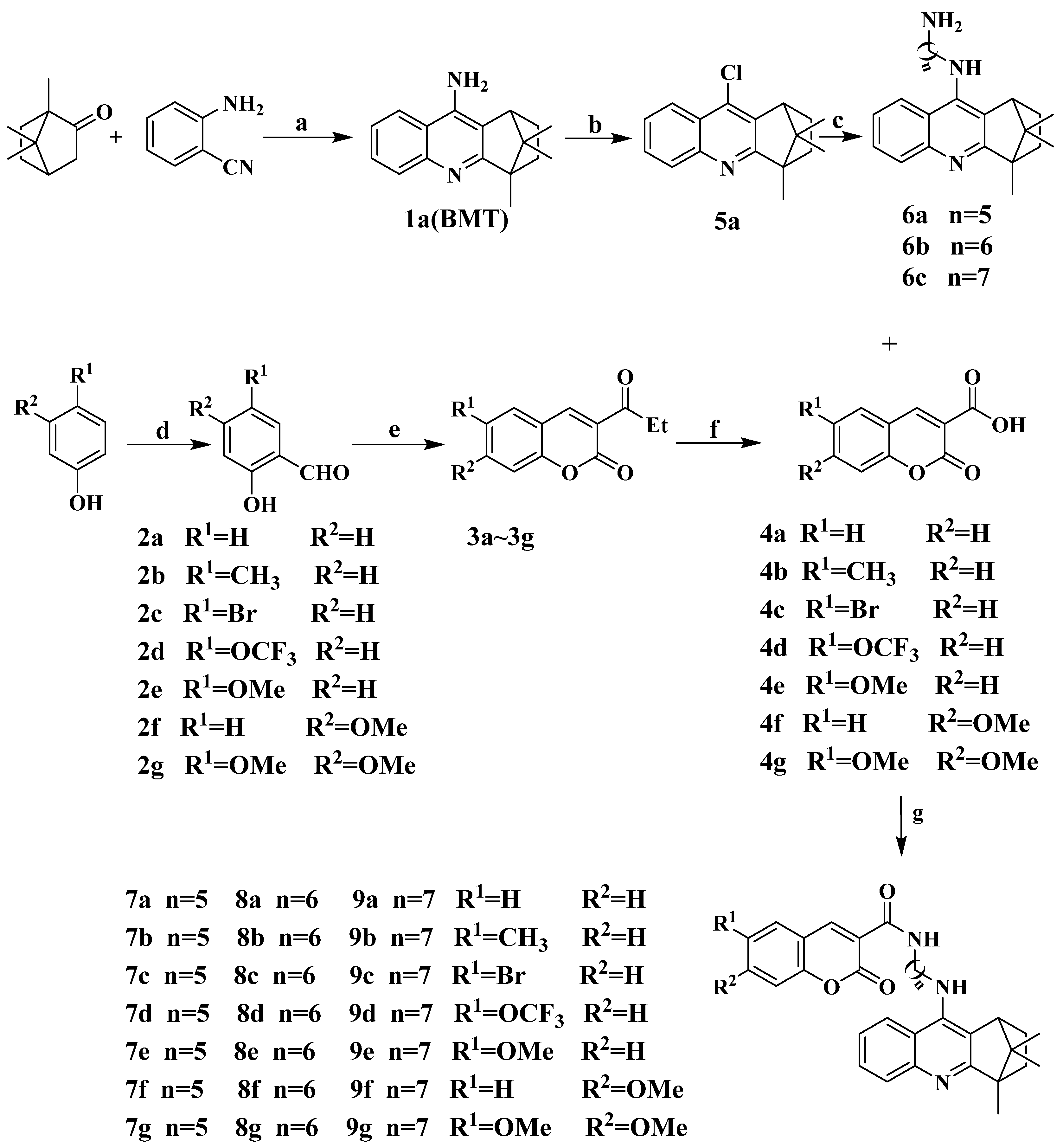
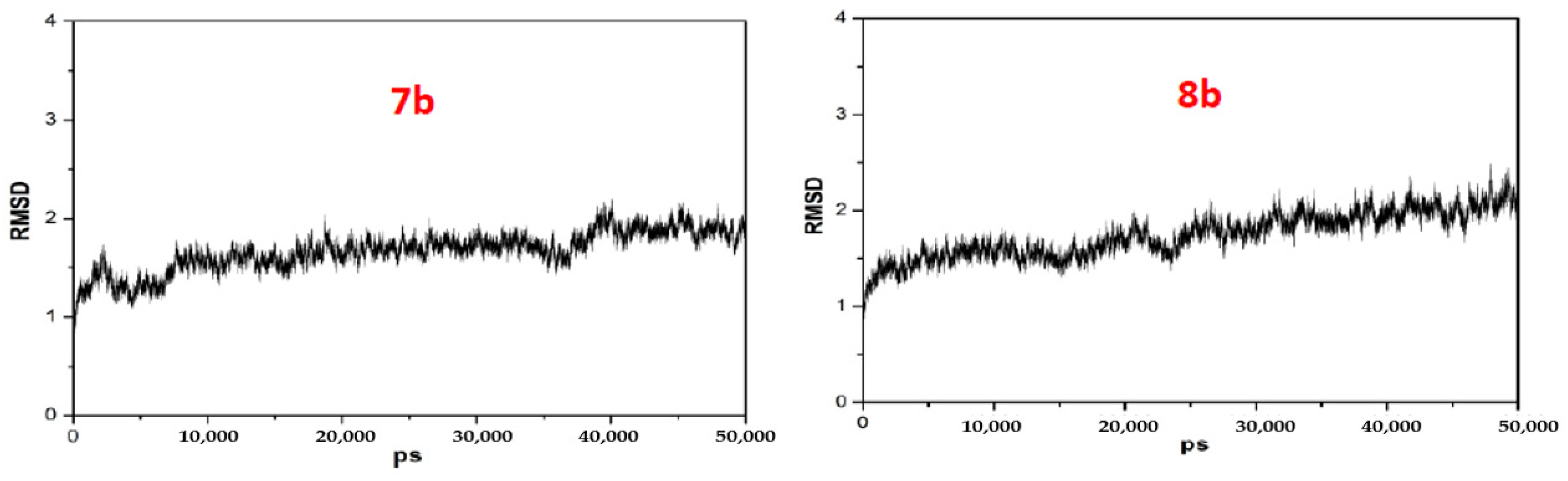
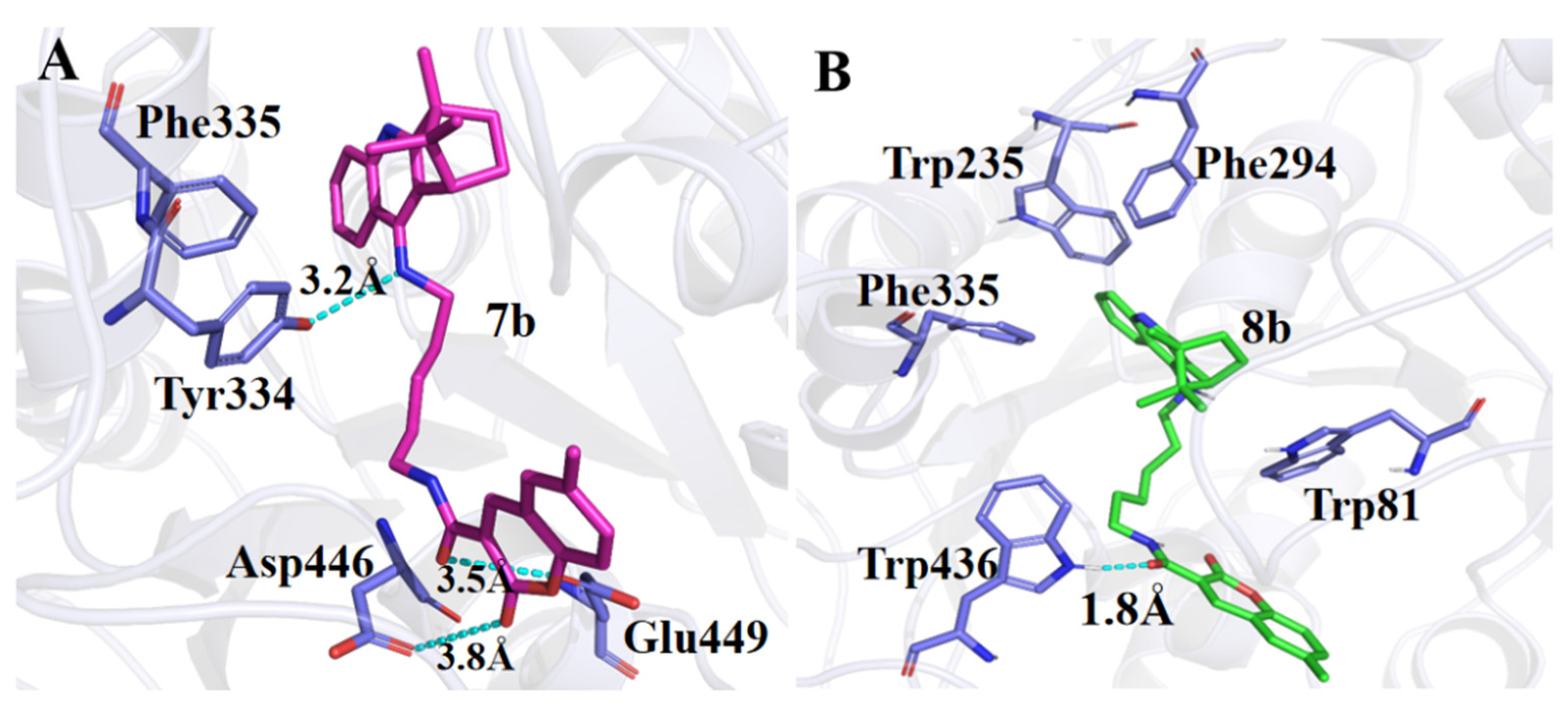
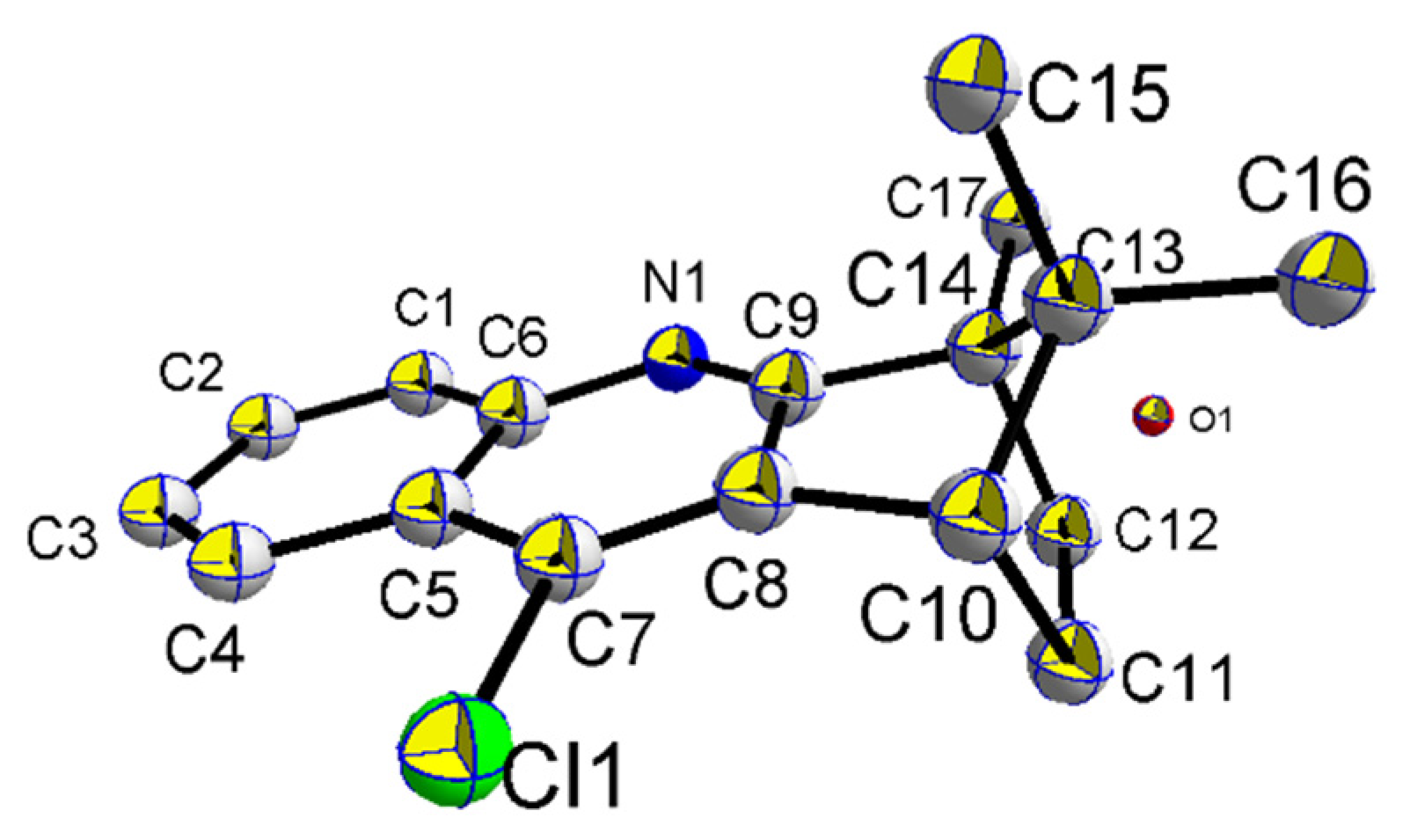
4.4.3. General Procedures for the Preparation of Coumarin–BMT Hybrids 7a~9g
To a solution of intermediate 6a~6c (1 mmol) and coumarin derivatives 4a~4g (1 mmol) in CH2Cl2 (20 mL), benzotriazole-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP, 1.3 mmol) and triethylamine (2.6 mmol) were added. The reaction system was stirred at room temperature until the reaction was fully carried out, then washed by 10% citric acid solution, 10% NaHCO3 solution and distilled water in turn, extracted by CH2Cl2 three times. The organic layer was dried over anhydrous MgSO4, concentrated in vacuo and purified by flash column chromatography with PE/EA/triethylamine.
