Granulation of Nickel–Aluminum–Zirconium Complex Hydroxide Using Colloidal Silica for Adsorption of Chromium(VI) Ions from the Liquid Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Chromium(VI) Ion Adsorption According to Particle Diameter
2.3. Chromium(VI) Ion Adsorption under Different Conditions
2.4. Chromium(VI) Ion Adsorption in a Complex Solution System
2.5. Recovery of Chromium(VI) Ions by Desorption
3. Results and Discussion
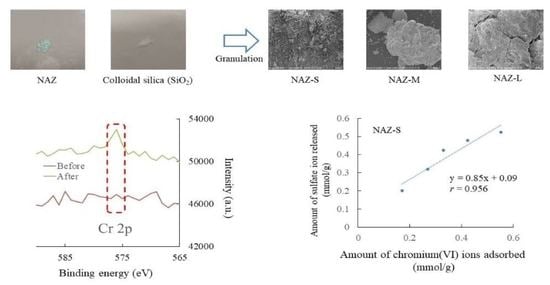
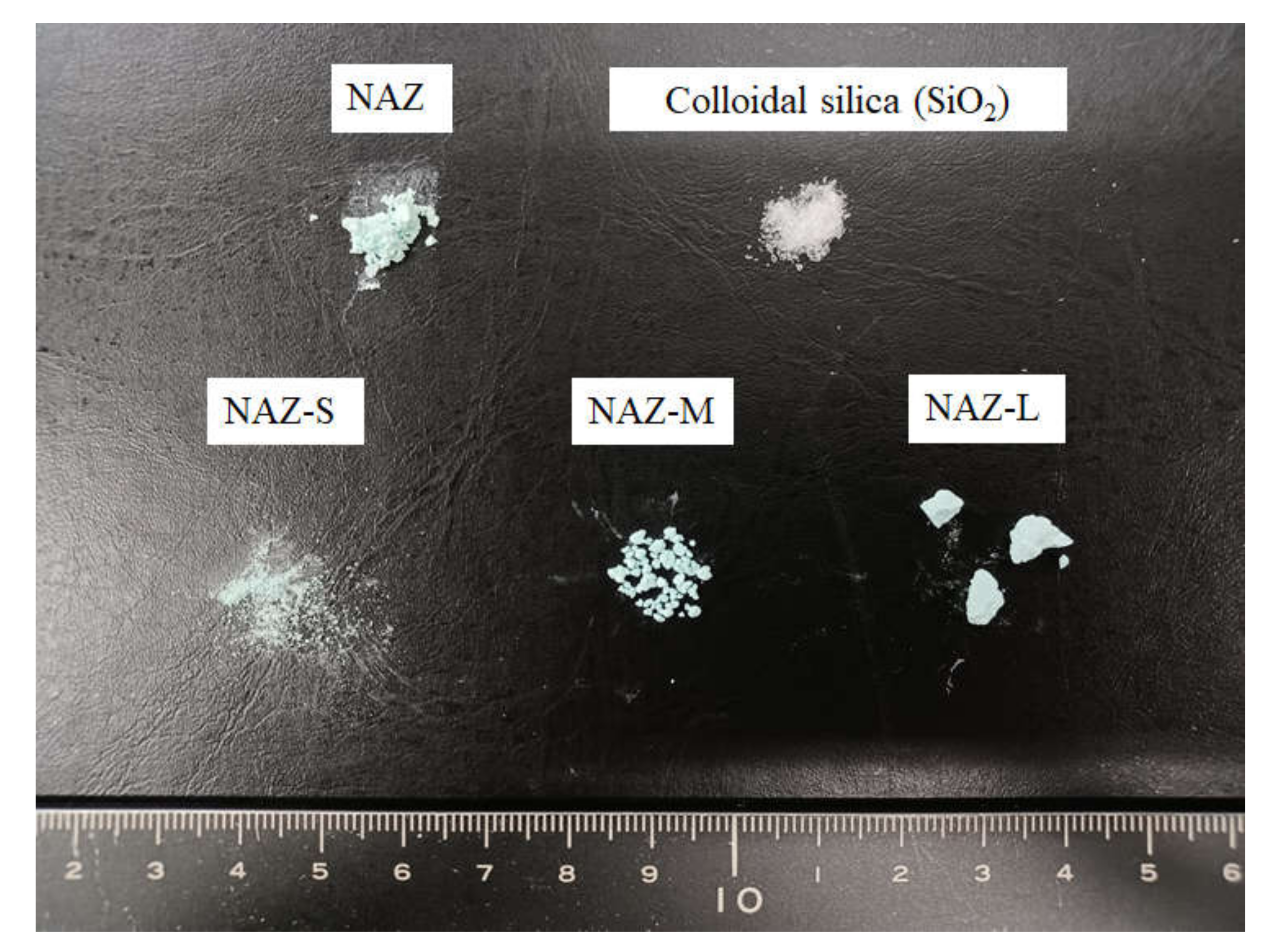
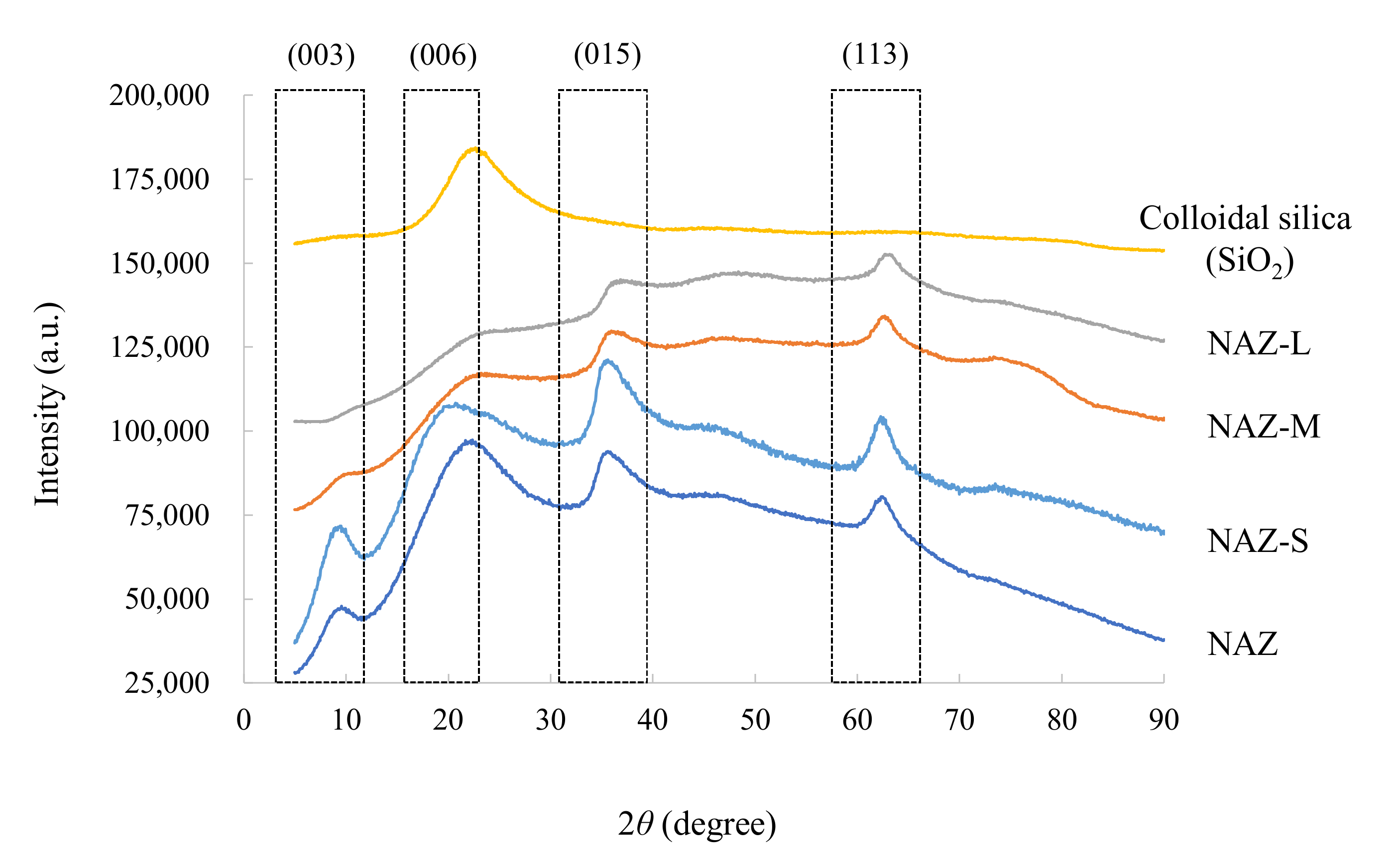
3.1. Characteristics of Prepared Samples
