Degradation and Pathways of Carvone in Soil and Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Preparation of Stock Standard Solutions
2.3. Collection and Characterization of Soil Samples
2.4. Degradation of Carvone in Different Soil Types
- (1)
- Aerobic treatment: 50 ± 0.1 g of soil sample (S1–S4) was weighed in a 250 mL conical flask; 250 μL of 1000 mg L−1 carvone ethanol solution was added into it and mixed evenly for 5 min to form an initial carvone concentration level of 5 mg kg−1. The soil sample was then covered with a sterile cotton plug, which was opened weekly to maintain the aerobic state of the soil.
- (2)
- Anaerobic treatment: Carvone was added according to the above description, and enough sterilized deionized water was added to the soil sample (S1–S4) to form a water layer of at least 2 cm on the soil surface. The soil sample was then covered with a sterile cotton plug and vacuum extracted in the conical flask to form anaerobic conditions.
- (3)
- Sterilized treatment: 50 ± 0.1 g of soil sample (S1–S4) was weighed in a 250 mL conical flask, plugged with a cotton stopper, and placed in a high-pressure sterilization pot at 121 °C and 200 kPa for 30 min. After the soil samples were cooled to room temperature, soil respiration was measured by alkali absorption method (AA-method) [29] to ensure complete sterilization. This procedure was repeated until no soil respiration was detected. After this, carvone was added according to the above description.
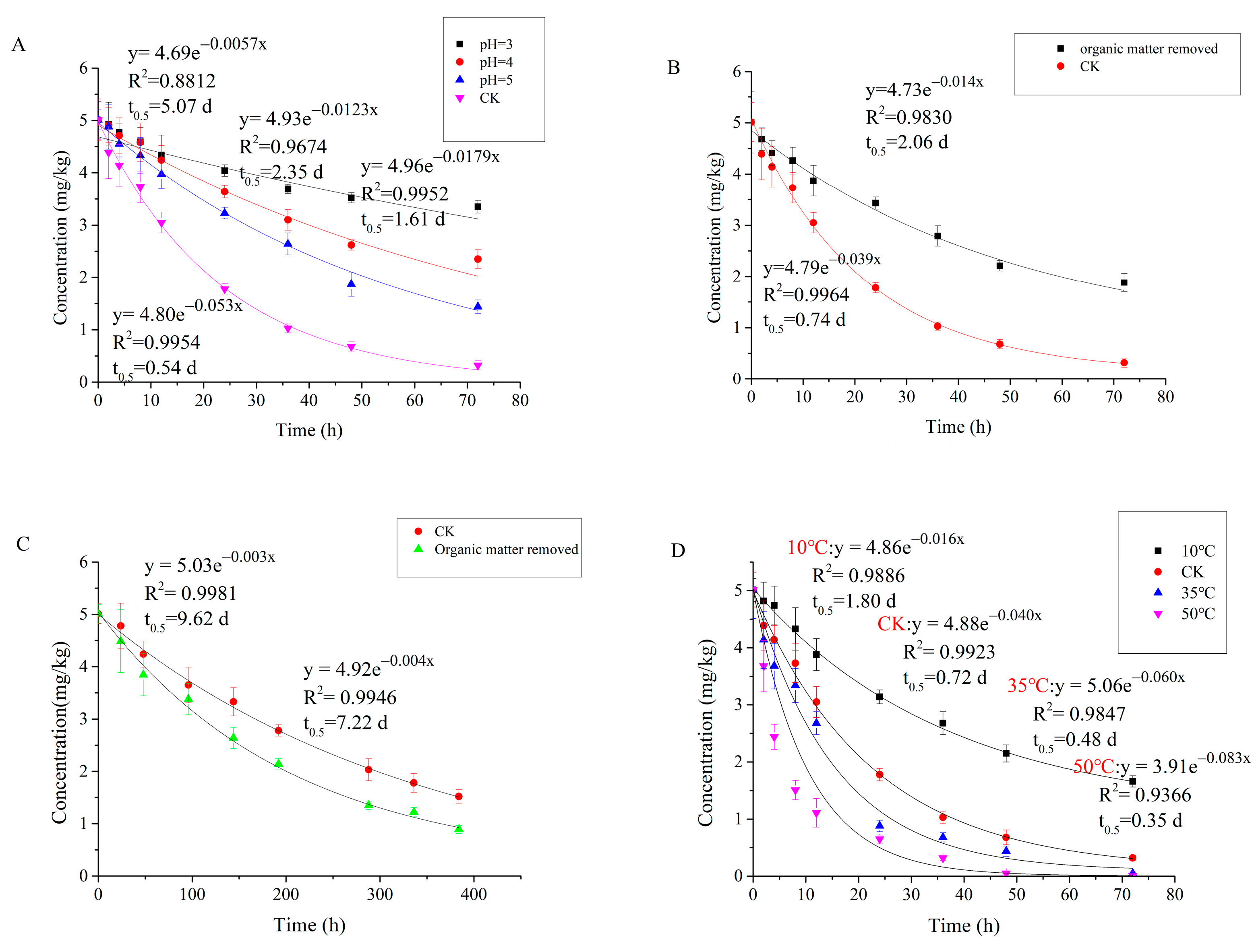
Degradation of Carvone in Different Soil Conditions
- (1)
- pH: We used 0.1 M hydrochloric acid (HCl) aqueous solution to adjust the pH of the soil to pH 3, 4, and 5. At the same time, a control group without HCl and only deionized water was created.