2-oxo-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide(7a): Intermediate 6a was treated with intermediate 4a according to general procedure to give the desired product 7a as a white solid (42%). mp: 100–102 °C. 1H NMR (500 MHz, CDCl3) δ 8.91 (s, 1H), 8.89 (s, 1H), 7.96 (d, J = 8.3 Hz, 1H), 7.74 (d, J = 7.6 Hz, 1H), 7.71–7.63 (m, 2H), 7.53–7.48 (m, 1H), 7.43–7.31 (m, 3H), 4.52 (bs, 1H), 3.65–3.56 (m, 1H), 3.55–3.47 (m, 3H), 3.27 (d, J = 4.0 Hz, 1H), 2.20–2.12 (m, 1H), 1.92–1.84 (m, 1H), 1.82–1.69 (m, 4H), 1.63–1.55 (m, 2H), 1.37 (s, 3H), 1.33 (d, J = 8.3 Hz, 2H), 1.02 (s, 3H), 0.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.23, 161.59, 161.52, 154.36, 148.34, 142.26, 134.04, 129.77, 129.39, 127.39, 125.30, 123.77, 120.00, 119.50, 118.61, 118.39, 118.01, 116.59,54.32, 53.55, 50.96, 45.98, 39.46, 32.08, 30.36, 29.36, 27.01, 24.34, 20.33, 19.18, 14.17, 10.86. HRMS(ESI) m/z [M + H]+: calcd for C32H35N3O3: 510.2712, found 510.2739.
