Identification of Stingless Bee Honey Adulteration Using Visible-Near Infrared Spectroscopy Combined with Aquaphotomics
Abstract
:1. Introduction
2. Results and Discussion
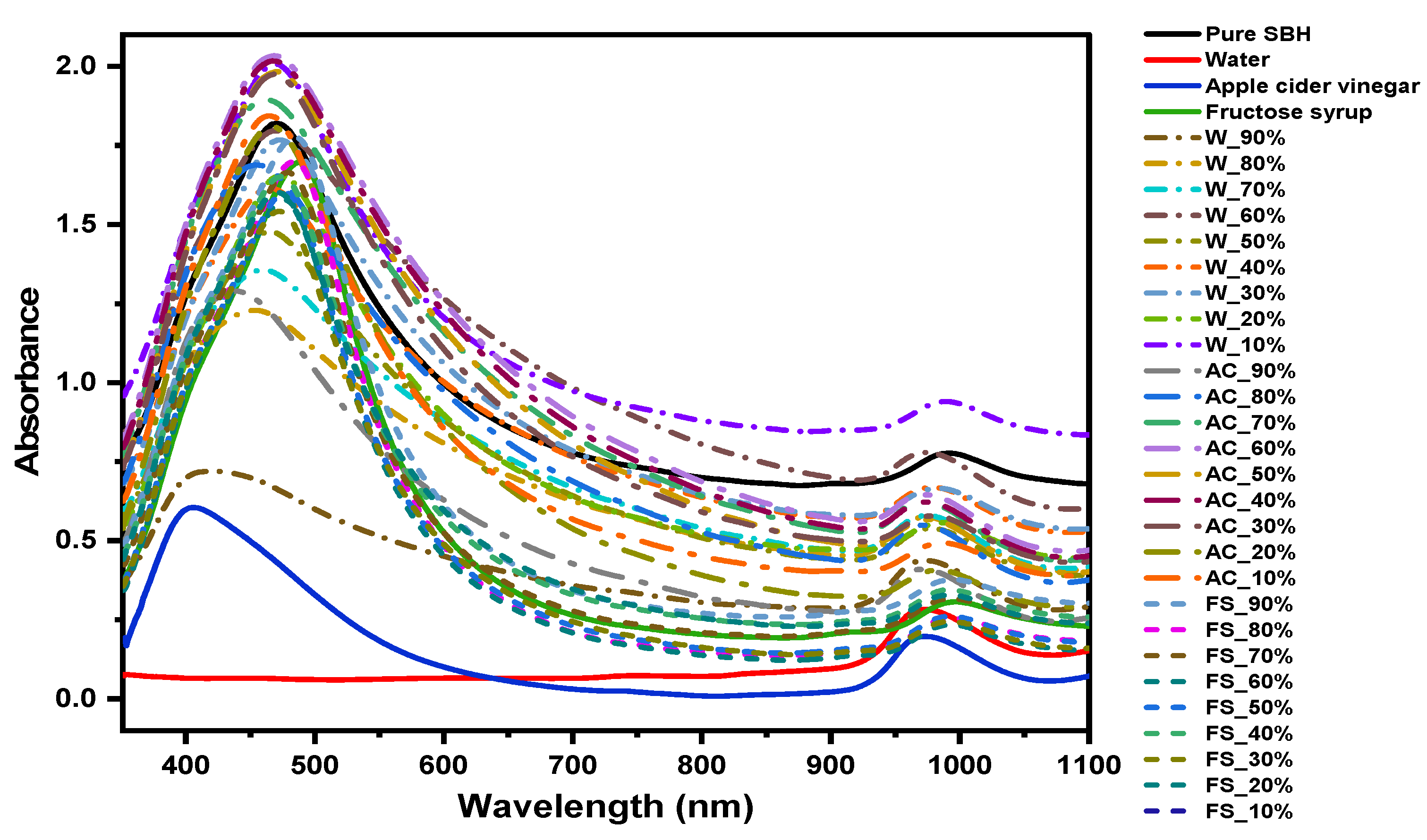
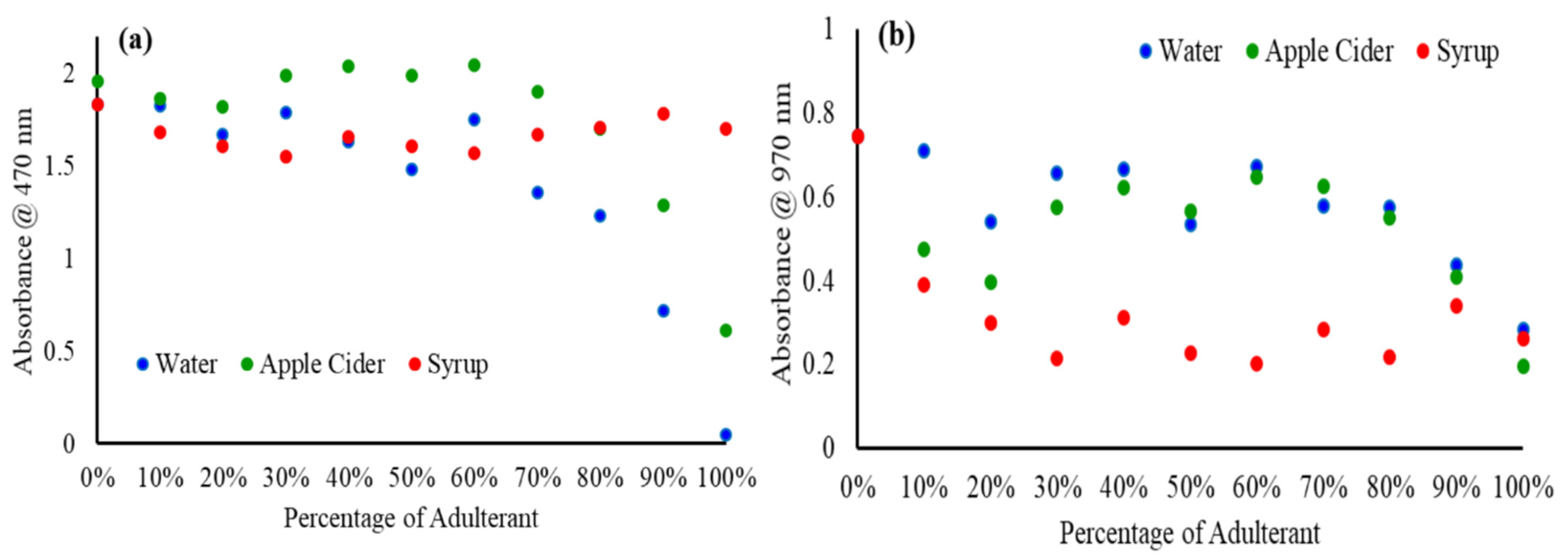
2.1. Vis-NIR Spectra of Pure and Adulterated SBH Samples
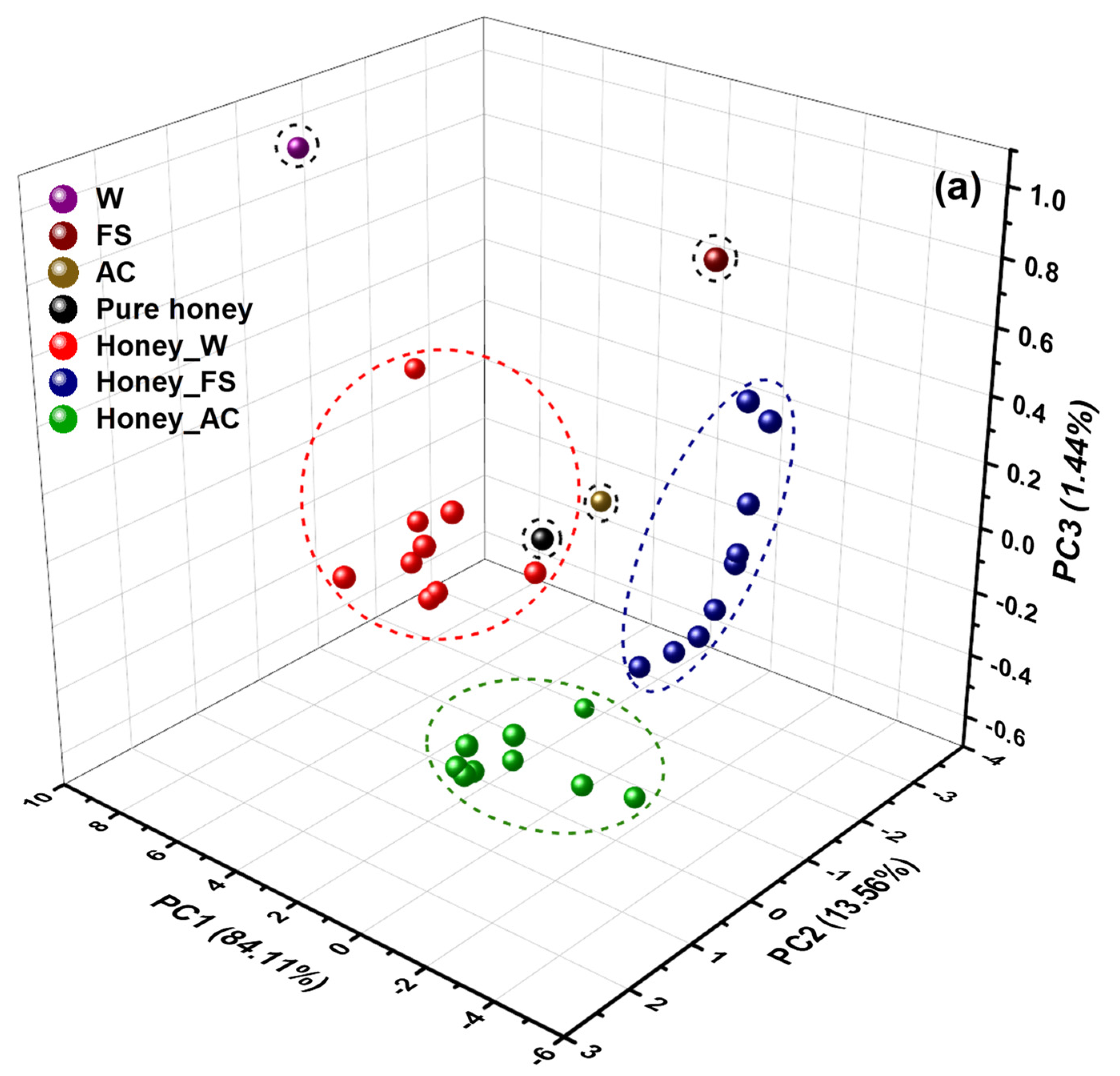
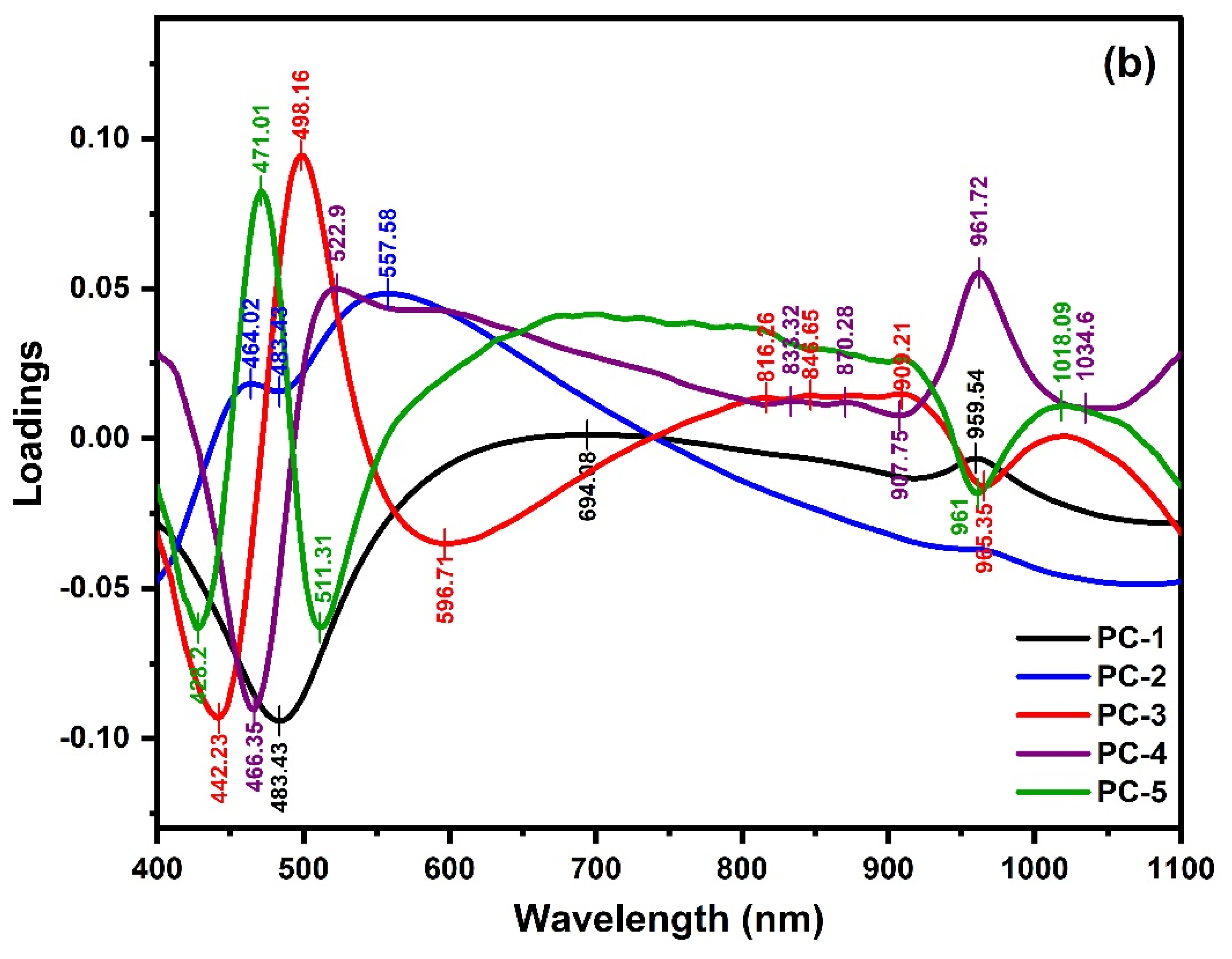
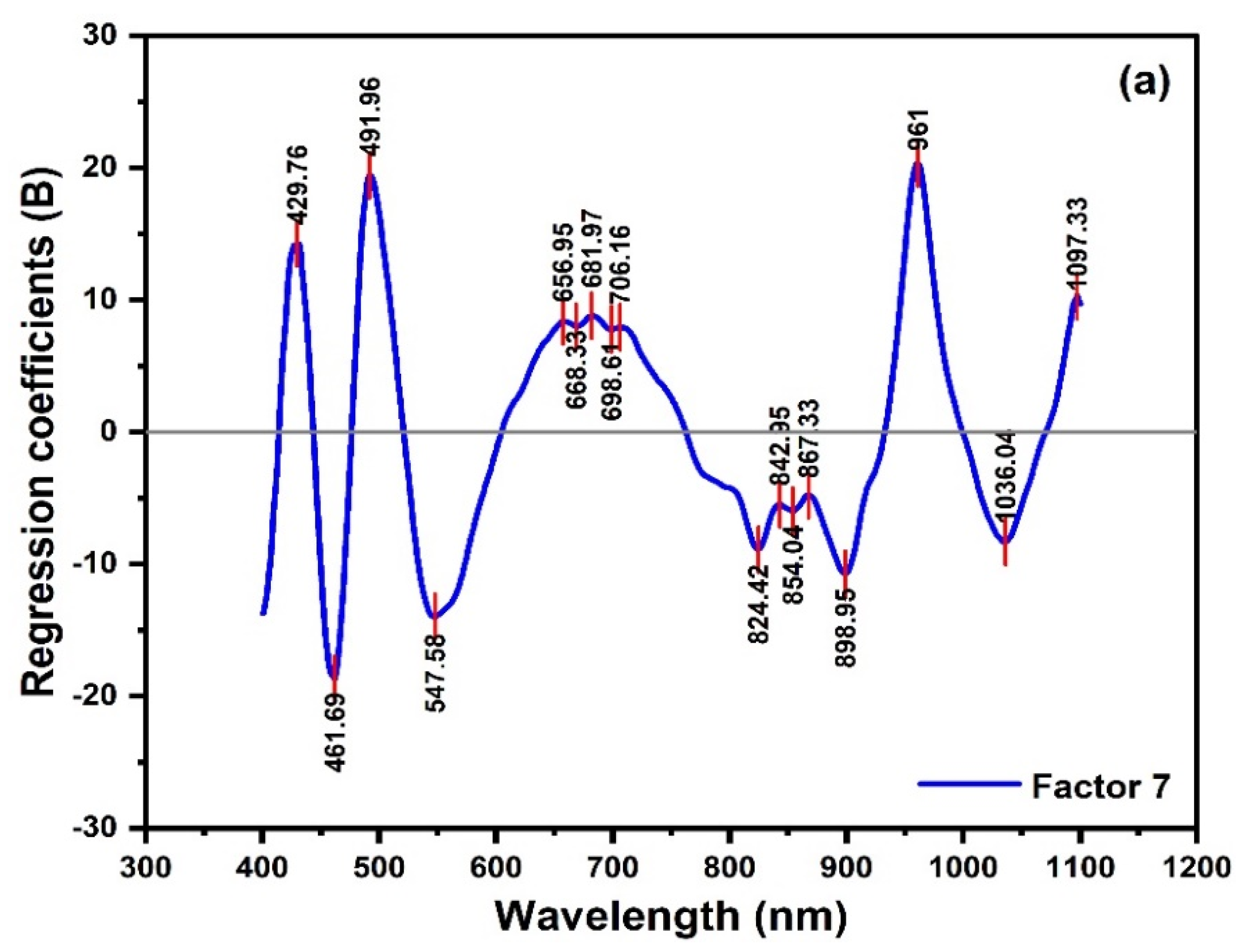
2.2. PCA Analysis and PLSR Modeling
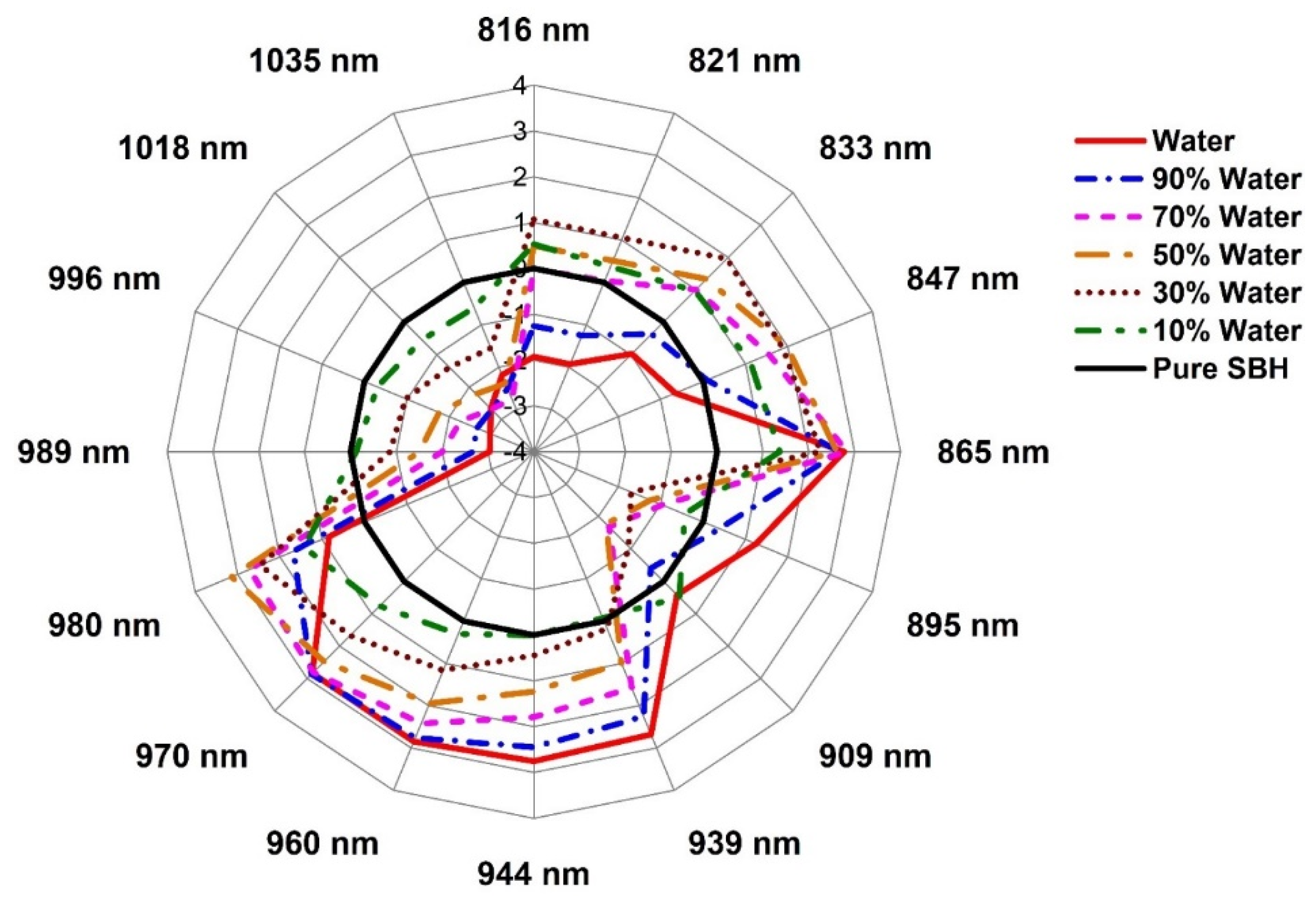
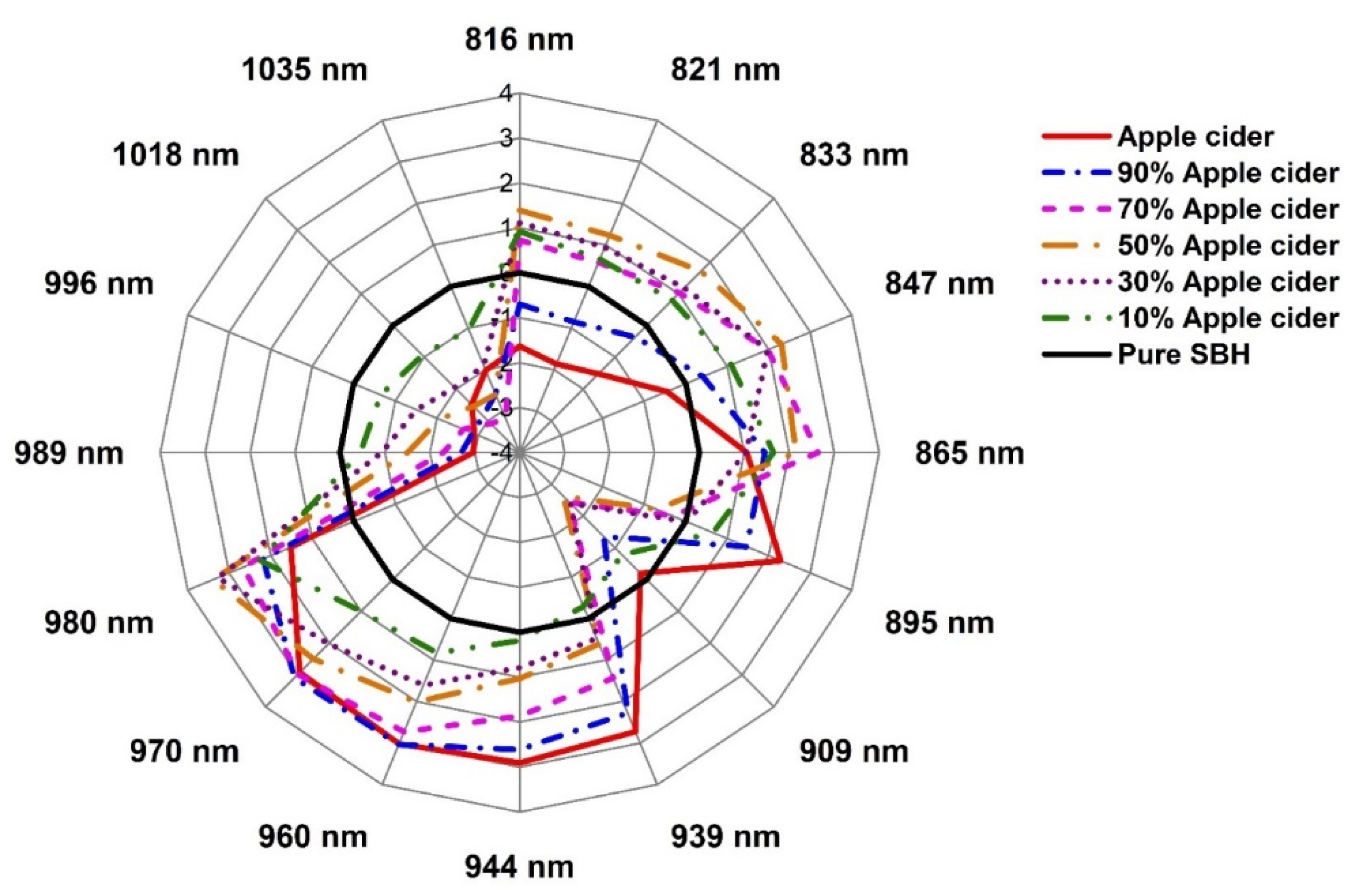
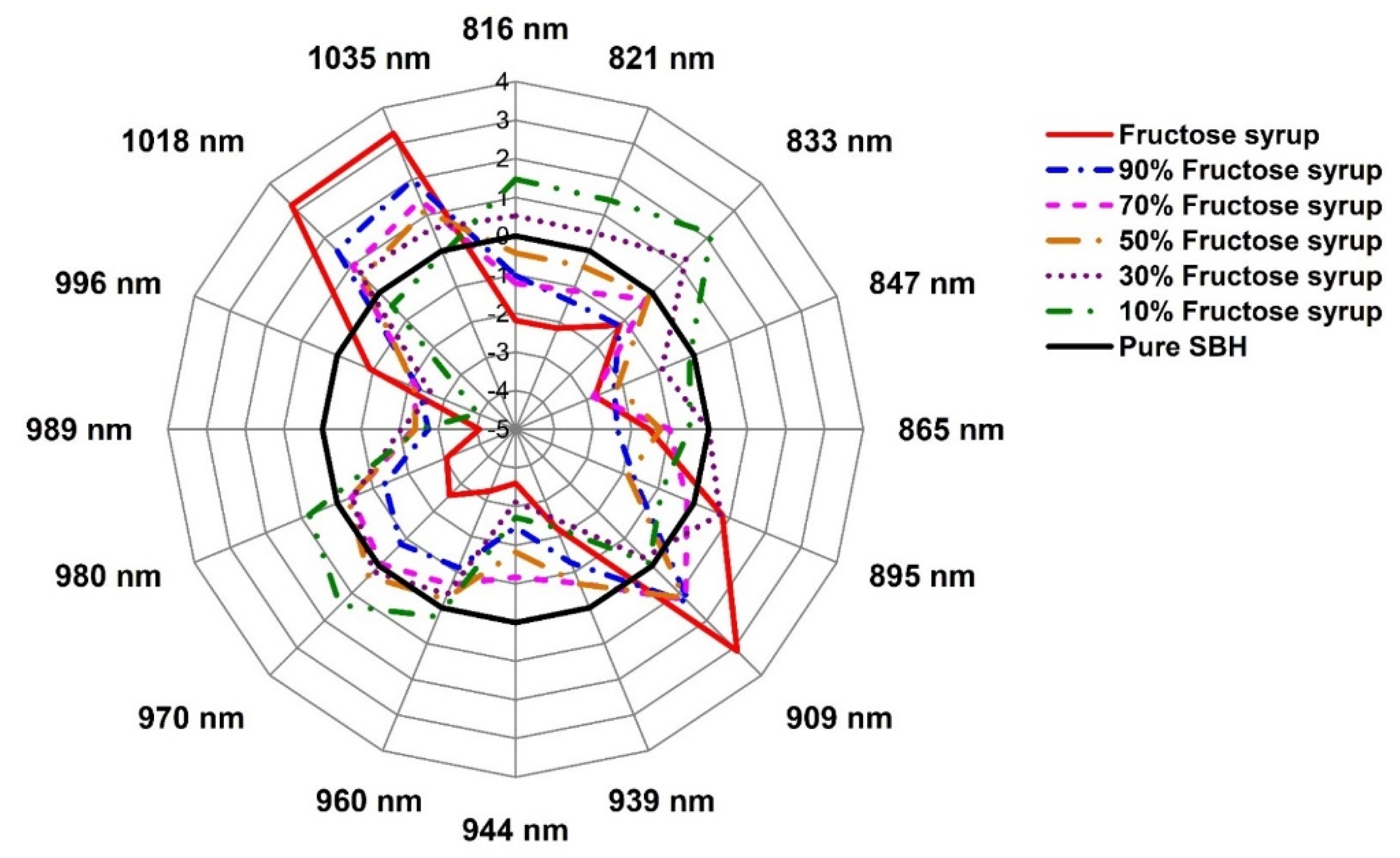
2.3. Evaluation of the Structural Changes of Adulterated SBH Based on Aquaphotomics
3. Materials and Methods
3.1. Honey Samples
3.2. Adulterants
3.3. Honey Adulteration Sample Preparation
3.4. Vis-NIR Spectroscopy
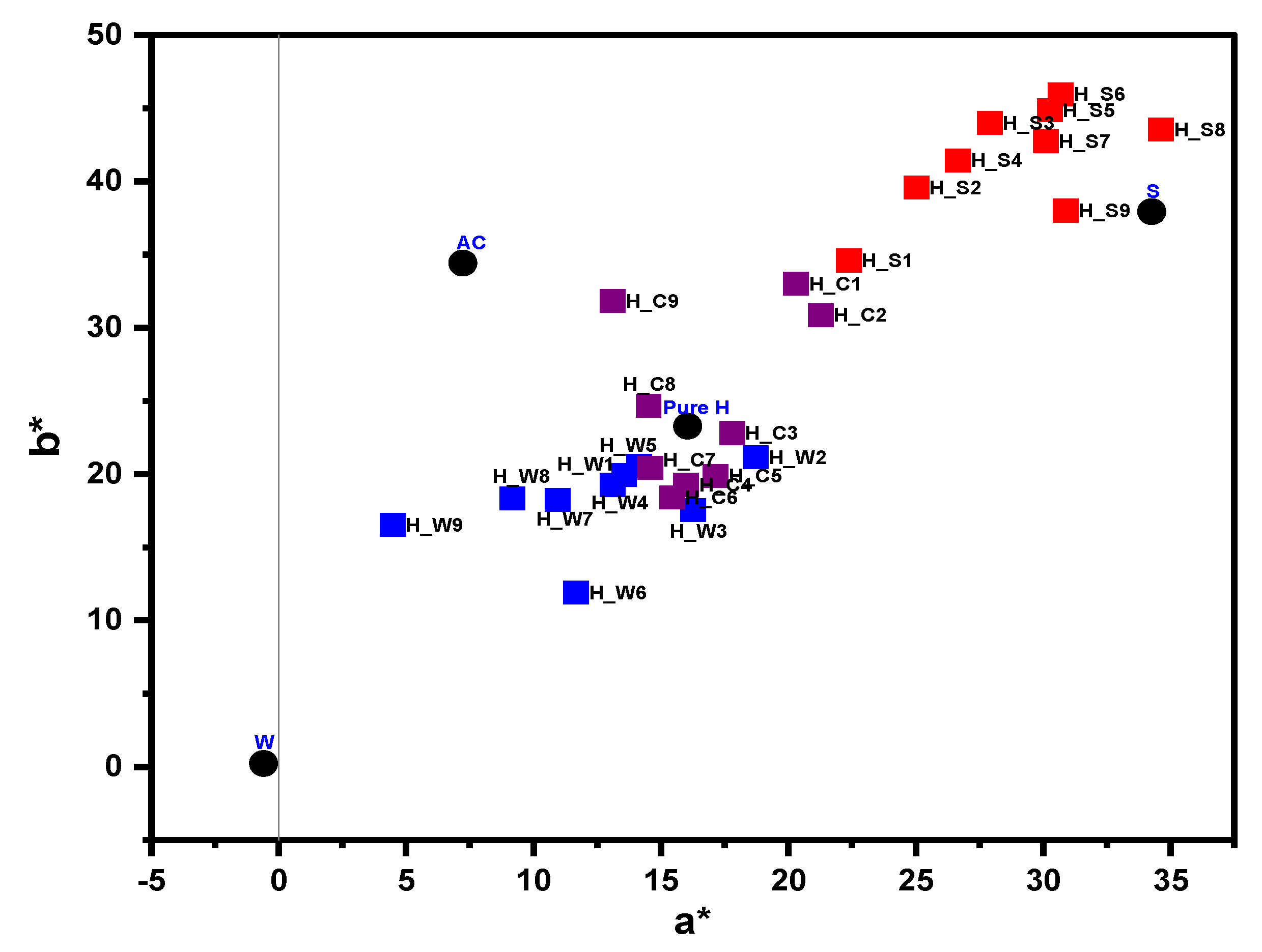
3.5. Color Analysis
3.6. Total Soluble Solids
3.7. Multivariate Analysis
3.8. Aquagrams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ávila, S.; Hornung, P.S.; Teixeira, G.L.; Beux, M.R.; Lazzarotto, M.; Ribani, R.H. A chemometric spproach for moisture control in stingless bee honey using near infrared spectroscopy. J. Near Infrared Spectrosc. 2018, 26, 379–388. [Google Scholar] [CrossRef]
- Valinger, D.; Longin, L.; Grbeš, F.; Benković, M.; Jurina, T.; Kljusurić, J.G.; Tušek, A.J. Detection of honey adulteration-The potential of UV-VIS and NIR spectroscopy coupled with multivariate analysis. LWT Food Sci. Technol. 2021, 145, 111316. [Google Scholar] [CrossRef]
- Lastra-Mejías, M.; Izquierdo, M.; González-Flores, E.; Cancilla, J.C.; Izquierdo, J.G.; Torrecilla, J.S. Honey Exposed to laser-induced breakdown spectroscopy for chaos-based botanical classification and fraud assessment. Chemom. Intell. Lab. Syst. 2020, 199, 103939. [Google Scholar] [CrossRef]
- Huang, F.; Song, H.; Guo, L.; Guang, P.; Yang, X.; Li, L.; Zhao, H.; Yang, M. Detection of adulteration in Chinese honey using NIR and ATR-FTIR spectral data fusion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118297. [Google Scholar] [CrossRef] [PubMed]
- Shiddiq, M.; Asyana, V.; Aliyah, H. Identification of Pure and Adulterated Honey Using Two Spectroscopic Methods. J. Physic Conf. Ser. 2019, 1531, 012022. [Google Scholar] [CrossRef]
