Abstract
Schiff bases are a vast group of compounds characterized by the presence of a double bond linking carbon and nitrogen atoms, the versatility of which is generated in the many ways to combine a variety of alkyl or aryl substituents. Compounds of this type are both found in nature and synthesized in the laboratory. For years, Schiff bases have been greatly inspiring to many chemists and biochemists. In this article, we attempt to present a new take on this group of compounds, underlining of the importance of various types of Schiff bases. Among the different types of compounds that can be classified as Schiff bases, we chose hydrazides, dihydrazides, hydrazones and mixed derivatives such as hydrazide–hydrazones. For these compounds, we presented the elements of their structure that allow them to be classified as Schiff bases. While hydrazones are typical examples of Schiff bases, including hydrazides among them may be surprising for some. In their case, this is possible due to the amide-iminol tautomerism. The carbon–nitrogen double bond present in the iminol tautomer is a typical element found in Schiff bases. In addition to the characteristics of the structure of these selected derivatives, and sometimes their classification, we presented selected literature items which, in our opinion, represent their importance in various fields well.
1. Introduction
The term Schiff’s base derives from the name of the German chemist Hugo Schiff, who, in 1864, was the first to describe the products resulting from the reaction of primary amines with carbonyl compounds [1].
Following the recommendation of IUPAC, Schiff bases are defined as chemical compounds (imines) bearing a hydrocarbyl group on the nitrogen atom R2C = NR′ (R′ ≠ H) (Figure 1). They are considered by many to be synonymous with azomethines [2].
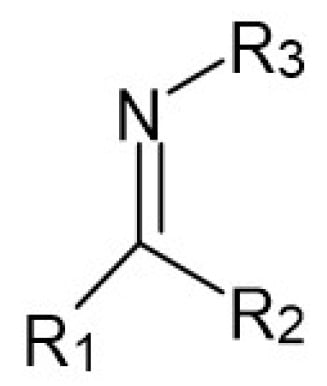
Figure 1.
The specific structure fragment characteristic of Schiff bases, where R1, R2 and R3 are alkyl or (more often) aryl groups. R1 or/and R2 may also be hydrogen atoms.
This common feature determines the ability of Schiff bases to form complexes with transition metal ions [3,4,5,6,7]. In these complexes, they function (like amines, amides and phosphines) as L-type ligands, that is, ligands containing two-electron donors, which do not undergo electron changes on their valence shells [8]. The complex formation takes place by coordinating the d-block metal ion by the electron-donating ligand atom and serves to modify the steric and electronic surrounding of the metal. As a consequence, this leads to the stabilization and regulation of the reactivity of the metal ion, which is especially useful for less stable ions at higher oxidation states [8,9]. Nitrogen, oxygen or sulfur atoms can participate in the coordination as donors. Multivalent Schiff base ligands (Figure 2) eagerly form complexes: bidentate and tridentate with Co(II), Ni(II) ions, as well as four-dentate, highly stabilizing metal ions at various oxidation states, starting with divalent Ni(II), Cu(II), Pd(II) or tetravalent V(IV), Ti(IV), up to uranium U(III,IV,V) [10,11]. The donor atoms (O, N, S) of bidentate ligands can occur, for example, in ON or NN sequences, tridentate ligands—ONO, NNN or ONS or NNS (where the sulfur atom comes from, for example, disulfide bridges) and in tetradentate ligands—NNNN, ONNO, NSNO [10,12,13].
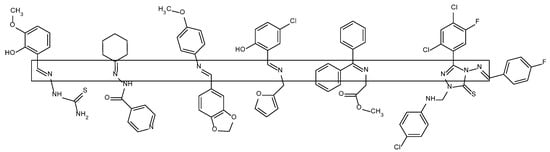
Figure 2.
Examples of Schiff bases—the imine fragments are framed [12].
Interest in the use of transition metal complexes with Schiff bases in medicine began to develop in the second half of the 19th century. Co(II), Ni(II), Cu(II) and Zn(II) complexes exhibit exceptional biological activity [14,15,16,17,18,19,20,21,22,23,24,25,26]. In recent years, there has been greater interest in the possibility of using metal ions such as Ag(I), Au(I) or Pt(II) in medicine [27,28]. Additionally, complexes of these metals with Schiff’s bases show interesting biological properties [29,30,31,32,33].
Schiff bases are called auxiliary ligands because they modulate the structure and reactivity of the transition metal ion in the center of the complex, while they do not undergo irreversible transformations themselves, unlike reactive ligands [8,9].
In the case of Schiff bases containing a benzene ring that is directly connected to an azomethine (imine) moiety, the presence of a hydroxyl group in the 2-position (ortho) to the moiety characteristic of Schiff bases may contribute to the formation of intramolecular resonance-stabilized hydrogen bonds (Figure 3), whereas their presence has a positive effect on the thermodynamic stability of the whole molecule [34].
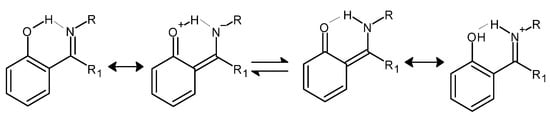
Figure 3.
An example of the formation of intra-molecular hydrogen bonds by Schiff bases in keto-enol equilibrium together with resonance structures of the corresponding forms (R and R1 = alkyl groups) [34].
Schiff bases have a number of applications as catalysts, including acid catalysts [33,35,36,37], reduction [38,39] or oxidation [40,41,42,43,44,45,46] catalysts.
Schiff bases are used, inter alia, in catalytic reactions, in crystal engineering, also as photo- or chemodetectors in biological systems (e.g., Al3+ ions in vivo) and most commonly, in medicine. Their most important medical applications include: antibacterial [47,48] and antifungal [49] (including anti-yeast) activity, antiviral [50,51], antitumor [52,53], anti-inflammatory [54], antipyretic, antimalarial [55], anticancer [56,57,58], anesthetic, oxytocin-imitating and oxytocin-inhibiting activity, as well as the selective inhibition of human tyrosine phosphatase 1B (PTP1B) or TCPTP and SHP-1 tyrosine phosphatases [12,34,59,60]. While there are indeed mentions of free ligands being more effective than their respective complexes [12], most often, it is the complexes of Schiff bases and metal ions that exhibit the strongest of the above-mentioned antimicrobial properties, creating favorable conditions for the penetration of microbial cell membranes by the metal ions they carry [12,61,62].
