Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crude Extraction
2.2. Sediments after Evaporation and Left Overnight
2.3. Precipitation Yield
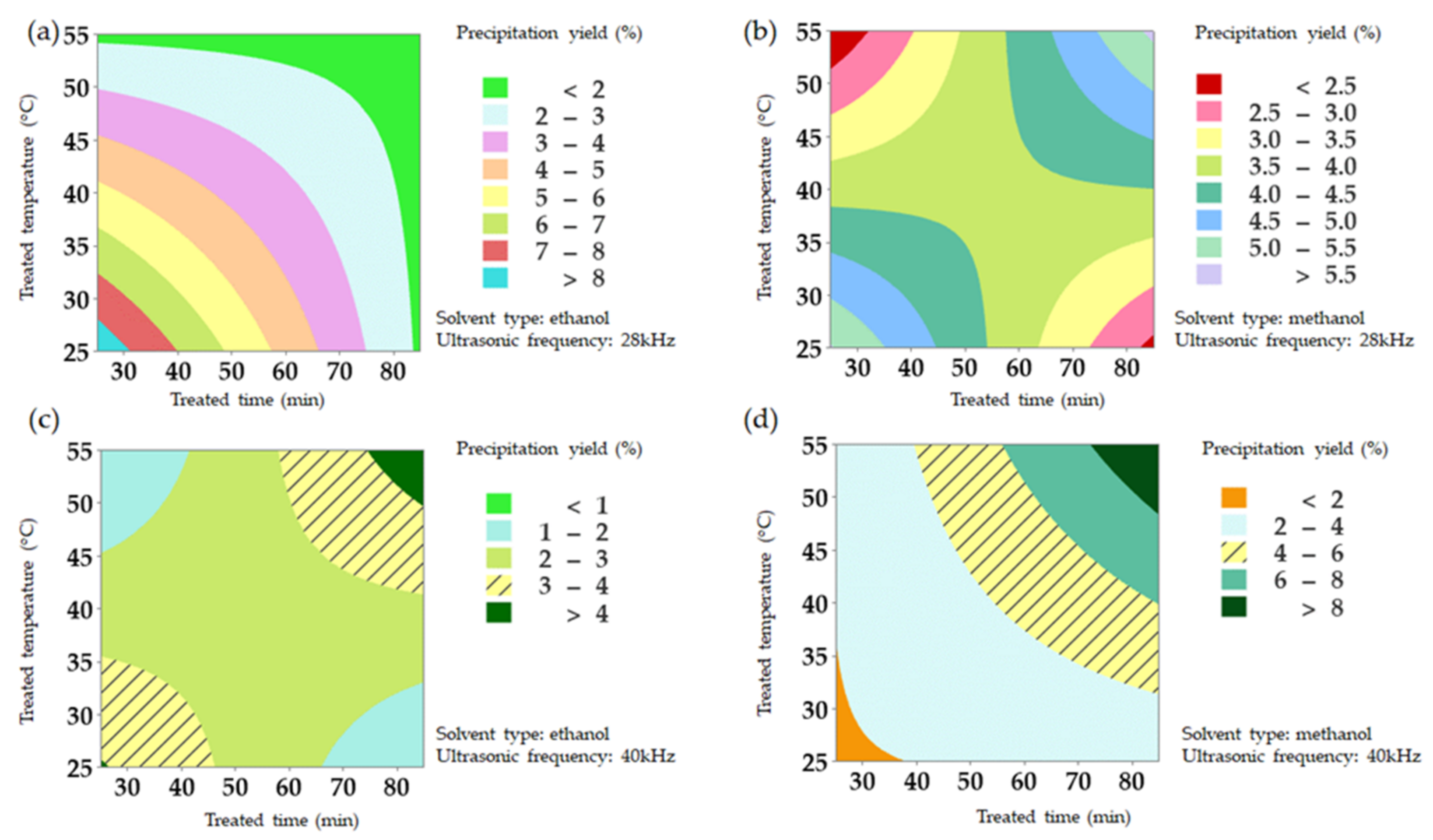
2.4. Processing Optimization
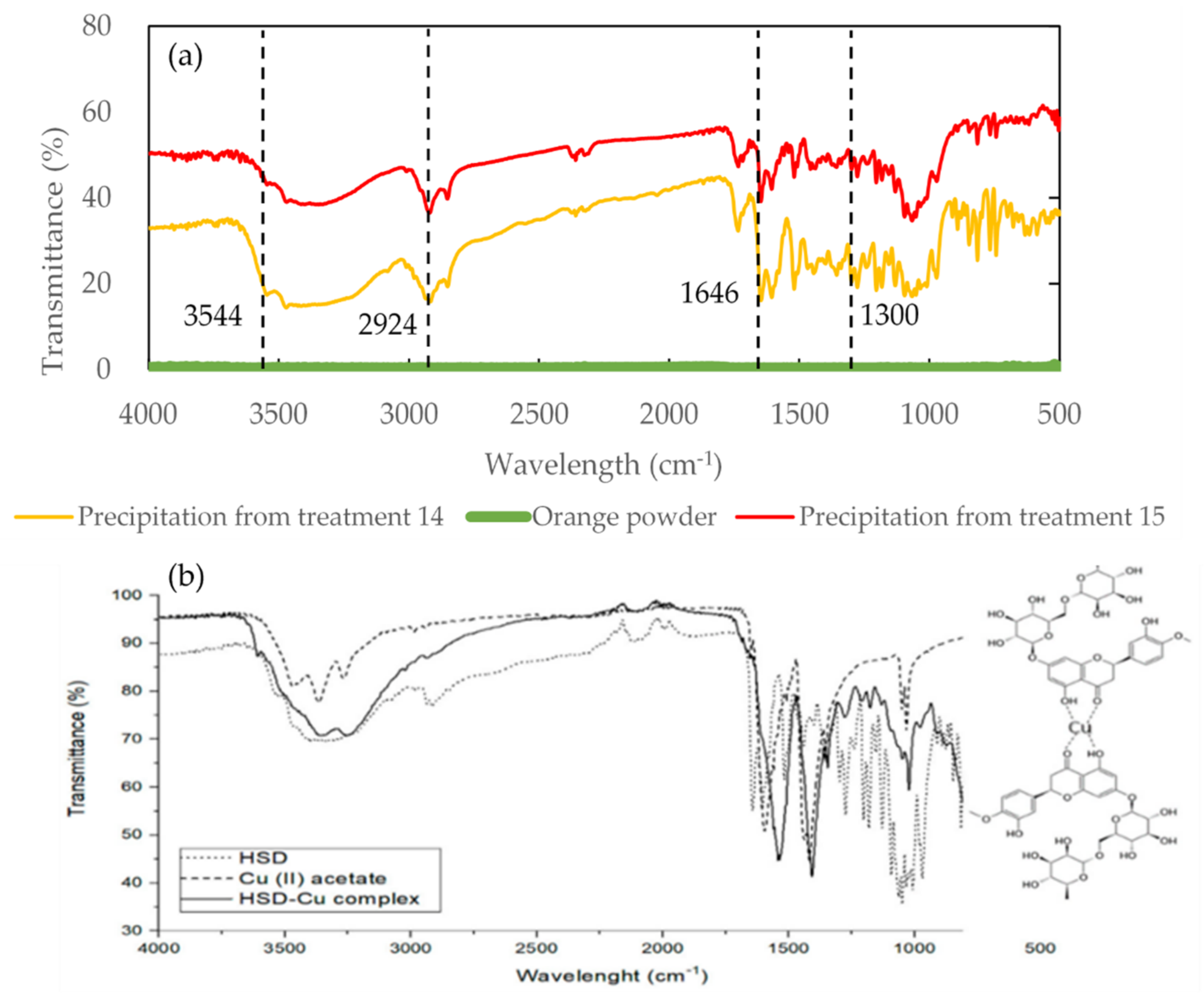
2.5. Spectral Characteristics Overview for the Sample with Different Treatments Measured by FTIR
3. Materials and Methods
3.1. Sample Preparation and Ultrasound-Assisted Extraction
3.2. Experiment Design
3.3. Compounds Pellets Preparation and FTIR Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sammani, M.S.; Cerdà, V. Sample pre-treatment and flavonoids analytical methodologies for the quality control of foods and pharmaceuticals matrices. In The Book of Flavonoids, 1st ed.; Feng, C.-H., García Martín, J.F., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2021; Chapter 1; pp. 1–129. [Google Scholar]
- Terahara, N. Flavonoids in Foods: A review. Nat. Prod. Commun. 2015, 10, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.R.; Liu, Y.; Yang, Y.Y.; Zhang, P.; Zhong, W.; Wang, Y.L.; Wang, Q.Q.; Xu, Y.; Li, M.X.; Li, X.Z.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Otani, C.; García-Martín, J.F. Flavonoids as a starting point for therapeutics against COVID-19: Current state-of-the art research advances. In The Book of Flavonoids, 1st ed.; Feng, C.-H., García Martín, J.F., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2021; Chapter 12; pp. 340–350. [Google Scholar]
- Feng, C.H.; Otani, C.; Ogawa, Y. Innovatively identifying naringin and hesperidin by using terahertz spectroscopy and evaluating flavonoids extracts from waste orange peels by coupling with multivariate analysis. Food Control 2022, 137, 108897. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Awad, O.M.; Shehata, M.G.; El-Sohaimy, S.A. Antioxidant and anti-acetylcholinesterase potential of artichoke phenolic compounds. Food Biosci. 2021, 41, 101006. [Google Scholar] [CrossRef]
- Feng, C.H.; García-Martín, J.F.; Lavado, M.B.; López-Barrera, M.C.; Álvarez-Mateos, P. Evaluation of different solvents on flavonoids extraction efficiency from sweet oranges and ripe and immature Seville oranges. Int. J. Food Sci. 2020, 55, 3123–3134. [Google Scholar] [CrossRef]