- Salvador, L.; Guijarro, M.; Rubio, D.; Aucatoma, B.; Guillén, T.; Vargas Jentzsch, P.; Ciobotă, V.; Stolker, L.; Ulic, S.; Vásquez, L.; et al. Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador. Foods 2019, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Guelpa, A.; Marini, F.; du Plessis, A.; Slabbert, R.; Manley, M. Verification of authenticity and fraud detection in South African honey using NIR spectroscopy. Food Control 2017, 73, 1388–1396. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef]
- Yang, X.; Guang, P.; Xu, G.; Zhu, S.; Chen, Z.; Huang, F. Manuka honey adulteration detection based on near-infrared spectroscopy combined with aquaphotomics. LWT 2020, 132, 109837. [Google Scholar] [CrossRef]
- Peng, J.; Xie, W.; Jiang, J.; Zhao, Z.; Zhou, F.; Liu, F. Fast Quantification of Honey Adulteration with Laser-Induced Breakdown Spectroscopy and Chemmometric Methods. Foods 2020, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Elhamdaoui, O.; El Orche, A.; Cheikh, A.; Mojemmi, B.; Nejjari, R.; Bouatia, M. Development of Fast Analytical Methods for the Detection and Quantification of Honey Adulteration Using Vibrational Spectroscopy and Chemometrics Tools. J. Anal. Methods Chem. 2020, 2020, 8816249. [Google Scholar] [CrossRef] [PubMed]
- Molnar, C.M.; Berghian-Grosan, C.; Magdas, D.A. An optimized green preparation method for the successful application of Raman spectroscopy in honey studies. Talanta 2020, 208, 120432. [Google Scholar] [CrossRef] [PubMed]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. NIR detection of honey adulteration reveals differences in water spectral pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Rosset, M.; de Sousa, J.M.B.; de Oliveira, L.I.G.; Mafaldo, I.M.; Pintado, M.M.E.; de Souza, E.L.; Magnani, M. Stingless bee honey: An overview of health benefits and main market challenges. J. Food Biochem. 2021, 46, e13883. [Google Scholar] [CrossRef]
- Chan, B.K.; Haron, H.; Talib, R.A.; Subramaniam, P. Physical properties, antioxidant content and anti-oxidative activities of Malaysian stingless kelulut (Trigona spp.) honey. J. Agric. Sci. 2017, 9, 32–40. [Google Scholar]
