The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria
Abstract
1. Introduction
2. Results
2.1. Characterization of the Bacterial Isolates Obtained and Their Antibiotic Bioassay
2.2. Phytochemical Screening of CFE and In Vitro Antibacterial Activity
2.3. Validation of Type 1 Diabetic and Diabetic Wound Onset
2.4. Effect of CFE and Cefepime on Wound Healing
2.5. Effect of CFE and Cefepime on the Expression of Wound Healing Markers and Collagen Deposition
2.6. Effect of Topical Application of Either CFE or Cefepime Hydrogel on the Wound Growth Factors Signaling Pathway
2.7. Effect of CFE and Cefepime on the Wound Oxidative Status
2.8. Effect of CFE and Cefipime on the Expression of Wound Inflammatory and Anti-Inflammatory Markers
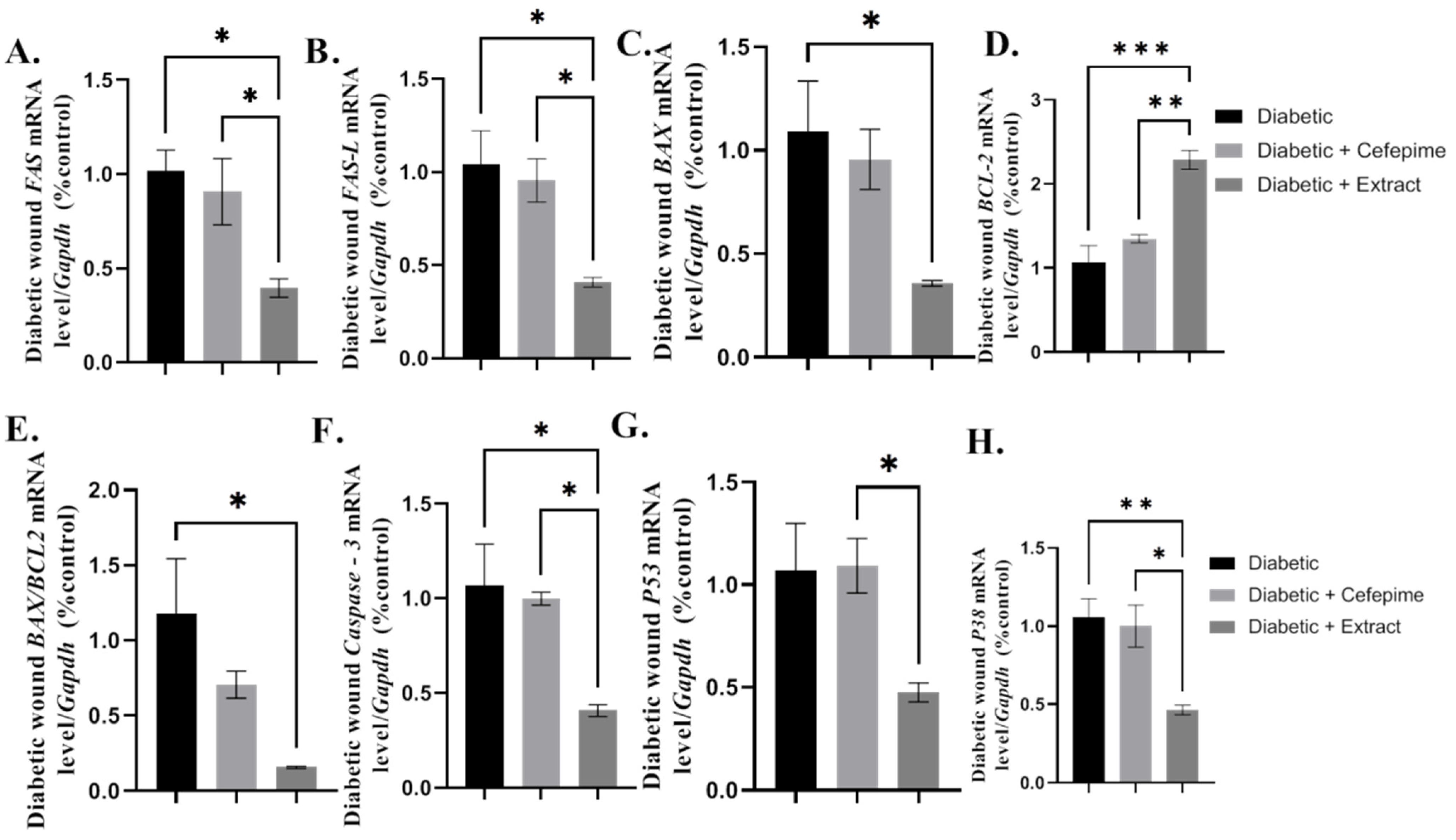
2.9. Effect of CFE and Cefepime on the Wound Apoptotic Signaling Pathway
2.10. Effect of CFE and Cefepime on the Histopathological Picture of Type 1 Diabetic Wound
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, and Plants
4.2. Pus Sample Collection and Bacterial Identification
4.3. Preparayion of the Clove Flower Extract CFE and Phytochemical Screening
4.4. Antibacterial Activity of CFE against MDR P. mirabilis
4.5. (MICs) of Both CFE and the Antibiotic Cefepime
4.6. CFE and Cefepime Hydrogel Preparation
4.7. Experimental Animals and Ethical Declaration
4.8. Experimental Design
4.9. Measuring Glycemic Parameters and Oxidant/Antioxidant Activity
4.10. Measuring Wound Diameter and Wound Index
4.11. Rt-qPCR for Wound Gene Expression
4.12. Determination of the Wound Total Bacterial and Coliform Count
4.13. Histopathological Examination for H&E and Masson Blue
4.14. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Core, D.; Ahn, J.; Robert, B.S.; Lewis, B.; Katherine, M.D.; Raspovic, M.; Trapper, D.P.M.; Lalli, A.J.; Dane, K.; Wukich, M.D. The Evaluation and Treatment of Diabetic Foot Ulcers and Diabetic Foot Infections. Foot Ankle Orthop. 2018, 3, 247301141878886. [Google Scholar] [CrossRef]
- Williams, D.T.; Hilton, J.R.; Harding, K.G. Diagnosing foot infection in diabetes. in Clinical Infectious Diseases. Clin. Infect. Dis. 2004, 39 (Suppl. 2), S83–S86. [Google Scholar] [CrossRef] [PubMed]
- Enan, G. Control of the regrowing bacteriocin resistance variants of Listeria monocytogenes LMG 10470 in Vitro and in Food by nisin-plantaricin UG1 mixture. Biotechnology 2006, 5, 143–147. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Ouda, S.M.; Elbalat, I.; Enan, G. Characterization and identification of multidrug resistant bacteria from some Egyptian patients. Biotechnology 2013, 12, 65–73. [Google Scholar] [CrossRef]
- Enan, G.; Abdel-Shafi, S.; Ouda, S.M.; El-Balat, I. Genetic linkage of the antibiotic resistance ability in the Escherichia coli UR4 strain isolated from urine. J. Med Sci. 2013, 13, 261–268. [Google Scholar] [CrossRef][Green Version]
- Ismaiel, A.A.; Ali, E.A.; Enan, G. Incidence of Listeria in Egyptian meat and dairy samples. Food Sci. Biotechnol. 2014, 234, 179–181. [Google Scholar] [CrossRef]
- El-Sayed, T.; Atef, D.; Amer, M.; Mahdy, A.; Enan, G. Molecular characterization and inhibition by natural agents of multidrug resistant Candida strains causing vaginal candidiasis. Res. J. Med Sci. 2015, 9, 1–7. [Google Scholar]
- Osman, A.; El-Didamony, G.; Sitohy, M.; Khalifa, M.; Enan, G. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int. J. Appl. Res. Nat. Prod. 2016, 9, 17–26. [Google Scholar]