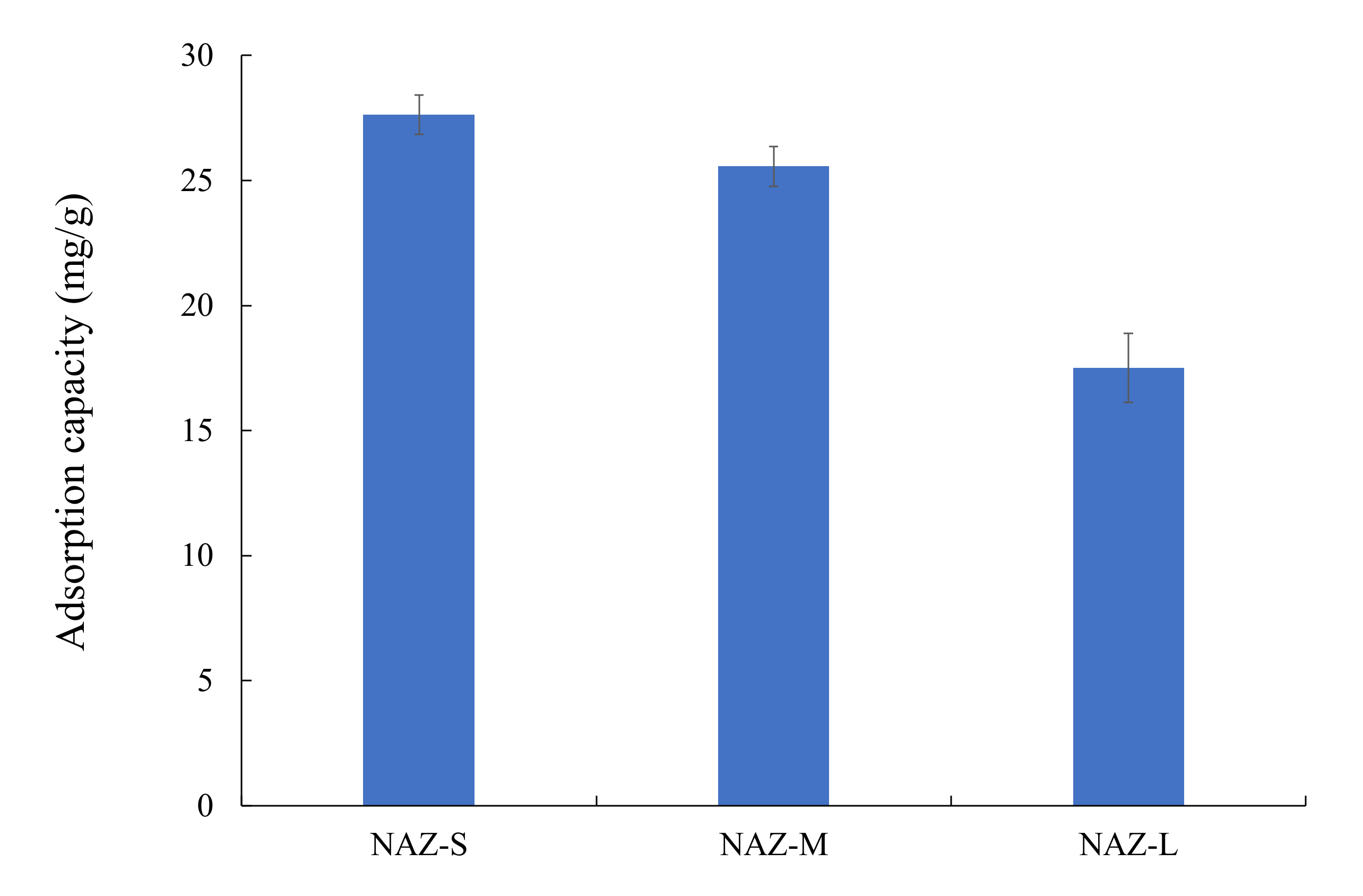
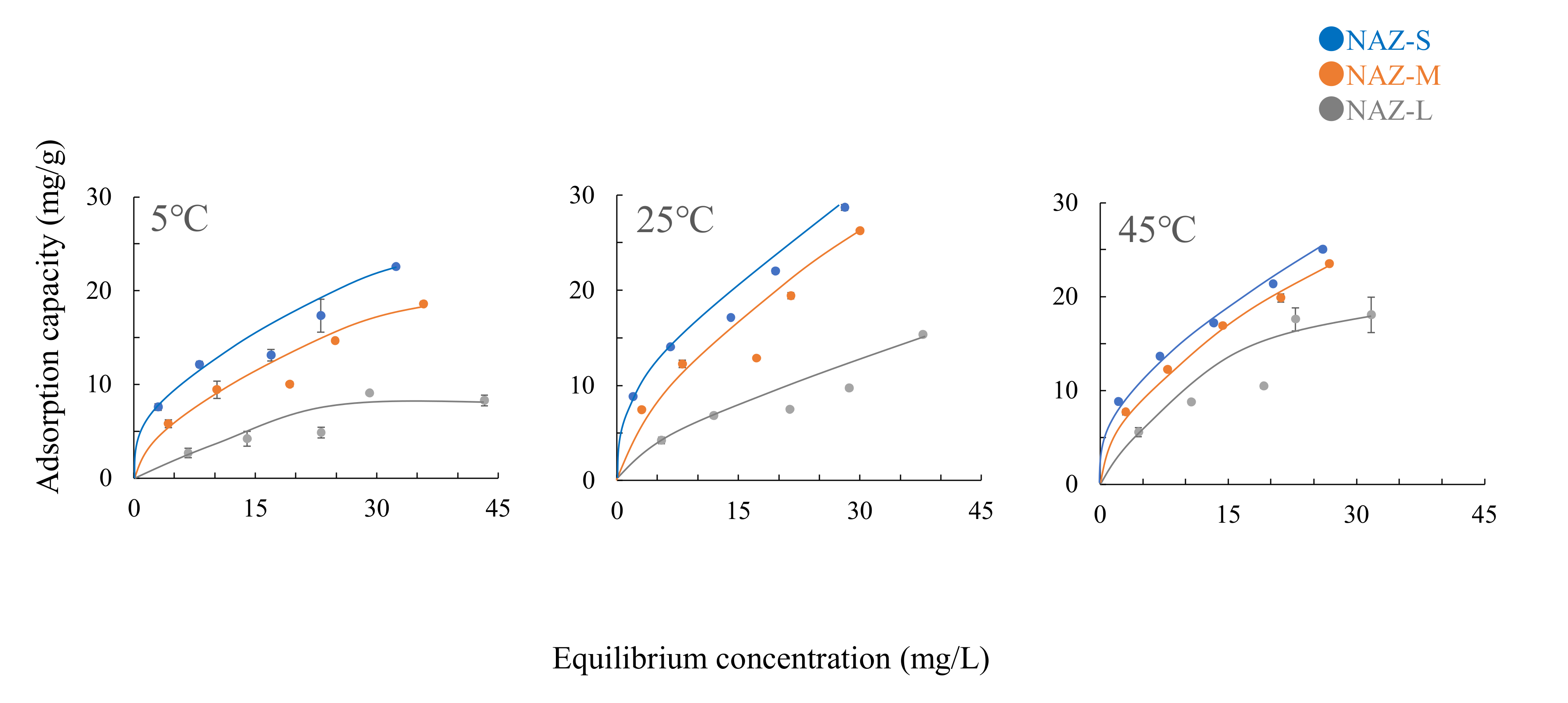
3.2. Adsorption Properties
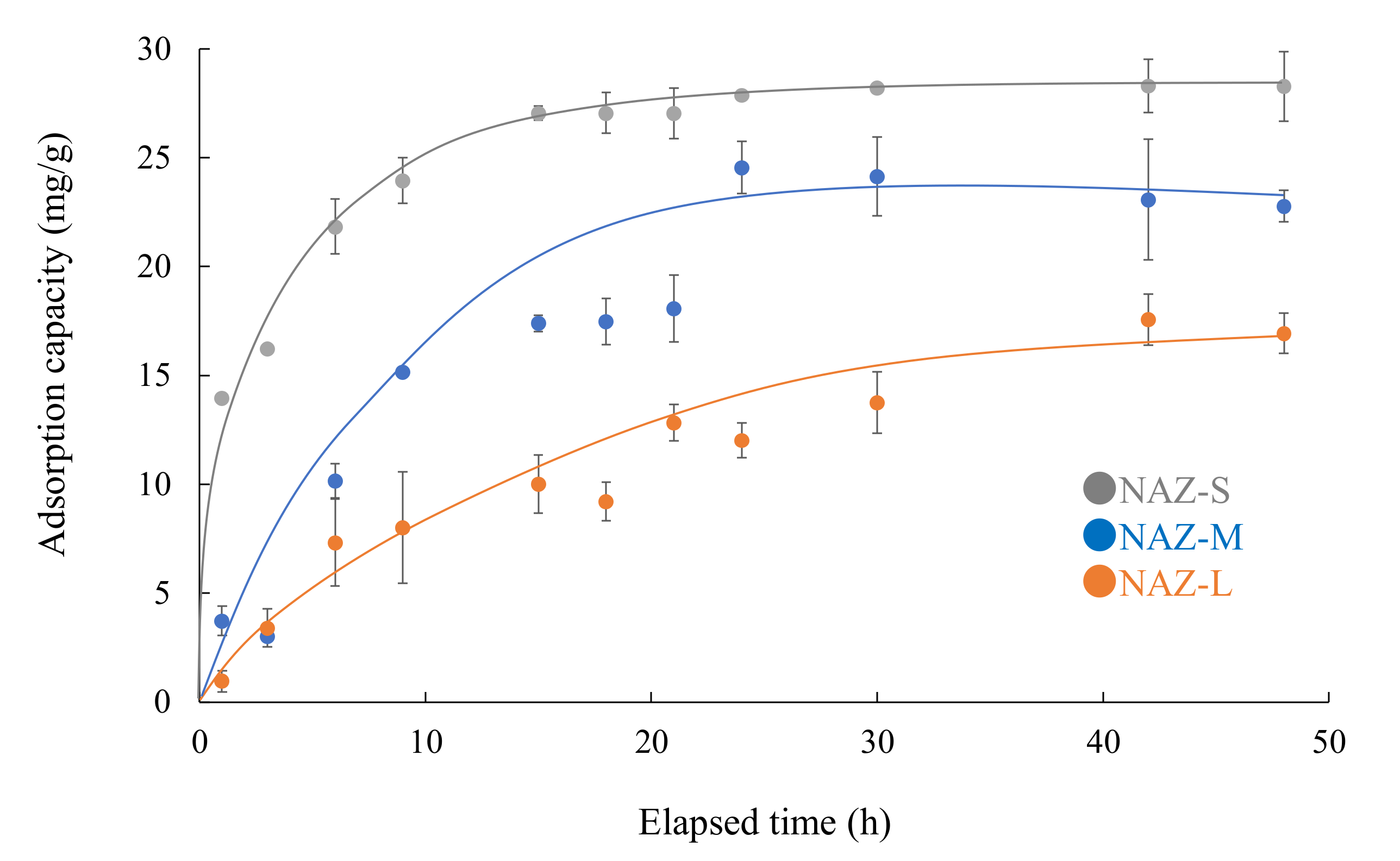
3.3. Effects of Parameters on Chromium(VI) Ion Adsorption
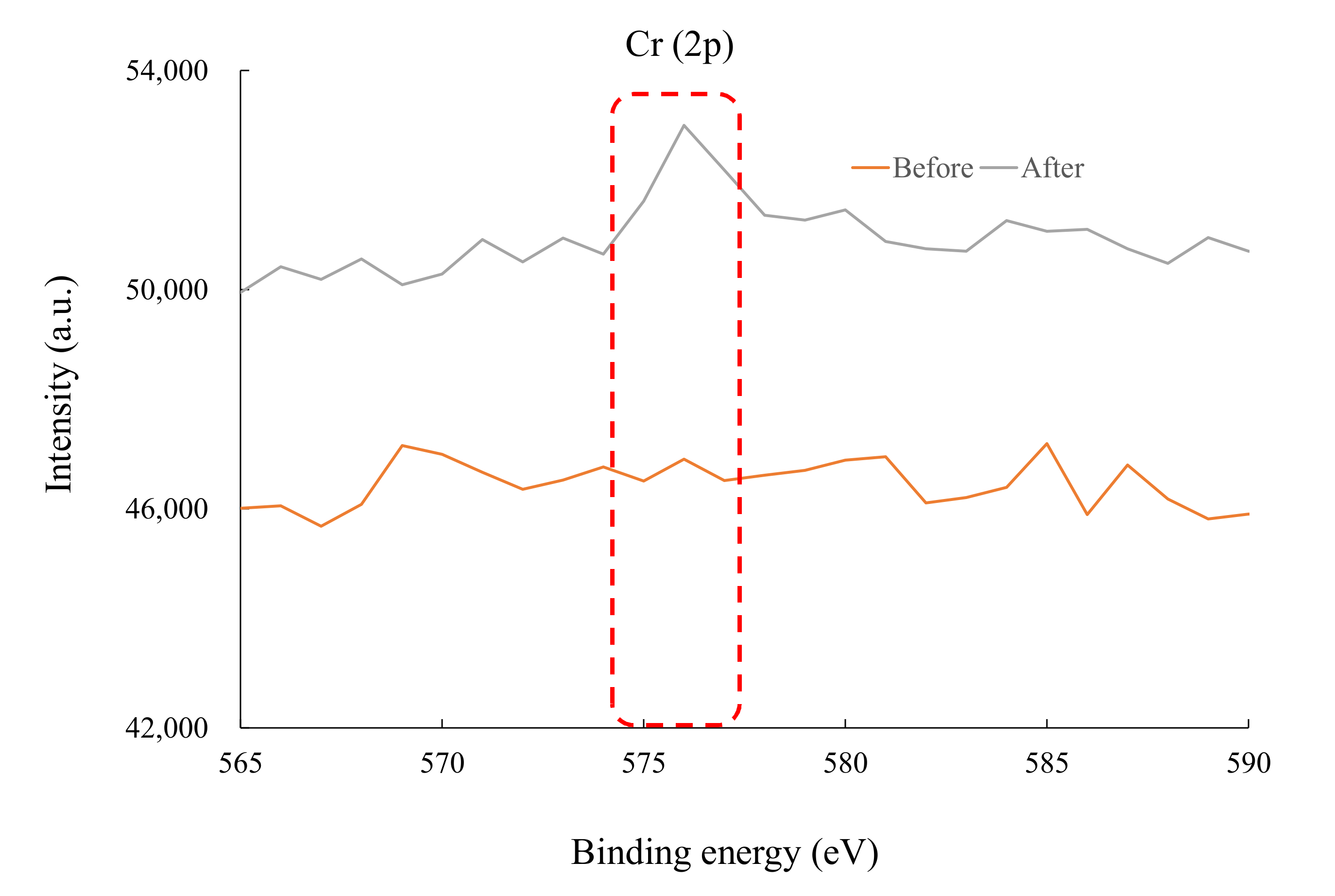
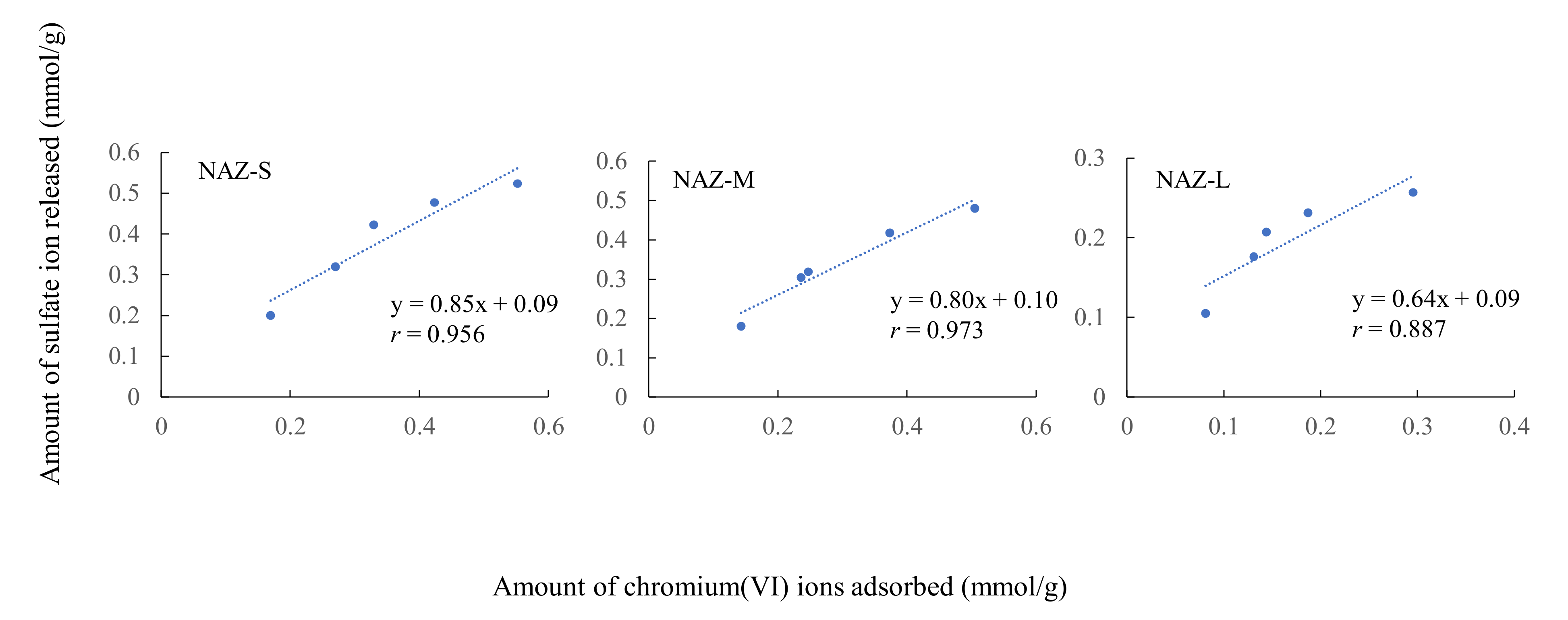
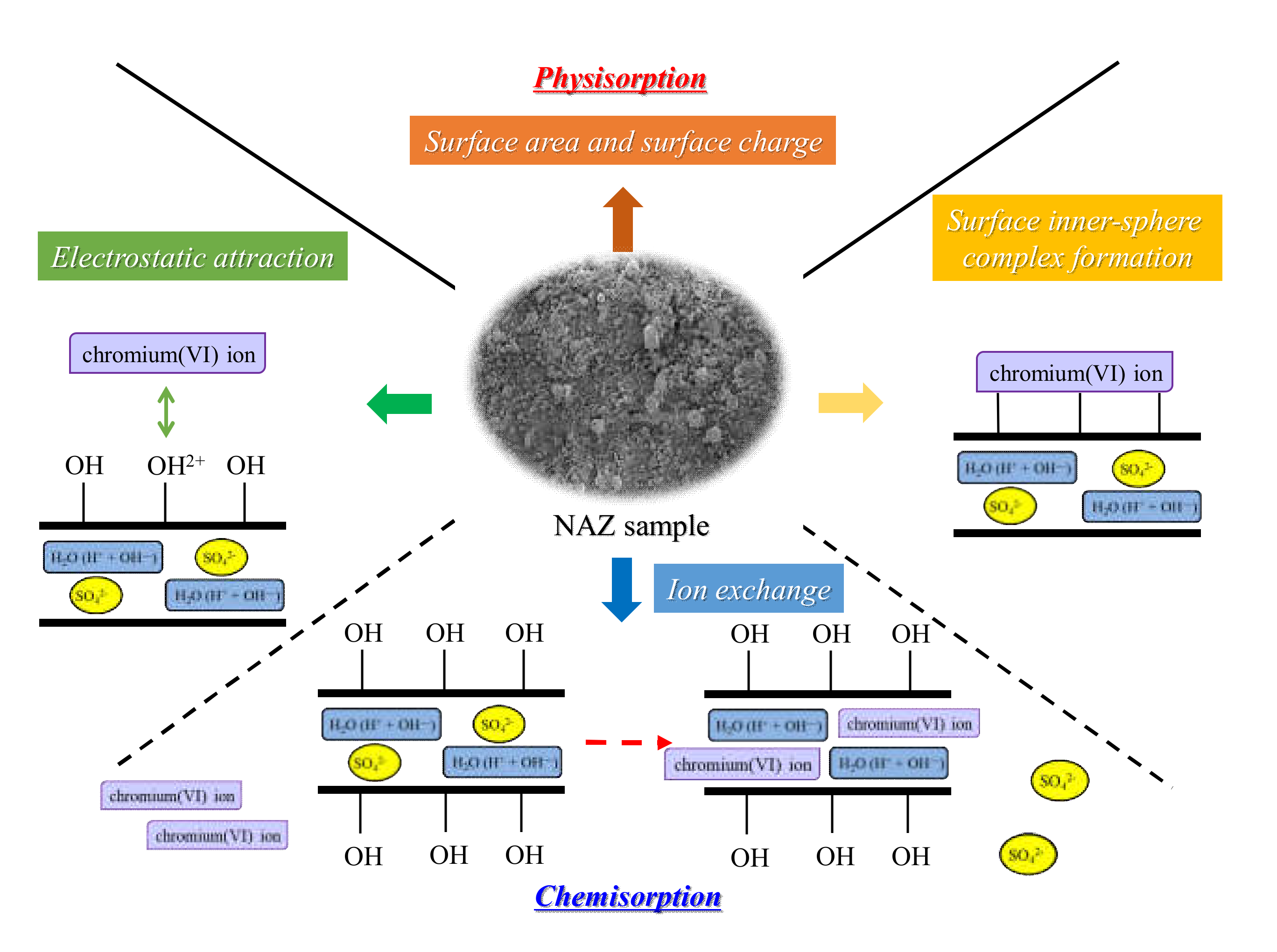
3.4. Chromium(VI) Ion Adsorption Mechanisms
3.5. Chromium(VI) Ion Adsorption in the Complex Solution System
3.6. Adsorption–Desorption of Chromium(VI) Ions in the Desorption Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Huang, S.; Ouyang, T.; Chen, J.; Wang, Z.; Liao, S.; Li, X.; Liu, Z.Q. Synthesis of nickel-iron layered double hydroxide via topochemical approach: Enhanced surface charge density for rapid hexavalent chromium removal. J. Colloid Interface Sci. 2022, 605, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.P.; Judd, R.J.; Lay, P.A.; Dixon, N.E.; Baker, R.S.; Bonin, A.M. Chromium(V)-induced cleavage of DNA: Are chromium(V) complexes the active carcinogens in chromium(VI)-induced cancers? Chem. Res. Toxicol. 1989, 2, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Chirwa, E.M.; Shen, H. Cr(VI) reduction in continuous-flow coculture bioreactor. J. Environ. Eng. 2000, 126, 300–306. [Google Scholar] [CrossRef]
- Belachew, N.; Hinsene, H. Preparation of cationic surfactant-modified kaolin for enhanced adsorption of hexavalent chromium from aqueous solution. App. Water Sci. 2020, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bhattacharyya, K.G. Adsorption of chromium(VI) on Azadirachta indica (Neem) leaf powder. Adsorption 2005, 10, 327–338. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- EPA (Environmental Protection Agency). Environmental Pollution Control Alternatives; EPA/625/4-89/023; U.S. Government Printing Office: Cincinnati, OH, USA, 1990.
