Beneficial Effects of Capsaicin in Disorders of the Central Nervous System
Abstract
:1. Introduction
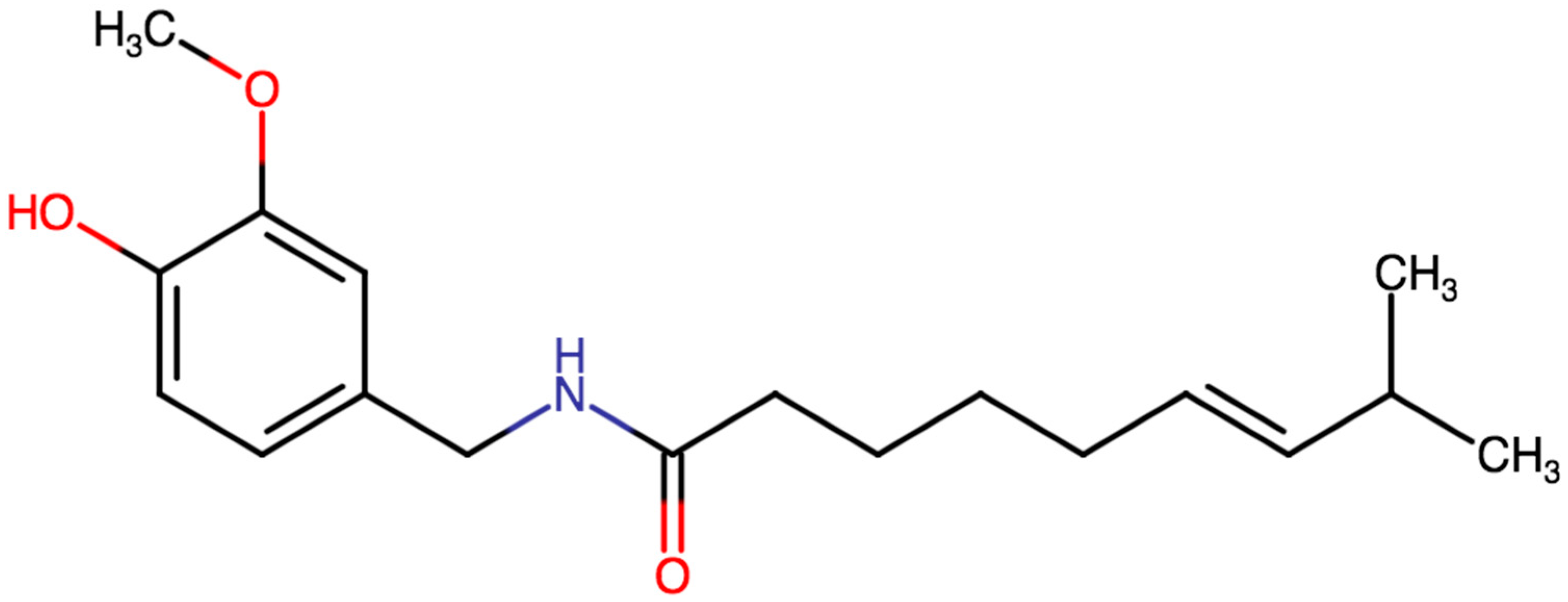
2. Physico-Chemical and Pharmacokinetic Properties of Capsaicin
3. Alzheimer’s Disease
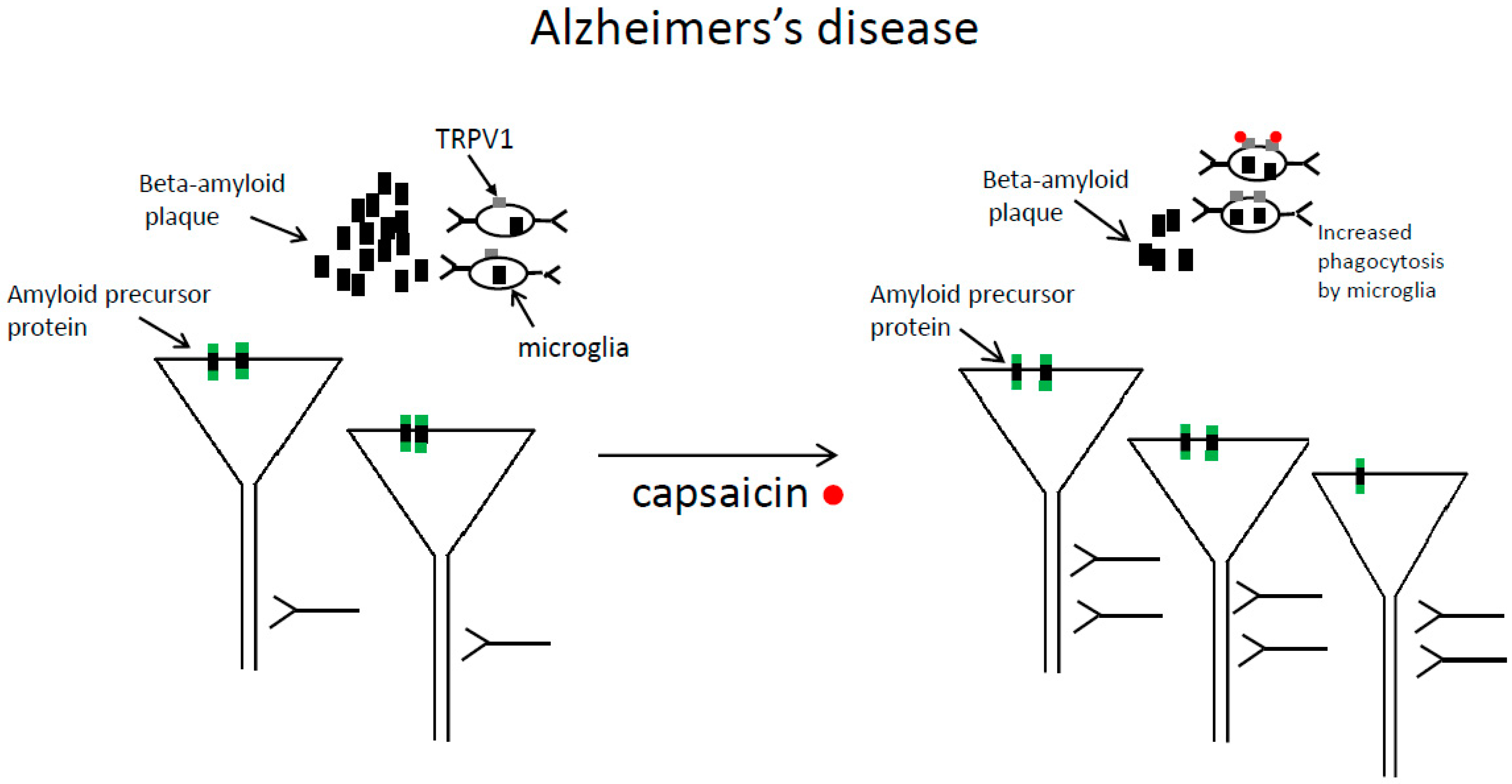
3.1. Beneficial Effects of Capsaicin in Animal Models of Alzheimer’s Disease
3.2. Capsaicin Reduces the Risk of Alzheimer’s Disease
4. Beneficial Effects of Capsaicin in Parkinson’s Disease
5. Effects of Capsaicin in Animal Models of Epilepsy
6. Stroke
6.1. Beneficial Effects of Capsaicin in Animal Models of Stroke
6.2. Capsaicin Is Helpful in Reducing Stroke Complications in Humans
7. Antidepressant Effects of Capsaicin
8. Beneficial Effects of Capsaicin in the Treatment of Headaches
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Estimation of Dietary Capsaicinoid Exposure in Korea and Assessment of Its Health Effects. Nutrients 2021, 13, 2461. [Google Scholar] [CrossRef]
- Pasierski, M.; Szulczyk, B. Capsaicin inhibits sodium currents and epileptiform activity in prefrontal cortex pyramidal neurons. Neurochem. Int. 2020, 135, 104709. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, S.; Onizuka, S.; Yonaha, T.; Tamura, R.; Hosokawa, N.; Kawasaki, Y.; Kashiwada, M.; Shirasaka, T.; Tsuneyoshi, I. Capsaicin indirectly suppresses voltage-gated Na+ currents through TRPV1 in rat dorsal root ganglion neurons. Anesthesia Analg. 2011, 112, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Tang, Y.; Yin, H.; Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 2020, 64, 64. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Laviada, I.; Rodríguez-Henche, N. The Potential Antitumor Effects of Capsaicin. Capsaicin A Ther. Mol. 2014, 68, 181–208. [Google Scholar]
- Mccarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health: Table 1. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, P.; Zhao, Z.; Cao, T.; He, H.; Luo, Z.; Zhong, J.; Gao, F.; Li, L.; Yan, Z.; et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke 2011, 42, 3245–3251. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Oortgiesen, M.; Li, L.; Simon, S.A. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J. Neurophysiol. 2001, 85, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Huang, W.; Lu, J.; Chen, H.; Yu, Z. TRPV1-Mediated Microglial Autophagy Attenuates Alzheimer’s Disease-Associated Pathology and Cognitive Decline. Front. Pharmacol. 2022, 12, 763866. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Jeong, J.Y.; Kim, K.I.; Won, S.-Y.; Chung, Y.C.; Nam, J.; Cho, E.J.; Ahn, T.-B.; Bok, E.; Shin, W.-H.; et al. Inhibition of Microglia-Derived Oxidative Stress by Ciliary Neurotrophic Factor Protects Dopamine Neurons In Vivo from MPP+ Neurotoxicity. Int. J. Mol. Sci. 2018, 19, 3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jittiwat, J.; Suksamrarn, A.; Tocharus, C.; Tocharus, J. Dihydrocapsaicin effectively mitigates cerebral ischemia-induced pathological changes in vivo, partly via antioxidant and anti-apoptotic pathways. Life Sci. 2021, 283, 119842. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Gu, L.; Guo, Y.; Feng, H.; Chen, S.; Jurat, J.; Fu, W.; Zhang, D. Gut Microbiota Mediates the Preventive Effects of Dietary Capsaicin Against Depression-Like Behavior Induced by Lipopolysaccharide in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 627608. [Google Scholar] [CrossRef]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [Green Version]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thail. 2009, 92, 108–113. [Google Scholar]
- Saria, A.; Skofitsch, G.; Lembeck, F. Distribution of capsaicin in rat tissues after systemic administration. J. Pharm. Pharmacol. 2011, 34, 273–275. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [Green Version]
- McGrattan, A.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Zhou, W.; Dou, F.; Wang, C.; Yu, Z. TRPV1 sustains microglial metabolic reprogramming in Alzheimer’s disease. EMBO Rep. 2021, 22, e52013. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019, 30, 493–507.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.-L.; Xiang, Y.; Tian, D.-Y.; Zhu, C.; Li, W.-W.; Liu, Y.-H.; Bu, X.-L.; Shen, L.-L.; Jin, W.-S.; et al. Capsaicin consumption reduces brain amyloid-beta generation and attenuates Alzheimer’s disease-type pathology and cognitive deficits in APP/PS1 mice. Transl. Psychiatry 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Z.; Du, Y.; Fu, M.; Han, H.; Wang, Y.; Dong, Z. Capsaicin Attenuates Amyloid-beta-Induced Synapse Loss and Cognitive Impairments in Mice. J. Alzheimer’s Dis. 2017, 59, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fu, M.; Huang, Z.; Tian, X.; Li, J.; Pang, Y.; Song, W.; Wang, Y.T.; Dong, Z. TRPV1 activation alleviates cognitive and synaptic plasticity impairments through inhibiting AMPAR endocytosis in APP23/PS45 mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13113. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.S.; Demiralp, T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef]
- Balleza-Tapia, H.; Crux, S.; Andrade-Talavera, Y.; Dolz-Gaiton, P.; Papadia, D.; Chen, G.; Johansson, J.; Fisahn, A. TrpV1 receptor activation rescues neuronal function and network gamma oscillations from Abeta-induced impairment in mouse hippocampus in vitro. Elife 2018, 7, e37703. [Google Scholar] [CrossRef]
- Grimm, M.; Blümel, T.; Lauer, A.A.; Janitschke, D.; Stahlmann, C.; Mett, J.; Haupenthal, V.J.; Miederer, A.-M.; Niemeyer, B.A.; Grimm, H.S.; et al. The impact of capsaicinoids on APP processing in Alzheimer’s disease in SH-SY5Y cells. Sci. Rep. 2020, 10, 9164. [Google Scholar] [CrossRef]
- Liu, C.-H.; Bu, X.-L.; Wang, J.; Zhang, T.; Xiang, Y.; Shen, L.-L.; Wang, Q.-H.; Deng, B.; Wang, X.; Zhu, C.; et al. The Associations between a Capsaicin-Rich Diet and Blood Amyloid-beta Levels and Cognitive Function. J. Alzheimer’s Dis. 2016, 52, 1081–1088. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J.; Ma, D.; Yuan, G.; Lu, Y.; Yang, Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS ONE 2017, 12, e0172477. [Google Scholar] [CrossRef]