- (2)
- Organic matter: In order to remove the organic matter, 100 g S2 soil was added to a 2 L beaker, mixed with deionized water, and stirred evenly. Then, 30% hydrogen peroxide was slowly added and vigorously stirred to prevent the bubbles from overflowing. After the foam subsided, the beaker was heated in a water bath at 70~80 °C, in order to speed and complete the reaction. This process was repeated until the soil no longer produced foam upon the addition of hydrogen peroxide. Then, 400 mL deionized water was added to the beaker and the mixture was boiled to remove all hydrogen peroxide. This process was repeated until no hydrogen peroxide remained. Finally, soil was dried in a 105 ℃ oven and screened to obtain organic-matter-free S2 soil; 50 g of treated soil was sterilized in an autoclave at 121 °C and 200 kPa for 30 min to ensure both sterilization and complete removal of organic material.
- (3)
- Temperature: S2 soil was cultured in incubators at 10 °C, 25 °C, 35 °C, and 50 °C, of which 25 °C was the control condition.
- (4)
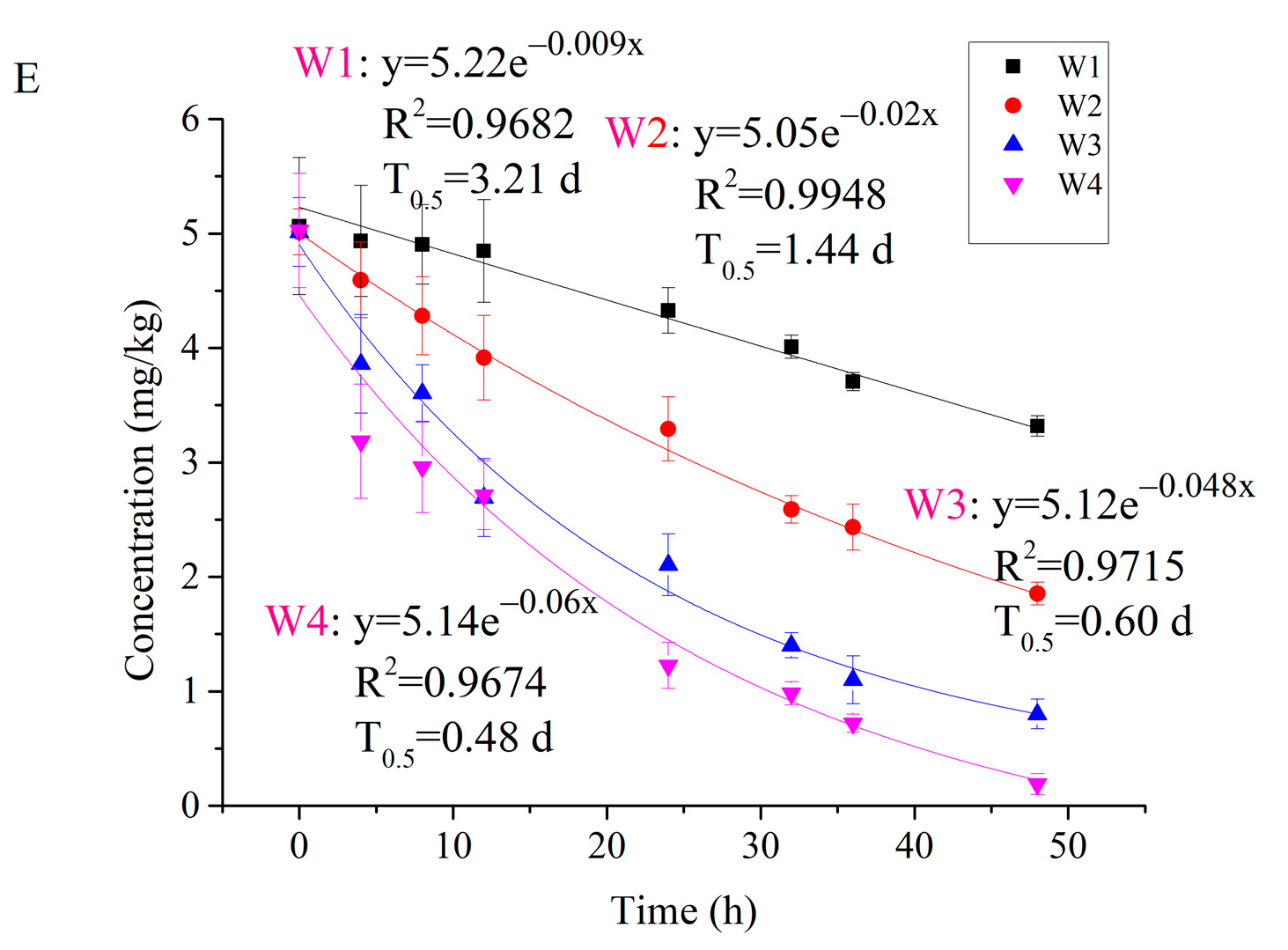
- Moisture: The water content of S2 was set to 0.1, 10, 20, and 30% (w/w) using deionized water, of which 25% level is the maximum water holding capacity of the soil.
2.5. Photolysis of Carvone
2.6. Sample Extraction and Purification
2.7. Instrumental Analysis
2.8. Method Validation and Data Processing
3. Results and Discussion
3.1. Sample Extraction and Purification
3.2. Validation of the Analytical Method
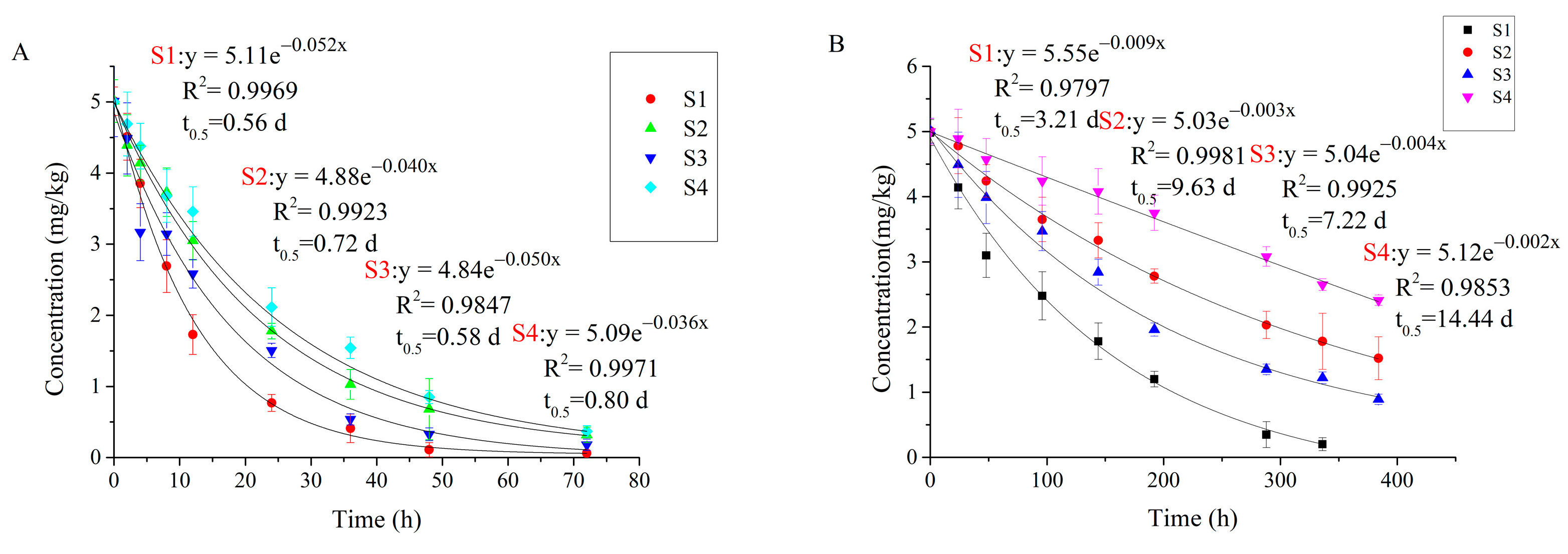
3.3. Degradation of Carvone in Different Soil Types
3.4. Degradation of Carvone in Different Soil Conditions