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef]
- Liu, W.; Yang, L.; Xu, S.; Chen, Y.; Liu, B.; Li, Z.; Jiang, C. Efficient removal of hexavalent chromium from water by an adsorption-reduction mechanism with sandwiched nanocomposite. RSC Adv. 2018, 8, 15087–15093. [Google Scholar] [CrossRef] [Green Version]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, Y.; Zheng, J.; Shang, L.; Shi, Y.; Wu, Q.; Liu, X.; Wang, Y.; Shi, L.; Shao, Q. Synthesis and characterization of ZnNiCr-layered double hydroxides with high adsorption activities for Cr(VI). Adv. Compos. Hybrid Mater. 2021, 4, 819–829. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Mao, W.; Guan, Y. Fast and efficient removal of Cr(VI) to ppb level together with Cr(VI) sequestration in water using layered double hydroxide interacted with dimethyldithiocarbamate. Sci. Total Environ. 2020, 727, 138701. [Google Scholar] [CrossRef]
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luio, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide interacted with ethylenediaminetetraacetic acid. Bioresour. Technol. 2019, 276, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.; Nagai, N.; Tabuchi, A.; Toda, M.; Otani, M.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of chromium(VI) ion from aqueous media using Ni-Al type and Ni-Al-Zr type hydroxides. Water 2021, 13, 551. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Martino, A.D.; Zednik, J.; Ozaltin, K.; Lovecka, L.; Bergerova, E.D.; Kimmer, D.; Svodoba, J.; Sedlarrik, V. Efficient Cu2+, Pb2+, and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-modifed magnetic Fe3O4@SiO2-based polyvinyl alcohol/chitosan composite material for efficient removal of heavy metal ions from water. Polymer 2022, 14, 1107. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Dey, R.; Eltaweil, S.; Abd El-Monaen, E.M.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Omer, A.M.; El-Monaen, A.M.S.; El-Latifa, A.M.M.; El-Subruiti, G.M.; Eltaweil, A.S. Facil fabrication of novel magnetic ZIF-67 MOF@ aminated chitosan composite beads for the adsorptive removal of Cr(VI) from aqueous solutions. Carbohydr. Polym. 2021, 265, 118084. [Google Scholar] [CrossRef]
- Ogata, F.; Ueta, E.; Kawasaki, N. Characteristics of a novel adsorbent Fe-Mg-type hydrotalcite and its adsorption capability of As(III) and Cr(VI) from aqueous solution. J. Ind. Eng. Chem. 2018, 59, 56–63. [Google Scholar] [CrossRef]
- Yue, Q.; Zhao, Y.; Li, Q.; Li, W.; Gao, B.; Han, S.; Qi, Y.; Yu, H. Research on the characteristics of red mud granular adsorbents (RMGA) for phosphate removal. J. Hazard. Mater. 2010, 176, 741–748. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Toda, M.; Otani, M.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of arsenic(III) ion from aqueous media using complex nickel-aluminum and nickel-aluminum-zirconium hydroxides. Water 2020, 12, 1697. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Orfao, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef]