6-methyl-2-oxo-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide(7b): Intermediate 6a was treated with intermediate 4b according to general procedure to obtain the desired product 7b as a yellow solid (33%). mp: 131–132 °C. 1H NMR (500 MHz, CDCl3) δ 8.92 (s, 1H), 8.86 (s, 1H), 7.97 (d, J = 8.3 Hz, 1H), 7.74 (d, J = 8.3 Hz, 1H), 7.54–7.42 (m, 3H), 7.35 (t, J = 7.6 Hz, 1H), 7.30 (d, J = 8.3 Hz, 1H), 4.55 (bs, 1H), 3.66–3.56 (m, 1H), 3.55–3.48 (m, 3H), 3.27 (d, J = 4.0 Hz, 1H), 2.44 (s, 3H), 2.19–2.13 (m, 1H), 1.91–1.84 (m, 1H), 1.83–1.68 (m, 4H), 1.63–1.54 (m, 2H), 1.38 (s, 3H), 1.33 (d, J = 8.6 Hz, 2H), 1.02 (s, 3H), 0.64 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.01,161.77, 161.74, 152.57, 148.34, 135.24, 135.20, 129.35,128.26, 127.47, 123.82, 119.96, 119.55, 118.38, 118.19, 117.94, 116.29, 54.36, 53.58, 50.96, 45.96, 39.42, 32.06, 30.90, 30.34, 29.36, 27.00, 26.88, 24.34, 20.74, 20.32, 19.17, 10.85. HRMS(ESI) m/z [M + H]+: calcd for C33H37N3O3: 524.2868, found 524.2875.