- Leila, M.; Ratiba, D.; Al-Marzouqi, A.H. Experimental and mathematical modelling data of green process of essential oil extraction: Supercritical CO2 extraction. Mater. Proc. 2022, 49, 1023–1029. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; West, C.; Lesellier, E. Supercritical Fluid Chromatography development of a predictive analytical tool to selectively extract bioactive compounds by supercritical fluid extraction and pressurised liquid extraction. J. Chromatogr. A 2020, 1632, 461582. [Google Scholar] [CrossRef]
- Liović, N.; Bratanić, A.; Zorić, Z.; Pedisić, S.; Režek Jambrak, A.; Krešić, G.; Bilušić, T. The effect of freeze-drying, pasteurisation and high-intensity ultrasound on gastrointestinal stability and antioxidant activity of blueberry phenolics. Int. J. Food Sci. 2021, 56, 1996–2008. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abd EI Aziz, M.N.; Youssef, M.M.; EI-Sohaimy, S.A. Optimization conditions of ultrasound-assisted extraction of phenolic compounds from orange peels using response surface methodology. J. Food Processing Preserv. 2021, 45, e15870. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C.M. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of ultrasound extraction of functional compound from makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason. Sonochem. 2022, 83, 105916. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Mohammad, N.A.; Zaidel, D.N.A.; Muhamad, I.I.; Hamid, M.A.; Yaakob, H.; Jusoh, Y.M.M. Optimization of the amtopxodamt-rich xanthone extract from mangosteen (Garcinia mangostana L.) pericarp via microwave-assisted extraction. Heliyon 2019, 5, e02571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.H.; Drummond, L.; Sun, D.W.; Zhang, Z.-H. Evaluation of natural hog casings modified by surfactant solutions combined with lactic acid by response surface methodology. LWT-Food Sci. Technol. 2014, 58, 427–438. [Google Scholar] [CrossRef]
- Fan, T.; Hu, J.G.; Fu, L.D.; Zhang, L.J. Optimization of enzymolysis-ultrasonic assisted extraction of polysaccharides from Momordica charabtia L. by response surface methodology. Carbohydr. Polym. 2015, 115, 701–706. [Google Scholar] [CrossRef]