3.5. Photolysis of Carvone
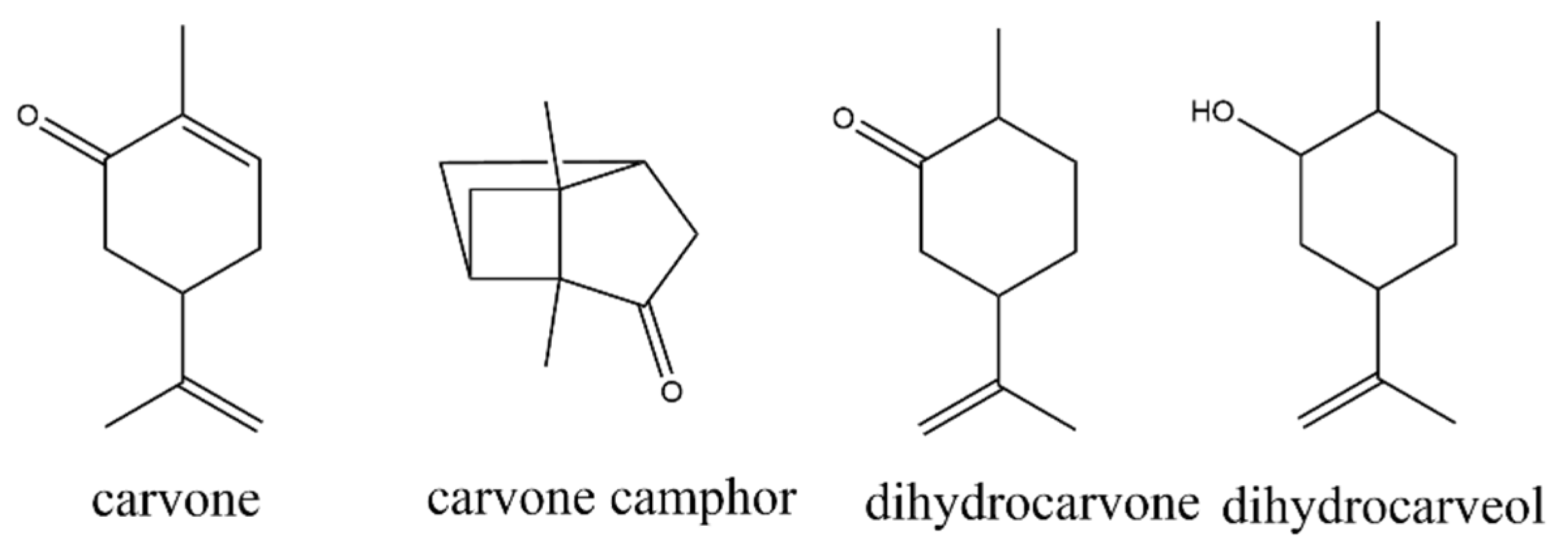
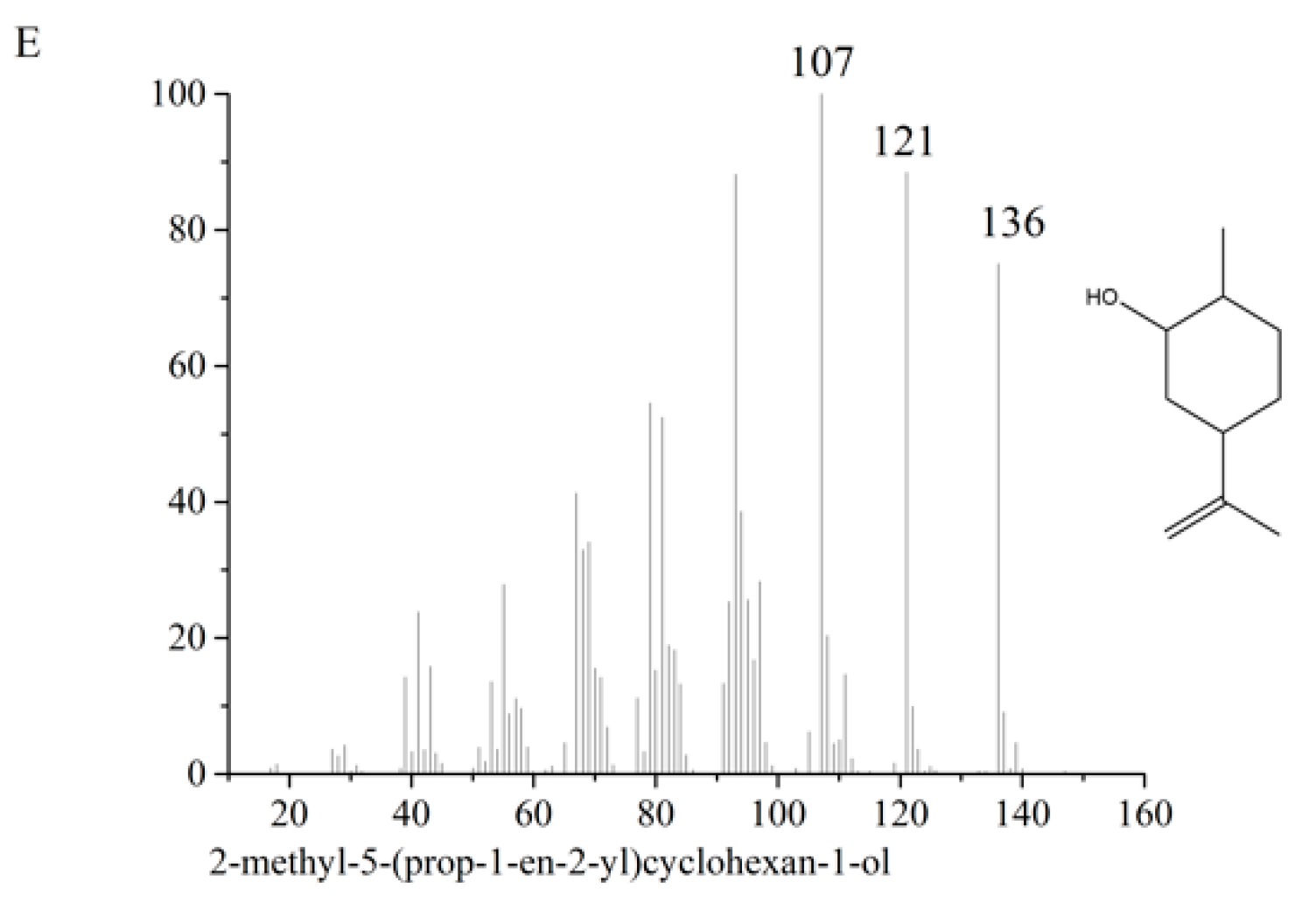
3.6. Identification and Quantification of Carvone Degradation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, K.; Khanuja, S.; Ahmad, A.; Santha Kumar, T.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils ofMentha spicata andAnethum sowa. Flavour. Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49. [Google Scholar]
- Barba, C.; Toledano, R.; Santa-María, G.; Herraiz, M.; Martínez, R.M. Enantiomeric analysis of limonene and carvone by direct introduction of aromatic plants into multidimensional gas chromatography. Talanta 2013, 106, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bekku, Y.; Koizumi, H.; Oikawa, T.; Iwaki, H. Examination of four methods for measuring soil respiration. Appl. Soil Ecol. 1997, 5, 247–254. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Chen, K.; Tian, F.; Wu, C.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Degradation products and pathway of ethiprole in water and soil. Water Res. 2019, 161, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Commission, E.E. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; Guidance SANTE/12682/2019; Accredia: Rome, Italy, 2019; p. 12682. [Google Scholar]
- Committee, E.S. Scientific Opinion on the safety assessment of carvone, considering all sources of exposure. EFSA J. 2014, 12, 3806. [Google Scholar] [CrossRef]