6-bromo-2-oxo-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide (7c): Intermediate 6a was treated with intermediate 4c according to general procedure to obtain the desired product 7c as a yellow solid (38%). mp: 143–145 °C. 1H NMR (500 MHz, CDCl3) δ 8.82 (s, 1H), 8.80 (s, 1H), 8.06–7.96 (m, 2H), 7.84–7.72 (m, 2H), 7.56–7.49 (m, 1H), 7.36 (d, J = 7.8 Hz, 1H), 7.30 (d, J = 8.9 Hz, 1H), 4.62 (bs, 1H), 3.59 (d, J = 6.4 Hz, 1H), 3.55–3.46 (m, 3H), 3.30–3.25 (m, 1H), 2.20–2.12 (m, 1H), 1.92–1.85 (m, 1H), 1.85–1.66 (m, 4H), 1.65–1.53 (m, 2H), 1.31 (s, 3H), 1.27 (d, J = 7.4 Hz, 2H), 1.02 (s, 3H), 0.66 (s, 3H).13C NMR (125 MHz, CDCl3) δ 173.20, 160.83, 153.07, 147.27, 146.82, 142.22, 136.63, 131.74, 129.43, 127.37, 123.74, 120.06, 119.98, 119.51, 119.38, 118.26, 117.97, 117.88,54.30, 53.53, 50.92, 46.04, 39.84, 32.07, 30.69, 29.17, 28.99, 26.97, 26.80, 20.31, 19.18, 10.84. HRMS (ESI) m/z [M + H]+: calcd for C32H34BrN3O3: 588.1862, found 588.1848.
2-oxo-6-(trifluoromethoxy)-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide(7d): Intermediate 6a was treated with intermediate 4d according to general procedure to obtain the desired product 7d as a faint yellow solid (42%). mp: 105–107 °C. 1H NMR (500 MHz, CDCl3) δ 8.87 (s, 1H), 8.80 (s, 1H), 7.97 (d, J = 8.3 Hz, 1H), 7.76–7.71 (m, 1H), 7.56–7.48 (m, 3H), 7.45 (d, J = 8.9 Hz, 1H), 7.37–7.32 (m, 1H), 4.51 (bs, 1H), 3.65–3.56 (m, 1H), 3.55–3.47 (m, 3H), 3.28 (t, J = 6.8 Hz, 1H), 2.20–2.12 (m, 1H), 1.91–1.85 (m, 1H), 1.83–1.69 (m, 4H), 1.62–1.55 (m, 2H), 1.38 (s, 3H), 1.33 (d, J = 8.6 Hz, 2H), 1.03 (s, 3H), 0.65 (s, 3H).13C NMR (125 MHz, CDCl3) δ 173.21, 160.92, 160.84, 152.33, 147.19, 145.62, 142.27, 129.37, 127.42, 126.93, 123.78, 121.28, 119.98, 119.70, 119.48, 119.23, 118.27, 118.02, 54.34, 53.57, 50.97, 45.94, 39.57, 32.08, 30.91, 30.33, 29.30, 27.01, 24.31, 20.33, 19.19,14.096, 10.86. HRMS(ESI) m/z [M + H]+: calcd for C33H34F3N3O4: 588.1862, found 588.1883.
6-methoxy-2-oxo-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide (7e): Intermediate 6a was treated with intermediate 4e according to general procedure to obtain the desired product 7e as a yellow solid (40%). mp: 127–129 °C. 1H NMR (500 MHz, CDCl3) δ 8.91 (s, 1H), 8.83 (s, 1H), 7.98 (d, J = 8.3 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.53 (d, J = 11.2, 1H), 7.40–7.34 (m, 1H), 7.33 (d, J = 9.1 Hz, 1H), 7.23 (d, J = 9.1 Hz, 1H), 7.03 (d, J = 2.9 Hz, 1H), 4.55 (bs, 1H), 3.87 (s, 3H), 3.62–3.54 (m, 1H), 3.52–3.45 (m, 3H), 3.27 (d, J = 4.0 Hz, 1H), 2.20–2.12 (m, 1H), 1.91–1.82 (m, 1H), 1.78–1.64 (m, 4H), 1.60–1.44 (m, 2H), 1.38 (s, 3H), 1.32 (t, J = 7.5 Hz, 2H), 1.02 (s, 3H), 0.64 (s, 3H).13C NMR (125 MHz, CDCl3) δ 173.142,161.62, 161.56, 156.58, 148.93, 148.06, 129.28, 127.47, 123.82, 122.55, 119.98, 119.52, 118.95, 118.55, 117.93, 117.66, 110.59, 55.90, 54.35, 53.57, 50.97, 45.82, 39.51, 32.07, 30.91, 30.64, 29.30, 26.99, 26.60, 26.43, 20.30, 19.18, 10.85. HRMS(ESI) m/z [M + H]+: calcd for C33H37N3O4: 540.2818, found 540.2850.
7-methoxy-2-oxo-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide (7f): Intermediate 6a was treated with intermediate 4f according to general procedure to obtain the desired product 7f as a white solid (38%). mp: 123–125 °C. 1H NMR (500 MHz, CDCl3) δ 8.83 (s, 1H), 8.82 (s, 1H),7.95 (d, J = 8.3, 1H), 7.74 (d, J = 7.7 Hz, 1H), 7.55 (d, J = 8.7 Hz, 1H), 7.53–7.48 (m, 1H), 7.36–7.31 (m, 1H), 6.92 (dd, J = 8.7, 2.4 Hz, 1H), 6.85 (d, J = 2.3 Hz, 1H), 4.53 (bs, 1H), 3.90 (s, 3H), 3.63–3.55 (m, 1H), 3.53–3.46 (m, 3H), 3.27 (d, J = 4.0 Hz, 1H), 2.19–2.13 (m, 1H), 1.87 (t, J = 9.5 Hz, 1H), 1.82–1.74 (m, 2H), 1.73–1.67 (m, 2H), 1.61–1.54 (m, 2H), 1.37 (s, 3H), 1.32 (d, J = 8.3 Hz, 2H), 1.02 (s, 3H), 0.64 (s, 3H).13C NMR (125 MHz, CDCl3) δ 173.21, 164.78, 162.09, 161.87, 156.55, 148.22, 147.28, 142.23, 130.86, 129.40, 127.33, 123.71, 119.99, 119.53, 117.95, 114.69, 113.98, 112.35, 100.24, 55.97, 54.28, 53.51, 50.93, 45.96, 39.32, 32.06, 30.33, 29.41, 26.99, 24.32, 20.31, 19.16, 10.84. HRMS(ESI) m/z [M + H]+: calcd for C33H37N3O4: 540.2818, found 540.2847.