- Mail, M.H.; Rahim, N.A.; Amanah, A.; Khawory, M.H.; Shahudin, M.A.; Seeni, A. FTIR and elementary analysis of Trigona honey, Apis honey and adulterated honey mixtures. Biomed. Pharmacol. J. 2019, 12, 2011–2017. [Google Scholar] [CrossRef]
- Do Nascimento, A.S.; Marchini, L.C.; de Carvalho, C.A.L.; Araújo, D.F.D.; de Olinda, R.A.; da Silveira, T.A. Physical-chemical parameters of honey of stingless bee (Hymenoptera: Apidae). Am. Chem. Sci. J. 2015, 7, 139–149. [Google Scholar] [CrossRef]
- Ramón-Sierra, J.M.; Ruiz-Ruiz, J.C.; de la Luz Ortiz-Vázquez, E. Electrophoresis characterisation of protein as a method to establish the entomological origin of stingless bee honeys. Food Chem. 2015, 183, 43–48. [Google Scholar] [CrossRef]
- Bijlsma, L.; de Bruijn, L.L.; Martens, E.P.; Sommeijer, M.J. Water content of stingless bee honeys (Apidae, Meliponini): Interspecific variation and comparison with honey of Apis mellifera. Apidologie 2006, 37, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Muncan, J.; Tsenkova, R. Aquaphotomics—From innovative knowledge to integrative platform in science and technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [Green Version]
- Tsenkova, R. Aquaphotomics: Dynamic spectroscopy of aqueous and biological systems describes peculiarities of water. J. Near Infrared Spectrosc. 2009, 17, 303–313. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Water in the biological and aqueous world scrutinised with invisible light. Spectrosc. Eur. 2010, 22, 6. [Google Scholar]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of aquaphotomics and its chemometrics approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Scalvenzi, L.; Sacchetti, G. Ecuadorian stingless bee (Meliponinae) honey: A chemical and functional profile of an ancient health product. Food Chem. 2009, 114, 1413–1420. [Google Scholar] [CrossRef]
- De Almeida-Muradian, L.B.; Stramm, K.M.; Estevinho, L.M. Efficiency of the FT-IR ATR spectrometry for the prediction of the physicochemical characteristics of M elipona subnitida honey and study of the temperature’s effect on those properties. Int. J. Food Sci. Technol. 2014, 49, 188–195. [Google Scholar] [CrossRef]
- Alimentarius, C. Revised codex standard for honey. Codex Stan 2001, 12, 1982. [Google Scholar]