- Murphy, S.C.; Gilroy, D.; Kerry, J.F.; Buckley, D.J.; Kerry, J.P. Evaluation of surimi, fat, and water content in a low/no added pork sausage formulation using response surface methodology. Meat Sci. 2004, 66, 689–701. [Google Scholar] [CrossRef]
- Feng, C.H.; Makino, Y. Colour analysis in sausages stuffed in modified casings with different storage days using hyperspectral imaging—A feasibility study. Food Control 2020, 111, 107047. [Google Scholar] [CrossRef]
- Feng, C.H.; Makino, Y.; García-Martín, J.F. Hyperspectral imaging coupled with multivariate analysis and image processing for detection and visualisation of colour in cooked sausages stuffed with different modified casings. Foods 2020, 9, 1089. [Google Scholar] [CrossRef]
- Feng, C.H.; Makino, Y.; Yoshimura, M.; Rodríguez-Pulido, F.J. Real-time prediction of pre-cooked Japanese sausages colour with different storage days using hyperspectral imaging. J. Sci. Food Agric. 2018, 98, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Makino, Y.; Yoshimura, M.; Rodríguez-Pulido, F.J. Estimation of adenosine triphosphate content in ready-to-eat sausages with different storage days, using hyperspectral imaging coupled with R statistics. Food Chem. 2018, 264, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Karacaglar, N.N.Y.; Bulat, T.; Boyaci, I.H.; Topcu, A. Raman spectroscopy coupled with chemometric methods for the discrimination of foreign fats and oils in cream and yogurt. J. Food Drug Anal. 2019, 27, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.H.; Otani, C.; Ogawa, Y.; García-Martín, J.F. Evaluation of properties in different casings modified by surfactants and lactic acid using terahertz spectroscopy—A feasibility study. Food Control 2021, 127, 108152. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Ngadi, M.O. Assessment of intramuscular fat content of pork using NIR hyperspectral images of rib end. J. Food Eng. 2017, 193, 29–41. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Fourier transform infrared (FT-IR) spectroscopy of nanoencapsulated food ingredients. In Characterization of Nanoencapsulated Food Ingredients; Afari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 347–410. [Google Scholar]