6,7-dimethoxy-2-oxo-N-(5-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)pentyl)-2H-chromene-3-carboxamide (7g): Intermediate 6a was treated with intermediate 4g according to general procedure to obtain the desired product 7g as a faint yellow solid (43%). mp: 86–88 °C. 1H NMR (500 MHz, CDCl3) δ 8.89 (s, 1H), 8.83 (s, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.53–7.48 (m, 1H), 7.39–7.32 (m, 1H), 6.98 (d, J = 4.4 Hz, 1H), 6.88 (s, 1H), 4.53 (bs, 1H), 3.99 (s, 3H), 3.94 (s, 3H), 3.63–3.56 (m, 1H), 3.55–3.47 (m, 3H), 3.28 (d, J = 4.0 Hz, 1H), 2.20–2.12 (m, 1H), 1.87 (t, J = 9.5 Hz, 1H), 1.83–1.68 (m, 4H), 1.62–1.55 (m, 2H), 1.40–1.36 (m, 3H), 1.32 (t, J = 8.6 Hz, 2H), 1.04 (s, 3H), 0.65 (s, 3H).13C NMR (125 MHz, CDCl3) δ 173.26, 162.24, 162.03, 155.13, 151.15, 148.07, 147.17, 142.29, 129.43, 127.34, 123.75, 120.04, 119.59, 118.00, 114.98, 111.50, 108.71, 99.33,56.60, 56.39, 54.32, 53.55, 50.97, 46.01, 39.31, 32.09, 30.91, 30.31, 29.45, 27.02, 24.34, 20.34, 19.19, 10.86. HRMS(ESI) m/z [M + H]+: calcd for C34H39N3O5: 571.3002, found 570.2987.
2-oxo-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8a): Intermediate 6b was treated with intermediate 4a according to general procedure to obtain the desired product 8a as a yellow solid (28%). mp: 103–105 °C. 1H NMR (500 MHz, CDCl3) δ 8.90 (s, 1H), 8.87 (s, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.67 (t, J = 7.9 Hz, 2H), 7.52 (t, J = 7.5 Hz, 1H), 7.43–7.34 (m, 3H), 7.19 (t, J = 7.6 Hz, 1H), 6.88 (t, J = 7.5 Hz, 1H), 4.58 (bs, 1H), 3.62 -3.56 (m, 1H), 3.54–3.45 (m, 3H), 3.28 (d, J = 4.0 Hz, 1H), 2.22–2.13 (m, 1H), 1.89 (t, J = 9.6 Hz, 1H), 1.79–1.64 (m, 4H), 1.59–1.44 (m, 4H), 1.39 (s, 3H), 1.33 (d, J = 6.8 Hz, 2H), 1.02 (s, 3H), 0.66 (s, 3H).13C NMR (125 MHz, CDCl3) δ 172.93,161.52, 161.50, 154.33, 148.30, 134.03, 129.76, 129.40, 127.76, 125.29, 123.99, 119.82,119.53, 118.58, 118.38, 117.77,116.56, 115.65, 54.47, 53.73, 50.98, 45.77, 39.55, 32.04, 30.90,30.62, 29.27, 26.95, 26.60, 26.43, 20.28, 19.14, 10.86. HRMS(ESI) m/z [M + H]+: calcd for C33H37N3O3: 524.2868, found 524.2898.
7-methyl-2-oxo-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8b): Intermediate 6b was treated with intermediate 4b according to general procedure to obtain the desired product 8b as a yellow solid (36%). mp: 131–133 °C. 1H NMR (500 MHz, CDCl3) δ 8.89 (s, 1H), 8.85 (s, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.47 (d, J = 8.6 Hz, 1H), 7.44 (s, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.30 (d, J = 8.5 Hz, 1H), 4.79 (bs, 1H), 3.67–3.56 (m, 1H), 3.55–3.43 (m, 3H), 3.28 (d, J = 4.0 Hz, 1H), 2.44 (s, 3H), 2.21–2.13 (m, 1H), 1.89 (t, J = 9.7 Hz, 1H), 1.78–1.65 (m, 4H), 1.59–1.44 (m, 4H), 1.40 (s, 3H), 1.34 (d, J = 9.0 Hz, 2H), 1.03 (s, 3H), 0.66 (s, 3H).13C NMR (125 MHz, CDCl3) δ 172.52,161.70, 161.65, 152.53, 148.26, 143.08, 135.20, 135.16, 133.85, 129.33, 128.50, 127.84, 124.06, 119.75, 118.34, 118.19, 117.62, 116.26, 54.57, 53.71, 51.03, 45.69, 39.47, 32.02, 30.59, 29.28, 26.93, 26.56, 26.38, 25.96, 20.73, 20.25, 19.12, 10.94. HRMS(ESI) m/z [M + H]+: calcd for C34H39N3O3: 538.3025, found 538.3056.
7-bromo-2-oxo-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8c): Intermediate 6b was treated with intermediate 4c according to general procedure to obtain the desired product 8c as a yellow solid (45%). mp: 130–132 °C.1H NMR (500 MHz, CDCl3) δ 8.88 (s, 1H),8.86 (s, 1H), 7.98 (d, J = 8.2 Hz, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.49–7.41 (m, 2H), 7.36 (t, J = 7.4 Hz, 1H), 7.28 (d, J = 2.9 Hz, 1H), 4.55 (bs, 1H), 3.62–3.53 (m, 1H), 3.51–3.41 (m, 3H), 3.28 (d, J = 3.6 Hz, 1H), 1.94–1.83 (m, 1H), 1.77–1.60 (m, 4H), 1.55–1.45 (m, 4H), 1.44–1.42 (m, 1H), 1.38 (s, 3H), 1.36–1.31 (m, 2H), 1.03 (s, 3H), 0.66 (s, 3H).13C NMR (125 MHz, CDCl3) δ 173.12, 161.71, 161.55, 152.53, 148.21, 135.15, 129.31, 127.44, 123.79, 119.45, 118.37, 118.26, 116.25,54.34, 53.55, 50.93, 46.25, 46.22, 46.02, 39.72, 32.06, 30.68, 29.20, 28.98, 26.96, 26.81, 26.78, 26.40, 26.34, 20.72, 20.29, 19.17, 10.83. HRMS(ESI) m/z [M + H]+: calcd for C33H36BrN3O3: 603.1920, found 603.1903.