2. Hydrazides
2.1. Structure
A particular example of Schiff bases are hydrazides in their iminol tautomeric form. Hydrazides are a group of unique monosubstituted hydrazine derivatives that not only retain their specific -NH-NH- nitrogen bridge but also contain a carbonyl or sulfonyl group linked directly to one of the nitrogen atoms (Figure 4). The distinctive terminally occurring hydrazide moiety is as follows: R-NH-NH2 [62].
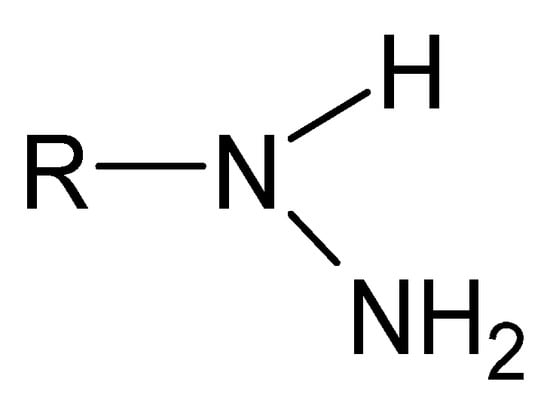
Figure 4.
A moiety characteristic of hydrazides, where R is -C(=O)- or -(O=)S(=O)-.
It should be noted that the illustrated hydrazide moiety may be partly analogous to the characteristic amide (peptide) moiety: (O=)C-NH-, the presence of which makes one think of hydrazides as potential peptidomimetics. An additional consequence of such a close proximity to oxygen and nitrogen atoms endowed with lone electron pairs, forming a torsion angle with the carbonyl atom and the hydrogen atom connected to the nitrogen atom, respectively, is the possibility of the migration of the double bond between the carbon and nitrogen atoms, with the formation of the hydroxyl group by the carbonyl oxygen atom [63,64,65].
The above-mentioned amide bond tautomerism characteristic of peptides also occurs in hydrazides (Figure 5).
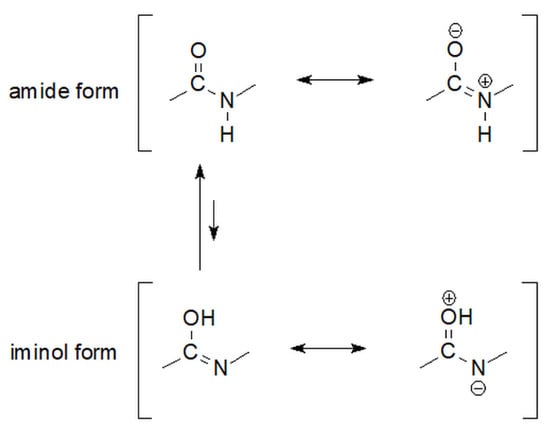
Figure 5.
The equilibrium of amide-iminol tautomers of carbonyl hydrazides [66].
The high rotation barrier around the C=N bond limits conformational changes within the molecule and results in the existence of iminol forms of hydrazides, similar to the existence of amides, in the form of E or Z isomers [64,67,68]. However, the differentia specifica, which places hydrazides in a category different from that of amides, is the already mentioned presence of a directly attached, subsequent Nβ atom. It is appropriate for E-iminols to be prevalent in amides, while the thermodynamic stability of hydrazides depends on additional factors, including the number and steric expansion of the β nitrogen atom, which prevents a universal isomer from being clearly indicated. The presence of the Nβ atom determines the possibility of hyper-couplings in hydrazide molecules due to the additional electron pair that adds to the existing electron density [69]. Moreover, in the case of at least monosubstituted hydrazides, it is perfectly distinguishable on a simple infrared spectrum, where these compounds present an additional intense signal shifted by approximately 100–150 cm−1 towards shorter waves—compared with their amide analogs with a -CH <group in place of the N atom [70].
Strong signals in the range of 2800–2700 cm−1, the so-called Bohlmann bands [71], named after their discoverer, are observed in response to the presence of a substituent of the amine nitrogen atom, in the form of a sp2 carbon atom (which is a ring member) or sp3. Originally, the study of these bands was used to clarify the stereochemistry of alkaloids, especially those containing the quinolizidine system [72,73]—found in the Egyptian water lily, called the tiger lotus, in the Scotch broom (Cytisus, Fabaceae) or in the Andean lupin (Lupinus, Fabaceae), such as lupinine or sparteine and its oxo-derivatives. In order to clearly illustrate the core of the Bohlmann effect, the commonly known anomeric effect should be recalled. In the classic approach, there is a preference for the electronegative substituent—attached to the atom adjacent to the heteroatom (most often, the oxygen atom), which is a member of the cyclohexane ring—to assume the axial orientation, despite the fact that if only steric aspects were considered, the equatorial orientation would present much less of a spatial hindrance. The anomeric effect may be regarded as a special type of the Bohlmann effect [73,74,75].
The generalized Bohlmann effect applies to the electron density transfer of lone pairs/lone pair of electrons of an oxygen atom or a nitrogen atom (their participation in the ring is not necessary), the acceptor of which is an anti-bonding CH σ* or CC σ* orbital, oriented antiperiplanarly (Figure 6) towards the orbital on which the lone electron pair is located. The importance of the Bohlmann effect in the context of IR spectroscopy may be seen in the case of amines rather than ethers or alcohols, due to the crucial, lower electronegativity of the nitrogen atom (E = 3.04) in comparison with the oxygen atom (E = 3.44). Electronegativity is defined as the ability of atoms of a given element to attract electrons, and undeniably, lone electron pairs of an oxygen atom are more strongly attracted than the lone electron pair of a nitrogen atom—so its relocation is easier to achieve and results in a greater shift in the infrared spectrum. Moreover, the stronger electronegativity of the elements—the aforementioned oxygen, or fluorine (E = 3.98)—causes a shift of the absorption bands towards higher frequencies, which for ethers or alcohols results in almost complete masking of it. This effect is competitive to the Bohlmann effect and is significantly greater than in the case of nitrogen. It should be mentioned that the antiperiplanarity towards the acceptor orbital for alcohols does not apply to the lone pair on the oxygen atom, but rather to the O-H bond in the hydroxyl group [70].