- Chung, Y.C.; Baek, J.Y.; Kim, S.R.; Ko, H.W.; Bok, E.; Shin, W.-H.; Won, S.-Y.; Jin, B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2017, 49, e298. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, J.; Wang, L.; Yao, X.; Liu, Y.; Li, Y.; Chen, S.; Yue, T.; Wang, X.; Yu, W.; et al. Capsaicin Protects Against Oxidative Insults and Alleviates Behavioral Deficits in Rats with 6-OHDA-Induced Parkinson’s Disease via Activation of TRPV1. Neurochem. Res. 2017, 42, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Zhao, Z.; Wang, J.; Guo, D.; Liu, Y. Regulation of Actg1 and Gsta2 is possible mechanism by which capsaicin alleviates apoptosis in cell model of 6-OHDA-induced Parkinson’s disease. Biosci. Rep. 2020, 40, BSR20191796. [Google Scholar] [CrossRef]
- Bok, E.; Chung, Y.C.; Kim, S.-K.; Baik, H.H.; Shin, W.-H.; Jin, B.K. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.I.; Baek, J.Y.; Jeong, J.Y.; Nam, J.; Park, E.S.; Bok, E.; Shin, W.-H.; Chung, Y.C.; Jin, B.K. Delayed Treatment of Capsaicin Produces Partial Motor Recovery by Enhancing Dopamine Function in MPP+-lesioned Rats via Ciliary Neurotrophic Factor. Exp. Neurobiol. 2019, 28, 289–299. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, S.R.; Jin, B.K. Transient receptor potential vanilloid subtype 1 contributes to mesencephalic dopaminergic neuronal survival by inhibiting microglia-originated oxidative stress. Brain Res. Bull. 2012, 89, 92–96. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Naz, F.; Jyoti, S. Effect of capsaicin on the oxidative stress and dopamine content in the transgenic Drosophila model of Parkinson’s disease. Acta Biol. Hung. 2018, 69, 115–124. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Mantegazza, M.; Curia, G.; Biagini, G.; Ragsdale, D.S.; Avoli, M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABAA Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Panzini, M.-A.; Carpentier, A.V.; Comtois, J.; Rioux, B.; Gore, G.; Bauer, P.R.; Kwon, C.-S.; Jetté, N.; Josephson, C.B.; et al. Incidence and Prevalence of Drug-Resistant Epilepsy: A Systematic Review and Meta-analysis. Neurology 2021, 96, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, M.; Elhamdani, A.; Camacho, S.; Meystre, J.; González, S.M.; Le Coutre, J.; Markram, H. Dampened neural activity and abolition of epileptic-like activity in cortical slices by active ingredients of spices. Sci. Rep. 2014, 4, 6825. [Google Scholar] [CrossRef] [Green Version]
- Carletti, F.; Gambino, G.; Rizzo, V.; Ferraro, G.; Sardo, P. Involvement of TRPV1 channels in the activity of the cannabinoid WIN 55,212-2 in an acute rat model of temporal lobe epilepsy. Epilepsy Res. 2016, 122, 56–65. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Nazıroglu, M. TRPV1 Channel: A Potential Drug Target for Treating Epilepsy. Curr. Neuropharmacol. 2015, 13, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Naziroğlu, M.; Kutluhan, S.; Yilmaz, N.; Yürekli, V.A.; Vural, H. Topiramate modulates hippocampus NMDA receptors via brain Ca2+ homeostasis in pentylentetrazol-induced epilepsy of rats. J. Recept. Signal Transduct. 2011, 31, 173–179. [Google Scholar] [CrossRef]