- de Carvalho, C.C. Adaptation of Rhodococcus erythropolis cells for growth and bioremediation under extreme conditions. Res. Microbiol. 2012, 163, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Decarvalho, C.C.; Dafonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Elmastaş, M.; Dermirtas, I.; Isildak, O.; Aboul-Enein, H.Y. Antioxidant activity of S-carvone isolated from spearmint (Mentha Spicata, L. Fam Lamiaceae). J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1465–1475. [Google Scholar] [CrossRef]
- FAO, I. Revised Legend of the FAO-UNESCO Soil Map of the World; International Soil Refererence and Information Centre: Wageningen The Netherlands, 1988; Volume 1. [Google Scholar]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating Pesticide Degradation in the Environment: Blind Spots and Emerging Opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gámiz, B.; Facenda, G.; Celis, R. Nanoengineered Sorbents to Increase the Persistence of the Allelochemical Carvone in the Rhizosphere. J. Agric. Food Chem. 2018, 67, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Gámiz, B.; Hermosín, M.C.; Celis, R. Appraising factors governing sorption and dissipation of the monoterpene carvone in agricultural soils. Geoderma 2018, 321, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Yan, D.; Wang, Q.; Fang, W.; Wang, X.; Li, J.; Wang, D.; Li, Y.; Ouyang, C.; Cao, A. Effects of soil type, temperature, moisture, application dose, fertilizer, and organic amendments on chemical properties and biodegradation of dimethyl disulfide in soil. Land Degrad. Dev. 2018, 29, 4282–4290. [Google Scholar] [CrossRef]