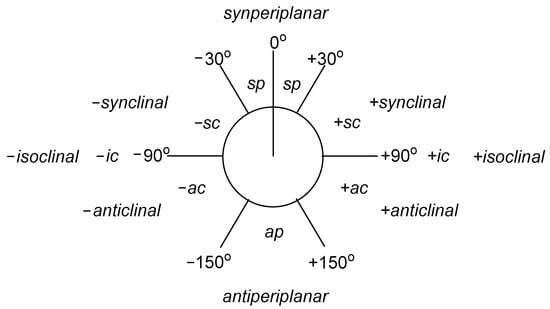
Figure 6.
Nomenclature in Newman’s projection depending on the value of the torsion angle [76].
If we take into account the most energetically stable conformers: antiperiplanar and gauche, then the lowest energetic conformation in at least a monosubstituted amino group means the axial arrangement of both the lone electron pair of the nitrogen atom and at least one C-H or C-C bond. The axial arrangement of the consecutive C-H or C-C bond of a disubstituted amino group (or a ring-closed nitrogen atom) increases the intensity of the Bohlmann bands, and sometimes also causes the appearance of an additional signal in the characteristic range and consequent shifting of the first signal towards waves of lower frequency. The contribution of the lone electron pair of the nitrogen atom to the Bohlmann effect is evidenced by the fact that the introduction of an additional substituent or the formation of an ammonium salt in the IR spectrum is visible as the disappearance of Bohlmann bands. The gauche conformation characterized by the presence of C-H stretching vibrations (not weakened as in the case of the axial position of substituent bonds) is not detectable in the Bohlmann bands region [70].
The topic of hydrazide isomerism must be supplemented with E/Z descriptors of CN/C=N bond [64,77]. Typically, hydrazides exist in an equilibrium between both isomeric forms (Figure 7), and its shift towards the formation of one of them depends on three overarching factors: the size of the substituents around the bond, the electrostatic repulsion of lone pairs on the oxygen and nitrogen atoms, and the possibility of hydrogen bonding in the folding macromolecules. On the 13C NMR spectrum, the isomers are easily recognizable on the basis of the value of the chemical shift δ of the carbonyl signal of the carbon atom, which for Z isomers does not exceed 170 ppm, while for E isomers, it reaches about 175 ppm. The percentage of isomers is easily calculated from the 1H NMR spectrum using the signal integrity of the -NHZ- and -NHE- groups, the former of which is shifted more strongly towards higher frequencies [64].
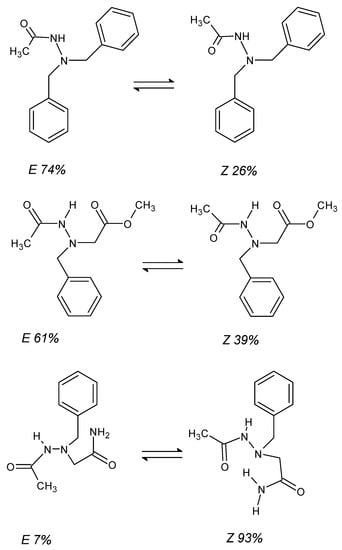
Figure 7.
Examples of isomeric equilibria of E (left) and Z (right) hydrazides. The Z isomer dominates when the folding of the molecule allows for the formation of multiple hydrogen bonds [64].
It is worth mentioning that the lone electron pair of the nitrogen atom of the primary amine group gives it the character of a Lewis base, making it able to participate in the formation of hydrogen bonds, intramolecular or with molecules of a polar, competing solvent, and that the iminol form promotes the formation of intramolecular hydrogen bonds, especially in the presence of appropriately non-polar solvents.
2.2. Importance
The documented biological activity of hydrazides confirms their functional affiliation to Schiff’s bases. Hydrazides exhibit broadly understood biocidal properties, including bactericidal properties. M. tuberculosis has received special attention since the 1950s. The effectiveness of the synthesized compounds against this bacterium is often compared with the isonidazid (Figure 8), pyridine-4-carboxylic acid hydrazide, which was the first to be studied regarding these properties. In addition, hydrazides also have virucidal (atazanavir is a popular antiretroviral agent used in HIV-1 infections, [65,78,79]), fungicidal and protozoicidal properties.
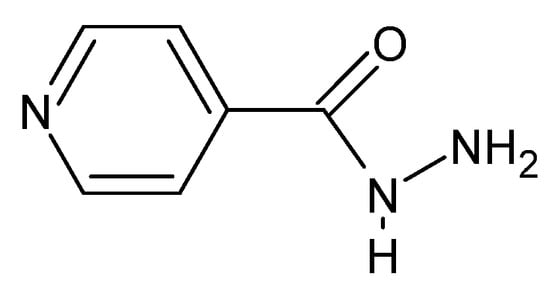
Figure 8.
Isonicotinic acid hydrazide (isonidazid).
The mechanism of action of isonidazid is not fully understood. Most likely, it becomes activated by CP-KatG—catalase-peroxidase—an enzyme inherent in some strains of bacteria, including M. tuberculosis, to the form of its acyl radical (Figure 9).
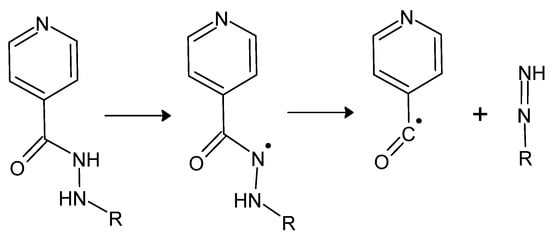
Figure 9.
Enzymatic activation of isonidazid or its derivative (where R is H, an alkyl or aryl substituent), resulting in the formation of a hydrazyl and ultimately acyl radical and a diazene derivative [80].
