Rapid Detection of Five Estrogens Added Illegally to Dietary Supplements by Combining TLC with Raman Imaging Microscope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Solutions Preparation
2.4. The TLC Method
2.5. The TLC-RIM Method
3. Results and Discussion
3.1. TLC Separation
3.2. The TLC-RIM Method
3.3. Correlation between Raman Spectra of Reference Powders and the Spectra by TLC-RIM
3.4. Analysis of Characteristic Peaks of Estrogens
3.5. Experiment of Simulated Positive Samples
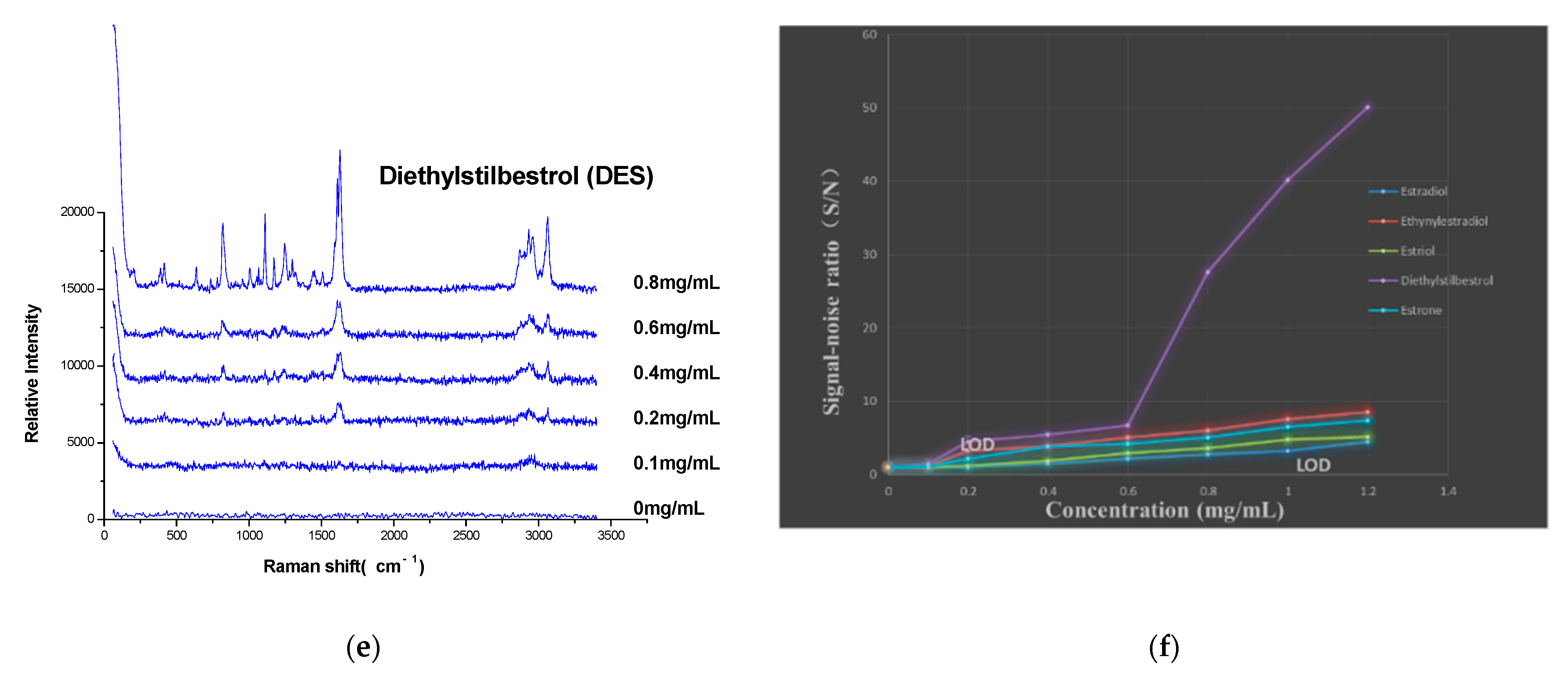
3.6. Detection of the Limit of Detection
3.7. Detection of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Panadda, P.; Uthai, S.; Tasamaporn, S.; Jariya, A.; Sarunyaporn, K. Quantitative determination of sibutramine in adulterated herbal slimming formulations by TLC image analysis method. Forensic Sci. Int. 2012, 219, 96–100. [Google Scholar]
- Lei, Y.P.; Yin, S.; Wu, G.P. Proprietary Chinese medicine and health food illegally added in adrenal cortical hormone drugs detection method research. J. Tradit. Chin. Med. Guides 2009, 15, 81–83. [Google Scholar]
- Xue, Y.; Sheng, Y.H.; Wang, J.; Huang, Q.; Zhang, F.Y.; Wen, Y.; Liu, S.h.; Jiang, Y.P. Fast Screening and Identification of Illegal Adulterated Glucocorticoids in Dietary Supplements and Herbal Products Using UHPLC-QTOF-MS with All-Ion Fragmentation Acquisition Combined with Characteristic Fragment Ion List Classification. Front. Chem. 2021, 9, 785475. [Google Scholar] [CrossRef]
