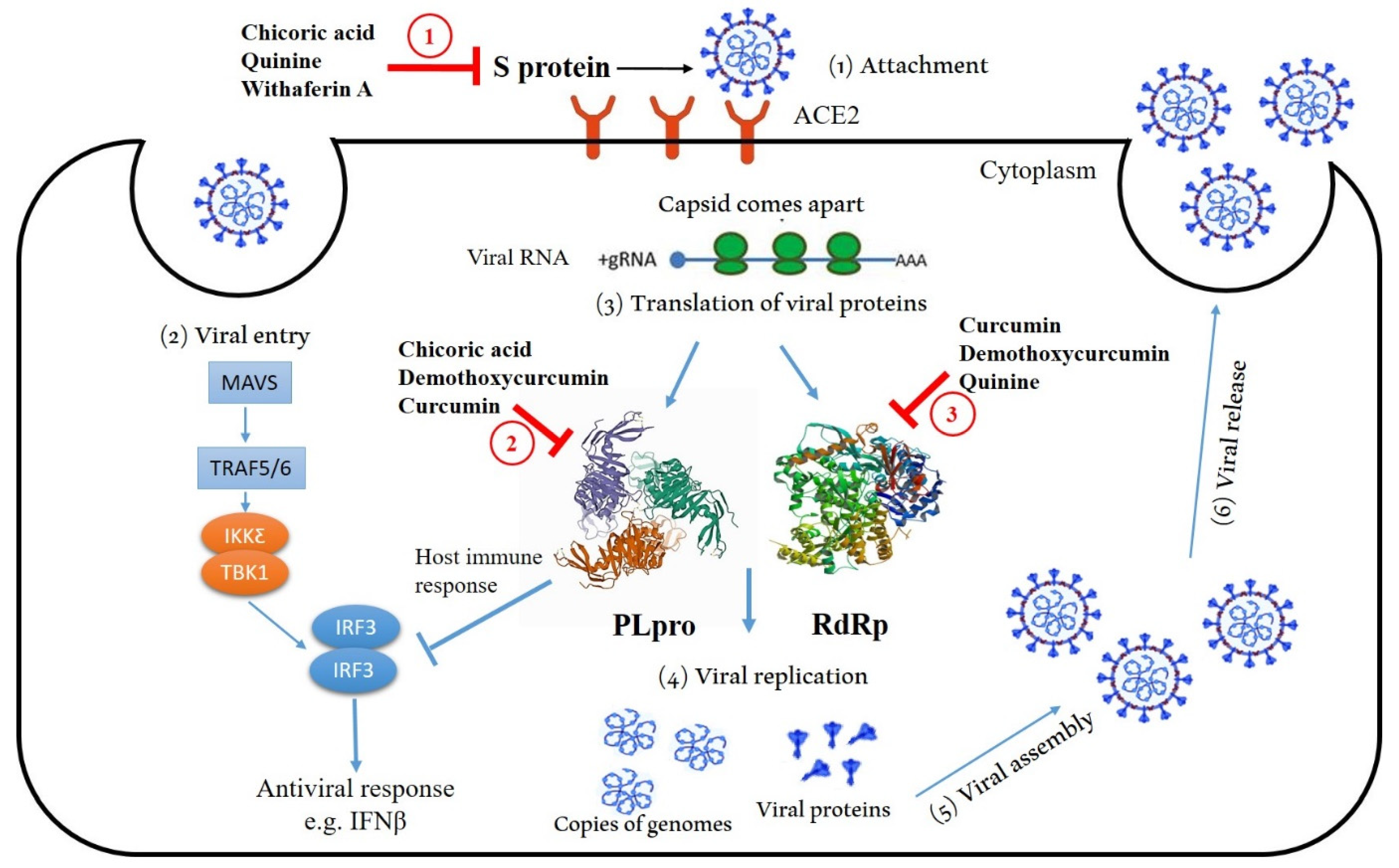
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Docked SARS-CoV-2 Protein Structures
3.2. Ligands and Drug Scan
3.3. Determination of SARS-CoV-2 Proteins Binding Hits
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elfiky, A.A. Zika Virus: Novel Guanosine Derivatives revealed strong binding and possible inhibition of the polymerase. Future Virol. 2017, 12, 721–728. [Google Scholar] [CrossRef]
- Hasan, A.; Paray, B.A.; Hussain, A.; Qadir, F.A.; Attar, F.; Aziz, F.M.; Sharifi, M.; Derakhshankhah, H.; Rasti, B.; Mehrabi, M.; et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2020, 39, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Jando, J.; Camargo, S.M.R.; Herzog, B.; Verrey, F. Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PLoS ONE 2017, 12, e0184845. [Google Scholar] [CrossRef]
- Baig, M.S.; Alagumuthu, M.; Rajpoot, S.; Saqib, U. Identification of a Potential Peptide Inhibitor of SARS-CoV-2 Targeting its Entry into the Host Cells. Drugs R&D 2020, 20, 161–169. [Google Scholar] [CrossRef]
- Harcourt, B.H.; Jukneliene, D.; Kanjanahaluethai, A.; Bechill, J.; Severson, K.M.; Smith, C.M. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004, 8, 13600–13612. [Google Scholar] [CrossRef] [Green Version]
- Wertz, I.E.; Murray, J.M. Structurally-defined deubiquitinase inhibitors provide opportunities to investigate disease mechanisms. Drug Discov. Today Technol. 2019, 31, 109–123. [Google Scholar]
- Baez-Santos, Y.M.; St John, S.E.; Mesecar, A.D. The SARS coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Sulea, T.; Lindner, H.A.; Purisima, E.O.; Ménard, R. Deubiquitination, a new function of the severe acute respiratory syndrome coronavirus papainlike protease. J. Virol. 2005, 79, 4550–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekes, M.; van Noort, G.J.V.; Ekkebus, R.; Ovaa, H.; Huang, T.T.; Lima, C.D. Recognition of Lys48-linked di-ubiquitin and deubiquitinating activities of the SARS coronavirus papain-like protease. Mol. Cell 2016, 62, 572–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfiky, A.A.; Ismail, A. Molecular dynamics and docking reveal the potency of novel GTP derivatives against RNA dependent RNA polymerase of genotype 4a HCV. Life Sci. 2019, 238, 116958. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.M.; Awad, O.M.; Shehata, M.S.; El-Sohaimy, S.A. Inhibition of the SARS-CoV-2 RNA-Dependent RNA Polymerase by Natural Bioactive Compounds: Molecular Docking Analysis. Egypt. J. Chem. 2021, 64, 1989–2001. [Google Scholar]