The pyridine-4-carbonyl radical is then coupled with NADH or NAD+, and the resulting adduct inhibits the action of InhA reductase (responsible for transferring enoyl and acyl groups). Thus, it hinders the synthesis of mycolic acids—α-branched and β-hydroxylated fatty acids, building bacterial cell walls—contributing to their decomposition and, consequently, also to the death of the bacterial cell [80,81].
Some hydrazides can be used to treat depression. Both the N′-isopropyl and the N′-benzyl isonidazid derivative are of importance as mood modulators (Figure 10). Both act as inhibitors of monoamine oxidase, which causes the deamination of serotonin and norepinephrine [82].
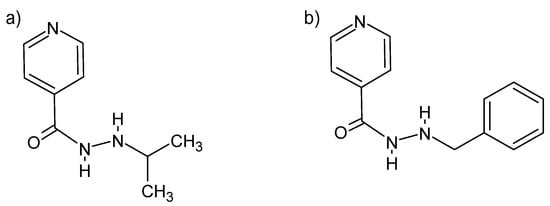
Figure 10.
Isonidazid derivatives: (a) N′-(propan-2-yl)-4-pyridinecarboxylic acid hydrazide and (b) N′-benzyl-4-pyridine carboxylic acid hydrazide [82].
Hydrazides also found chemical application inter alia as catalysts of enantioselective reactions in asymmetric aldol reactions [83], as substrates in regioselective reactions in the preparation of azoheterocycles, such as 1-aryl-1H-indazoles [84], or 1,2-disubstituted (in Pd/Cu catalyzed reactions) [85] and multisubstituted alkenes (Mizoroki–Heck reactions) [86] and as a sulfenylating reagents in reactions used to obtain indole thioethers [87]. Hydrazides are also used as protein markers in SSPL (Site-Specific Protein Labeling) methods, in which analyses are performed using spectrometric and electrophoretic (SDS-PAGE) techniques [88] and as modifiers of magnetic beads in the profiling of surface proteins or mapping N-glycosylation sites in A. nige [89,90]. Primary and secondary hydrazides containing alkene fragments facilitate cyclization in hydroamination and aminocarbonylation reactions [91].
Some hydrazides exhibit chemiluminescence (Figure 11) [92], which allows for their use in the labeling of, e.g., chromate(VI) ions without reducing them to Cr(III)—rhodamine B and rhodamine G6 hydrazides [93], or deoxyribonucleic acid (Figure 12)—biotin hydrazide [94].
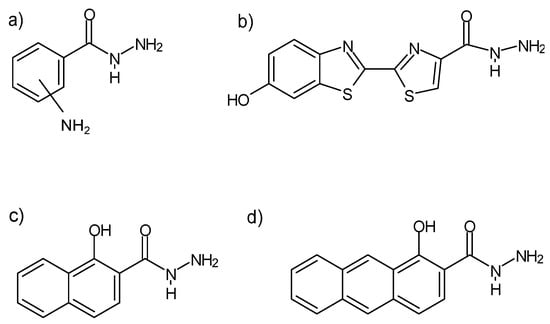
Figure 11.
Examples of monosubstituted, hydrazides of (a) aminobenzoic acid, (b) luciferin, (c) 1-hydroksy-2-naphthoic acid, (d) 1-hydroxyanthracene-2-carboxylic acid, exhibiting chemiluminescence. The -NH2 substituent in the Markush structure (a) can assume “ortho” and “meta” positions without losing its chemiluminescent properties [92].
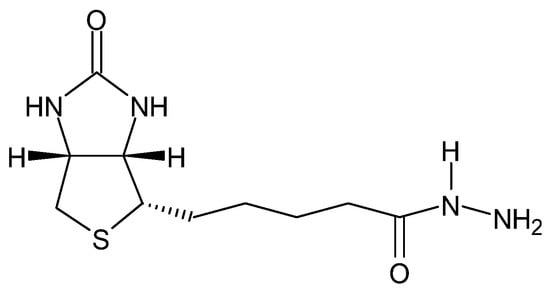
Figure 12.
Biotin hydrazide.
As aza-peptides, they find use as inhibitors of serine and cysteine proteases due to their lower susceptibility to enzymatic hydrolysis in comparison to their peptide analogs and greater metabolic stability [95,96]. Moreover, the appropriate inclusion of an aza-amino acid in the synthetic peptide chain stabilizes its β-turns [97].
A useful example of a hydrazine derivative is carbidopa hydrazine acid (Figure 13), co-administered with the dopamine precursor (S) 3,4-dihydroxy-L-phenylalanine (levodopa), in the treatment of Parkinson’s disease. This hydrazine acid regulates the rate of dopamine release, prolonging the effect of the drugs [82].
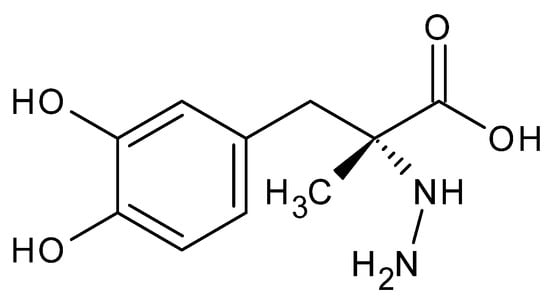
Figure 13.
Carbidopa-N-amino-α-methyl-3-hydroxy-L-tyrosine.
Interesting results of research on the synthesis, structure and biological activity of hydrazine and hydrazide derivatives of 3-formylchromone were presented by Słomiak et al. [98]. They synthesized a number of hydrazine derivatives and a hydrazide derivative which they complexed with Cu (II) (Figure 14).
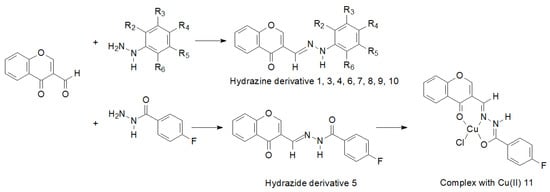
Figure 14.