- Souza, B.A.; Roubik, D.W.; Barth, O.M.; Heard, T.A.; Enríquez, E.; Carvalho, C.; Villas-Bôas, J.; Marchini, L.; Locatelli, J.; Persano-Oddo, L.; et al. Composition of stingless bee honey: Setting quality standards. Interciencia 2006, 31, 867–875. [Google Scholar]
- Shenk, J.S.; Workman, J.J., Jr.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007; pp. 365–404. [Google Scholar]
- Rust, A.; Marini, F.; Allsopp, M.; Williams, P.J.; Manley, M. Application of ANOVA-simultaneous component analysis to quantify and characterise effects of age, temperature, syrup adulteration and irradiation on near-infrared (NIR) spectral data of honey. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119546. [Google Scholar] [CrossRef]
- Tsenkova, R.N.; Iordanova, I.K.; Toyoda, K.; Brown, D.R. Prion protein fate governed by metal binding. Biochem. Biophys. Res. Commun. 2004, 325, 1005–1012. [Google Scholar] [CrossRef]
- Chatani, E.; Tsuchisaka, Y.; Masuda, Y.; Tsenkova, R. Water molecular system dynamics associated with amyloidogenic nucleation as revealed by real time near infrared spectroscopy and aquaphotomics. PLoS ONE 2014, 9, e101997. [Google Scholar] [CrossRef]
- Martin, K. In vivo measurements of water in skin by near-infrared reflectance. Appl. Spectrosc. 1998, 52, 1001–1007. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.; Ziena, H.; Youssef, M. Adulteration of honey with high-fructose corn syrup: Detection by different methods. Food Chem. 1993, 48, 209–212. [Google Scholar] [CrossRef]
- Bidin, N.; Zainuddin, N.H.; Islam, S.; Abdullah, M.; Marsin, F.M.; Yasin, M. Sugar detection in adulterated honey via fiber optic displacement sensor for food industrial applications. IEEE Sens. J. 2015, 16, 299–305. [Google Scholar] [CrossRef]
- Nikolova, K.; Panchev, I.; Sainov, S.; Gentscheva, G.; Ivanova, E. Selected physical properties of lime bee honey in order to discriminate between pure honey and honey adulterated with glucose. Int. J. Food Prop. 2012, 15, 1358–1368. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Lanza, E.; Li, B. Application for near infrared spectroscopy for predicting the sugar content of fruit juices. J. Food Sci. 1984, 49, 995–998. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A screening method based on Visible-NIR spectroscopy for the identification and quantification of different adulterants in high-quality honey. Talanta 2019, 203, 235–241. [Google Scholar] [CrossRef]