- Harder, J.; Probian, C. Microbial degradation of monoterpenes in the absence of molecular oxygen. Appl. Environ. Microbiol. 1995, 61, 3804–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmans, K.J.; Diepenhorst, P.; Bakker, W.; Gorris, L.G. The use of carvone in agriculture: Sprout suppression of potatoes and antifungal activity against potato tuber and other plant diseases. Ind. Crop. Prod. 1995, 4, 3–13. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Lazari, D.M. Decomposition process in the Mediterranean region. Chemical compounds and essential oil degradation from Myrtus communis. Int. Biodeterior. Biodegrad. 2010, 64, 356–362. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial Degradation of Pesticide Residues and an Emphasis on the Degradation of Cypermethrin and 3-phenoxy Benzoic Acid: A Review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isman, M.B. Pesticides Based on Plant Essential Oils: Phytochemical and Practical Considerations. In Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization; ACS Publications: Washington, DC, USA, 2016; pp. 13–26. [Google Scholar] [CrossRef]
- Jäger, W.; Mayer, M.; Platzer, P.; Reznicek, G.; Dietrich, H.; Buchbauer, G. Stereoselective Metabolism of the Monoterpene Carvone by Rat and Human Liver Microsomes. J. Pharm. Pharmacol. 2000, 52, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Kuk, M.; Hron Sr, R. Cottonseed extraction with a new solvent system: Isohexane and alcohol mixtures. J. Am. Oil Chem. Soc. 1998, 75, 927–930. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Jia, H.; Zhou, W.; Li, B.; Huang, H. Residue analysis of tetraniliprole in rice and related environmental samples by HPLC/MS. Microchem. J. 2019, 150, 104168. [Google Scholar] [CrossRef]