- Kline, D.D.; Wang, S.; Kunze, D.L. TRPV1 channels contribute to spontaneous glutamate release in nucleus tractus solitarii following chronic intermittent hypoxia. J. Neurophysiol. 2019, 121, 881–892. [Google Scholar] [CrossRef]
- Shoudai, K.; Peters, J.H.; McDougall, S.; Fawley, J.; Andresen, M.C. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J. Neurosci. 2010, 30, 14470–14475. [Google Scholar] [CrossRef] [Green Version]
- Chávez, A.E.; Hernández, V.M.; Rodenas-Ruano, A.; Chan, C.S.; Castillo, P.E. Compartment-specific modulation of GABAergic synaptic transmission by TRPV1 channels in the dentate gyrus. J. Neurosci. 2014, 34, 16621–16629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.S.; Umathe, S.N. Involvement of transient receptor potential vanilloid type 1 channels in the pro-convulsant effect of anandamide in pentylenetetrazole-induced seizures. Epilepsy Res. 2012, 100, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, M.D.; Smith, B.N. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp. Neurol. 2010, 223, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saffarzadeh, F.; Eslamizade, M.; Mousavi, S.; Abraki, S.; Hadjighassem, M.; Gorji, A. TRPV1 receptors augment basal synaptic transmission in CA1 and CA3 pyramidal neurons in epilepsy. Neurosci. 2016, 314, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Reyes, L.E.; Ladas, T.P.; Chiang, C.-C.; Durand, D. TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 2013, 250, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Kamiński, K.; Mogilski, S.; Abram, M.; Rapacz, A.; Latacz, G.; Szulczyk, B.; Walczak, M.; Kuś, K.; Matyjaszczyk, K.; Kamiński, R.M. KA-104, a new multitargeted anticonvulsant with potent antinociceptive activity in preclinical models. Epilepsia 2020, 61, 2119–2128. [Google Scholar] [CrossRef]
- Jones, P.J.; Merrick, E.C.; Batts, T.W.; Hargus, N.J.; Wang, Y.; Stables, J.P.; Bertram, E.H.; Brown, M.L.; Patel, M.K. Modulation of sodium channel inactivation gating by a novel lactam: Implications for seizure suppression in chronic limbic epilepsy. J. Pharmacol. Exp. Ther. 2008, 328, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Szulczyk, B.; Pasierski, M.; Nurowska, E. Valproic acid potently inhibits interictal-like epileptiform activity in prefrontal cortex pyramidal neurons. Neurosci. Lett. 2019, 708, 134350. [Google Scholar] [CrossRef]
- Lee, T.-H.; Lee, J.-G.; Yon, J.-M.; Oh, K.-W.; Baek, I.-J.; Nahm, S.-S.; Lee, B.J.; Yun, Y.W.; Nam, S.-Y. Capsaicin prevents kainic acid-induced epileptogenesis in mice. Neurochem. Int. 2011, 58, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Avoli, M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2013, 37, 2887–2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cheng, G.; Tan, H.; Qin, R.; Zou, Y.; Wang, Y.; Zhang, Y. Capsaicin protects cortical neurons against ischemia/reperfusion injury via down-regulating NMDA receptors. Exp. Neurol. 2017, 295, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, N.H.; Jadhav, V.; Charles, S.; Chiu, J.; Buchholz, J.; Tang, J.; Zhang, J.H. Capsaicin pre-treatment provides neurovascular protection against neonatal hypoxic-ischemic brain injury in rats. Acta Neurochir. Suppl. 2011, 111, 225–230. [Google Scholar] [PubMed] [Green Version]