- Lenhardt, L.; Bro, R.; Zeković, I.; Dramićanin, T.; Dramićanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef]
- Peters, R.D.; Noble, S.D. Using near infrared measurements to evaluate NaCl and KCl in water. J. Near Infrared Spectrosc. 2019, 27, 147–155. [Google Scholar] [CrossRef]
- Li, H.; Yang, W.; Lei, J.; She, J.; Zhou, X. Estimation of leaf water content from hyperspectral data of different plant species by using three new spectral absorption indices. PLoS ONE 2021, 16, e0249351. [Google Scholar] [CrossRef]
- Das, B.; Sahoo, R.N.; Pargal, S.; Krishna, G.; Verma, R.; Viswanathan, C.; Sehgal, V.K.; Gupta, V.K. Evaluation of different water absorption bands, indices and multivariate models for water-deficit stress monitoring in rice using visible-near infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119104. [Google Scholar] [CrossRef] [PubMed]
- Vembadi, A.; Menachery, A.; Qasaimeh, M.A. Cell cytometry: Review and perspective on biotechnological advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.; Sainik, N.Q.A.V.; Chowdhury, S.R.; Saim, A.B.; Idrus, R.B.H. Physicochemical properties of stingless bee honey from around the globe: A comprehensive review. J. Food Compos. Anal. 2018, 73, 91–102. [Google Scholar] [CrossRef]
- Pontis, J.A.; Costa, L.A.M.A.D.; Silva, S.J.R.D.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Belay, A.; Solomon, W.K.; Bultossa, G.; Adgaba, N.; Melaku, S. Botanical origin, colour, granulation, and sensory properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2015, 167, 213–219. [Google Scholar] [CrossRef]
- Bodor, Z.; Benedek, C.; Urbin, Á.; Szabó, D.; Sipos, L. Colour of honey: Can we trust the Pfund scale?–An alternative graphical tool covering the whole visible spectra. LWT 2021, 149, 111859. [Google Scholar] [CrossRef]
- Shamsudin, S.; Selamat, J.; Sanny, M.; Abd Razak, S.B.; Jambari, N.N.; Mian, Z.; Khatib, A. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int. J. Food Prop. 2019, 22, 239–264. [Google Scholar] [CrossRef] [Green Version]
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.Á.; Heredia, F.J. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef]