- Altunayar-Unsalan, C.; Unsalan, O.; Mavromoustakos, T. Insights into molecular mechanism of action of citrus flavonoids hesperidin and naringin on lipid bilayers using spectroscopic, calorimetric, microscopic and theoretical studies. J. Mol. Liq. 2022, 347, 118411. [Google Scholar] [CrossRef]
- Tunchaiyaphum, S.; Eshtiaghi, M.; Yoswathana, N. Extraction of bioactive compounds from mango peels using green technology. Int. J. Chem. Eng. Appl. 2013, 4, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Nutr. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Feng, C.H. Quality evaluation and mathematical modelling approach to estimate the growth parameters of total viable count in sausages with different casings. Foods 2022, 11, 634. [Google Scholar] [CrossRef]
- Iqbal, A.; Sun, D.-W.; Allen, P. Prediction of moisture, colour and pH in cooked, pre-sliced turkey hams by NIR hyperspectral imaging system. J. Food Eng. 2013, 117, 42–51. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bio, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef] [Green Version]
- Du Preez, B.V.P.; Beer, D.D.; Joubert, E. By-product of honeybush (Cyclopia maculata) tea processing as source of hesperidin-enriched nutraceutical extract. Ind. Crops Prod. 2016, 87, 132–141. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbasa, P.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids—An application to orange peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredio, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Zhang, G.W.; He, L.; Hu, M.M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Stanisic, D.; Liu, L.H.B.; Santos, R.V.D.; Costa, A.F.; Durán, N.; Tasic, L. New Sustainable Process for Hesperidin Isolation and Anti-Ageing Effects of Hesperidin Nanocrystals. Molecules 2020, 25, 4534. [Google Scholar] [CrossRef]
- Li, Y.S.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2019, 105, 77–85. [Google Scholar] [CrossRef]
- Nabil, M.; Donia, T.; Mohamed, T.M. Antioxidant effect of hesperidin isolated from orange peels. Appl. Sci. Eng. Prog. 2016, 35, 1–5. [Google Scholar]