- Saltoglu, N.; Ergonul, O.; Tulek, N.; Yemisen, M.; Kadanali, A.; Karagoz, G.; Batirel, A.; Ak, O.; Sonmezer, C.; Eraksoy, H.; et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int. J. Infect. Dis. 2018, 70, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Enan, F.; Osman, M.E.; Abdel-Haliem, M.E.F.; Abdel-Ghany, S.E. Advanced in microbial and nucleic acids biotechnology. Biomed Res. Int. 2018, 2018, 3102374. [Google Scholar] [CrossRef] [PubMed]
- Enan, F.; Al-Mohammadi, A.R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.F.; Taha, M.A.; El-Gazzar, N. Inhibition of Staphylococcus aureus LC554891 by Moringa oleifera seed extract either singly or in combination with antibiotics. Molecules 2020, 25, 4583. [Google Scholar] [CrossRef] [PubMed]
- Fattah Hamid, S.; Bahadeen Taha, A.; Jamel Abdulwahid, M. Distribution of blaTEM, blaSHV, blaCTX-M, blaOXA, and blaDHA in Proteus mirabilis Isolated from Diabetic Foot Infections in Erbil, Iraq. Cell. Mol. Biol. 2020, 66, 88. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.-R.; El-Didamony, G.; Abdel-Haliem, M.E.F. Antimicrobial activity of Enterococcus faecium NM2 isolated from urine: Purification, characterization and bactericidal action of enterocin NM2. Asian J. Appl. Sci. 2014, 7, 721–734. [Google Scholar] [CrossRef]
- Enan, G.; El-Didamony, G.; Mohamed, E.H.; Zakaria, A. Novel antibacterial activity of Enterococcus faecium NM2 isolated from urine of healthy people. Asian J. Appl. Sci. 2014, 7, 66–78. [Google Scholar] [CrossRef]
- Ouda, S.M.; Debevere, J.; Enan, G. Purification and biochemical characterization of plantaricin UG1, a bacteriocin produced by Lactobacillas plantarum UG1 isolated from dry sausage. Life Sci. J. 2014, 11, 271–279. [Google Scholar]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Negm, S.; Enan, G. Antibacterial activity of Lactobacillas delbreukii subsp. bulgaricus isolated from Zabady. Life Sci. J. 2014, 11, 264–270. [Google Scholar]
- Osman, A.; Abdel-Shafi, S.; Al-Mohammadi, A.R.; Kamal, N.; Enan, G.; Sitohy, M. Catifish glycoprotein, a highhly powerful safe preservatyive of minced beef stored at 4 °C for 15 days. Foods 2020, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Osman, A.; Enan, G.; El-Nemer, M.; Sitohy, M. Antibacterial activity of methylated egg white proteins against pathogenic G+ and G− bacteria matching antibiotics. Springeplus 2016, 5, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Enan, G.; Al-Mohammadi, A.; Abdel-Shafi, S.; Abdel-Hameid, S.; Sitohy, M.; El-Gazzar, N. Antibacterial peptides produced by Alcalase from cowpea seed proteins. Antibiotics 2021, 10, 870. [Google Scholar] [CrossRef]
- Sitohy, M.; Al-Mohammadi, A.R.; Osman, A.; Abdel-Shafi, S.; El-Gazzar, N.; Hamdi, S.; Ismail, S.H.; Enan, G. Silver-protein nanocomposites as antimicrobial agents. Nanomaterials 2021, 11, 3006. [Google Scholar] [CrossRef]
- Askoura, M.; Saed, N.; Enan, G.; Askora, A. Characterization of polyvalent bacteriophages targetng multidrug-resistant Klebsiella pneumonia with enhanced anti-biofilm activity. Appl. Biochem. Micrbiol. 2021, 57, 117–126. [Google Scholar] [CrossRef]