- Long, F.; Gong, J.L.; Zeng, G.M.; Chen, L.; Wang, X.Y.; Deng, J.H.; Niu, Q.Y.; Zhang, H.Y.; Zhang, X.R. Removal of phosphate from aqueous solution by magnetic Fe–Zr binary oxide. Chem. Eng. J. 2011, 171, 448–455. [Google Scholar] [CrossRef]
- Ogata, F.; Iijima, S.; Toda, M.; Otani, M.; Nakamura, T.; Kawasaki, N. Characterization and phosphate adsorption capability of novel nickel-aluminum-zirconium complex hydroxide. Chem. Pharm. Bull. 2020, 68, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khitous, M.; Salem, Z.; Halliche, D. Effect of interlayer anions on chromium removal using Mg-Al layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. Chin. J. Chem. Eng. 2016, 24, 433–445. [Google Scholar] [CrossRef]
- Hilbrandt, I.; Ruhl, A.S.; Zietzschmann, F.; Molkenthin, M.; Jekel, M. Competition in chromate adsorption onto micro-sized granular ferric hydroxide. Chemosphere 2019, 218, 749–757. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Jekel, M.; Zouboulis, A.I. Removal of Cr(VI), Mo(VI), and V(V) ions from single metal aqueous solutions by sorption or nanofiltration. Sep. Sci. Technol. 2003, 38, 2201–2219. [Google Scholar] [CrossRef]
- Abo El-Reesh, G.Y.; Farghali, A.A.; Taha, M.; Mahmoud, R.K. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal. Sci. Rep. 2020, 10, 587. [Google Scholar]
- Li, Y.; Gao, B.; Wu, T.; Sun, D.; Li, X.; Wang, B.; Lu, F. Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Res. 2009, 43, 3067–3075. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M. Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- Leite, V.d.S.A.; Jesus, B.G.L.d.; Duarte, V.G.d.O.; Constantino, V.R.L.; Izumi, C.M.S.; Tronto, J.; Pinto, F.G. Determination of chromium (VI) by dispersive solid-phase extraction using dissolvable Zn-Al layered double hydroxide intercalated with l-Alanine as adsorbent. Microchem. J. 2019, 146, 650–657. [Google Scholar] [CrossRef]
- Donat, R.; Akdogan, A.; Erdem, E.; Cetisli, H. Thermodynamic of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J. Colloid Interf. Sci. 2005, 286, 43–52. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Das, R.; Ho, W.H.; Srinivasu, V.; Maity, A. A novel method for removal of Cr(VI) using polypyrrole magnetic nanocomposite in the presence of unsteady magnetic fields. Sep. Purif. Technol. 2018, 194, 377–387. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Onyancha, R.B.; Ukhurebor, K.E.; Obodo, K.O. Removal of fluoride ions using polypyrrole magnetic nanocomposite influenced by rotating magnetic field. RSC Adv. 2020, 10, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Ayawei, N.; Angaye, S.S.; Wankasi, D.; Dikio, E.D. Synthesis, characterization and application of Mg/Al layered double hydroxide for the degradation of Congo red in aqueous solution. Open J. Phys. Chem. 2015, 5, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Long, F.L.; Niu, C.G.; Tang, N.; Guo, H.; Li, Z.W.; Yang, Y.Y.; Lin, L.S. Highly efficient removal of hexavalent chromium from aqueous solution by calcined Mg/Al-layered double hydroxides/polyaniline composites. Chem. Eng. J. 2021, 404, 127084. [Google Scholar] [CrossRef]