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid™. Available online: https://www.fda.gov/media/155050/download (accessed on 22 December 2021).
- Mahase, E. COVID-19: UK becomes first country to authorise antiviral molnupiravir. BMJ 2021, 375, n2697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Traditional Chinese medicine contributes to the treatment of COVID-19 patients. Chin. Herb. Med. 2020, 12, 95–96. [Google Scholar] [CrossRef]
- Gautam, K.; Adhikari, R.P.; Gupta, A.S.; Shrestha, R.K.; Koirala, P.; Suraj, K.S. Self-reported psychological distress during the COVID-19 outbreak in Nepal: Fndings from an online survey. BMC Psychol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Singh, P.; Guleri, R.; Singh, V.; Kaur, G.; Kataria, H.; Singh, B.; Kaur, G.; Kaul, S.C.; Wadhwa, R.; Pati, P.K. Biotechnological interventions in Withania somnifera (L.) dunal. Biotechnol. Genet. Eng. Rev. 2015, 31, 1–20. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.; Latheef, S.K.; Ahmed, I.; Iqbal, H.M.N.; Bule, M.H.; Dhama, K.; Samad, H.A.; Karthik, K.; Alagawany, M.; El-Hack, M.E.A.; et al. Herbal Immunomodulators—A remedial panacea for designing and developing effective drugs and medicines: Current scenario and future prospects. Curr. Drug Metab. 2018, 19, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Dubuisson, J.; Rouille, Y.; Seron, K. Hepatitis C virus and natural compounds: A new antiviral approach? Viruses 2012, 4, 2197–2217. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; He, Z.D.; Jiang, R.W.; Ye, W.C.; Xu, H.X.; But, P.P.H. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 2003, 62, 1235–1238. [Google Scholar] [CrossRef]
- Luganini, M.E.; Terlizzi, G.; Catucci, G.; Gilardi, M.E.; Maffei, G.G. The cranberry extract oximacro exerts in vitro virucidal activity against influenza virus by interfering with hemagglutinin. Front. Microbiol. 2018, 9, 1826. [Google Scholar]
- Xu, H.X.; Ming, D.S.; Dong, H.; But, P.P. A new anti-HIV triterpene from Geum japonicum. Chem. Pharm. Bull. 2000, 48, 1367–1369. [Google Scholar] [CrossRef] [Green Version]