- Payam, A.; Natalia, G.B.; Hilary, M.; Mattia, P.; Luca, V.; Valentina, E.V.F.; Willem, H.; Michel, W.F.N. Receptor-based high-throughput screening and identification of estrogens in dietary supplements using bioaffinity liquid-chromatography ion mobility mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 9427–9436. [Google Scholar]
- Ilse, B.; Christof, V.P.; Carlos, V.P. An LC–MS screening method with library identification for the detection of steroids in dietary supplements. J. Mass. Spectrom. 2011, 46, 327–335. [Google Scholar]
- He, X.Q.; Xi, C.X.; Tang, B.; Wang, G.M.; Chen, D.D.; Peng, T.; Mu, Z.D. Simultaneous determination of 30 hormones illegally added to anti-ageing functional foods using UPLC-MS/MS coupled with SPE clean-up. Food Addit. Contam. Part A 2014, 31, 1625–1638. [Google Scholar] [CrossRef]
- Fan, W.T.; Gao, X.N.; Ding, C.H.; Lv, Y.A.; Shen, T.T.; Ma, G.P.; Yan, L.P.; Song, S.Q. Estrogen receptors participate in carcinogenesis signaling pathways by directly regulating NOD-like receptors. Biochem. Biophys. Res. Commun. 2019, 511, 468–475. [Google Scholar] [CrossRef]
- Shen, M.Q.; Cao, J.Y.; Shi, H.F. Effects of Estrogen and Estrogen Receptors on Transcriptomes of HepG2 Cells: A Preliminary Study Using RNA Sequencing. Int. J. Endocrinol. 2018, 28, 5789127. [Google Scholar] [CrossRef] [Green Version]