- Omar, A.F.; Yahaya, O.K.M.; Tan, K.C.; Mail, M.H.; Seeni, A. The influence of additional water content towards the spectroscopy and physicochemical properties of genus Apis and stingless bee honey. In Optical Sensing and Detection IV; International Society for Optics and Photonics: Bellingham, WA, USA, 2016. [Google Scholar]
- Omar, A.F.; Atan, H.; MatJafri, M.Z. Peak response identification through near-infrared spectroscopy analysis on aqueous sucrose, glucose, and fructose solution. Spectrosc. Lett. 2012, 45, 190–201. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Hungerford, N.L.; Webber, D.; Carpinelli de Jesus, M.; Zhang, J.; Stone, I.S.; Blanchfield, J.T.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Giangiacomo, R. Study of water–sugar interactions at increasing sugar concentration by NIR spectroscopy. Food Chem. 2006, 96, 371–379. [Google Scholar] [CrossRef]
- Bakier, S. Application of NIR spectroscopy for the analysis of water-carbohydrate interactions in water solutions. Acta Agrophys 2008, 11, 7–21. [Google Scholar]
- Kovacs, Z.; Bázár, G.; Oshima, M.; Shigeoka, S.; Tanaka, M.; Furukawa, A.; Nagai, A.; Osawa, M.; Itakura, Y.; Tsenkova, R. Water spectral pattern as holistic marker for water quality monitoring. Talanta 2016, 147, 598–608. [Google Scholar] [CrossRef]
- Šašić, S.; Ozaki, Y. Short-wave near-infrared spectroscopy of biological fluids. 1. Quantitative analysis of fat, protein, and lactose in raw milk by partial least-squares regression and band assignment. Anal. Chem. 2001, 73, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Tsenkova, R.; Muncan, J. Aquaphotomics for Bio-Diagnostics in Dairy: Applications of Near-Infrared Spectroscopy; Springer: Singapore, 2021. [Google Scholar]
- Bakhsheshi, M.F.; Lee, T.-Y. Non-Invasive Monitoring of Brain Temperature by Near-Infrared Spectroscopy; Taylor & Francis: Milton Park, UK, 2015; Volume 2, pp. 31–32. [Google Scholar]
- Osborne, B.G. Near-infrared spectroscopy in food analysis. Encycl. Anal. Chem. Appl. Theory Instrum. 2006, 2, 31–32. [Google Scholar]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.; Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists: St. Paul, MN, USA, 1987. [Google Scholar]