- Yan, C.; Gao, N.; Sun, H.; Yin, J.; Lee, P.; Zhou, L.; Fan, X.; Yu, F.S. Targeting Imbalance between IL-1β and IL-1 Receptor Antagonist Ameliorates Delayed Epithelium Wound Healing in Diabetic Mouse Corneas. Am. J. Pathol. 2016, 186, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Zubair, M.; Malik, A. Plasma adiponectin, IL-6, hsCRP, and TNF-α levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian J. Endocrinol. Metab. 2012, 16, 769. [Google Scholar] [CrossRef] [PubMed]
- Portou, M.J.; Yu, R.; Baker, D.; Xu, S.; Abraham, D.; Tsui, J. Hyperglycaemia and Ischaemia Impair Wound Healing via Toll-like Receptor 4 Pathway Activation in vitro and in an Experimental Murine Model. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Van Asten, S.A.; Nichols, A.; La Fontaine, J.; Bhavan, K.; Peters, E.J.; Lavery, L.A. The value of inflammatory markers to diagnose and monitor diabetic foot osteomyelitis. Int. Wound J. 2017, 14, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, L.; Nabzdyk, C.; Andersen, N.D.; LoGerfo, F.W.; Veves, A. Inflammation and neuropeptides: The connection in diabetic wound healing. Expert Rev. Mol. Med. 2009, 11, e2. [Google Scholar] [CrossRef]
- Seyed, M.A.; Ayesha, S. Modern Phytomedicine in Treating Diabetic Foot Ulcer: Progress and Opportunities. In Diabet Foot Ulcer; Springer: Singapore, 2021; pp. 281–313. [Google Scholar] [CrossRef]
- Faujdar, S.; Bisht, D.; Sharma, A. Antibacterial activity of Syzygium aromaticum (clove) against uropathogens producing ESBL, MBL, and AmpC beta-lactamase: Are we close to getting a new antibacterial agent? J. Fam. Med. Prim. Care 2020, 9, 180. [Google Scholar] [CrossRef]
- Rodríguez, J.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv. Pharmacol. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Tayebwa, D.S.; Shaheen, H.M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick-Borne Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Lin, Q.; Gu, Y.; Yu, W. Eugenol protects cells against oxidative stress via Nrf2. Exp. Ther. Med. 2020, 21, 2. [Google Scholar] [CrossRef]
- Loose, M.; Pilger, E.; Wagenlehner, F. Anti-bacterial effects of essential oils against uropathogenic bacteria. Antibiotics 2020, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Antonescue, I.A.; Antonescu, A.; Miere, F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of wound healing potential of novel hydrogel based on Ocimum basilicum and Trifolium pratense extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Antonescu, A.-I.; Miere, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the combined effects of Ocimum basilicum and Trifolium pratense extracts in terms of phytochemical profile and pharmacological effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.J.; Leandro, C.I.; Bonaparte, D.P.; Pinto, A.L. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp. Med. Am. Assoc. Lab. Anim. Sci. 2012, 62, 37–48. [Google Scholar]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilback, C.; Sandgren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathgens 2011, 7, 1002158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Shier, K.L.; Graber, G.J. Determining a clinical frameworkj for use of cefepime and β-lactam/β-lactamase inhibitors iun the treatment of infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2014, 69, 871–880. [Google Scholar] [CrossRef]