- Kohei, T.; Rintaro, K.; Liu, X.H.; Kaoru, U.; Tatsuya, K.; Kenji, W.; Tohru, O.; Hiroshi, N.; Yasuyuki, S. Design and synthesis of benzoacridines as estrogenic and anti-estrogenic agents. Bioorg. Med. Chem. 2017, 25, 5216–5237. [Google Scholar]
- Engler-Chiurazzi, E.B.; Brown, C.M.; Povroznik, J.M.; Simpkins, J.W. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog. Neurobiol. 2017, 157, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Maria, A.F.; Elisa, A.; Marta, P.; Lucia, D.M.; Evandro, A.; Ines, V.L.; Ann, H.P.; Matteo, L. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar]
- Thomas, F.W. Hypertriglyceridemia: An Infrequent, Difficult-to-Predict, Severe Metabolic and Vascular Problem Associated with Estrogen Administration. Curr. Vasc. Pharmacol. 2020, 18, 254–261. [Google Scholar]
- Linda, T. Evidence of intergenerational transmission of diethylstilbestrol health effects: Hindsight and insight. Biol. Reprod. 2021, 105, 681–686. [Google Scholar]
- Zhao, C.Q.; Li, Y.H.; Liu, Z.; Luo, J.W. Determination of 8 estrogen residues in milk powder by UPLC-MS/MS. Chin. J. Pharm. Anal. 2020, 40, 253–259. [Google Scholar]
- Jaque, J.; Macdonald, H.; Brueggmann, D.; Patel, S.K.; Azen, C.; Clarke, N.; Stanczyk, F.Z. Deficiencies in immunoassay methods used to monitor serum Estradiol levels during aromatase inhibitor treatment in postmenopausal breast cancer patients. SpringerPlus 2013, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Clavijo, S.; Cerdà, V. Estrogens determination exploiting a SIA-LOV system prior in-port derivatization-large volume injection-programmable temperature vaporization-gas chromatography. Talanta 2019, 194, 852–858. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, Y. Pitfalls and solution for simultaneous determination of estrone and 17α-ethinylestradiol by gas chromatography–mass spectrometry after derivatization with N, O-bis (trimethylsilyl) trifluoroacetamide. Anal. Chim. Acta 2005, 554, 190–196. [Google Scholar] [CrossRef]
- Wang, X.J.; Ding, H.; Yu, X.R.; Shi, X.Z.; Sun, A.L.; Li, D.X.; Zhao, J. Characterization and application of molecularly imprinted polymer-coated quantum dots for sensitive fluorescent determination of diethylstilbestrol in water samples. Talanta 2019, 197, 98–104. [Google Scholar] [CrossRef]
- Jayson, V.; Elmer-Rico, E.M.; Ruel, Z.B.D. Raman Spectroscopic Discrimination of Estrogens. Vib. Spectrosc. 2018, 96, 93–100. [Google Scholar]
- Jin, S.; Pang, W.; Zhao, L.; Zhao, Z.; Mei, S. Review of HPLC-MS methods for the analysis of nicotine and its active metabolite cotinine in various biological matrices. Biomed. Chromatogr. 2022, e5351. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, L.L.; Liu, J.C.; Li, L.; Jia, S.S. Rapid Detection of Four Chemical Components Added Illegally in Slimming Health Food with TLC Situ Raman Spectroscopy. Spectrosc. Spectr. Anal. 2018, 38, 830–836. [Google Scholar]