- Rekha, S.S.; Pradeepkiran, J.A.; Bhaskar, M. Bioflavonoid hesperidin possesses the anti-hyperglycemic and hypolipidemic property in STZ induced diabetic myocardial infarction (DMI) in male Wister rats. J. Nutr. Intermed. Metab. 2019, 15, 58–64. [Google Scholar] [CrossRef]
- Wang, X.S.; Wu, Q.N.; Wu, Y.F.; Chen, G.Y.; Yue, W.; Liang, Q.L. Response surface optimized ultrasonic-assisted extraction of flavonoids from sparganii rhizoma and evaluation of their in vitro antioxidant activities. Molecules 2012, 17, 6769–6783. [Google Scholar] [CrossRef] [PubMed]

| Source of Variation | Df | Response Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude Extraction (Y1, g) | Sediments after Evaporation and Left Overnight (Y2, g) | Precipitation Yield (Y3, %) | ||||||||
| Source | SSS | Contribution (%) | F | SSS | Contribution (%) | F | SSS | Contribution (%) | F | |
| Model | 15 | 11,284.10 | 80.49 | 12.10 ** | 10,569.60 | 77.01 | 9.83 ** | 2.64 | 52.95 | 3.30 ** |
| Linear | 5 | 9820.60 | 70.05 | 31.60 ** | 9546.90 | 69.56 | 26.63 ** | 0.81 | 16.34 | 3.07 * |
| X1 (%, v/v) | 1 | 9248.00 | 65.97 | 148.78 ** | 9035.00 | 65.83 | 126.02 ** | 0.67 | 13.56 | 12.71 ** |
| X2 (min) | 1 | 12.90 | 0.09 | 0.21 ns | 0.10 | 0.00 | 0.00 ns | 0.00 | 0.08 | 0.09 ns |
| X3 (°C) | 1 | 38.90 | 0.28 | 0.63 ns | 0.30 | 0.00 | 0 ns | 0.00 | 0.01 | 0.01 ns |
| X4 (methanol or ethanol) | 1 | 189.40 | 1.35 | 3.05 ns | 166.30 | 1.21 | 2.32 ns | 0.12 | 2.33 | 2.20 ns |
| X5 (kHz) | 1 | 331.40 | 2.36 | 5.33 * | 345.10 | 2.51 | 4.81 * | 0.02 | 0.36 | 0.33 ns |
| 2-Way Interaction | 10 | 1463.50 | 10.40 | 2.35* | 1022.70 | 7.45 | 1.43 ns | 1.82 | 36.72 | 3.42 ** |
| X1×X2 | 1 | 5.00 | 0.04 | 0.08 ns | 1.10 | 0.01 | 0.01 ns | 0.03 | 0.57 | 0.54 ns |
| X1×X3 | 1 | 72.70 | 0.52 | 1.17 * | 43.60 | 0.32 | 0.61 ns | 0.01 | 0.12 | 0.12 ns |
| X1×X4 | 1 | 92.50 | 0.66 | 1.49 * | 15.80 | 0.12 | 0.22 ns | 0.18 | 3.53 | 3.33 ns |
| X1×X5 | 1 | 167.40 | 1.19 | 2.69 ns | 4.60 | 0.03 | 0.06 ns | 0.00 | 0.01 | 0.01 ns |
| X2×X3 | 1 | 196.00 | 1.40 | 3.15 ns | 229.50 | 1.67 | 3.20 ns | 0.46 | 9.25 | 8.61 ** |
| X2×X4 | 1 | 40.30 | 0.29 | 0.65 ns | 2.40 | 0.02 | 0.03 ns | 0.28 | 5.57 | 5.22 * |