- Enan, G.; El-Essawy, A.A.; Uytendaele, M.; Debevere, J. Antibacterial activity of Lactobacillus plantarum UG1: Characterization, production and bactericidal action of plantarcin UG1. Int. J. Food Microbiol. 1996, 30, 189–215. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Enan, G.; Osman, A.; Kamal, N.; Sitohy, M. Characterization and antibacterial activity of both 7S and 11S globulins isolated from cowpea seed protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef]
- Sautter, N.B.; Delaney, K.L.; Hausman, F.A.; Trune, D.R. Tissue remodeling in the acute otitis media mouse model. Int. J. Pediatric Otorhinolaryngol. 2011, 75, 1368–1371. [Google Scholar] [CrossRef]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.T.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Artif. Cells Nanomed. Biotechnol. 2017, 45, 591–597. [Google Scholar] [CrossRef]
- Galehdari, H.; Negahdari, S.; Kesmati, M.; Rezaie, A.; Shariati, G. Effect of the herbal mixture composed of Aloe Vera, Henna, Adiantum capillus-veneris, and Myrrha on wound healing in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Meisser, A.; Bischof, P. Metalloproteinases and Human Placental Invasiveness. Placenta 2006, 27, 783–793. [Google Scholar] [CrossRef]
- Ekmektzoglou, K.A.; Zografos, G.C. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J. Gastroenterol. 2006, 12, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Chamberland, J.P.; Mantzoros, C.S. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metab. Clin. Exp. 2013, 62, 1279–1286. [Google Scholar] [CrossRef]
- Buteau, J.; Foisy, S.; Joly, E.; Prentki, M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003, 52, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, G.Y.; Maeng, H.J.; Kim, H.; Bae, J.H.; Kim, K.M.; Lim, S. Effects of glucagon-like peptide-1 analogue and fibroblast growth factor 21 combination on the atherosclerosis-related process in a type 2 diabetes mouse model. Endocrinol. Metabolism. 2021, 36, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Icre, G.; Wahli, W.; Michalik, L. Functions of the peroxisome proliferator-activated receptor (PPAR) α and β in skin homeostasis, epithelial repair, and morphogenesis. J. Investig. Dermatol. Symp. Proc. 2006, 11, 30–35. [Google Scholar] [CrossRef]
- Coll, T.; Barroso, E.; Álvarez-Guardia, D.; Serrano, L.; Salvadó, L.; Merlos, M.; Palomer, X.; Vázquez-Carrera, M. The role of peroxisome proliferator-activated receptor β/δ on the inflammatory basis of metabolic disease. PPAR Res. Vol. 2010, 2010, 368467. [Google Scholar] [CrossRef]
- Leask, A. ‘The Contribution of Peroxisome Proliferator–Activated Receptor Gamma to Cutaneous Wound Healing. Adv. Wound Care. 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Lamers, M.L.; Almeida, M.E.; Vicente-Manzanares, M.; Horwitz, A.F.; Santos, M.F. High glucose-mediated oxidative stress impairs cell migration. PLoS ONE 2011, 6, e22865. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Y.; Ardans, J.A.; Wahl, L.M. Interferon-γ Differentially Regulates Monocyte Matrix Metalloproteinase-1 and -9 through Tumor Necrosis Factor-α and Caspase 8. J. Biol. Chem. 2003, 278, 45406–45413. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef]