- Shen, Z.D.; Fan, Q.Z.; Qian, Y.; Wang, R.; Wang, H.; Kong, X. Facile detection of carbendazim in food using TLC-SERS on diatomite thin layer chromatography. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119037. [Google Scholar] [CrossRef]
- Tan, A.; Zhao, Y.; Sivashanmugan, K.; Squire, K.; Wang, A.X. Quantitative TLC-SERS detection of histamine in seafood with support vector machine analysis. Food Control 2019, 103, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.L.; Jia, Q.; Liu, C.Y.; Wang, W.; Duan, L.F.; Yang, G.H.; Han, C.Q.; Li, H.T. Thin layer chromatography combined with surface-enhanced raman spectroscopy for rapid sensing aflatoxins. J. Chromatogr. A 2018, 1579, 115–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, A.L.; Squire, K.; Sivashanmugan, K.; Wang, A.X. Quaternion-based Parallel Feature Extraction: Extending the Horizon of Quantitative Analysis using TLC-SERS Sensing. Sens. Actuators B Chem. 2019, 299, 126902. [Google Scholar] [CrossRef]
- Li, L.; Xu, F.; Sun, G.; Sun, M.; Jia, S.; Li, H.; Xu, T.; Zhang, H.; Wang, Y.; Guo, Y.; et al. Identification of N-methylaniline based on azo coupling reaction by combining TLC with SERRS. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119490. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Zhang, Y.Y.; Kou, Q.W.; Zhang, Y.J.; Wang, Y.X.; Chen, L.; Sun, Y.T.; Zhang, H.L.; Young, M.J. Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Molecules 2018, 23, 1330. [Google Scholar] [CrossRef] [Green Version]