Synthesis of hydrazine and hydrazide derivatives of 3-formylchromone. Hydrazine derivatives of 3-formylchromone: 1: R2=R5=F, R3=R4=R6=H; 3: R4=CF3, R2=R3=R5=R6=H; 4: R3=R5=CF3, R2=R4=R6=H; 6: R2=R3=R5=R6=F, R4=H; 7: R2=CH3, R5=F, R3=R4=R6=H; 8: R2=R4=R6=F, R3=R5=H; 9: R2=R3=R4=R5=R6= F, 10: R2=CF3, R3=R4=R5=R6=H.
They found that concentrations of 0.01–1250 μmol/L influenced cell proliferation. In the case of two cell lines: the L929 cell line (mouse fibroblast cell line) and the EA.hy926 cell line (human umbilical cord vein, somatic cell hybrid), these compounds demonstrated antiproliferative activity and stimulation of proliferation.
2.3. Classification
The simplest criterion for the classification of unsubstituted hydrazides, i.e., those containing a free amino group, is to consider the type of atom connected directly to the nitrogen atom of the secondary amino group. The most commonly studied are carbonyl-type hydrazides (Figure 15), obtained by the nucleophilic substitution of an ester of a suitable carboxylic acid with hydrazine. The reaction is sometimes referred to as the hydrazinolysis of carboxylic acid esters [99].
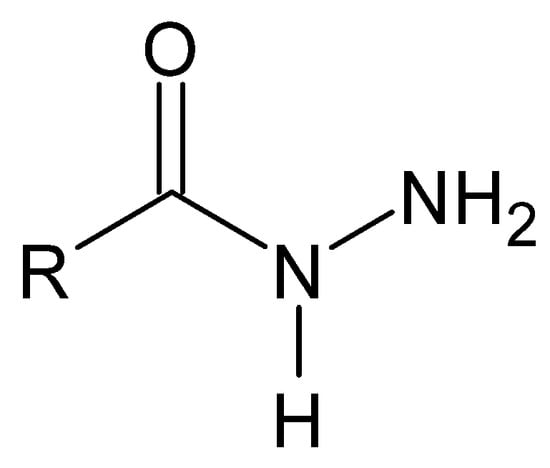
Figure 15.
Structure characteristic for carbonyl hydrazides, where R is an alkyl or aryl substituent.
The obtainment of the first hydrazides was reported by German scientists as early as 1892, as a result of the reaction of fatty acid esters with hydrazine (G. Schöfer) and the reaction of glycolic acid ester with hydrazine (N. Schwan), and more in 1895 by the aforementioned authors and T. Curtius, including the simplest hydrazide, i.e., formic acid hydrazide [100]. In 1968, R. Slagel obtained them through a less conventional method, in addition to the carboxylic acid ester, using a disubstituted, unsymmetrical hydrazine and an epoxide [101].
Of lesser interest are sulfonyl hydrazides (Figure 16), in which the connection with the R group is most often formed through a sulfonyl group, with the sulfur atom bonded directly to the aromatic ring.
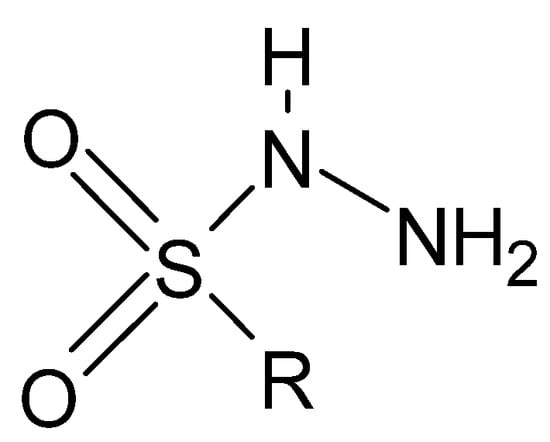
Figure 16.
Basic structure of sulfonyl hydrazides, where R is an aryl group.
However, the simplest criterion is not the only one. Some hydrazides can be called aza-amino acids, that is, amino acids enriched with an additional amino group, simultaneously directly connected to the amino N atom and carbonyl C atom, that replace the α-CH moiety. If said aza-amino acid is a part of a poly-chain of at least 10 members, it forms the aza-peptide (Figure 17 and Figure 18) [95].
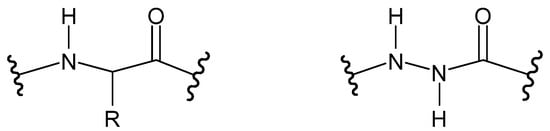
Figure 17.
Peptide (left) and aza-peptide (right), where R is a side chain.
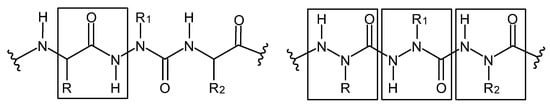
Figure 18.
Aza-peptide (left) and azatide (right), where R, R1 and R2 are side chains—hydrazide bonds are framed.
A special type of aza-peptide, called an azatide, is a biopolymer made exclusively of α-aza-amino acids [102].
Hydrazine acids (Figure 19) are derivatives of amino acids in which the amino group -NH2 is replaced with the hydrazine group -NH-NH2 [103].
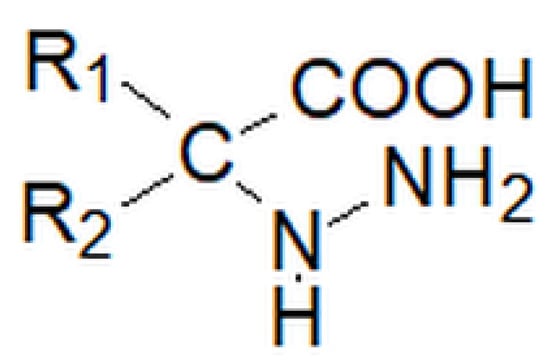
Figure 19.
Structure of hydrazine acid, where R1 and R2 may be a hydrogen atom, an alkyl group or an aryl group.
It is worth mentioning that due to the possibility of folding and the formation of hydrogen bonds between non-adjacent hydrogen atom acceptors and hydrogen atoms derived from -OH and -NH- groups, the share of the E isomer in the isomeric equilibrium decreases with the elongation of the chain [64,104]. Additionally, in the case of participation in the hydrogen bond of the H atom coming from the primary amine group, two singlets (of a significant chemical shift in relation to each other, e.g., δ 2.60 ppm) may appear in the 1H NMR spectrum, one on each side of the -NHZ- signal [64]. Participation in the hydrogen bond causes the hydrogen atoms of the -NH2 group to appear in the spectrum as separate signals.