- Williams, P.; Manley, M.; Antoniszyn, J. Near Infrared Technology: Getting the Best out of Light; African Sun Media: Stellenbosch, South Africa, 2019. [Google Scholar]
- Bázár, G.; Kovacs, Z.; Tanaka, M.; Furukawa, A.; Nagai, A.; Osawa, M.; Itakura, Y.; Sugiyama, H.; Tsenkova, R. Water revealed as molecular mirror when measuring low concentrations of sugar with near infrared light. Anal. Chim. Acta 2015, 896, 52–62. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.; Lawson, P. Short-wavelength near-infrared spectra of sucrose, glucose, and fructose with respect to sugar concentration and temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kitano, Y. Non-destructive determination of sugar content in satsuma mandarin fruit by near infrared transmittance spectroscopy. J. Near Infrared Spectrosc. 1995, 3, 227–237. [Google Scholar] [CrossRef]
- Guthrie, J.; Reid, D.; Walsh, K.B. Assessment of internal quality attributes of mandarin fruit. 2. NIR calibration model robustness. Aust. J. Agric. Res. 2005, 56, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, B.K.; Yang, W.; Hsieh, T.H.; Liaw, S.K.; Yang, F.L. Accurate prediction of glucose concentration and identification of major contributing features from hardly distinguishable near-infrared spectroscopy. Biomed. Signal Processing Control 2020, 59, 101923. [Google Scholar] [CrossRef]
- McGlone, V.A.; Kawano, S. Firmness, dry-matter and soluble-solids assessment of postharvest kiwifruit by NIR spectroscopy. Postharvest Biol. Technol. 1998, 13, 131–141. [Google Scholar] [CrossRef]
- Headrick, J.M.; Diken, E.G.; Walters, R.S.; Hammer, N.I.; Christie, R.A.; Cui, J.; Myshakin, E.M.; Duncan, M.A.; Johnson, M.A.; Jordan, K.D. Spectral signatures of hydrated proton vibrations in water clusters. Science 2005, 308, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Workman, J., Jr. The Handbook of Organic Compounds, Three-Volume Set: Nir, ir, r, and Uv-Vis Spectra Featuring Polymers and Surfactants; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Illumination, I.C.o. CIE 15: Technical Report: Colorimetry; CIE Technical Committee: Vienna, Austria, 2004. [Google Scholar]
- Tigabu, M.; Odén, P.C. Multivariate classification of sound and insect-infested seeds of a tropical multipurpose tree, Cordia africana, with near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 45–51. [Google Scholar] [CrossRef]