- Yehseen, T.; Sun, D.W.; Cheng, J.H. Raman Imaging for Food Quality and Safety Evaluation: Fundamentals and Applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Shona, S.; Ryan, J.P.; Matthew, P.N.; Patrick, J.T. Raman Imaging. Annu. Rev. Anal. Chem. 2012, 5, 337–360. [Google Scholar]
- Marlen, P.; Yu, Z.L.; Lu, X.N. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187. [Google Scholar]
- Paul, F.; Jianying, O.; Jacques, L. Full Spectrum Raman Excitation Mapping Spectroscopy. Sci. Rep. 2020, 10, 9172. [Google Scholar]
- Enza, F.; Carmelo, C.; Domenico, M. Paper aging and degradation monitoring by the non-destructive two-dimensional micro-Raman mapping. Sectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117660. [Google Scholar]
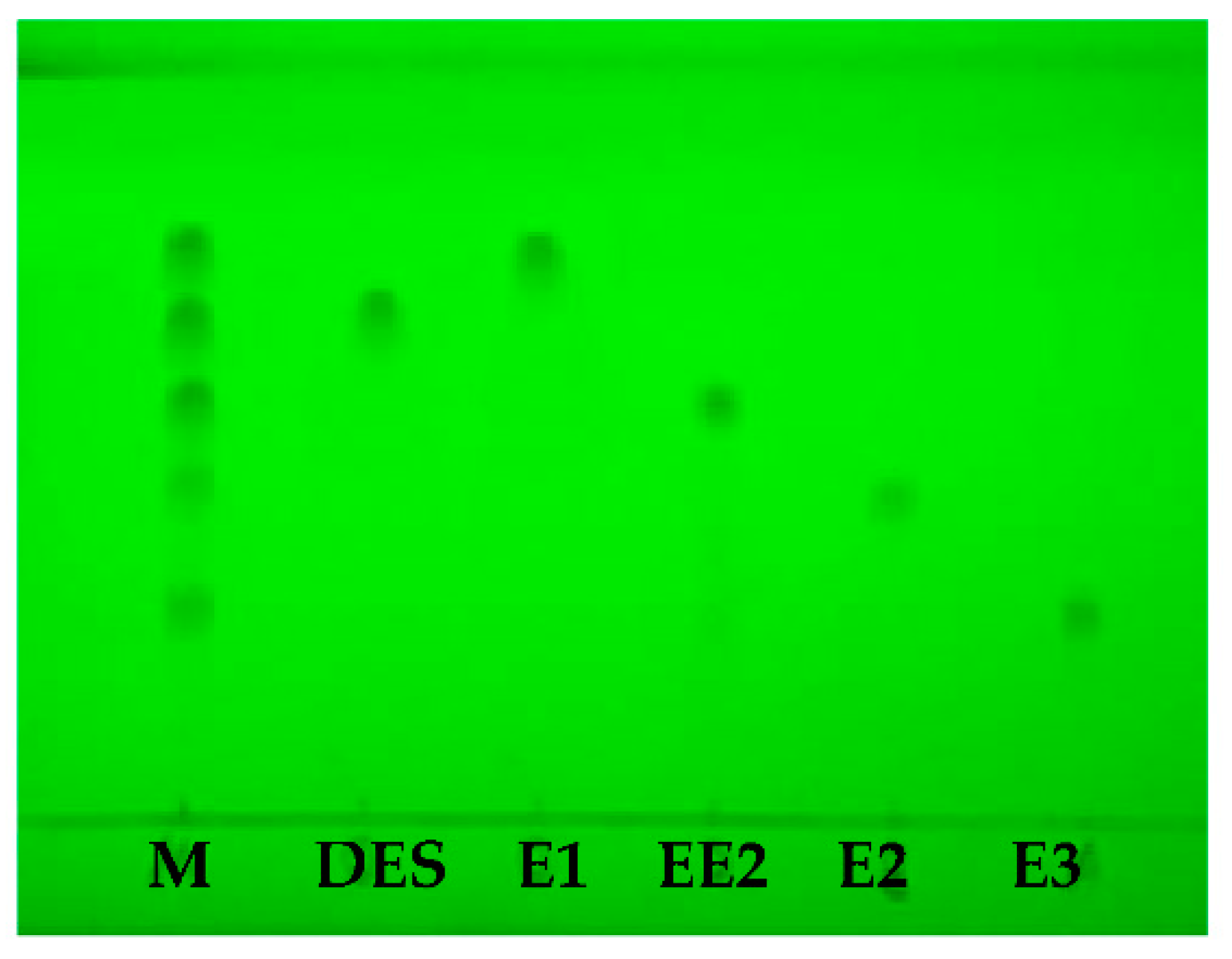
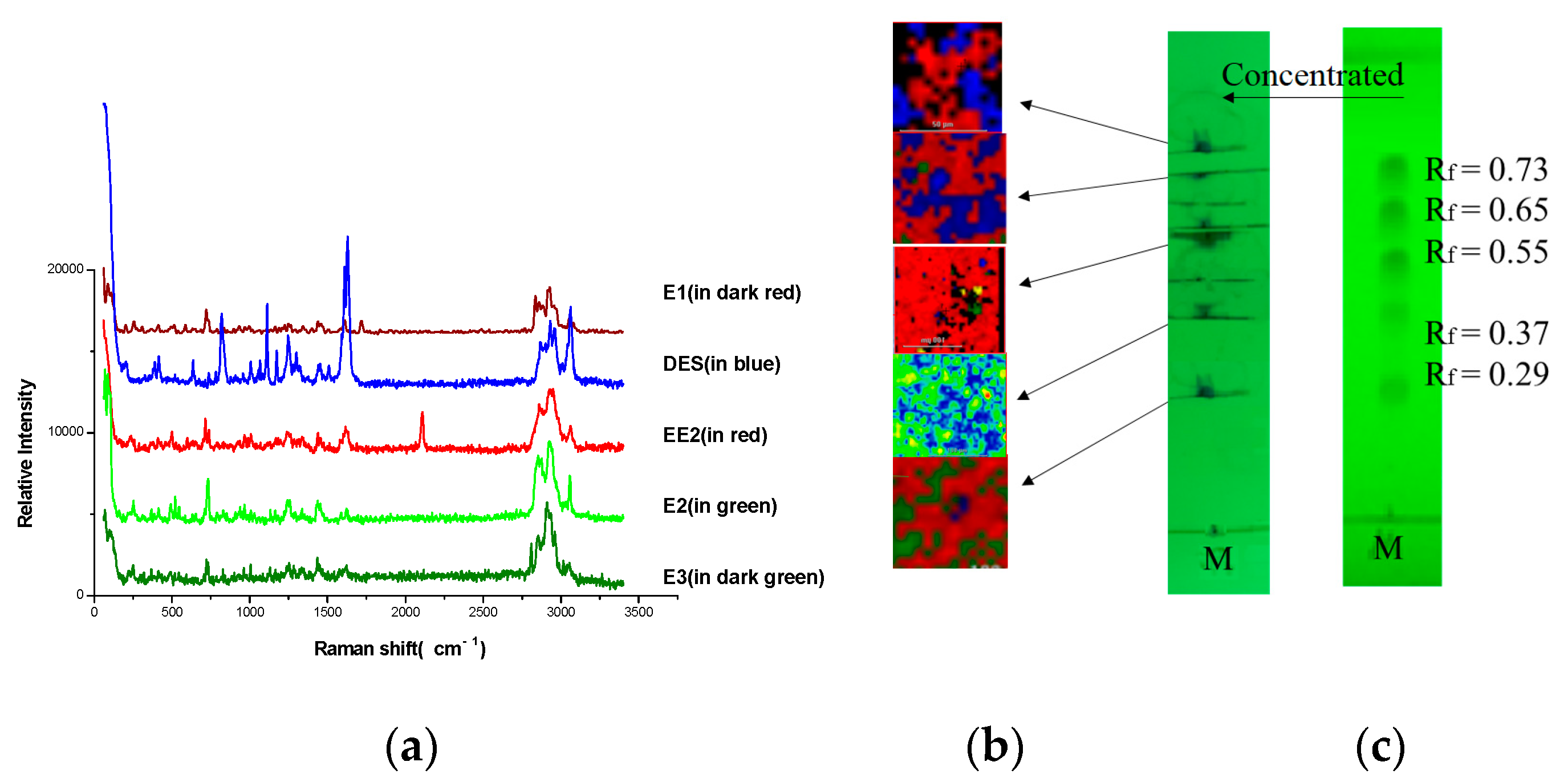
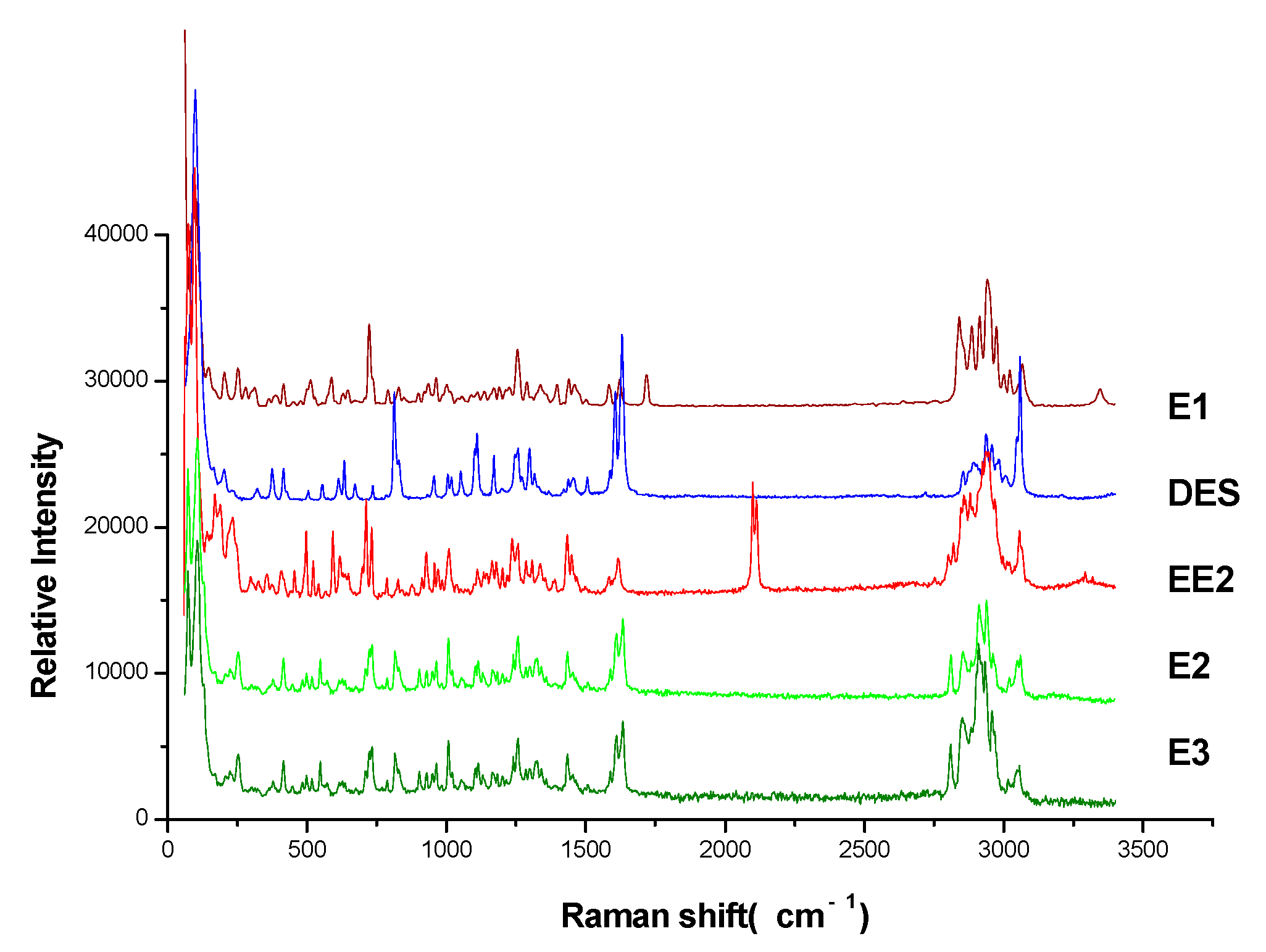
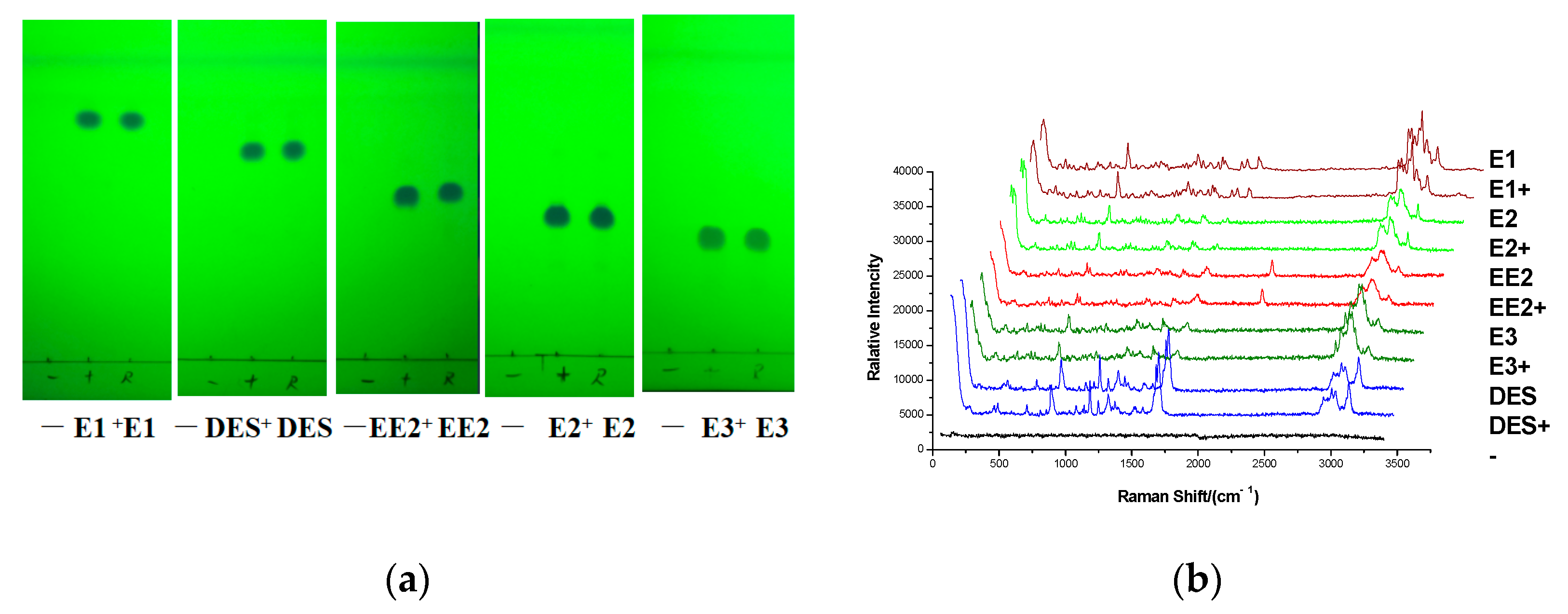


| Chemical Structure | Raman Shift (cm−1) of Estrogen Powders (Relative Intensity) | Raman Shift (cm−1) of Estrogen by TLC-RIM (Relative Intensity) | Assignments |
|---|---|---|---|
E1 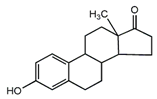 | 3068 (1.0) 2974, 2939 (1.8, 3.0) 2914, 2885 (2.0, 1.7) 1719 (0.8) 1590, 1615 (0.9, 1.0) 735, 723(1.4, 2.2) | 3058 (1.0) 2973, 2938 (2.0, 2.2) 2915, 2883 (1.2, 1.1) 1709 (0.5) 1589, 1612 (1.0, 1.1) 732, 721(1.0, 1.7) | ν=CH within phenyl rings νasCH3, νasCH2 νsCH3,νsCH2 νC=O νC=C γ=C-H within phenyl rings |