| X2×X5 | 1 | 689.10 | 4.92 | 11.09 ** | 547.00 | 3.99 | 7.63 * | 0.30 | 6.02 | 5.55 * |
| X3×X4 | 1 | 82.90 | 0.59 | 1.33 ns | 18.90 | 0.14 | 0.26 ns | 0.24 | 4.91 | 4.56 * |
| X3×X5 | 1 | 106.20 | 0.76 | 1.71 ns | 88.40 | 0.64 | 1.23 ns | 0.30 | 6.02 | 5.58 * |
| X4×X5 | 1 | 11.40 | 0.08 | 0.18 ns | 71.50 | 0.52 | 1.00 ns | 0.04 | 0.72 | 0.65 ns |
| Error | 44 | 2734.90 | 19.50 | 3154.70 | 22.99 | 2.33 | 46.94 | |||
| Lack-of-Fit | 36 | 2518.90 | 17.97 | 2.59 ns | 2750.40 | 20.04 | 1.51 ns | 2.32 | 46.62 | 31.75 ** |
| Pure Error | 8 | 216.10 | 1.54 | 404.30 | 2.95 | 0.02 | 0.32 | |||
| Total | 59 | 14,019.00 | 100.00 | 13,724.30 | 100.00 | 4.97 | 100.00 | |||
| R2(%) | 80.49 | 77.01 | 53.06 | |||||||
| Treatment | X1 (%) | X2 (min) | X3 (°C) | X4 | X5 (kHz) | Y1 (g) | Y2 (g) | Y3 (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | ||||||
| 1 | 100 | 25 | 40 | Ethanol | 40 | 24.30 | 33.80 | 1.50 | 16.45 | 3.71 | 2.78 |
| 2 | 75 | 25 | 25 | Methanol | 40 | 53.40 | 51.21 | 22.90 | 26.12 | 2.61 | 1.86 |
| 3 | 50 | 85 | 40 | Methanol | 28 | 66.30 | 67.92 | 40.00 | 41.84 | 1.66 | 1.35 |
| 4 | 50 | 25 | 40 | Methanol | 40 | 67.50 | 73.92 | 40.20 | 45.33 | 2.32 | 0.32 |
| 5 | 75 | 85 | 55 | Ethanol | 28 | 44.00 | 46.43 | 32.30 | 24.76 | 2.50 | 1.65 |
| 6 | 75 | 85 | 25 | Methanol | 40 | 65.00 | 52.44 | 31.30 | 24.79 | 2.30 | 2.50 |
| 7 | 75 | 55 | 40 | Ethanol | 40 | 46.90 | 46.81 | 30.00 | 30.17 | 2.61 | 2.69 |
| 8 | 50 | 55 | 25 | Ethanol | 40 | 77.60 | 65.64 | 55.00 | 48.74 | 2.03 | 1.70 |
| 9 | 50 | 85 | 40 | Methanol | 40 | 66.30 | 67.04 | 36.80 | 36.94 | 1.39 | 3.48 |
| 10 | 100 | 55 | 25 | Ethanol | 40 | 30.90 | 26.2 | 37.40 | 16.27 | 2.13 | 3.41 |
| 11 | 100 | 55 | 55 | Methanol | 40 | 22.60 | 28.31 | 4.40 | 5.74 | 5.32 | 7.85 |
| 12 | 50 | 55 | 25 | Methanol | 40 | 68.10 | 74.94 | 44.60 | 40.28 | 1.25 | -0.16 |
| 13 | 75 | 25 | 25 | Methanol | 28 | 36.30 | 34.45 | 13.70 | 11.91 | 4.14 | 5.54 |
| 14 | 100 | 55 | 25 | Methanol | 40 | 34.50 | 28.7 | 13.40 | 10.63 | 3.36 | 4.52 |
| 15 | 75 | 85 | 25 | Ethanol | 28 | 51.00 | 44.36 | 43.30 | 30.35 | 1.96 | 1.84 |