2-oxo-7-(trifluoromethoxy)-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8d): Intermediate 6b was treated with intermediate 4d according to general procedure to obtain the desired product 8d as a yellow solid (37%). mp: 106–108 °C. 1H NMR (500 MHz, CDCl3) δ 8.87 (s, 1H), 8.78 (t, J = 5.3 Hz, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.74 (dd, J = 7.1, 6.3 Hz, 1H), 7.58–7.49 (m, 3H), 7.45 (d, J = 9.0 Hz, 1H), 7.40–7.34 (m, 1H), 4.55 (bs, 1H), 3.63–3.56 (m, 1H), 3.54–3.45 (m, 3H), 3.28 (d, J = 4.0 Hz, 1H), 1.89 (t, J = 9.5 Hz, 1H), 1.84–1.80 (m, 1H), 1.79–1.65 (m, 4H), 1.60–1.45 (m, 4H), 1.39 (s, 3H), 1.34 (d, J = 8.6 Hz, 2H), 1.04 (s, 3H), 0.66 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.14, 160.81, 160.78, 152.31, 147.10, 145.59, 142.32, 129.32, 127.46, 126.87, 123.82, 121.27, 119.97, 119.75, 119.44, 119.23, 118.25, 117.97, 54.35, 53.57, 50.96, 46.27, 46.24, 45.86, 39.67, 32.07, 30.64, 29.24, 26.98, 26.64, 26.48, 20.30, 19.18, 10.83. HRMS(ESI) m/z [M + H]+: calcd for C34H36F3N3O4: 608.2691, found 608.2722.
6-methoxy-2-oxo-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8e): Intermediate 6b was treated with intermediate 4e according to general procedure to obtain the desired product 8e as a yellow solid (38%). mp: 107–109 °C. 1H NMR (500 MHz, CDCl3) δ 8.91 (s, 1H), 8.84 (s, 1H), 7.98 (d, J = 8.3 Hz, 1H), 7.75 (d, J = 8.3 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.37 (t, J = 7.6 Hz, 1H), 7.33 (d, J = 9.1 Hz, 1H), 7.23 (dd, J = 9.1, 2.8 Hz, 1H), 7.03 (d, J = 2.7 Hz, 1H), 4.51 (bs, 1H), 3.86 (s, 3H), 3.64–3.54 (m, 1H), 3.54–3.44 (m, 3H), 3.27 (d, J = 3.9 Hz, 1H), 2.22–2.10 (m, 1H), 1.92–1.83 (m, 1H), 1.78–1.63 (m, 4H), 1.60–1.43 (m, 4H), 1.37 (s, 3H), 1.35–1.30 (m, 2H), 1.02 (s, 3H), 0.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.09, 161.60, 161.54, 156.56, 148.91, 148.05, 142.40, 129.21, 127.47, 123.82, 122.53, 119.96, 119.53, 118.92, 118.52, 117.88, 117.64, 110.57, 55.88, 54.34, 53.55, 50.95, 45.78, 39.48, 32.05, 30.61, 29.28, 26.96, 26.57, 26.40, 20.27, 19.15, 10.82, 8.19. HRMS(ESI) m/z [M + H]+: calcd for C34H39N3O4: 554.2974, found 554.2987.
7-methoxy-2-oxo-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8f): Intermediate 6b was treated with intermediate 4f according to general procedure to obtain the desired product 8f as a white solid (39%). mp: 103–105 °C. 1H NMR (500 MHz, CDCl3) δ 8.84 (s, 1H), 8.80 (s, 1H), 7.98 (d, J = 8.3 Hz, 1H), 7.74 (dd, J = 8.3, 0.8 Hz, 1H), 7.57 (d, J = 8.7 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.37 (t, J = 7.6 Hz, 1H), 6.94 (dd, J = 8.7, 2.4 Hz, 1H), 6.86 (d, J = 2.3 Hz, 1H), 4.51 (bs, 1H), 3.92 (s, 3H), 3.65–3.55 (m, 1H), 3.52–3.46 (m, 3H), 3.28 (d, J = 4.0 Hz, 1H), 1.88 (t, J = 9.5 Hz, 1H), 1.78–1.64 (m, 4H), 1.60–1.46 (m, 5H), 1.43 (s, 1H), 1.39 (s, 3H), 1.34 (d, J = 8.7 Hz, 2H), 1.03 (s, 3H), 0.66 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.19, 164.77, 161.99, 161.89, 156.57, 148.18, 142.29, 130.87, 129.40, 127.40, 123.78, 120.01, 119.47, 117.97, 114.79, 113.98, 112.39, 100.26, 55.99, 54.33, 53.55, 50.95, 45.89, 39.47, 32.09, 30.67, 29.35, 26.99, 26.88, 26.66, 26.50, 20.31, 19.19, 10.85. HRMS(ESI) m/z [M + H]+: calcd for C34H39N3O4: 554.2974, found 554.3013.