- Pegorini, S.; Braida, D.; Verzoni, C.; Guerini-Rocco, C.; Consalez, G.G.; Croci, L.; Sala, M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. J. Cereb. Blood Flow Metab. 2005, 144, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wu, L.; Fang, Q.; Shen, M.; Zhang, L.; Liu, X. Effects of capsaicin on swallowing function in stroke patients with dysphagia: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Yin, Q.; Wu, C.; Shen, M.; Zhang, Y.; Ma, C.; Zhang, H.; Shen, F. Capsaicin combined with ice stimulation improves swallowing function in patients with dysphagia after stroke: A randomised controlled trial. J. Oral Rehabil. 2020, 47, 1297–1303. [Google Scholar] [CrossRef]
- Ortega, O.; Rofes, L.; Martin, A.; Arreola, V.; López, I.; Clavé, P. A Comparative Study Between Two Sensory Stimulation Strategies After Two Weeks Treatment on Older Patients with Oropharyngeal Dysphagia. Dysphagia 2016, 31, 706–716. [Google Scholar] [CrossRef]
- Cabib, C.; Nascimento, W.; Rofes, L.; Arreola, V.; Tomsen, N.; Mundet, L.; Palomeras, E.; Michou, E.; Clavé, P.; Ortega, O. Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2020, 32, e13887. [Google Scholar] [CrossRef]
- Kulnik, S. Could reflex cough induced through nebulized capsaicin achieve airway clearance in patients with acute retention of lung secretions? Med. Hypotheses 2018, 119, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, Y.; Yang, L.; Shen, F.; Ma, C.; Shen, M. Effect of Capsaicin Atomization–Induced Cough on Sputum Excretion in Tracheotomized Patients After Hemorrhagic Stroke: A Randomized Controlled Trial. J. Speech Lang. Hear. Res. 2021, 64, 4085–4095. [Google Scholar] [CrossRef]
- Marquez-Romero, J.M.; Huerta-Franco, M.R.; Vargas-Luna, M.; Madrigal-Gutiérrez, C.A.; Esparza-Hernández, J.M.; Velázquez-Barcena, M.G. Dose Escalation and Safety of Capsaicin for Cerebral Perfusion Augmentation: A Pilot Study. Stroke 2021, 52, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Ekong, M.B.; Iniodu, C.F. Nutritional therapy can reduce the burden of depression management in low income countries: A review. IBRO Neurosci. Rep. 2021, 11, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Hayase, T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Mendez, M.E.; Castro-Sánchez, L.A.; Dagnino-Acosta, A.; Aguilar-Martínez, I.; Pérez-Burgos, A.; Vázquez-Jiménez, C.; Moreno-Galindo, E.G.; Álvarez-Cervera, F.J.; Góngora-Alfaro, J.L.; Navarro-Polanco, R.A.; et al. Capsaicin produces antidepressant-like effects in the forced swimming test and enhances the response of a sub-effective dose of amitriptyline in rats. Physiol. Behav. 