- Nguyen, J.; Gogusev, J.; Knapnougel, P.; Bauvois, B. Protein tyrosine kinase and p38 MAP kinase pathways are involved in stimulation of matrix metalloproteinase-9 by TNF-α in human monocytes. Immunol. Lett. 2006, 106, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawaf, H.A.; Gabr, S.A.; Alghadir, A.H. Molecular Changes in Diabetic Wound Healing following Administration of Vitamin D and Ginger Supplements: Biochemical and Molecular Experimental Study. Evid. Based Complement. Altern. Med. 2019, 2019, 4352470. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.-T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-kB Pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol potentiates cisplatin anti-cancer activity through inhibition of ALDH-positive breast cancer stem cells and the NF-κB signaling pathway. Mol. Carcinog. 2018, 57, 333–346. [Google Scholar] [CrossRef]
- Roncon, F.P.; Silva, R.C.; Ponci, V.; Oliv, C.R.; Arantes-Costa, F.M.; Caperuto, L.C.; Tiberio, I.C.; Lago, J.H.G.; Prado, C.M. Anti-Inflammatory effects of eugenol and dehydrodieugenol in a murine model of allergic asthma. In Prcoceedings of the American Thoracic Society International Conference Meetings Abstracts, Dallas, TX, USA, 17–22 May 2019; Dallas, TX, USA, American Thoracic Society; p. A2184. [CrossRef]
- Han, X.; Parker, T.L. ‘Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; Dipietro, L.A. Diabetes and wound angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, S.; Wadsworth, S.; Cullen, B.; Silcock, D.; Ma, J.Y.; Mangadu, R.; Kerr, I.; Chakravarty, S.; Luedtke, G.L.; Dugar, S.; et al. p38 MAPK inhibition reduces diabetes-induced impairment of wound healing. Diabetes Metab. Syndr. Obes. Targets Ther. 2009, 2, 91–100. [Google Scholar] [CrossRef][Green Version]
- Nuñez, L.; D’Aquino, M. Microbicide activity of clove essential oil (Eugenia Caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.J.; Rumpf, D.A.H. ‘Chlorhexidine-Induced Changes to Human Gingival Fibroblast Collagen and Non-Collagen Protein Production. J. Periodontol. 1999, 70, 1443–1448. [Google Scholar] [CrossRef]
- Philip, R.; Dinsuhaimi, S.; Rosdan, S.; Samsudin, A.R.; Shamsuria, O.; Zaki, S.M.; Jamalulail, S.M.S. In vitro evaluation of the growth enhancing or cytotoxic effect of Sticophus species (Gamat) on established human fibroblast cell lines and antimicrobial activity. Med. J. Malays. 2004, 59 (Suppl. B), 95–96. [Google Scholar]
- Hirsch, T.; Koerber, A.; Jacobsen, F.; Dissemond, J.; Steinau, H.U.; Gatermann, S.; Al-Benna, S.; Kesting, M.; Seipp, H.M.; Steinstraesser, L. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J. Surg. Res. 2010, 164, 344–350. [Google Scholar] [CrossRef]
- Altoé, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Gonçalves, R.V. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS ONE 2019, 14, e0223511. [Google Scholar] [CrossRef] [PubMed]
- Beloeil, P.; Guerra, B.; Stoicescu, A. Manual for Reporting on Antimicrobial Resistance within the Framework of Directive 2003/99/EC And Decision 2013/652/EU for Information Derived from the Year 2019; EFSA Supporting Publications; Wiley: Hoboken, NJ, USA, 2020; Volume 17, p. 1794E. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standfards for Antimicrobial Susceptibility Testing: Twenty-Fourth Information Supplement. CLSI 2014, 34, 100–122. [Google Scholar]
- Ligozzi, M.; Bernini, C.; Bonora, M.G.; De Fatima, M.; Zuliani, J.; Fontana, R. Evaluation of the VITEK 2 system for identification and antimicrobial susceptibility testing of medically relevant gram-positive cocci. J. Clin. Microbiol. 2002, 40, 1681–1686. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D. Molecular Cloning: A Labpratory Mannual, 3rd ed.; Cold Springs Harbour Laboratory Press: Woodburry NY, USA, 2001. [Google Scholar]