One cannot fail to mention the interesting combinations called metallohydrazides: precursors of hydrazides, which can also be considered their isolobal analogues. The concept of isolobality introduced by Roald Hoffmann is based on the theory of frontier (border) orbitals HOMO and LUMO by Kenichi Fukui, assuming that, in simplified terms, the reactivity of a molecule or its fragment results from the properties of its frontier orbitals, i.e., valence active orbitals. It describes similarity in the number, symmetry, approximate energy and shape of orbitals and in the number of their electrons between organometallic compounds and known organic ligands, helping to determine the electronic structure and, consequently, the reactivity of the former [105,106,107].
Essential is the presence of an even-electron Lewis base as the electron donor in the ligand [106]. Naturally, in the organometallic complex the transition metal atom also participates —in the described complexes, ones belonging in the 6th–8th groups in the periodic table. Tetra- and pentacarbonyl hydrazinecarbonyl complexes (Fisher carbene type) lead to the production of corresponding hydrazides due to the isolobality of the metal = carbon double bond as regards the double bond of the carbonyl group in the hydrazide even as a result of mild oxidation. The complexation of a metal atom with carbonyl ligands is twofold in nature, i.e., simultaneously of a coordinate σ-bond and a π-type return (redonor) bond. The first and most important of them arises as a result of overlapping of the empty hybridized orbitals (formed from the s, d and p orbitals) on the metal atom and the filled hybridized HOMO orbital on the carbon atom in the carbon monoxide(II) molecule with the donation of a lone electron pair by the latter. The second, on the other hand, is the consequence of overlapping of the filled dπ orbitals or hybridized dpπ orbitals on the metal atom and the unfilled LUMO orbital on the carbon monoxide(II) carbon atom after the initial transfer of electrons from the filled π orbital of the CO molecule to the unfilled d-orbital of the CO molecule and their subsequent redonation to the available π* orbital on the carbon atom. Thus, a resonance hybrid is formed (Figure 20), in which the multiplicity of the bond between the metal and carbon atoms is between 1 and 2, and the bond between the carbon and oxygen atoms—between 2 and 3 [8,107,108,109,110].

Figure 20.
Resonance forms of the M–C–O bond [55].
Transition metal carbonyls easily give Fisher-type carbenes with organolithium compounds, and in a two-step substitution reaction at a carbon atom with sp2 hybridization (that consists of a nucleophilic addition followed by elimination) with an amine (in this case, hydrazine), leading through a tetrahedral intermediate—hydrazinecarbenes [110]. These compounds can be used in RCM (Ring-Closing Metathesis) reactions [111] and in reactions with peptide nucleic acid monomers—DNA and RNA mimetics, in which the main phosphate-sugar chain is replaced with a polyamide chain composed of N-(2-aminoethyl)-glycine units [112]—in order to modify their properties, including increasing their lipophilicity and thus, the ability to penetrate cell membranes, which may increase their use in molecular biology and medicine [113].
The reaction to obtain hydrazides should not be carried out without considering the sensitivity of the expected products to further oxidation (Figure 21). Molecular iodine obtained in situ can be a safe, mild oxidant. The reagent kit used for this purpose is an iodide anion oxidized with sodium borate in a neutral pH water–ethyl acetate buffer with the addition of potassium bicarbonate, which results in the gradual release of molecular iodine into the reaction mixture [107].
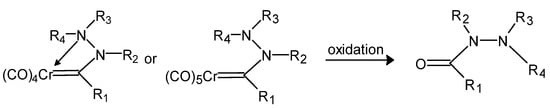
Figure 21.
Tetra- and pentacarbonyl hydrazinecarbonyl complexes and their oxidation product carbonyl hydrazid (R1, R2, R3 and R4 are an alkyl group) [107].
3. Dihydrazides
3.1. Structure
The plurality of dihydrazide compounds (Figure 22), similar to that of hydrazide–hydrazones, results from the variety of alkyl or aryl fragments and their substituents that can be linked by a diamide bridge. The symmetrical structure determines the nomenclature: each time, we start naming with the carbonyl group, invariably obtaining a monosubstituted hydrazide. Thus, including both reading possibilities, the name mentioned above can be adopted.
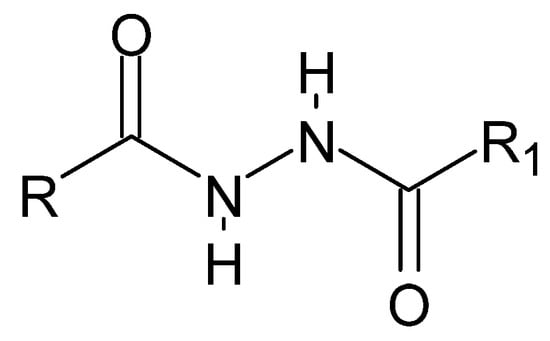
Figure 22.
The basic structure of dihydrazides, where R and R1 is an alkyl, aryl, or hydrogen group.
The synthesis of dihydrazides—methanoic and ethanoic acid dihydrazides—was first described by T. Curtis, N. Schwann and G. Schöfer in 1895 [100].
3.2. Importance
Dihydrazides are important as antibacterial, antifungal and antiparasitic agents. The fact that it is the hydrazide moiety, which is the key to the biological activity of similar compounds, was already reported in the research by Raymond Cavier and Richard Rips in 1965, in which they observed that the replacement of the diisopropylidenemalonyl hydrazide moiety with an amide moiety reduces the compound’s activity against the nematode S. obvelata [114].