6,7-dimethoxy-2-oxo-N-(6-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)hexyl)-2H-chromene-3-carboxamide (8g): Intermediate 6b was treated with intermediate 4g according to general procedure to obtain the desired product 8g as a faint yellow solid (41%). mp: 96–98 °C. 1H NMR (500 MHz, CDCl3) δ 8.86 (d, J = 5.3 Hz, 1H), 8.79 (s, 1H), 7.99 (d, J = 8.3 Hz, 1H), 7.76 (d, J = 8.2 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.37 (dd, J = 8.1, 7.0 Hz, 1H), 6.96 (s, 1H), 6.88 (s, 1H), 4.23 (bs, 1H), 3.99 (s, 3H), 3.95 (s, 3H), 3.58 (dd, J = 12.5, 6.3 Hz, 1H), 3.53–3.44 (m, 3H), 3.27 (d, J = 3.9 Hz, 1H), 1.87 (t, J = 9.5 Hz, 1H), 1.81 (d, J = 6.4 Hz, 1H), 1.78–1.63 (m, 4H), 1.60–1.44 (m, 4H), 1.37 (s, 3H), 1.33 (d, J = 9.0 Hz, 2H), 1.03 (s, 3H), 0.64 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.12, 162.12, 162.00, 155.08, 151.11, 148.01, 147.14, 142.44, 129.21, 127.49, 123.84, 119.99, 119.56, 117.91, 115.00, 111.48, 108.71, 99.31, 56.59, 56.39, 54.36, 53.58, 50.97, 46.26, 45.78, 39.39, 32.06, 30.63, 29.36, 26.98, 26.56, 26.40, 20.29, 19.17, 10.86. HRMS(ESI) m/z [M + H]+: calcd for C35H41N3O5: 584.3080, found 584.3113.
2-oxo-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9a): Intermediate 6c was treated with intermediate 4a according to general procedure to obtain the desired product 9a as a yellow solid (42%). mp: 99–101 °C. 1H NMR (500 MHz, CDCl3) δ 8.90 (s, 1H), 8.83 (s, 1H), 8.00 (d, J = 7.1 Hz, 1H), 7.79–7.70 (m, 1H), 7.70–7.60 (m, 1H), 7.48 (m, 1H), 7.43–7.29 (m, 3H), 4.79 (bs, 1H), 3.63–3.52 (m, 1H), 3.50–3.43 (m, 3H), 3.27 (s, 1H), 1.92–1.80 (m, 1H), 1.75–1.60 (m, 4H), 1.53–1.40 (m, 6H), 1.41–1.36 (m, 4H), 1.33 (s, 3H), 1.27–1.22 (m, 2H), 1.01 (s, 3H), 0.65 (s, 3H).13C NMR (125 MHz, CDCl3) δ172.23, 161.49, 161.39, 154.32, 148.22, 133.97, 129.73, 127.76, 125.26, 123.99, 119.75, 119.63, 118.59, 118.44, 116.55, 60.34, 58.43, 54.53, 53.67, 50.99, 45.92,39.75, 32.02, 30.65, 29.19, 28.97, 26.92, 26.80, 26.76, 21.01, 20.26, 19.13, 14.15, 10.83. HRMS(ESI) m/z [M + H]+: calcd for C34H39N3O3: 538.3025, found 538.3057.
6-methyl-2-oxo-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9b): Intermediate 6c was treated with intermediate 4b according to general procedure to obtain the desired product 9b as a faint yellow solid (37%). mp: 113–114 °C. 1H NMR (500 MHz, CDCl3) δ 8.87 (s, 1H), 8.86 (s, 1H), 7.99 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 8.3 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.48–7.44 (m, 2H), 7.41–7.33 (m, 1H), 7.29 (d, J = 8.3 Hz, 1H), 4.59 (bs, 1H), 3.61–3.53 (m, 1H), 3.52–3.42 (m, 3H), 3.28 (d, J = 4.0 Hz, 1H), 2.44 (s, 3H), 1.92–1.85 (m, 1H), 1.85–1.78 (m, 1H), 1.76–1.62 (m, 4H), 1.54–1.42 (m, 6H), 1.39 (s, 3H), 1.34 (d, J = 8.7 Hz, 2H), 1.02 (s, 3H), 0.66 (s, 3H). 13C NMR (125 MHz, CDCl3) δ173.01, 161.70, 161.55, 152.52, 148.21, 143.08, 135.15, 129.31, 128.24, 127.55, 123.86, 119.87, 119.49, 118.37, 118.25, 117.82, 116.25, 54.40, 53.60, 50.95, 46.00, 39.73, 32.05, 30.67, 29.20, 28.98, 26.96, 26.82, 26.78, 25.96, 24.26, 20.73, 20.29, 19.16, 10.83. HRMS(ESI) m/z [M + H]+: calcd for C35H41N3O3: 552.3181, found 552.3220.
6-bromo-2-oxo-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9c): Intermediate 6c was treated with intermediate 4c according to general procedure to obtain the desired product 9c as a faint yellow oil (30%). 1H NMR (500 MHz, CDCl3) δ 8.82 (s, 1H), 8.75 (s, 1H), 7.97 (d, J = 8.3 Hz, 1H), 7.80 (s, 1H), 7.76–7.70 (m, 1H), 7.70 (d, J = 8.5 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.30–7.26 (m, 1H), 4.47 (s, 1H), 3.56 (d, J = 6.5 Hz, 1H), 3.46 (dd, J = 13.1, 6.7 Hz, 3H), 3.26 (d, J = 3.9 Hz, 1H), 2.21–2.11 (m, 2H), 1.87 (t, J = 9.5 Hz, 1H), 1.74–1.62 (m, 4H), 1.51–1.40 (m, 6H), 1.37 (s, 3H), 1.33 (d, J = 8.7 Hz, 2H), 1.02 (s, 3H), 0.64 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.23, 160.85, 153.10, 147.29, 146.84, 142.23, 136.65, 131.76, 129.46, 127.39, 123.76, 120.08, 120.00, 119.53, 119.39, 118.29, 118.01, 117.91, 54.32, 53.55, 50.94, 46.07, 39.86, 32.09, 30.71, 29.19, 29.01, 26.99, 26.84, 26.81,26.498, 20.32, 19.20, 10.86. HRMS(ESI) m/z [M + H]+: calcd for C34H38BrN3O3:618.2154, found 618.2177.