- El Ghallab, Y.; Al Jahid, A.; Eddine, J.J.; Said, A.A.H.; Zarayby, L.; Derfoufi, S. Syzygium aromaticum L.: Phytochemical investigation and comparison of the scavenging activity of essential oil, extracts and eugenol. Orient. Pharm. Exp. Med. 2019, 20, 153–158. [Google Scholar] [CrossRef]
- Okur, M.E.; Karadağ, A.E.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Kültür, S.; Demirci, B.; Demirci, F. Anti-inflammatory, analgesic and in vivo-in vitro wound healing potential of the Phlomis rigida Labill. extract. J. Ethnopharmacol. 2021, 266, 113408. [Google Scholar] [CrossRef]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly Developed Topical Cefotaxime Sodium Hydrogels: Antibacterial Activity and in Vivo Evaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef]
- Muthachan, T.; Tewtrakul, S. Anti-inflammatory and wound healing effects of gel containing Kaempferia marginata extract. J. Ethnopharmacol. 2019, 240, 111964. [Google Scholar] [CrossRef]
- Tasić-Kostov, M.; Arsić, I.; Pavlović, D.; Stojanović, S.; Najman, S.; Naumović, S.; Tadić, V. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J. Ethnopharmacol. 2019, 238, 111789. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Arulselvan, P.; Cheah, P.S.; Abas, F.; Fakurazi, S. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. Dev. Ther. 2016, 10, 1715–1730. [Google Scholar] [CrossRef]
- Solarek, W.; Koper, M.; Lewicki, S.; Szczylik, C.; Czarnecka, A.M. Insulin and insulin-like growth factors act as renal cell cancer intratumoral regulators. J. Cell Commun. Signal. 2019, 13, 381–394. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, D.; Wang, C.; Garzotto, M.; Kopp, R.; Wilmot, B.; Thuillier, P.; Dang, A.; Palma, A.; Farris, P.E.; et al. Polymorphisms in oxidative stress pathway genes and prostate cancer risk. Cancer Causes Control 2019, 30, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Khamis, T.; Abdelalim, A.F.; Saeeda, A.A.; Edress, N.M.; Nafea, A.; Ebian, H.F.; Algendy, R.; Hendawy, D.M.; Arisha, A.H.; Abdallah, S.H. Breast milk MSCs upregulated β-cells PDX1, Ngn3, and PCNA expression via remodeling ER stress /inflammatory /Apoptotic signaling pathways in type 1 diabetic rats. Eur. J. Pharmacol. 2021, 776, 174188. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mclennan, S.V.; Lo, L.; Natfaji, A.; Bolton, T.; Liu, Y.; Twigg, S.M.; Yue, D.K. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care. Am. Diabetes Assoc. 2007, 30, 378–380. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. Bancroft’s Theory and Practice of Histological Techniques, Chapter: 10 The Hematoxylins and Eosin, Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]







| No | Biochemical Tests | Abbreviations | Morganella monganii | Proteus mirabilis | Serratia fonticola | Escherichia coli |
|---|---|---|---|---|---|---|
| 1- | Ala-phe-pro-arylamidase | APPA | −ve | −ve | −ve | −ve |
| 2- | Hydrogen sulfide production | H2S | +ve | +ve | +ve | −ve |
| 3- | Beta-glucosidase | BGLu | −ve | −ve | +ve | −ve |
| 4- | L-proline arylamidase | ProA | −ve | −ve | −ve | +ve |
| 5- | Saccharose/Sucralose | SAC | −ve | −ve | +ve | +ve |
| 6- | L-Lactate alkalinization | LLATK | +ve | +ve | +ve | +ve |
| 7- | Glycine arylamidase | GlyA | −ve | −ve | −ve | −ve |
| 8- | O/129 Resistance | O129R | +ve | +ve | +ve | +ve |
| 9- | Adonitol | ADO | −ve | −ve | +ve | −ve |
| 10- | Beta-N-Acetyl-glucose aminidase | BNAG | −ve | −ve | −ve | −ve |