| 16 | 75 | 85 | 55 | Methanol | 40 | 47.00 | 40.78 | 20.70 | 15.63 | 13.81 | 9.59 |
| 17 | 100 | 85 | 40 | Methanol | 40 | 15.50 | 26.18 | 3.70 | 3.47 | 9.18 | 8.60 |
| 18 | 75 | 55 | 40 | Ethanol | 40 | 37.80 | 46.81 | 14.80 | 30.17 | 3.33 | 2.69 |
| 19 | 50 | 55 | 25 | Ethanol | 28 | 48.80 | 51.85 | 36.30 | 37.68 | 1.52 | 4.34 |
| 20 | 50 | 25 | 40 | Ethanol | 40 | 67.80 | 70.09 | 50.40 | 51.71 | 1.41 | 2.30 |
| 21 | 50 | 85 | 40 | Ethanol | 40 | 52.30 | 58.72 | 39.60 | 44.4 | 1.93 | 1.75 |
| 22 | 50 | 55 | 55 | Methanol | 40 | 63.60 | 66.02 | 45.20 | 41.99 | 2.36 | 3.96 |
| 23 | 100 | 85 | 40 | Ethanol | 40 | 15.00 | 24.65 | 1.40 | 8.12 | 4.62 | 3.92 |
| 24 | 75 | 25 | 25 | Ethanol | 40 | 49.70 | 47.55 | 41.80 | 32.63 | 2.02 | 4.08 |
| 25 | 100 | 55 | 25 | Ethanol | 28 | 25.10 | 21.56 | 1.60 | 6.72 | 3.50 | 6.23 |
| 26 | 100 | 25 | 40 | Methanol | 28 | 23.70 | 22.3 | 5.80 | 2.77 | 7.82 | 5.62 |
| 27 | 75 | 25 | 55 | Ethanol | 28 | 41.90 | 45.12 | 28.80 | 23.62 | 3.52 | 1.80 |
| 28 | 75 | 55 | 40 | Methanol | 28 | 59.80 | 45.67 | 33.30 | 22.05 | 2.57 | 3.90 |
| 29 | 50 | 25 | 40 | Methanol | 28 | 66.00 | 56.24 | 36.50 | 33.7 | 1.65 | 2.01 |
| 30 | 75 | 85 | 55 | Methanol | 28 | 47.20 | 49.88 | 22.80 | 24.61 | 3.03 | 5.63 |
| 31 | 75 | 55 | 40 | Methanol | 28 | 51.20 | 45.67 | 21.00 | 22.05 | 2.97 | 3.90 |
| 32 | 75 | 85 | 25 | Ethanol | 40 | 39.70 | 44.29 | 21.20 | 32.38 | 3.43 | 1.02 |
| 33 | 100 | 25 | 40 | Ethanol | 28 | 19.30 | 23.52 | 3.20 | 1.96 | 2.98 | 5.59 |
| 34 | 75 | 55 | 40 | Methanol | 40 | 52.80 | 49.49 | 8.80 | 24.66 | 2.75 | 4.04 |
| 35 | 100 | 55 | 25 | Methanol | 28 | 18.30 | 25.81 | 3.30 | 5.44 | 5.66 | 6.38 |
| 36 | 100 | 25 | 40 | Methanol | 40 | 27.10 | 30.83 | 11.50 | 12.89 | 5.13 | 3.76 |
| 37 | 75 | 25 | 55 | Methanol | 28 | 30.30 | 44.09 | 15.30 | 24.55 | 1.95 | 2.08 |
| 38 | 75 | 85 | 25 | Methanol | 28 | 52.40 | 54.25 | 27.30 | 27.12 | 3.55 | 2.36 |
| 39 | 100 | 55 | 55 | Ethanol | 28 | 38.10 | 34.89 | 3.80 | 5.41 | 2.21 | 2.28 |
| 40 | 100 | 55 | 55 | Methanol | 28 | 32.80 | 32.7 | 11.60 | 7.21 | 5.07 | 5.88 |
| 41 | 75 | 55 | 40 | Ethanol | 40 | 49.80 | 46.81 | 32.90 | 30.17 | 1.98 | 2.69 |
| 42 | 100 | 85 | 40 | Methanol | 28 | 36.50 | 36.21 | 3.90 | 9.88 | 9.94 | 6.64 |