An example of another property of some dihydrazides is chemiluminescence, i.e., the emission of radiation manifested by the light effect (ultraviolet, visible light and infrared), which results from the recombination of the electron–hole pair in an electronically excited product or an intermediate product, resulting from the action of an external factor, which in this case is a chemical reaction, on a given compound [115]. One of the earliest described chemiluminophores—as early as 1928 [116], commonly called luminol, is 3-aminophthalic acid hydrazide, with the capacity to emit light at λ = 425 nm (blue light) [92]. The exchange of the carbonyl groups with the secondary amino groups while maintaining the symmetry (amide groups connected through the carbon atoms of carbonyl groups) causes the compounds to lose their chemiluminescent properties. They are also negatively affected by the presence of electron-withdrawing substituents (-Cl, -NO2), while electron-donating substituents (-NH2, -OH) strengthen them [92]. The chemiluminescent properties allow the use of dihydrazides, as they are highly sensitive to pH changes and are indicators of the endpoint of acid–base titration (N′-formyl-rhodamine B dihydrazide) [117], as DNA probes and in immunoassays (luminol) [118].
The presence of the amide-analogous -C(=O)-NH-NH- group enables the occurrence of the aforementioned amide-iminol tautomerism, presented below (Figure 23 and Figure 24) [92].
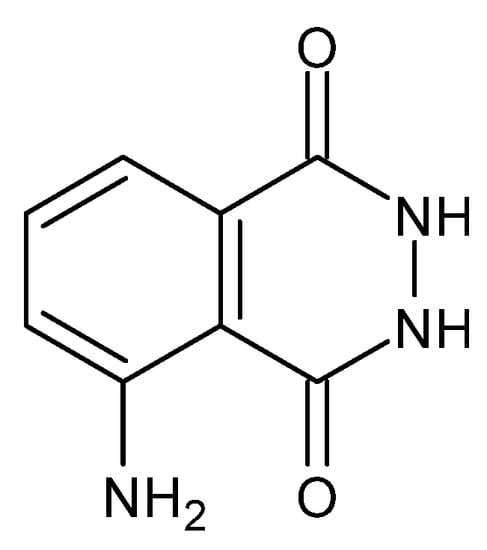
Figure 23.
Structure of 3-aminophthalic acid hydrazide (luminol) [92].
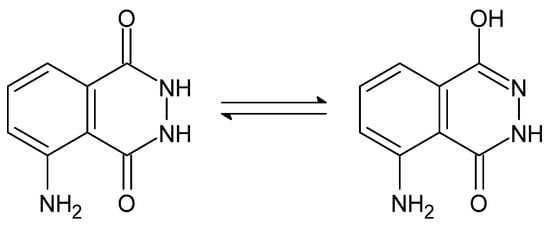
Figure 24.
Amide-iminol tautomerism of luminol [92].
Dihydrazides are also used as catalysts in highly enantioselective reactions leading to the formation of asymmetric aldols [83].
4. Hydrazones
4.1. Structure
A special type of hydrazides are hydrazones, the main distinguishing feature of which is the presence of an imine bond in their moiety (Figure 25), which in turn participates in imine–enamine tautomerization (Figure 26) due to the presence of the α-hydrogen atom.
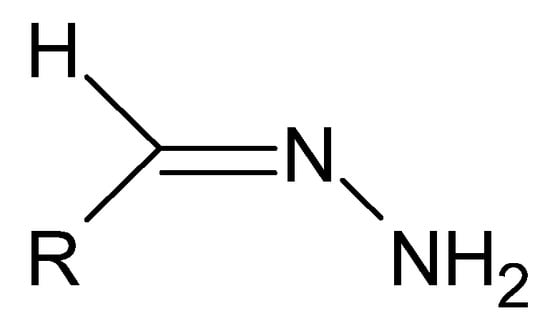
Figure 25.
The basic structure of hydrazones, where R is an alkyl or aryl group.
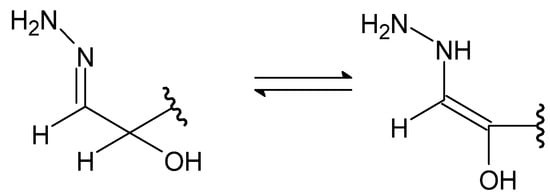
Figure 26.
The imine–enamine tautomerization of hydrazones.
Characteristic signals are visible in the 1H NMR spectrum in the form of a singlet with a chemical shift of δ 8.16–8.67 ppm for -CH= and a singlet with a chemical shift of δ 10.45 12.25 ppm for -NH- [119].
The N-N bond can be reduced to the -NH2 group [120] or the whole hydrazone molecule can be reduced to a hydrazide by reductive acylation [121]. The C=N bond is susceptible to a nucleophilic attack. It can be hydrolyzed, oxidized or reduced—it willingly restores the C=O carbonyl group. It can undergo the nucleophilic addition of an organometallic compound (containing Li, Mg, Ce and Yb atoms), as well as of the intramolecular group -SH [107,122]. Under appropriate conditions, hydrazones can react with α,β-unsaturated aldehydes, giving interesting dihydrazide connections with a lactam ring [123].
4.2. Importance
Hydrazones have uses, among others, as pesticides, insecticides, nematicides, rodenticides or plant-growth regulators [124].
Sulfonyl hydrazones are important as antidepressants, analgesics, anti-inflammatory, anti-cancer, antifungal, and antibacterial agents—they can act as strong inhibitors of tyrosine phosphatase B (PtpB) of bacteria from the M. tuberculosis strain [125] and are antidiabetic [126]. This particular type of hydrazone is often tested for antimicrobial properties alongside its analogues—sulfonamides [125]. Equally common is the use of aryl hydrazones, in which the hydrazone moiety is directly linked to the aromatic ring, in medicine [127].
As with Schiff bases, hydrazones are considered multidentate ligands for their chelating capacity. Complexes of hydrazones with transition metal ions may show increased antimicrobial activity compared to uncomplexed ligands, where it is the metal ion that has the capacity to disturb cellular activity, while the ligand “assists”, increasing the lipophilicity of the ion’s environment, thus allowing for its penetration of the cell membrane [61,128,129,130].
Less frequently, the opposite phenomenon occurs—a decrease in the antibacterial activity of the sulfonyl hydrazones and their complexes (Figure 27) in relation to their ligands (Table 1), which is explained by the fact that the electron density is reduced on the donor (O, N) atoms involved in the formation of coordination bonds with the tran-sition metal ion in the complex [131].