- Sahuc, M.E.; Sahli, R.; Riviere, C.; Pene, V.; Lavie, M.; Vandeputte, A. Dehydrojuncusol, a natural phenanthrene compound extracted from Juncus maritimus, is a new inhibitor of hepatitis C virus RNA replication. J. Virol. 2019, 93, e02009–e02018. [Google Scholar] [CrossRef] [Green Version]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evid.-Based Complement. Altern. Med. 2018, 1463579, 13. [Google Scholar]
- Wang, Z.; Song, Q.; Su, J.; Tang, W.; Song, J.; Huang, X.; An, J.; Li, Y.; Ye, W.; Wang, Y. Caffeic acid oligomers from Mesona chinensis and their in vitro antiviral activities. Fitoterapia 2020, 144, 104603. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Li, W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016, 7, 40800–40815. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine 2019, 62, 152956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Wang, Y.F.; Liu, X.J.; Lu, J.H.; Qian, C.W.; Wan, Z.Y.; Yan, X.G.; Zheng, H.Y.; Zhang, M.Y.; Xiong, S.; et al. Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 2005, 20, 493–496. [Google Scholar]
- Okamoto, M.; Ono, M.; Baba, M. Potent inhibition of HIVtype 1 replication by an antiinflammatory alkaloid cepharanthinein chronically infected monocytic cells. AIDS Res. Hum. 1998, 14, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M.; Gallori, S.; Romani, A.; LaMarca, G.; Vincieri, F.F. Optimization of the chromatographic determination of polyphenols in the aerial parts of Cichoriumintybus L. Chromatographia 2001, 24, 455–461. [Google Scholar] [CrossRef]
- Ziamajidi, N.; Khagjani, S.; Hassanzadeh, G.; Vardasbi, S.; Ahmadian, S.; Nowrouzi, A. Amelioration by chicory seed extract of diabetes-and oleic acid-inducednon-alcoholicfattyliverdisease(NAFLD)/non-alcoholicsteato- hepatitis (NASH) via modulkation of PPARα and SREBP-1. Food Chem. Toxicol. 2013, 58, 198–209. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. The Bioactive Effects of Chicoric Acid As a Functional Food Ingredient. J. Med. Food. 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem Inst. Oswaldo Cruz. 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.K.; Mehta, A.; Ratre, Y.K.; Tiwari, A.K.; Amit, A.; Singh, R.P.; Sonkar, S.C.; Chaturvedi, N.; Shukla, D.; Vishvakarma, N.K. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur. J. Pharmacol. 2020, 886, 173551. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef]