- Abe, I.; Hayashi, K.; Kitagawa, M. Studies on the adsorption of surfactants on activated carbons. I. Adsorption of nonionic surfactants. Yukagaku 1976, 25, 145–150. [Google Scholar]
- Wu, P.; Xia, L.; Liu, Y.; Wu, J.; Chen, Q.; Song, S. Simultaneous sorption of arsenate and fluoride on calcined Mg-Fe-La hydrotalcite-like compound from water. ACS Sustain. Chem. Eng. 2018, 6, 16287–16297. [Google Scholar] [CrossRef]
- Aregay, G.G.; Jawad, A.; Du, Y.; Shahzad, A.; Chen, Z. Efficient and selective removal of chromium(VI) by sulfide assembled hydrotalcite compounds through concurrent reduction and adsorption processes. J. Mol. Lip. 2019, 294, 111532. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z.; Wang, L.; Zhou, S. Fabrication of a novel NiFe2O4/Zn-Al layered double hydroxide inter-calated with EDTA composite and its adsorption behavior for Cr(VI) from aqueous solution. J. Phys. Chem. Solids 2017, 104, 79–90. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42− ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, Z.G.; Gong, W.B.; Zhang, L.D.; Xu, Z.L.; Su, G.B.; Han, D.G. The mechanism study of trace Cr(VI) removal from water using Fe0 nanorods modified with chitosan in porous anodic alumina. Appl. Surf. Sci. 2015, 328, 606–613. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and reduction of chromium(VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Akpotu, S.O.; Moodley, B. Application of as-synthesised MCM-41 and MCM-41 wrapped with reduced graphene oxide/graphene oxide in the remediation of acetaminophen and aspirin from aqueous system. J. Environ. Manag. 2018, 209, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Shi, Z.; Li, B.; Yang, L.F.; Luo, L.; Yang, X.Z. Adsorption of Cr(VI) and phosphate on Mg-Al hydrotalcite supported kaolin clay prepared by ultrasoundassisted coprecipitation method using batch and fixed-beb systems. Ind. Eng. Chem. Res. 2014, 53, 7746–7757. [Google Scholar] [CrossRef]
- Zhang, B.; Luan, L.Y.; Gao, R.T.; Li, F.; Li, Y.J.; Wu, T. Rapid and effective removal of Cr(VI) from aqueous solution using exfoliated LDH nanosheets. Colloid Surf. A 2017, 520, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Das, D.; Dash, G.P.; Parida, K.M. Studies on Mg/Fe hydrotalcite-like-compound (HTlc): I. Removal of inorganic selenite (SeO32−) from aqueous medium. J. Colloid Interface Sci. 2002, 251, 26–32. [Google Scholar] [CrossRef]

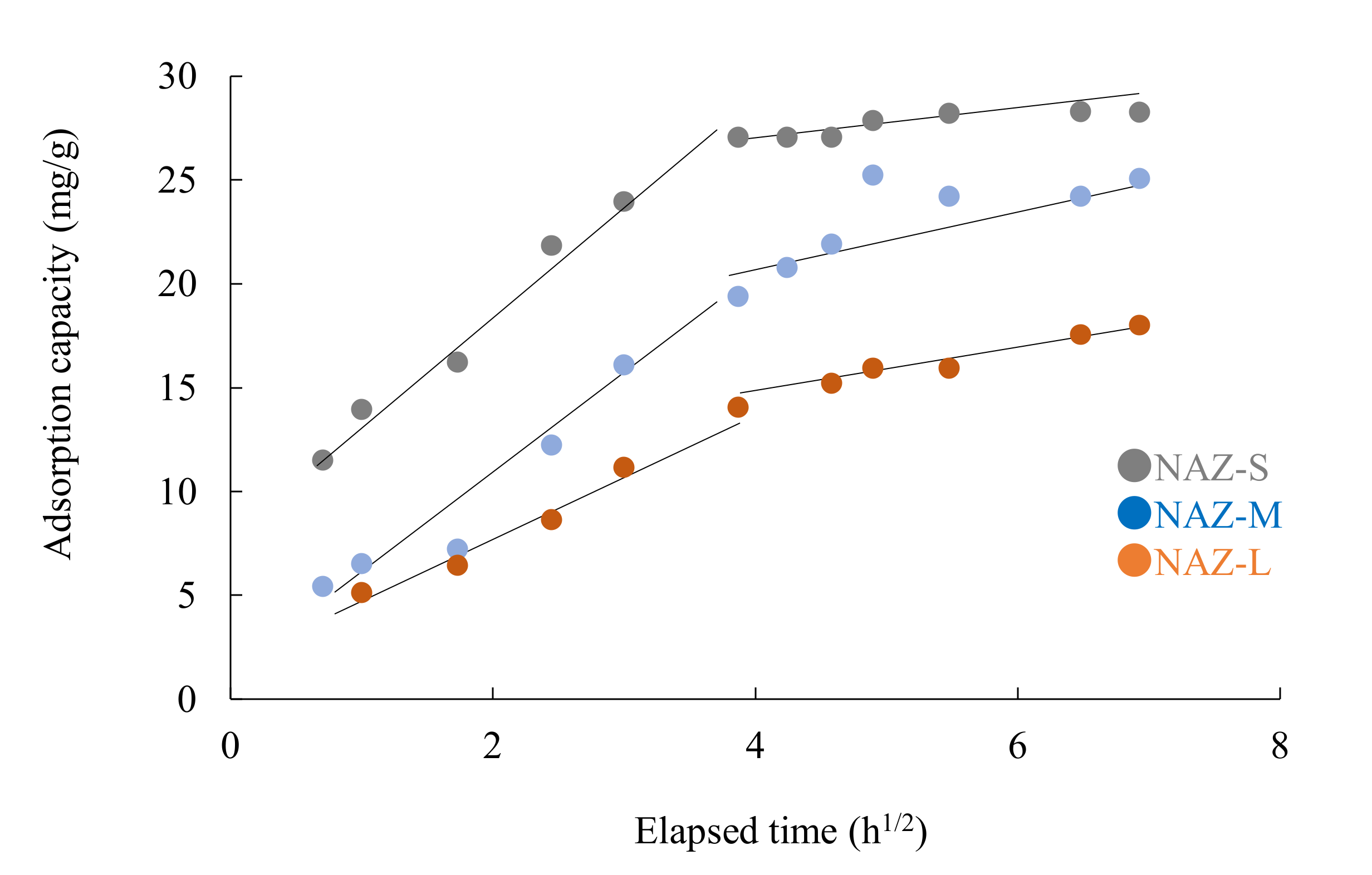
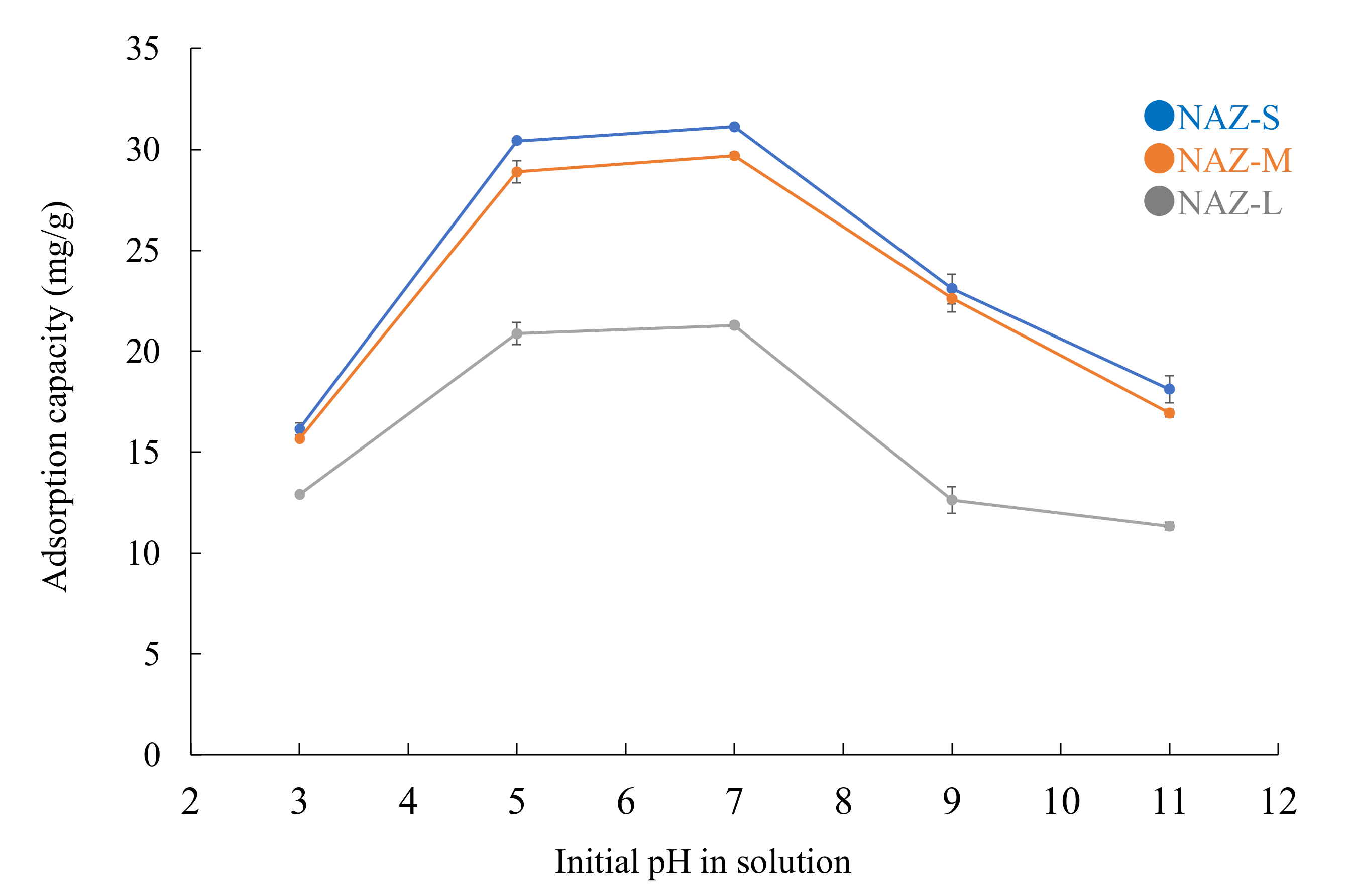
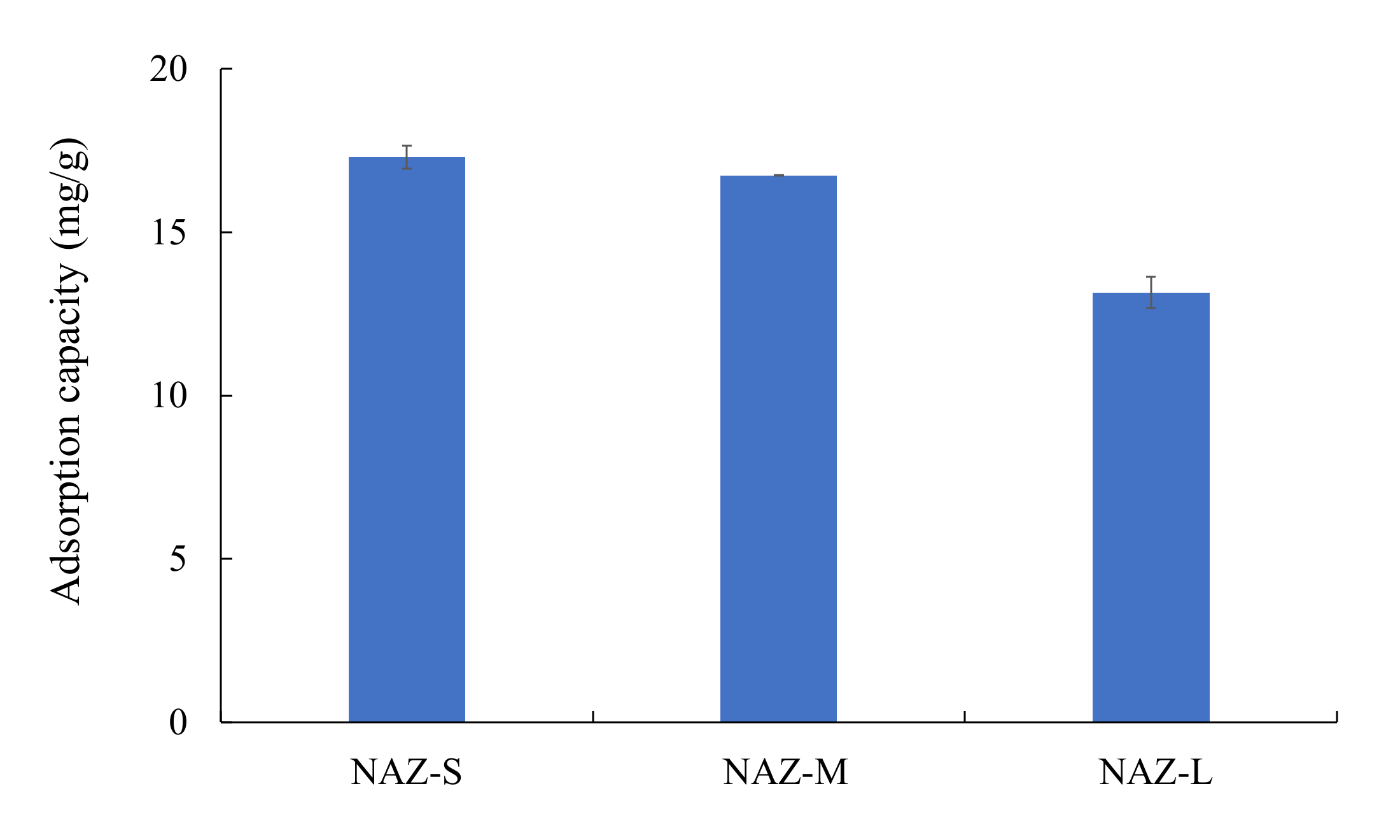
 : adsorption; □: desorption; adsorbent: NAZ-S, NAZ-M, and NAZ-L.
: adsorption; □: desorption; adsorbent: NAZ-S, NAZ-M, and NAZ-L.
 : adsorption; □: desorption; adsorbent: NAZ-S, NAZ-M, and NAZ-L.