2018, 195, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Martinez, I.S.; Reyes-Mendez, M.E.; Herrera-Zamora, J.M.; Osuna-Lopez, F.; Virgen-Ortiz, A.; Mendoza-Munoz, N.; Gongora-Alfaro, J.L.; Moreno-Galindo, E.G.; Alamilla, J. Synergistic antidepressant-like effect of capsaicin and citalopram reduces the side effects of citalopram on anxiety and working memory in rats. Psychopharmacology 2020, 237, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Alijanpour, S.; Tirgar, F.; Haj-Mirzaian, A.; Amini-Khoei, H.; Rahimi-Balaei, M.; Rastegar, M.; Ghaderi, M.; Ghazi-Khansari, M.; Zarrindast, M.-R. NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience 2016, 329, 122–133. [Google Scholar] [CrossRef]
- Li, H.-B.; Mao, R.-R.; Zhang, J.-C.; Yang, Y.; Cao, J.; Xu, L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol. Psychiatry 2008, 64, 286–292. [Google Scholar] [CrossRef]
- Iglesias, L.P.; Aguiar, D.C.; Moreira, F.A. TRPV1 blockers as potential new treatments for psychiatric disorders. Behav. Pharmacol. 2020, 33, 2–14. [Google Scholar] [CrossRef]
- Charles, A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef]
- Fusco, B.M.; Marabini, S.; Maggi, C.A.; Fiore, G.; Geppetti, P. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain 1994, 59, 321–325. [Google Scholar] [CrossRef]
- Fusco, B.M.; Fiore, G.; Gallo, F.; Martelletti, P.; Giacovazzo, M. “Capsaicin-sensitive” sensory neurons in cluster headache: Pathophysiological aspects and therapeutic indication. Headache 1994, 34, 132–137. [Google Scholar] [CrossRef]
- Fusco, B.; Barzoi, G.; Agrò, F. Repeated intranasal capsaicin applications to treat chronic migraine. Br. J. Anaesth. 2003, 90, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicuteri, F.; Fusco, B.M.; Marabini, S.; Campagnolo, V.; Maggi, C.A.; Geppetti, P.; Fanciullacci, M. Beneficial Effect of Capsaicin Application to the Nasal Mucosa in Cluster Headache. Clin. J. Pain 1989, 5, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.R.; Rapoport, A.; Padla, D.; Weeks, R.; Rosum, R.; Sheftell, F.; Arrowsmith, F. A Double-Blind Placebo-Controlled Trial of Intranasal Capsaicin for Cluster Headache. Cephalalgia 1993, 13, 114–116. [Google Scholar] [CrossRef]
- Cianchetti, C. Capsaicin jelly against migraine pain. Int. J. Clin. Pr. 2010, 64, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasierski, M.; Szulczyk, B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules 2022, 27, 2484. https://doi.org/10.3390/molecules27082484
Pasierski M, Szulczyk B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules. 2022; 27(8):2484. https://doi.org/10.3390/molecules27082484
Chicago/Turabian StylePasierski, Michał, and Bartłomiej Szulczyk. 2022. "Beneficial Effects of Capsaicin in Disorders of the Central Nervous System" Molecules 27, no. 8: 2484. https://doi.org/10.3390/molecules27082484
APA StylePasierski, M., & Szulczyk, B. (2022). Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules, 27(8), 2484. https://doi.org/10.3390/molecules27082484