- Sivaprabha, J.; Dharani, B.; Padma, P.R.; Sumathi, S. Induction of DNA damage by the leaves and rhizomes of Curcuma amada Roxb in breast cancer cell lines. J. Acute Dis. 2015, 4, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Mahadevi, R.; Kavitha, R. Phytochemical and pharmacological properties of Curcuma amada: A Review. Int. J. Res. Pharm. Sci. 2020, 11, 3546–3555. [Google Scholar] [CrossRef]
- Gurung, P.; De, P. Spectrum of biological properties of cinchona alkaloids: A brief review. J. Pharmacogn. Phytochem. 2017, 6, 162–166. [Google Scholar]
- O’Neill, E.J.; Termini, D.; Albano, A.; Tsiani, E. Anti-Cancer Properties of Theaflavins. Molecules 2021, 26, 987. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Y.; Kang, J.; Zhang, C.; Kang, S. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Wang, G.; Xu, L.; Liu, W.; Xu, W.; Mu, Y.; Wang, Z.; Huang, X.; Li, L. New anti-inflammatory withanolides from Physalis pubescens fruit. Fitoterapia 2020, 146, 104692. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Liu, Y.; Cheng, Y.; Sun, Y.; Pan, J.; Guan, W.; Li, X.; Huang, J.; Jiang, P.; Guo, S.; et al. New withanolides with anti-inflammatory activity from the leaves of Datura metel L. Bioorganic Chem. 2020, 95, 103541. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, J.; Kim, H.K.; Moon, B.C.; Ji, Y.; Ryu, D.H.; Hwang, G.S. Metabolite profiling of Curcuma species grown in different regions using 1HNMR spectroscopy and multivariate analysis. Analyst 2012, 137, 5597–5606. [Google Scholar] [CrossRef]
- Kamazeri, T.S.A.T.; Samah, O.A.; Taher, M.; Susanti, D.; Qaralleh, H. Antimicrobial activity and essential oils of Curcuma aeruginosa, Curcuma mangga, and Zingiber cassumunar from Malaysia. Asian Pac. J. Trop. Med. 2012, 5, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Crosby, D.C.; Lei, X.Y.; Gibbs, C.G.; McDougall, B.R.; Robinson, W.E.; Reinecke, M.G. Design, synthesis, and biological evaluation of novel hybrid dicaffeoyltartaric/diketo acid and tetrazole-substituted lchicoric acid analogue inhibitors of human immunodeficiency virus type 1 integrase. J. Med. Chem. 2010, 53, 8161–8175. [Google Scholar] [CrossRef] [PubMed]
- Hazuda, D.J.; Felock, P.; Witmer, M.; Wolfe, A.; Stillmock, K.; Grobler, J.A.; Espeseth, A.; Gabryelski, L.; Schleif, W.; Blau, C. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000, 287, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Reinke, R.A.; Lee, D.J.; McDougall, B.R. l-Chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV-1 integrase in vitro. Virology 2004, 326, 203–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshe, R.; Paul, O.; Igor, K. Cepharanthine: A review of the antiviral potential of a Japanese-approved alopecia drug in COVID-19. Pharmacol. Rep. 2020, 72, 1509–1516. [Google Scholar]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S.; et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience 2021, 23, 102367. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Deguchi, A. Curcumin targets in inflammation and cancer. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 88–96. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Clifton, P. Curcumin, Cardiometabolic Health and Dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J. Curcumin and autoimmune disease. Adv. Exp. Med. Biol. 2007, 595, 425–451. [Google Scholar]
- Sordillo, P.P.; Helson, L. Curcumin Suppression of Cytokine Release and Cytokine Storm. A Potential Therapy for Patients with Ebola and Other Severe Viral Infections. In Vivo 2015, 29, 1–4. [Google Scholar]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25, reprinted in Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena 1. Prediction of intestinal absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar]
- Chang, L.C.W.; Spanjersberg, R.F.; von Frijtag Drabbe, K.; Unzel, J.K.; Mulder-Krieger, T.; van den Hout, G.; Beukers, M.W.; Brussee, J.; Ijzerman, A.P. 2,4,6-Trisubstituted pyrimidines as a new class of selective adenosine A, receptor antagonists. J. Med. Chem. 2004, 47, 6529–6540. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Andersson, S.; Antonsson, M.; Elebring, M.; Jansson-Lofmark, R.; Weidolf, L. Drug metabolism and pharmacokinetic strategies for oligonucleotide- and mRNA-based drug development. Drug Discov. Today 2018, 23, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Z.; Tay-Sontheime, J.; Levy, R.H.; Ragueneau-Majlessi, I. Intestinal Drug Interactions Mediated by OATPs: A Systematic Review of Preclinical and Clinical Findings. J. Pharm. Sci. 2017, 106, 2312–2325. [Google Scholar] [CrossRef] [Green Version]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 1, 391–396. [Google Scholar]
- Paz, S.; Vilasco, M.; Werden, S.J.; Arguello, M.; Joseph-Pillai, D.; Zhao, T.; Nguyen, T.L.; Sun, Q.; Meurs, E.F.; Lin, R. A functional C-terminal TRAF3- binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res. 2011, 21, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Battistuz, T.; Bhat, T. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Giménez, B.G.; Santos, M.S.; Ferrarin, M.; Dos Santos Fernandes, J.P. Evaluation of blockbuster drugs under the rule-of-five. Pharmazie 2010, 65, 148–152. [Google Scholar]