E2 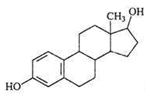 | 3060 (1.0) 2964, 2939 (1.0, 2.0) 2913, 2858 (2.0, 1.0) 1619, 1612 (1.7, 1.4) 732, 725 (1.2, 0.6) | 3060 (1.0) 2960, 2936 (1.0, 1.6) 2911, 2853 (1.4, 1.3) 1622, 1609 (0.5, 0.4) 732, 722 (1.0, 0.4) | ν=CH within phenyl rings νasCH3, νasCH2 νsCH3,νsCH2 νC=C γ=C-H within phenyl rings |
E3  | 3060 (1.0) 2964, 2939 (1.0, 2.2) 2913, 2858 (1.3, 1.3) 1614 (0.7) 723, 709 (1.3, 0.7) | 3060 (1.0) 2960, 2936 (1.0, 1.6) 2911, 2853 (1.2, 1.2) 1613 (0.6) 732, 722 (1.2, 0.5) | ν=CH within phenyl rings νasCH3, νasCH2 νsCH3,νsCH2 νC=C γ=C-H within phenyl rings |
EE2  | 3056 (1.0) 2965, 2941 (1.4, 2.2) 2910, 2860 (1.6, 1.5) 2113, 2102 (1.4, 1.5) 1617, 1602 (0.7, 0.5) 735, 713 (1.0, 1.4) | 3063 (1.0) 2957, 2935 (1.5, 2.3) 2873, 2861 (1.8, 1.8) 2111 (1.7) 1615, 1601 (0.9, 0.6) 733, 716 (0.7, 1.2) | ν=CH within phenyl rings νasCH3, νasCH2 νsCH3,νsCH2 νC≡C νC=C γ=C-H within phenyl rings |
DES 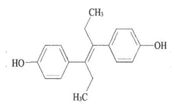 | 3058 (1.0) 2960, 2937 (0.3, 0.4) 2913, 2878 (0.3, 0.2) 1633 (1.2) 1608, 1600 (0.7, 0.1) 817 (0.8) | 3061 (1.0) 2960, 2930 (0.8, 0.7) 2902, 2870 (0.6, 0.6) 1629 (1.4) 1608, 1593 (1.2, 0.8) 821 (1.0) | ν=CH within phenyl rings νasCH3, νasCH2 νsCH3,νsCH2 νC=C symmetric vibration νC=C within phenyl rings γ=C-H within phenyl rings |
| Drugs | The minimum Therapeutic Dosage in Oral Medications (mg/a Day) | Potentially Abused Dosages of Drugs in BDS (mg/a Single Dose) | The Possible Concentration of the Drug in Sample Solution (mg/mL) | LOD (mg/mL) |