2-oxo-6-(trifluoromethoxy)-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9d): Intermediate 6c was treated with intermediate 4d according to general procedure to obtain the desired product 9d as a faint yellow solid (33%). mp: 89–91 °C. 1H NMR (500 MHz, CDCl3) δ 8.87 (s, 1H), 8.75 (s, 1H), 8.00 (d, J = 9.5 Hz, 1H), 7.71 (d, J = 8.5 Hz, 1H), 7.58–7.48 (m, 3H), 7.44 (d, J = 9.0 Hz, 1H), 7.40–7.32 (m, 1H), 4.56 (bs, 1H), 3.62–3.53 (m, 1H), 3.52–3.42 (m, 3H), 3.29–3.24 (m, 1H), 1.96–1.90 (m, 1H), 1.89–1.84 (m, 1H), 1.76–1.61 (m, 4H), 1.54–1.41 (m, 6H), 1.38 (s, 3H), 1.33 (d, J = 8.5 Hz, 2H), 1.02 (s, 3H), 0.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ172.97, 160.82, 160.72, 152.32, 147.08, 127.56, 126.85, 123.87, 121.26, 119.89, 119.80, 119.45, 119.24, 118.24, 117.89, 109.99, 54.41, 53.61, 50.96, 47.02, 46.98, 46.00, 39.87, 32.05, 30.68, 29.17, 28.99, 26.96, 26.82, 26.79, 26.34, 26.27, 20.29, 19.16, 10.82. HRMS(ESI) m/z [M + H]+: calcd for C35H38F3N3O4: 622.2848, found 622.2868.
6-methoxy-2-oxo-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9e): Intermediate 6c was treated with intermediate 4e according to general procedure to obtain the desired product 9e as a faint yellow solid (46%). mp: 104–106 °C. 1H NMR (500 MHz, CDCl3) δ 8.88 (s, 1H), 8.84 (s, 1H), 8.11 (d, J = 7.7 Hz, 1H), 7.84 (dd, J = 21.8, 8.3 Hz, 1H), 7.55–7.46 (m, 1H), 7.39–7.28 (m, 2H), 7.25–7.19 (m, 1H), 7.08–7.01 (m, 1H), 4.11 (bs, 1H), 3.85 (s, 3H), 3.63–3.55 (m, 1H), 3.54–3.41 (m, 3H), 3.29–3.24 (m, 1H), 1.94–1.83 (m, 1H), 1.77–1.57 (m, 4H), 1.54–1.38 (m, 7H), 1.31 (s, 3H), 1.30–1.25 (m, 2H), 1.01 (s, 3H), 0.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ171.92, 161.58, 161.46, 156.54, 148.88, 148.00, 128.20, 124.27, 122.48, 120.07, 119.49, 118.91, 118.53, 117.61, 117.22, 110.59,58.45, 55.86, 54.78, 53.82, 51.08, 45.72,43.17, 39.70, 31.95, 30.58, 29.15, 28.91, 26.85, 26.76, 26.69, 20.19, 19.05, 14.05, 10.87. HRMS(ESI) m/z [M + H]+: calcd for C35H41N3O4: 568.3131, found 568.3158.
7-methoxy-2-oxo-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9f): Intermediate 6c was treated with intermediate 4f according to general procedure to obtain the desired product 9f as a white solid (38%). mp: 99–101 °C. 1H NMR (500 MHz, CDCl3) δ 8.83 (s, 1H), 8.78 (s, 1H), 7.98 (d, J = 8.3 Hz, 1H), 7.70 (d, J = 9.0 Hz, 1H), 7.56 (dd, J = 8.7, 4.0 Hz, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 6.93 (dt, J = 8.7, 2.9 Hz, 1H), 6.85 (d, J = 2.6 Hz, 1H), 4.50 (bs, 1H), 3.90 (s, 3H), 3.60–3.52 (m, 1H), 3.51–3.42 (m, 3H), 3.27 (d, J = 3.9 Hz, 1H), 2.21–2.12 (m, 1H), 1.90–1.83 (m, 1H), 1.76–1.60 (m, 4H), 1.53–1.40 (m, 6H), 1.37 (s, 3H), 1.32 (t, J = 8.8 Hz, 2H), 1.02 (s, 3H), 0.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.13, 161.62, 161.56, 156.58, 148.93, 148.06, 147.11, 142.38, 129.28, 127.47, 123.82, 122.55, 119.98, 119.52, 118.95, 118.55, 117.93, 117.66, 110.59, 55.90, 54.35, 53.57, 50.97, 45.82, 39.51, 32.07, 30.91, 30.64, 29.30, 26.99, 26.60, 26.43, 20.30, 19.18, 10.85. HRMS(ESI) m/z [M + H]+: calcd for C35H41N3O4: 568.3131, found 568.3156.
6,7-dimethoxy-2-oxo-N-(7-((4,11,11-trimethyl-1,2,3,4-tetrahydro-1,4-methanoacridin-9-yl)amino)heptyl)-2H-chromene-3-carboxamide (9g): Intermediate 6c was treated with intermediate 4g according to general procedure to obtain the desired product 9g as a yellow solid (36%). mp: 83–86 °C. 1H NMR (500 MHz, CDCl3) δ 8.83 (s, 1H), 8.80 (s, 1H), 7.97 (dd, J = 8.0, 2.8 Hz, 1H), 7.71 (d, J = 8.3 Hz, 1H), 7.57–7.47 (m, 1H), 7.40–7.30 (m, 1H), 6.96 (d, J = 7.5 Hz, 1H), 6.86 (d, J = 6.6 Hz, 1H), 4.52 (bs, 1H), 4.00 (s, 3H), 3.92 (s, 3H), 3.63–3.52 (m, 1H), 3.51–3.39 (m, 3H), 3.29–3.23 (m, 1H), 1.91–1.83 (m, 1H), 1.75–1.59 (m, 4H), 1.55–1.39 (m, 7H), 1.37 (s, 3H), 1.32 (dd, J = 8.4, 4.0 Hz, 2H), 1.01 (s, 3H), 0.64 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 173.16, 162.02, 155.06, 151.10, 147.95, 147.13, 142.35, 129.36, 127.42, 123.78, 119.98, 119.46, 117.95, 115.07, 111.49, 108.70, 99.32, 56.59, 56.38, 54.34, 53.56, 50.95, 46.25, 46.05, 39.66, 32.09, 30.69, 29.26, 28.99, 26.99, 26.69, 26.43, 26.36, 20.32, 19.19, 10.87. HRMS(ESI) m/z [M + H]+: calcd for C36H43N3O5: 598.3236, found 598.3258.