- Afonine, P.V.; Klaholz, B.P.; Moriarty, N.W.; Poon, B.K.; Sobolev, O.V.; Terwilliger, T.C.; Adams, P.D.; Urzhumtsev, A. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Bio. 2018, 74, 814–840. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couc, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
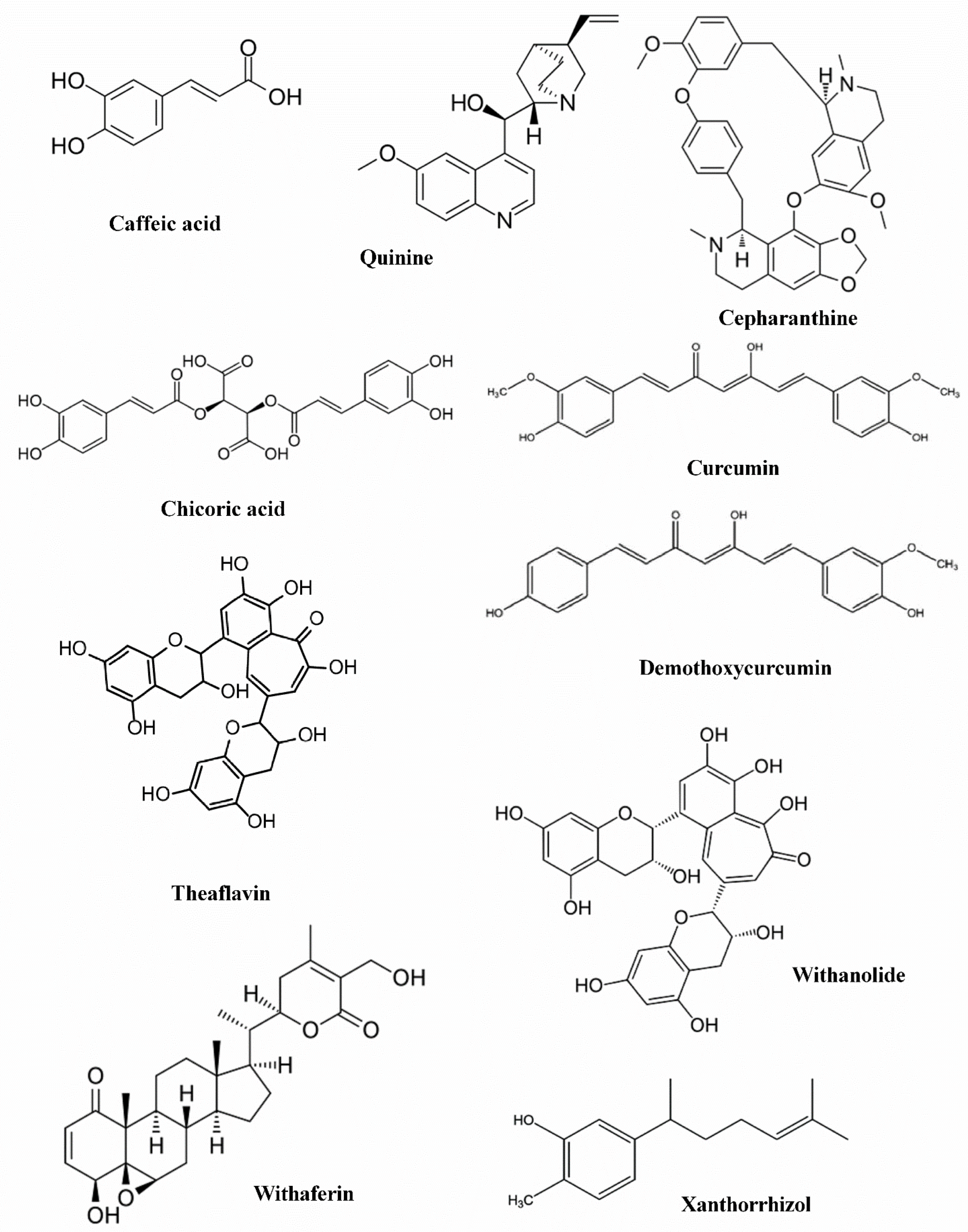

| Ligands | Traditional Medicine Source | Region | Reported Biological Activity | References |
|---|---|---|---|---|
| 1 | Citrullus colocynthis (fruit coat), C. colocynthis (Seeds), Paraguariensis Mesona chinensis Echinacea purpurea | Egypt, Poland, Argentina, China | Antiviral activities Antioxidant activity | [30] [31] |
| 2 | Stephania cepharantha Stephania rotunda | Japan | Anti-inflammatory activity, Antiparasitic activities, Anti-oxidative properties, Antiviral activities and Anti-HIV activity | [32,33,34,35] |
| 3 | Sickle seagrass, starflower, sweet basil, African basil, Chicory and Echinacea purpurea | Egypt, China, India, and North America | Antivirus, anti- inflammation, glucose and lipid homeostasis, Neuroprotection, antioxidation effects. Antimicrobial activity and Antioxidant activity | [36,37,38] |
| 4 | Curcuma longa L. | Southern Asia, China, India, Indonesia, Indochina | Anti-inflammatory activity, Antioxidant activity, Anti-bacteria activity Chemopreventive and Chemotherapeutic activity Anti-HIV activity, and Nematocidal activities | [39,40,41] |
| 5 | Curcuma longa L. | Southern Asia, China, India, Indonesia, Indochina, | Antimicrobial activity, Anti-inflammatory activity, Antioxidant activity, Platelet aggregation inhibitory activity, Antiallergy activity, Anticancer activity | [42,43,44] |
| 6 | Chinchona bark | Peru, Bolivia, Colombia, Ecuador, India, and Sri Lanka. | Antimalarial activity, Antioxidant activity, Anti-cancer agent, Anti-inflammatory, Antiparasitic activity and Antimicrobial property | [45] |
| 7 | Black tea | Asia and Europe | Antioxidant activity and Anti-cancer activity | [46,47] |
| 8 | Withania somnifera L. | Sri Lanka | Anti-cancer activity and Anti-COVID activity | [48] |