- Li, Y.; Su, P.; Li, Y.; Wen, K.; Bi, G.; Cox, M. Adsorption-desorption and degradation of insecticides clothianidin and thiamethoxam in agricultural soils. Chemosphere 2018, 207, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rao, L.; Li, W.; Zhou, W.; Li, B.; Tang, L. Detection of Glyamifop residues in rice and its environment by the QuEChERS method combined with HPLC–MS. Microchem. J. 2020, 158, 105157. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Margaris, N.; Vokou, D. Microbial decomposition of smog organic pollutants. Bull. Environ. Contam. Toxicol. 1986, 36, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.A.; Nachtergaele, F. World Reference Base for Soil Resources: Introduction; Acco: Lake Zurich, IL, USA, 1998. [Google Scholar]
- Noma, Y.; Asakawa, Y. Biotransformation of Monoterpenoids by Microorganisms, Insects, and Mammals. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 613–767. [Google Scholar] [CrossRef]
- Obalum, S.; Chibuike, G.; Peth, S.; Ouyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef] [PubMed]
- Ranby, B.G.; Rabek, J.F. Photodegradation, Photo-Oxidation, and Photostabilization of Polymers; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Refsgaard, H.H.; Nielsen, B.R.; Skibsted, L.H. Sensory Importance and Mechanism of Photochemical Conversion of Carvone to Carvonecamphor in Ethanol-Water Mixtures. J. Agric. Food Chem. 1995, 43, 1177–1183. [Google Scholar] [CrossRef]