: adsorption; □: desorption; adsorbent: NAZ-S, NAZ-M, and NAZ-L.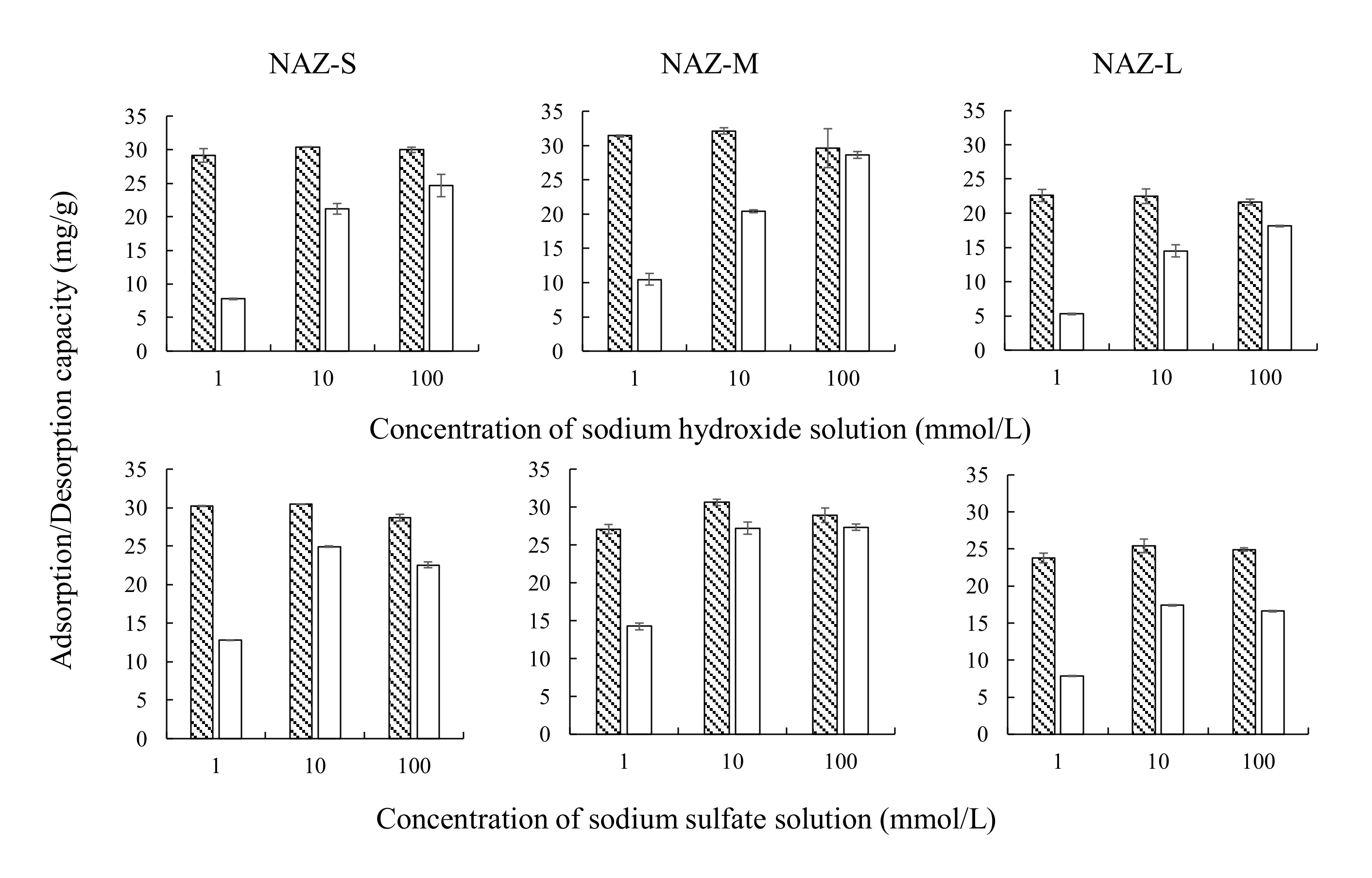
| Adsorbents | NAZ | Colloidal Silica | NAZ-S | NAZ-M | NAZ-L |
|---|---|---|---|---|---|
| pHpzc | 6.2 | 5.6 | 6.9 | 6.8 | 6.7 |
| Specific surface area (m2/g) | 23.8 | 199.7 | 101.6 | 69.5 | 66.8 |
| Number of hydroxyl groups (mmol/g) | 1.08 | 0.18 | 1.09 | 1.08 | 0.94 |
| Adsorbents | Adsorption Capacity (mg/g) | pH | Temp. (°C) | Initial Concentration (mg/L) | Contact Time(h) | Adsorbent (g/L) | Ref. |
|---|---|---|---|---|---|---|---|
| Micro-sized granular ferric hydroxide | 5.8 | 7 | 20 | 1.2 | 24 | 0.04–0.5 | [26] |
| Granular ferric hydroxide | 17.0 | 7 | 20 | 20 | 1 | 0.2 | [27] |
| ZnNiCr-LDHs | 28.2 | 2 | 25 | 30 | 0.83 | 0.5 | [11] |
| Ni/Fe LDH | 50.43 | 5 | 23–55 | 50 | 24 | 0.5 | [28] |
| Aluminum-magnesium mixed hydroxide | 105.3–112 | 2.5–5 | 30 | 200 | 1.5 | 2 | [29] |
| Maghemite nanoparticle | 19.2 | 2.5 | 22.5 | 50 | 0.25 | 5 | [30] |
| Zn/Al/Ala LDH | 12.25 | 5.2 | 25 | 2 | 0.25 | 0.4 | [31] |
| NAZ-S | 27.6 | 7 | 25 | 50 | 24 | 1.0 | This study |
| NAZ-M | 25.6 | 7 | 25 | 50 | 24 | 1.0 | This study |
| NAZ-L | 17.5 | 7 | 25 | 50 | 24 | 1.0 | This study |
| Adsorbents | Temperatures (°C) | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| Ws (mg/g) | a (L/mg) | r | logk | 1/n | r | ||
| NAZ-S | 5 | 20.75 | 5.33 | 0.964 | 0.67 | 0.42 | 0.968 |
| 25 | 24.81 | 3.69 | 0.965 | 0.81 | 0.42 | 0.983 | |
| 45 | 23.92 | 3.78 | 0.974 | 0.80 | 0.41 | 0.995 | |
| NAZ-M | 5 | 18.87 | 9.82 | 0.962 | 0.43 | 0.51 | 0.964 |
| 25 | 23.20 | 6.59 | 0.946 | 0.62 | 0.49 | 0.940 | |
| 45 | 26.32 | 7.41 | 0.989 | 0.64 | 0.50 | 0.999 | |
| NAZ-L | 5 | 12.53 | 25.21 | 0.963 | −0.12 | 0.65 | 0.938 |
| 25 | 15.43 | 14.96 | 0.962 | 0.17 | 0.59 | 0.949 | |
| 45 | 22.32 | 13.91 | 0.962 | 0.33 | 0.61 | 0.954 | |
| Adsorbent | qe,exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe,cal (mg/g) | r | k2 (g/mg/h) | qe,cal (mg/g) | r | ||
| NAZ-S | 28.3 | 0.14 | 15.0 | 0.980 | 0.02 | 29.5 | 1.000 |
| NAZ-M | 24.6 | 0.07 | 19.4 | 0.838 | 3.1 × 10−3 | 27.5 | 0.984 |
| NAZ-L | 18.0 | 0.06 | 18.8 | 0.968 | 2.1 × 10−3 | 24.0 | 0.978 |
| Samples | k1 (mg/g h1/2) | C1 | r | k2 (mg/g h1/2) | C1 | r |
|---|---|---|---|---|---|---|
| NAZ-S | 5.0 | 5.8 | 0.991 | 0.5 | 25.2 | 0.858 |
| NAZ-M | 4.6 | 1.3 | 0.980 | 1.2 | 17.2 | 0.696 |
| NAZ-L | 3.2 | 1.3 | 0.991 | 1.6 | 10.0 | 0.979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabuchi, A.; Ogata, F.; Uematsu, Y.; Toda, M.; Otani, M.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Granulation of Nickel–Aluminum–Zirconium Complex Hydroxide Using Colloidal Silica for Adsorption of Chromium(VI) Ions from the Liquid Phase. Molecules 2022, 27, 2392. https://doi.org/10.3390/molecules27082392
Tabuchi A, Ogata F, Uematsu Y, Toda M, Otani M, Saenjum C, Nakamura T, Kawasaki N. Granulation of Nickel–Aluminum–Zirconium Complex Hydroxide Using Colloidal Silica for Adsorption of Chromium(VI) Ions from the Liquid Phase. Molecules. 2022; 27(8):2392. https://doi.org/10.3390/molecules27082392
Chicago/Turabian StyleTabuchi, Ayako, Fumihiko Ogata, Yugo Uematsu, Megumu Toda, Masashi Otani, Chalermpong Saenjum, Takehiro Nakamura, and Naohito Kawasaki. 2022. "Granulation of Nickel–Aluminum–Zirconium Complex Hydroxide Using Colloidal Silica for Adsorption of Chromium(VI) Ions from the Liquid Phase" Molecules 27, no. 8: 2392. https://doi.org/10.3390/molecules27082392
APA StyleTabuchi, A., Ogata, F., Uematsu, Y., Toda, M., Otani, M., Saenjum, C., Nakamura, T., & Kawasaki, N. (2022). Granulation of Nickel–Aluminum–Zirconium Complex Hydroxide Using Colloidal Silica for Adsorption of Chromium(VI) Ions from the Liquid Phase. Molecules, 27(8), 2392. https://doi.org/10.3390/molecules27082392