| 9 | Withania somnifera L. Datura metel L. leaves | India | Anti-inflammatory and Antioxidant activity | [49,50] |
| 10 | Java turmeric Curcuma species (C. zedoaria, C. xanthorrhiza, C. aeruginosa and C. mangga) | India and Southeast Asia | Anti-inflammatory, Antioxidant, and Anti-cancer activities | [51,52] |
| Ligand Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular formula | C9H8O4 | C37H38N2O6 | C22H18O12 | C21H20O6 | C20H18O5 | C20H24N2O2 | C29H24O12 | C28H38O6 | C28H38O6 | C15H22O |
| Classification | Phenolics | Alkaloid | Phenylpropanoid | Phenolics | Phenolics | Alkaloid | Phenolics | Phenolics | Phenolics | Sesquiterpenoid |
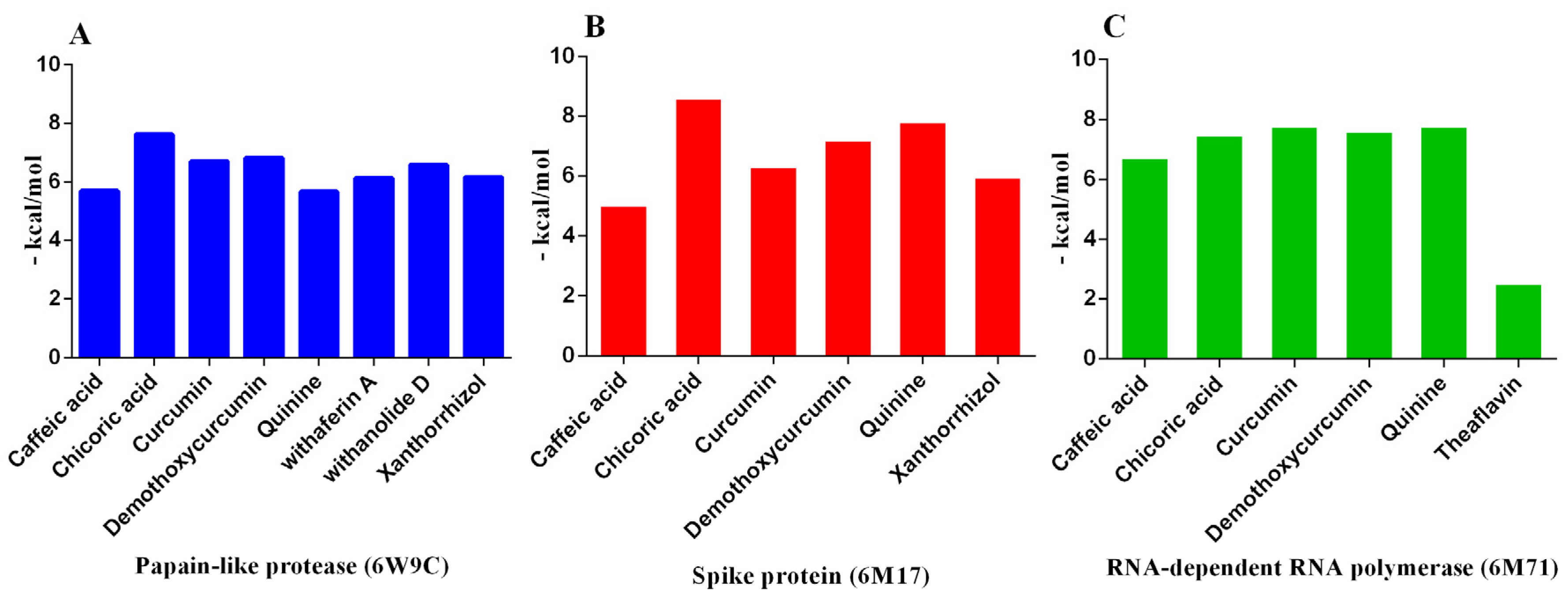
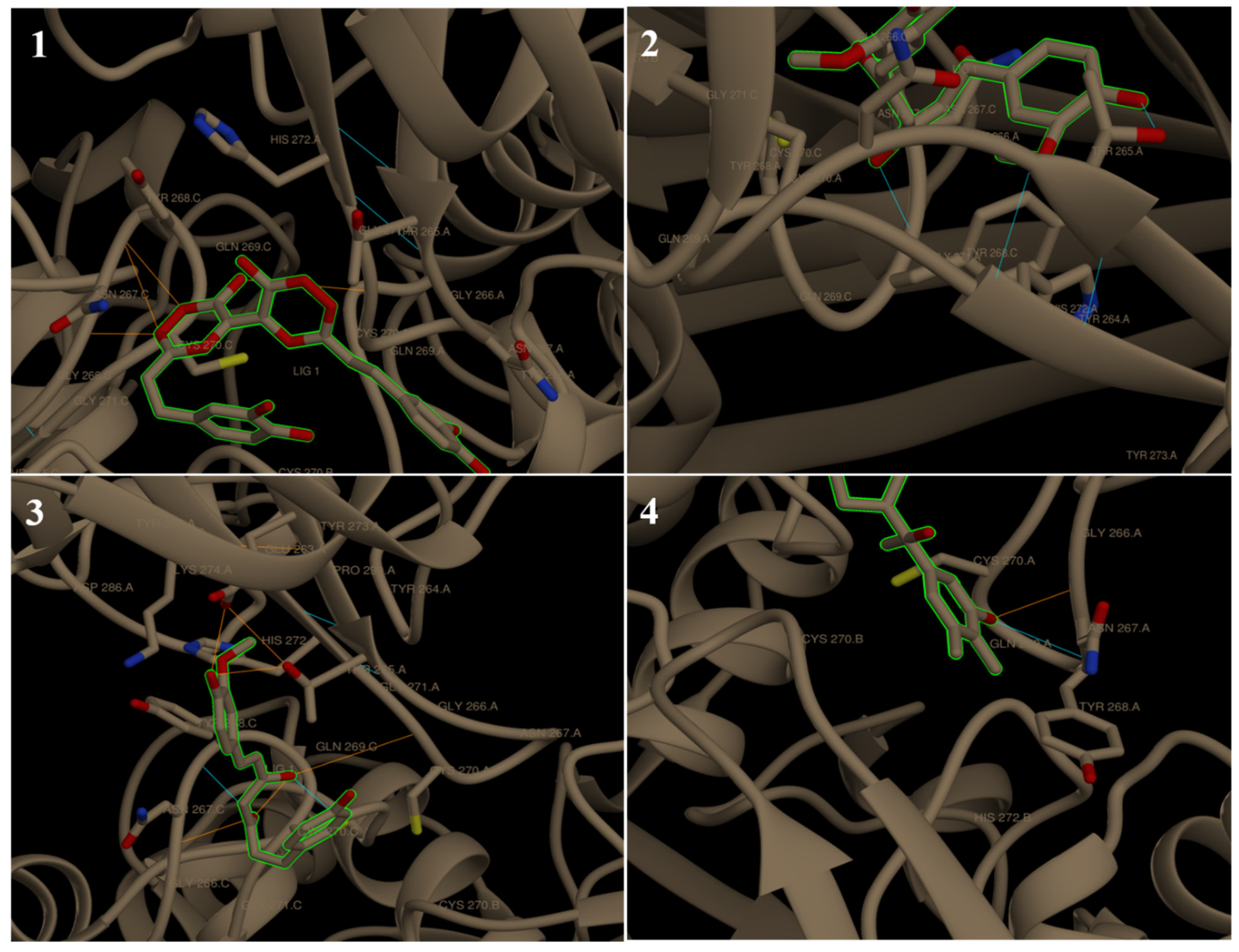
| 6W9C | ||||||||||
| Binding energy ΔG | −5.70 | - | −7.62 | −6.70 | −6.81 | −5.67 | - | −6.13 | −6.58 | −6.16 |
| No. of H bonding | 6 | - | 4 | 3 | 6 | 2 | - | 1 | 2 | 2 |
| Binding sites | CYS 270, GLN 269, TYR 268, ASN 267 and THR 265 | - | CYS 270, TYR 268, ASN 267 and ASP 286 | THR 265 and TYR 268 | CYS 270, TYR 268, ASN 267, THR 265 and GLU 263 | ASP 286 and LEU 290 | - | TYR 264 | ASN 267 and TYR 268 | ASN 267 and TYR 268 |
| 6M71 | ||||||||||
| Binding energy ΔG | −6.75 | - | −7.50 | −7.80 | −7.64 | −7.80 | −2.55 | −6.27 | −6.10 | −5.33 |
| No. of H bonding | 6 | - | 4 | 11 | 8 | 4 | 7 | 4 | 7 | 2 |
| Binding sites | THR 680, ARG 555, ARG 553, THR 556, and SER 682 | - | ALA 554, ARG 553 and ARG 555 | ARG 555, ARG 553, THR 556, and LYS 545 | ARG 555, and THR 556, | ARG 555, THR 556, SER 682 and ALA 554 | ARG 553, THR 556, and SER 682 | THR 556, and SER 682 | THR 556, SER 682 and ASP 623 | TYR 455 and ALA 554 |
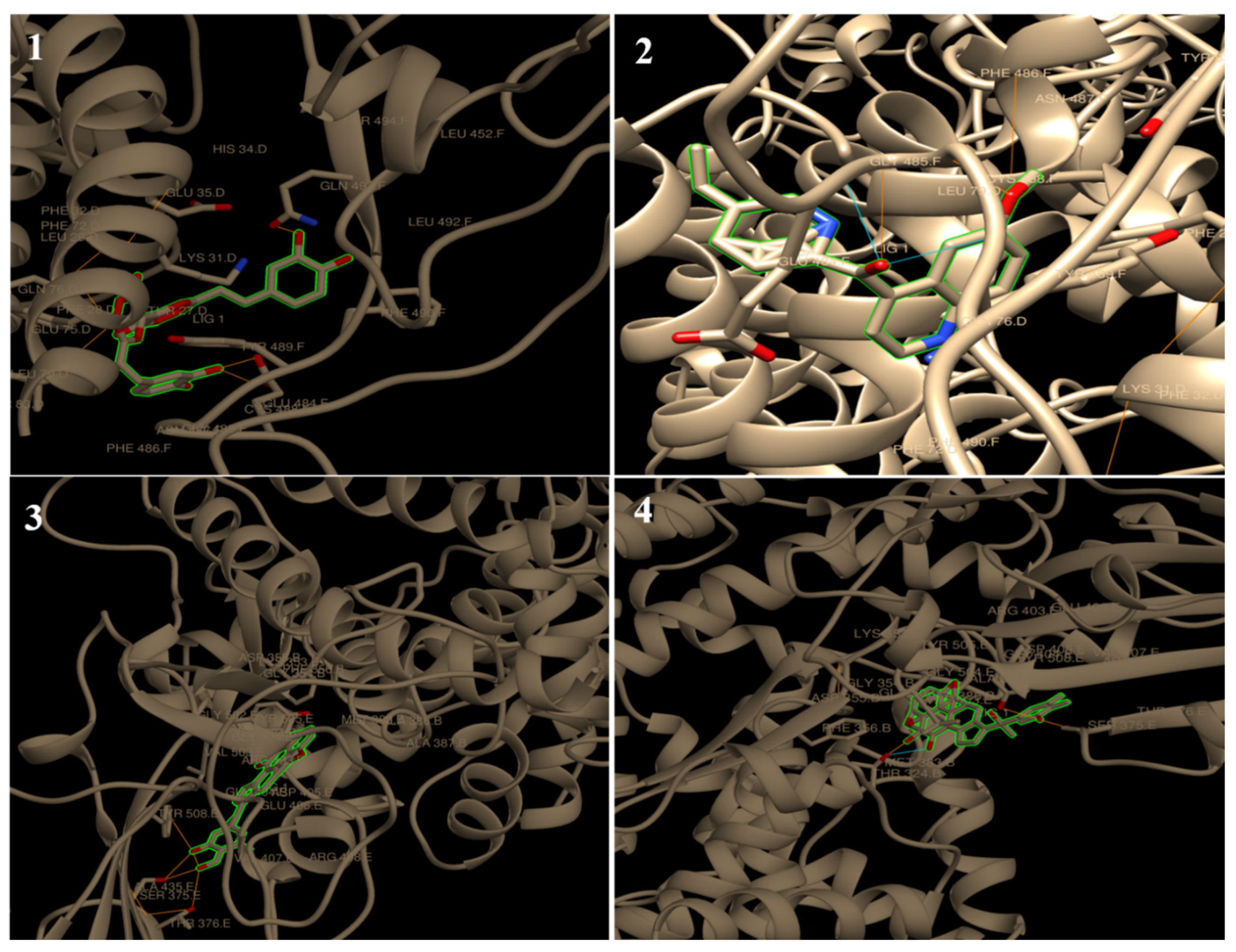
| 6M17 | ||||||||||
| Binding energy ΔG | −5.06 | −5.26 | −8.63 | −6.34 | −7.23 | −7.85 | −7.43 | −7.85 | −7.78 | −6.00 |
| No. of H bonding | 2 | 1 | 4 | 4 | 1 | 4 | 7 | 12 | 7 | 3 |
| Binding sites | ASN 487 | GLN 76 | GLU 484, CYS 488, GLN 493 and GLN 76 | ASP 355, THR 500 and THR 324 | ASN 487 | CYS 488, GLY 484 and ASN 487 | THR 324, GLY 404, ARG 408 and THR 508 | THR 324, SER 375, TYR 376 and TYR 508 | THR 324, SER 375, and TYR 508 | THR 324 and VAL 503 |
| Ligands Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Lipinski’s Rule of Five | ||||||||||
| Molecular weight (<500 Da) | 180.16 | 606.71 | 474.37 | 368.38 | 338.35 | 324.42 | 564.49 | 470.60 | 470.60 | 218.33 |
| LogP (<5) | 0.93 | 5.35 | 1.01 | 3.03 | 3.00 | 2.81 | 1.31 | 2.29 | 2.36 | 4.34 |