- Rhodes, A.H.; Owen, S.M.; Semple, K.T. Biodegradation of 2,4-dichlorophenol in the presence of volatile organic compounds in soils under different vegetation types. FEMS Microbiol. Lett. 2007, 269, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandín-España, P.; Sevilla-Morán, B.; Calvo, L.; Mateo-Miranda, M.; Alonso-Prados, J.L. Photochemical behavior of alloxydim herbicide in environmental waters. Structural elucidation and toxicity of degradation products. Microchem. J. 2013, 106, 212–219. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Sikora, F.; Moore, K. Soil test methods from the Southeastern United States. South. Coop. Ser. Bull. 2014, 419, 54–58. [Google Scholar]
- Tsipi, D.; Gegiou, D.; Hadjoudis, E. The intramolecular photocycloaddition carvone → carvonecamphor. J. Photochem. 1987, 37, 159–166. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Uclés, S.; Lozano, A.; Sosa, A.; Vázquez, P.P.; Valverde, A.; Fernández-Alba, A. Matrix interference evaluation employing GC and LC coupled to triple quadrupole tandem mass spectrometry. Talanta 2017, 174, 72–81. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Boot, A.M. Metabolism of carveol and dihydrocarveol in Rhodococcus erythropolis DCL14. Microbiology 2000, 146, 1129–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, E. Impacto Ambiental em Área com Aplicação de Agrotóxicos no Município de Brotas, SP. Ph.D. Thesis, Universidade Estadual Paulista, Faculdade de Ciências Agronômicas de Botucatu, São Paulo, Brasil, 2012. [Google Scholar]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Yamada, H. Behaviour, occurrence, and aquatic toxicity of new antifouling biocides and preliminary assessment of risk to aquatic ecosystems. Bull. Fish. Res. Agency 2007, 21, 31–45. [Google Scholar]