- Barnes, R.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Dotto, A.C.; Dalmolin, R.S.D.; Grunwald, S.; ten Caten, A.; Pereira Filho, W. Two preprocessing techniques to reduce model covariables in soil property predictions by Vis-NIR spectroscopy. Soil Tillage Res. 2017, 172, 59–68. [Google Scholar] [CrossRef]
- Wang, L.; Lee, F.S.; Wang, X.; He, Y. Feasibility study of quantifying and discriminating soybean oil adulteration in camellia oils by attenuated total reflectance MIR and fiber optic diffuse reflectance NIR. Food Chem. 2006, 95, 529–536. [Google Scholar] [CrossRef]


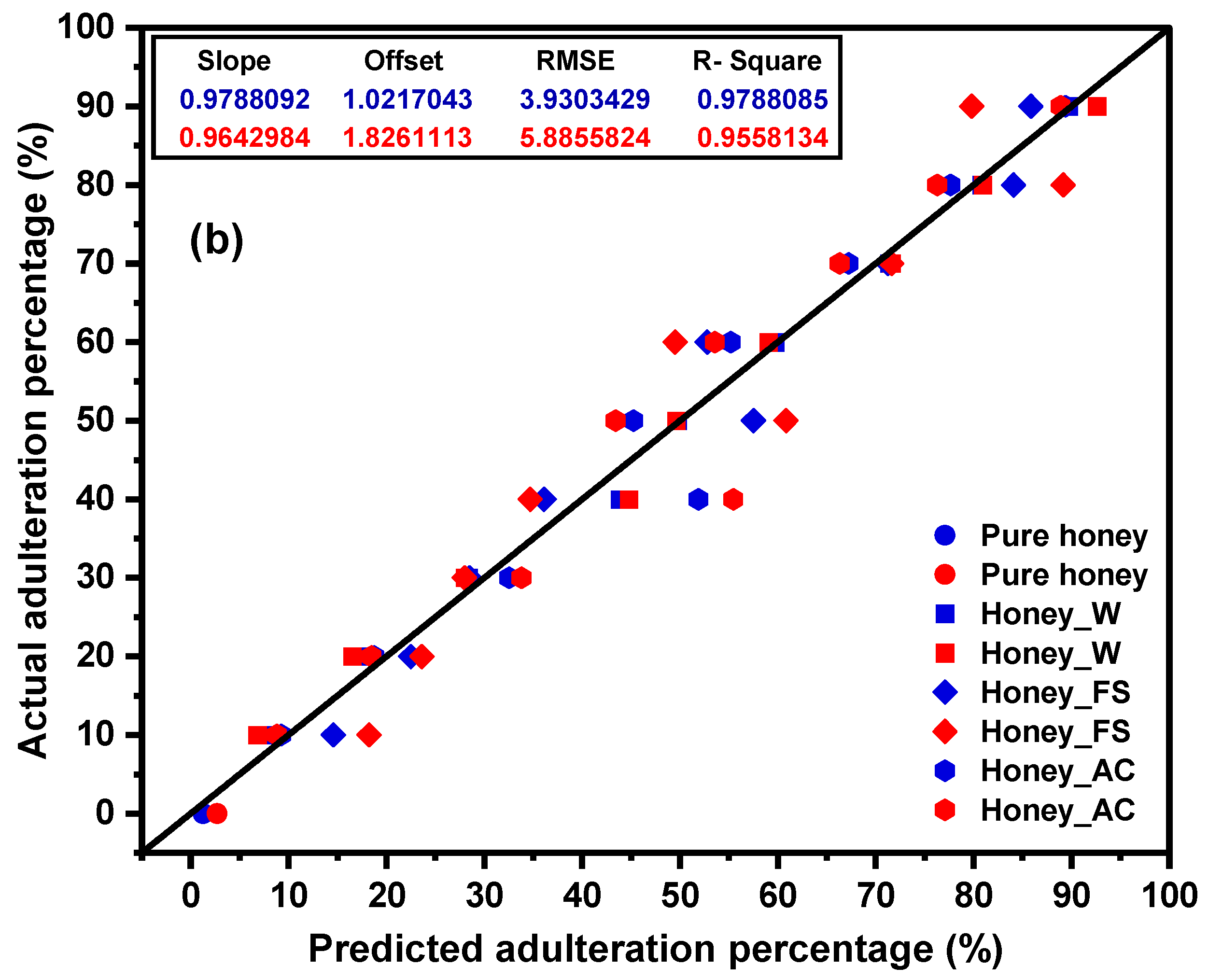
| Pre-Processing Methods | LVs | ||||
|---|---|---|---|---|---|
| Raw absorbance | 7 | 3.65 | 6.43 | 0.98 | 0.95 |
| Smoothing | 7 | 4.20 | 6.49 | 0.97 | 0.95 |
| Detrend 1st polynomial | 7 | 2.34 | 6.33 | 0.99 | 0.95 |
| 1st derivative SG | 6 | 3.85 | 6.29 | 0.98 | 0.95 |
| Smoothing, Detrend 1st Polynomial | 7 | 3.93 | 5.88 | 0.98 | 0.96 |
| Smoothing, detrend 1st polynomial, 1st derivative SG | 6 | 4.08 | 6.58 | 0.98 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raypah, M.E.; Omar, A.F.; Muncan, J.; Zulkurnain, M.; Abdul Najib, A.R. Identification of Stingless Bee Honey Adulteration Using Visible-Near Infrared Spectroscopy Combined with Aquaphotomics. Molecules 2022, 27, 2324. https://doi.org/10.3390/molecules27072324
Raypah ME, Omar AF, Muncan J, Zulkurnain M, Abdul Najib AR. Identification of Stingless Bee Honey Adulteration Using Visible-Near Infrared Spectroscopy Combined with Aquaphotomics. Molecules. 2022; 27(7):2324. https://doi.org/10.3390/molecules27072324
Chicago/Turabian StyleRaypah, Muna E., Ahmad Fairuz Omar, Jelena Muncan, Musfirah Zulkurnain, and Abdul Rahman Abdul Najib. 2022. "Identification of Stingless Bee Honey Adulteration Using Visible-Near Infrared Spectroscopy Combined with Aquaphotomics" Molecules 27, no. 7: 2324. https://doi.org/10.3390/molecules27072324
APA StyleRaypah, M. E., Omar, A. F., Muncan, J., Zulkurnain, M., & Abdul Najib, A. R. (2022). Identification of Stingless Bee Honey Adulteration Using Visible-Near Infrared Spectroscopy Combined with Aquaphotomics. Molecules, 27(7), 2324. https://doi.org/10.3390/molecules27072324