| No. rotatable bonds (<15) | 2 | 2 | 11 | 8 | 7 | 4 | 2 | 3 | 2 | 4 |
| No. H-Bond donors (5) | 3 | 0 | 6 | 2 | 2 | 1 | 9 | 4 | 4 | 1 |
| No. H-bond acceptors (<10) | 4 | 8 | 12 | 6 | 5 | 4 | 12 | 6 | 6 | 1 |
| TPSA Å | 77.76 | 61.86 | 208.12 | 93.06 | 83.83 | 45.59 | 217.60 | 115.06 | 115.06 | 20.23 |
| Violations | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Pharmacokinetics | ||||||||||
| GI absorption | High | High | Low | High | High | High | Low | High | High | High |
| BBB | No | No | No | No | No | Yes | No | No | No | Yes |
| P-gp substrate | No | No | Yes | No | No | No | No | Yes | Yes | No |
| CYP1A2 inhibitor | No | No | No | No | Yes | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | Yes | Yes | No | Yes | No | No | Yes |
| CYP2D6 inhibitor | No | No | No | No | No | Yes | No | No | No | Yes |
| CYP3A4 inhibitor | No | No | No | Yes | Yes | No | Yes | No | No | No |
| Amino Acids Hits | Papain-Like Protease (PLpro) (6W9C) | RNA Dependent RNA Polymerase (RdRp) (6M71) | Spike Protein (S Protein) (6M17) |
|---|---|---|---|
| ASP 286, HIS 272, and CYS 111 | ARG 553, ARG 555, and LYS 545 | GLY 502, TYR 489, and TYR 505 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Aziz, N.M.; Khalifa, I.; Darwish, A.M.G.; Badr, A.N.; Aljumayi, H.; Hafez, E.-S.; Shehata, M.G. Docking Analysis of Some Bioactive Compounds from Traditional Plants against SARS-CoV-2 Target Proteins. Molecules 2022, 27, 2662. https://doi.org/10.3390/molecules27092662
Abd El-Aziz NM, Khalifa I, Darwish AMG, Badr AN, Aljumayi H, Hafez E-S, Shehata MG. Docking Analysis of Some Bioactive Compounds from Traditional Plants against SARS-CoV-2 Target Proteins. Molecules. 2022; 27(9):2662. https://doi.org/10.3390/molecules27092662
Chicago/Turabian StyleAbd El-Aziz, Nourhan M., Ibrahim Khalifa, Amira M. G. Darwish, Ahmed N. Badr, Huda Aljumayi, El-Sayed Hafez, and Mohamed G. Shehata. 2022. "Docking Analysis of Some Bioactive Compounds from Traditional Plants against SARS-CoV-2 Target Proteins" Molecules 27, no. 9: 2662. https://doi.org/10.3390/molecules27092662
APA StyleAbd El-Aziz, N. M., Khalifa, I., Darwish, A. M. G., Badr, A. N., Aljumayi, H., Hafez, E.-S., & Shehata, M. G. (2022). Docking Analysis of Some Bioactive Compounds from Traditional Plants against SARS-CoV-2 Target Proteins. Molecules, 27(9), 2662. https://doi.org/10.3390/molecules27092662