| 11- | D-Maltose | dMAL | −ve | −ve | +ve | +ve |
| 12- | Lipase | LiP | −ve | −ve | −ve | −ve |
| 13- | d-Tagatose | dTAG | −ve | −ve | −ve | −ve |
| 14- | Alpha-glucosidase | AGlu | −ve | −ve | +ve | −ve |
| 15- | Ornithine decarboxylase | ODC | +ve | +ve | −ve | −ve |
| 16- | Glu-Gly-Arg-Arylamidase | GGAA | −ve | −ve | −ve | −ve |
| 17- | L-pyrrolydonyl-arilamidase | PyrA | −ve | −ve | −ve | −ve |
| 18- | Glutamyl arylamidase PNA | AGLTP | −ve | −ve | −ve | −ve |
| 19- | D-Mannitol | dMAN | −ve | −ve | +ve | +ve |
| 20- | Palatinose | PLE | −ve | −ve | +ve | −ve |
| 21- | D-Trehalose | dTRE | −ve | +ve | −ve | +ve |
| 22- | Succinate alkalinization | SuCT | +ve | +ve | +ve | +ve |
| 23- | Lysine decarboxylase | LDC | −ve | −ve | −ve | +ve |
| 24- | I-Malate assimilation | IMLTa | −ve | −ve | −ve | −ve |
| 25- | L-Arabitol | lARL | −ve | −ve | +ve | −ve |
| 26- | D-Glucose | dGLu | +ve | +ve | +ve | +ve |
| 27- | D-Mannose | dMNE | +ve | −ve | +ve | +ve |
| 28- | Tyrosine arylamidase | TyrA | +ve | +ve | +ve | +ve |
| 29- | Citrate (Sodium) | CIT | −ve | +ve | +ve | −ve |
| 30- | Beta-N-Acetyl-galactosaminidase | NAGA | −ve | −ve | −ve | −ve |
| 31- | L-histidine assimilation | LHISa | −ve | −ve | −ve | −ve |
| 32- | ELLMAN | ELLM | +ve | +ve | +ve | −ve |
| 33- | D-Cellobiose | dCEL | −ve | −ve | −ve | −ve |
| 34- | Gamma-Glutamyl transferase | GGT | +ve | +ve | +ve | −ve |
| 35- | Beta-Xylosidase | Bxyl | −ve | −ve | −ve | −ve |
| 36- | Urease | URE | +ve | +ve | +ve | −ve |
| 37- | Malonate | MNT | −ve | −ve | −ve | −ve |
| 38- | Alpha-galactosidase | AGAL | −ve | −ve | −ve | +ve |
| 39- | Coumarate | CMT | +ve | +ve | +ve | +ve |
| 40- | L-lactate assimilation | ILATa | −ve | −ve | −ve | −ve |
| 41- | Beta-galactosidase | BGAL | −ve | −ve | −ve | +ve |
| 42- | Fermentation glycose | OFF | +ve | +ve | +ve | +ve |
| 43- | Beta-alanine arylamidase PNA | BALaP | −ve | −ve | −ve | −ve |
| 44- | D-Sorbitol | dSOR | −ve | −ve | −ve | +ve |
| 45- | 5-Keto-D-gluconate | 5KG | −ve | −ve | −ve | −ve |
| 46- | Phosphatase | PHOS | +ve | +ve | +ve | −ve |
| 47- | Beta-Glucoronidase | BGUR | −ve | −ve | −ve | −ve |
| Concentration of the Antimicrobial Agents | Growth of P. mirabilis |
|---|---|
| Cefepime (μg/mL) | |
| 10 | Positive |
| 20 | Positive |
| 30 | Positive |
| 40 | Positive |
| 50 | No growth |
| CFE (μg/mL) | |
| 2 | Positive |
| 4 | Positive |
| 6 | Positive |
| 8 | Negative |
| 10 | Negative |
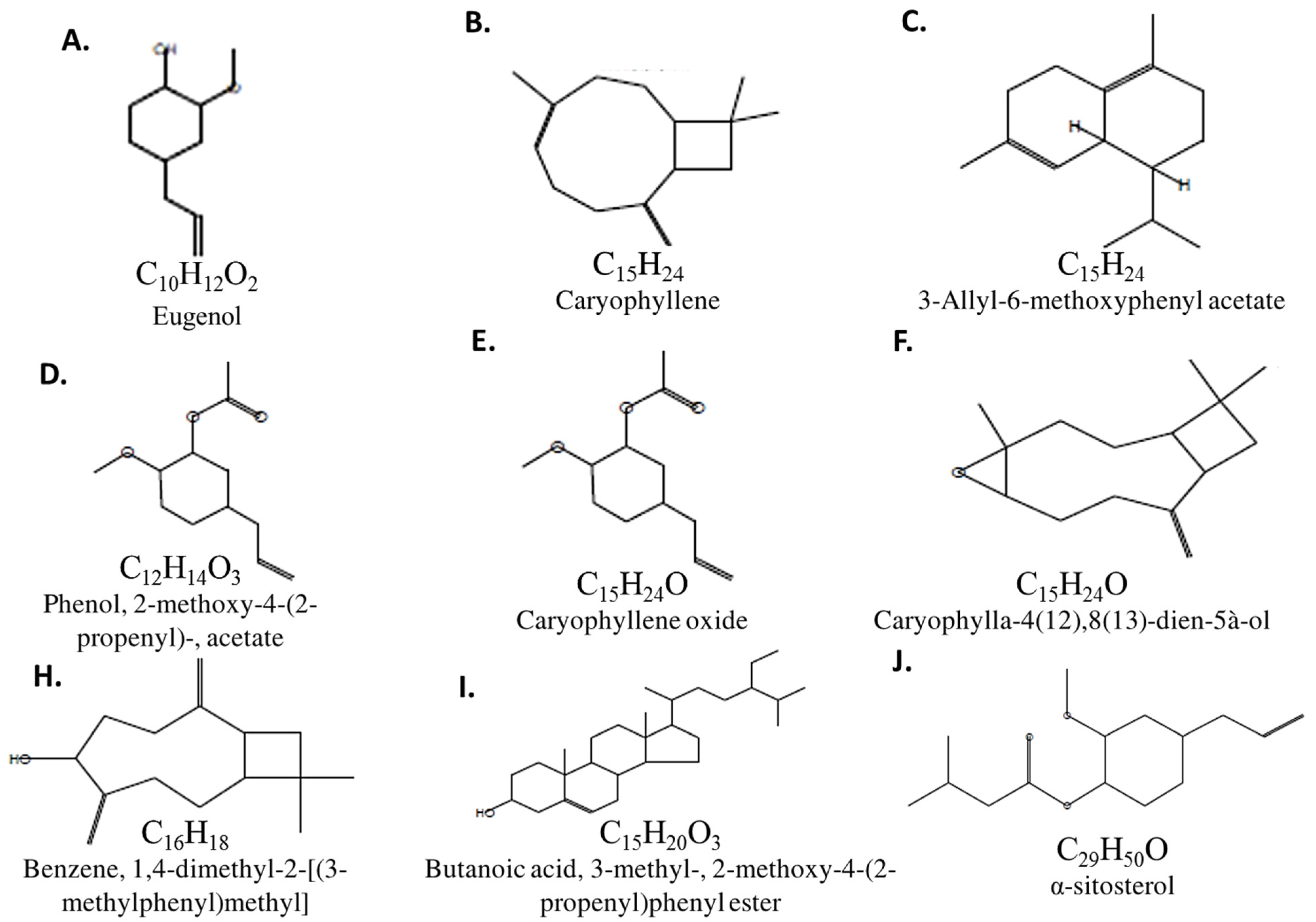
| RT/min | Name & Class | Mol. Formula | Mol. wt | Area | Base Peak (100%) |
|---|---|---|---|---|---|
| 13.78 | Eugenol (Phenol) | C10H12O2 | 164.0 | 82.34 | 77.00 |
| 14.40 | Caryophyllene (Bicyclic) | C15H24 | 204.0 | 3.20 | 133.0 & 93.00 |
| 16.47 | 3-Allyl-6-methoxyphenyl acetate | C15H24 | 204.0 | 0.26 | 161.0 |
| 17.04 | Phenol, 2-methoxy-4-(2-propenyl)-, acetate | C12H14O3 | 206.0 | 0.24 | 164.0 |
| 17.79 | Caryophyllene oxide (Bicyclic) | C15H24O | 220.0 | 0.48 | 41.00 |
| 19.06 | Caryophylla-4(12),8(13)-dien-5à-ol | C15H24O | 220.0 | 0.42 | 136.0 |
| 20.15 | Benzene, 1,4-dimethyl-2-[(3-methylphenyl)methyl] | C16H18 | 210 | 0.12 | 195 |
| 43.05 | Butanoic acid, 3-methyl-, 2-methoxy-4-(2-propenyl)phenyl ester | C15H20O3 | 248.0 | 0.27 | 164.0 |
| 49.51 | α-sitosterol (steroid) | C29H50O | 414.0 | 0.84 | 107.0 |
| Primer | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Size | Accession No. |