| 43 | 50 | 25 | 40 | Ethanol | 28 | 51.00 | 50.66 | 34.70 | 35.71 | 1.52 | 4.93 |
| 44 | 75 | 55 | 40 | Ethanol | 28 | 49.50 | 41.24 | 30.30 | 23.19 | 2.29 | 3.50 |
| 45 | 50 | 55 | 55 | Ethanol | 40 | 56.90 | 63.16 | 40.70 | 47.38 | 2.27 | 2.36 |
| 46 | 50 | 55 | 25 | Methanol | 28 | 49.90 | 62.89 | 33.60 | 33.59 | 1.28 | 1.53 |
| 47 | 50 | 55 | 55 | Methanol | 28 | 62.50 | 61.26 | 38.30 | 41.95 | 4.63 | 1.82 |
| 48 | 75 | 55 | 40 | Ethanol | 28 | 50.60 | 41.24 | 23.00 | 23.19 | 3.10 | 3.50 |
| 49 | 100 | 55 | 55 | Ethanol | 40 | 41.60 | 32.25 | 4.30 | 8.31 | 3.91 | 3.29 |
| 50 | 75 | 55 | 40 | Methanol | 40 | 41.90 | 49.49 | 17.40 | 24.66 | 3.40 | 4.04 |
| 51 | 75 | 55 | 40 | Methanol | 28 | 51.80 | 45.67 | 24.30 | 22.05 | 2.61 | 3.90 |
| 52 | 75 | 55 | 40 | Methanol | 40 | 53.30 | 49.49 | 23.30 | 24.66 | 2.95 | 4.04 |
| 53 | 75 | 55 | 40 | Ethanol | 28 | 46.60 | 41.24 | 25.30 | 23.19 | 2.48 | 3.50 |
| 54 | 75 | 25 | 25 | Ethanol | 28 | 21.80 | 29.05 | 5.70 | 14.05 | 17.76 | 8.72 |
| 55 | 75 | 85 | 55 | Ethanol | 40 | 50.30 | 39.08 | 37.00 | 20.15 | 2.59 | 4.65 |
| 56 | 100 | 85 | 40 | Ethanol | 28 | 23.10 | 32.94 | 1.50 | 10.17 | 3.05 | 2.92 |
| 57 | 75 | 25 | 55 | Methanol | 40 | 63.80 | 53.55 | 45.70 | 32.11 | 2.50 | 2.22 |
| 58 | 75 | 25 | 55 | Ethanol | 40 | 61.60 | 56.33 | 44.60 | 35.54 | 2.35 | 0.99 |
| 59 | 50 | 55 | 55 | Ethanol | 28 | 53.60 | 56.66 | 40.10 | 42.97 | 1.83 | 1.17 |
| 60 | 50 | 85 | 40 | Ethanol | 28 | 54.20 | 57.85 | 38.00 | 44.94 | 2.32 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.-H. Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology. Molecules 2022, 27, 2268. https://doi.org/10.3390/molecules27072268
Feng C-H. Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology. Molecules. 2022; 27(7):2268. https://doi.org/10.3390/molecules27072268
Chicago/Turabian StyleFeng, Chao-Hui. 2022. "Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology" Molecules 27, no. 7: 2268. https://doi.org/10.3390/molecules27072268
APA StyleFeng, C.-H. (2022). Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology. Molecules, 27(7), 2268. https://doi.org/10.3390/molecules27072268