|---|---|---|---|---|
| E1 | 1 | 1 | 10 | 0.4 |
| E2 | 1 | 1 | 10 | 1.0 |
| E3 | 1 | 1 | 10 | 0.8 |
| EE2 | 0.02 | 0.02 | 0.2 | 0.2 |
| DES | 0.25 | 0.25 | 2.5 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Li, L.; Dong, Y.; Dong, W.; Cui, H.; Xia, C.; Xu, T.; Wang, C.; Zhang, J.; Liu, T.; et al. Rapid Detection of Five Estrogens Added Illegally to Dietary Supplements by Combining TLC with Raman Imaging Microscope. Molecules 2022, 27, 2650. https://doi.org/10.3390/molecules27092650
Liang X, Li L, Dong Y, Dong W, Cui H, Xia C, Xu T, Wang C, Zhang J, Liu T, et al. Rapid Detection of Five Estrogens Added Illegally to Dietary Supplements by Combining TLC with Raman Imaging Microscope. Molecules. 2022; 27(9):2650. https://doi.org/10.3390/molecules27092650
Chicago/Turabian StyleLiang, Xin, Li Li, Yan Dong, Wei Dong, Hongxia Cui, Chunhui Xia, Tao Xu, Chaozhong Wang, Jie Zhang, Tingting Liu, and et al. 2022. "Rapid Detection of Five Estrogens Added Illegally to Dietary Supplements by Combining TLC with Raman Imaging Microscope" Molecules 27, no. 9: 2650. https://doi.org/10.3390/molecules27092650
APA StyleLiang, X., Li, L., Dong, Y., Dong, W., Cui, H., Xia, C., Xu, T., Wang, C., Zhang, J., Liu, T., Sui, H., & Gao, C. (2022). Rapid Detection of Five Estrogens Added Illegally to Dietary Supplements by Combining TLC with Raman Imaging Microscope. Molecules, 27(9), 2650. https://doi.org/10.3390/molecules27092650