|---|---|---|---|---|
| PCNA | ATCTAGACGTCGCAACTCCG | GCTGCACTAAGGAGACGTGA | 173 | NM_022381.3 |
| Mmp9 | GATCCCCAGAGCGTTACTCG | GTTGTGGAAACTCACACGCC | 132 | NM_031055.2 |
| Collagen | GCAATGCTGAATCGTCCCAC | CAGCACAGGCCCTCAAAAAC | 176 | NM_053304.1 |
| Fibronectin | GGATCCCCTCCCAGAGAAGT | GGGTGTGGAAGGGTAACCAG | 188 | NM_019143.2 |
| NF-κβ1 | CCACTGTCAACAGATGGCCC | CTTTGCAGGCCCCACATAGT | 177 | NM_001276711.1 |
| TNF-α | GGCTTTCGGAACTCACTGGA | GGGAACAGTCTGGGAAGCTC | 164 | NM_012675.3 |
| IL-10 | GCTCAGCACTGCTATGTTGC | TTGTCACCCCGGATGGAATG | 76 | NM_012854.2 |
| IL-8 | ACAGGCAGGCTGTAGTTGTC | ATCACCAGCGAGTTTCCCAG | 70 | NM_019310.1 |
| IL-4 | CGTGATGTACCTCCGTGCTT | GTGAGTTCAGACCGCTGACA | 88 | NM_201270.1 |
| IL-1β | GAGTCTGCACAGTTCCCCAA | TCCTGGGGAAGGCATTAGGA | 158 | NM_031512.2 |
| MCP-1 | TAGCATCCACGTGCTGTCTC | CAGCCGACTCATTGGGATCA | 94 | NM_031530.1 |
| TGF-β1 | AGGGCTACCATGCCAACTTC | CCACGTAGTAGACGATGGGC | 168 | NM_021578.2 |
| IL-1β | GAGTCTGCACAGTTCCCCAA | TCCTGGGGAAGGCATTAGGA | 158 | NM_031512.2 |
| Gapdh | GCATCTTCTTGTGCAGTGCC | GGTAACCAGGCGTCCGATAC | 91 | NM_017008.4 |
| GLP1 | CACCTCCTCTCAGCTCAGTC | CGTTCTCCTCCGTGTCTTGA | 128 | NM_012707.2 |
| GLPr1 | CTTGGAGACATAGAAGGGGGAC | AGGAGCATGCCTCTGGGTAG | 128 | NM_172091.2 |
| EGF-β1r | GGCATCATGGGGGAGAACAA | GGATCTTTGGCCCATAGGTACAG | 100 | NM_001393707.1 |
| EGF-β1 | GGTCCACCCATTGGCAAAAC | CACGAATCCTTCCCGACACA | 118 | NM_012842.2 |
| PPAR-α | GTCCTCTGGTTGTCCCCTTG | GTCAGTTCACAGGGAAGGCA | 176 | NM_013196.2 |
| PGC-1α | TTCAGGAGCTGGATGGCTTG | GGGCAGCACACTCTATGTCA | 70 | NM_031347.1 |
| FGF | GAGCGACCCTCACATCAA | CGTTTCAGTGCCACATACC | 222 | NM_019305.2 |
| VEGF | GATCCAGTACCCGAGCAGTCA | TCTCCTTTCTTTTTGGTCTGCAT | 83 | NM_053549.1 |
| Bax | CGAATTGGCGATGAACTGGA | CAAACATGTCAGCTGCCACAC | 109 | NM_017059.2 |
| Bcl-2 | GACTGAGTACCTGAACCGGCATC | CTGAGCAGCGTCTTCAGAGACA | 135 | NM_016993.1 |
| Fas | GAGCGTTCGTGAAACCGACA | AGGTTGGTGCACCTCCACTTG | 128 | NM_139194.2 |
| FasL | CACCAACCACAGCCTTAGAGTATCA | CACTCCAGAGATCAAAGCAGTTCC | 172 | NM_012908.1 |
| P38 | CGGCTTGCTCATGTCCTCAGAAC | GGAGGGCGGCTGCACATACAC | 214 | NM_031020.2 |
| P53 | CATGAGCGTTGCTCTGATGGT | GATTTCCTTCCACCCGGATAA | 67 | NM_030989.3 |
| Casp-3 | GAGACAGACAGTGGAACTGACGATG | GGCGCAAAGTGACTGGATGA | 147 | NM_012922.2 |
| 16s rRNA | AGAGTTTGATCCTGGCTCAG | CTACGGCTACCTTGTTACGA | 1507 | NR_043997.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, R.; Khamis, T.; Enan, G.; El-Didamony, G.; Sitohy, B.; Abdel-Fattah, G. The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria. Molecules 2022, 27, 2270. https://doi.org/10.3390/molecules27072270
Ali R, Khamis T, Enan G, El-Didamony G, Sitohy B, Abdel-Fattah G. The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria. Molecules. 2022; 27(7):2270. https://doi.org/10.3390/molecules27072270
Chicago/Turabian StyleAli, Rewaa, Tarek Khamis, Gamal Enan, Gamal El-Didamony, Basel Sitohy, and Gamal Abdel-Fattah. 2022. "The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria" Molecules 27, no. 7: 2270. https://doi.org/10.3390/molecules27072270
APA StyleAli, R., Khamis, T., Enan, G., El-Didamony, G., Sitohy, B., & Abdel-Fattah, G. (2022). The Healing Capability of Clove Flower Extract (CFE) in Streptozotocin-Induced (STZ-Induced) Diabetic Rat Wounds Infected with Multidrug Resistant Bacteria. Molecules, 27(7), 2270. https://doi.org/10.3390/molecules27072270