- Yankova, R.; Dimov, M.; Dobreva, K.; Stoyanova, A. Electronic structure, reactivity, and Hirshfeld surface analysis of carvone. J. Chem. Res. 2019, 43, 319–329. [Google Scholar] [CrossRef]
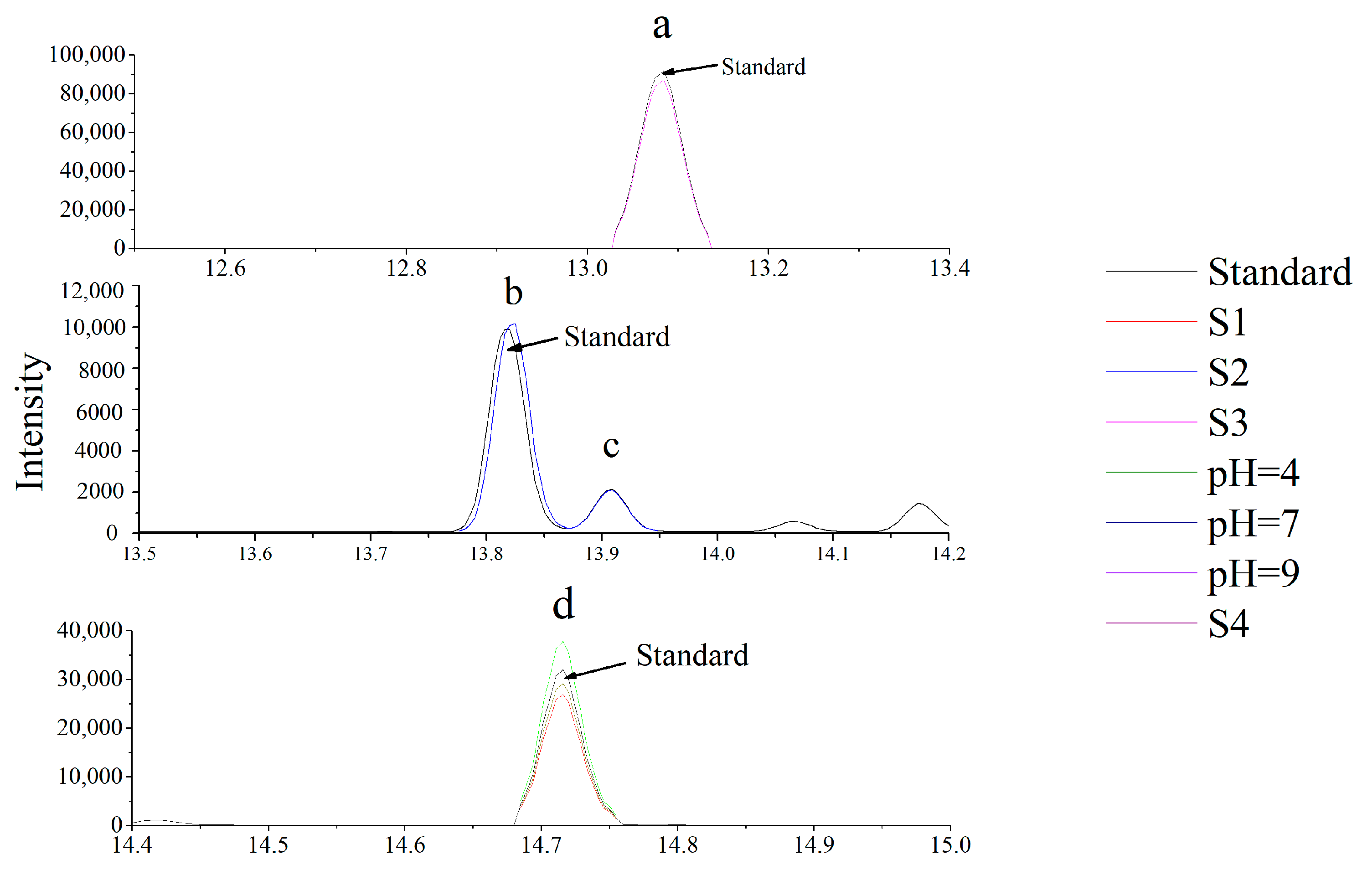




| Soils | Site | Texture | PH | CEC a (cmol kg−1) | OC b (%) | OM c (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Texture Class | ||||||
| S1 | Yichang, Hubei (N33°06′, E111°25′) | 88.17 | 7.91 | 3.92 | Sand | 7.32 | 12.1 | 0.5G | 0.86 |
| S2 | Chengdu, Sichuan (N30°56′, E105°51′) | 51.88 | 23.35 | 24.77 | sandy clay loam | 7.35 | 25.4 | 1.0 | 1.72 |
| S3 | Tiangang, Jiling (N43°91′, E126°88′) | 17.17 | 61.66 | 21.17 | Silty loam | 6.86 | 15.7 | 1.5 | 2.58 |
| S4 | Ningbo, Zhejiang (N29°14′, E121°48′) | 42.15 | 42.83 | 15.02 | loam | 7.65 | 12.9 | 0.98 | 1.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhou, W.; Bian, C.; Wang, L.; Li, Y.; Li, B. Degradation and Pathways of Carvone in Soil and Water. Molecules 2022, 27, 2415. https://doi.org/10.3390/molecules27082415
Huang C, Zhou W, Bian C, Wang L, Li Y, Li B. Degradation and Pathways of Carvone in Soil and Water. Molecules. 2022; 27(8):2415. https://doi.org/10.3390/molecules27082415
Chicago/Turabian StyleHuang, Chenyu, Wenwen Zhou, Chuanfei Bian, Long Wang, Yuqi Li, and Baotong Li. 2022. "Degradation and Pathways of Carvone in Soil and Water" Molecules 27, no. 8: 2415. https://doi.org/10.3390/molecules27082415
APA StyleHuang, C., Zhou, W., Bian, C., Wang, L., Li, Y., & Li, B. (2022). Degradation and Pathways of Carvone in Soil and Water. Molecules, 27(8), 2415. https://doi.org/10.3390/molecules27082415