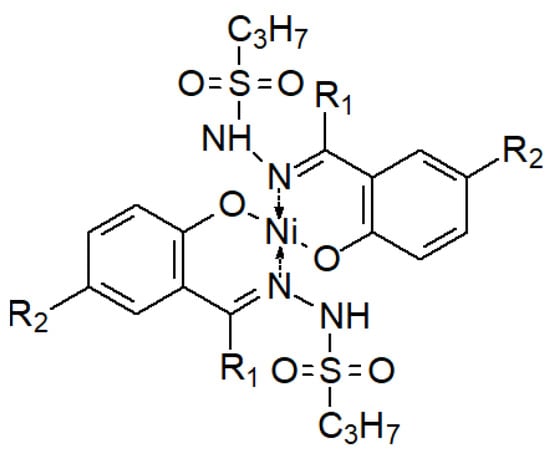
Figure 27.
A complex of two hydrazone ligands and one nickel(II) ion [131]. R1 and R2 are substituents as described in Table 1.

Table 1.
Summary of MIC values (μg/mL) of selected hydrazone derivatives (Figure 27 below) and their complexes with the Ni(II) ion against selected bacterial strains [131].
4.3. Classification
Among hydrazones, as well as among hydrazides, besides carbonyl, one can distinguish sulfonyl hydrazones. However, the sulfonyl group does not replace the nitrogen–carbon bond inherent to hydrazones, but is attached to the amino group, which is terminal in classic hydrazones (Figure 28 below).
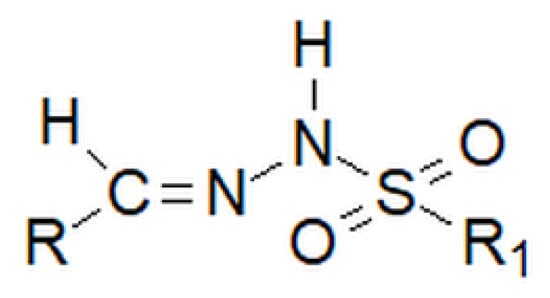
Figure 28.
Basic structure of the sulfonyl hydrazone, where R and R1 are alkyl or aryl groups.
The oxidation of thiol leads to sulfonyl acid, and with chlorinating reagent (SOCl2, POCl3, PCl5 and cyanuric acid chloride)—to sulfonyl acid chloride, which can further react with hydrazine and aldehyde or ketone to give sulfonyl hydrazone [126]. A special type of sulfonyl hydrazone is 4-toluenesulfonylhydrazone, obtained by reacting an aldehyde or ketone with tosylhydrazide, which is the product of the reaction of tosyl chloride and hydrazine [132].
5. Hydrazide–Hydrazones
5.1. Structure
Hydrazide–hydrazones are a numerous group of hybrid molecules which can connect diametrically different alkyl or aryl fragments through an unsymmetrical amide–imine bridge -C(=O)-NH-N=CH-, which builds the heterogeneity of this group of compounds (Figure 29). Its name unsurprisingly comes from the dual possibility of reading the order of atoms in a moiety. If the nomenclature begins with the carbonyl group, we can say that we are dealing with monosubstituted carbonyl hydrazides, while if we regard the imine bond as the beginning, the—monosubstituted—hydrazone clearly appears in front of us.
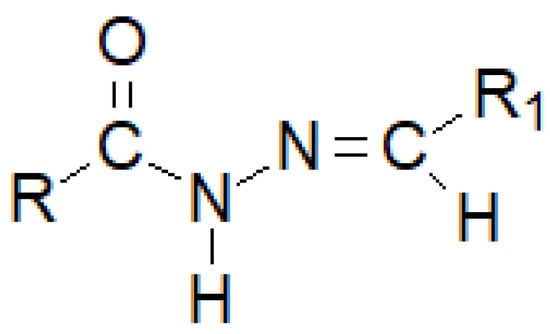
Figure 29.
The basic structure of a hydrazide–hydrazone, where R and R1 are either alkyl or an aryl groups.
The hydrazide–hydrazone moiety can be identified via spectroscopic methods. The IR spectrum shows signals at around 3050 cm−1, 1650 cm−1 and 1550 cm−1, corresponding to the -NH-, C=O and C=N group, respectively. Two singlets appear in the 1H NMR spectrum, one in the range of δ 8–9 ppm and the other in the range of δ 10–13 ppm, signals corresponding to the -CH= and -NH- group, respectively. In the 13C NMR spectrum, there are signals of carbon atoms from the CH= and C=O group in the range of δ 145–160 ppm and δ 160–170 ppm, respectively [133].
5.2. Importance
Hydrazide–hydrazones are synthesized in search of effective antibacterial and antifungal agents—especially due to the growing problem of antibiotic resistance [134]. In many cases, it is the presence of electron-withdrawing aromatic ring substituents that is essential for the antimicrobial activity of hydrazide–hydrazones. Studies on the biological activity of, among others, the following compound (Figure 30) provide an example of the mentioned relationship. The lowest MIC values (μg/mL) are shown for the chlorine substituent in position 2, against E. coli, S. aureus and B. subtilis, 0.31, 0.62 and 0.31, respectively. They were compared with the MIC values of ciprofloxacin (an organic antimicrobial compound inhibiting bacterial DNA topoisomerase) of 0.01, 0.15 and 0.12, respectively. On the other hand, the highest efficacy among the tested derivatives against C. albicans was recorded for the -NO2 substituent in position 2 (MIC = 0.31 μg/mL). The value was compared with clotrimazole (an organic compound with antifungal activity that inhibits the biosynthesis of sterols that build fungal cell membranes), for which the MIC value is 0.10 μg/mL [135].
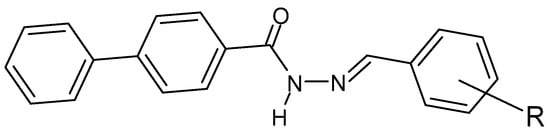
Figure 30.
Hydrazide–hydrazone derivative of biphenyl-4-carboxylic acid, where R = NO2, -Cl or -Br [135].
The following compounds (Figure 31) were tested against streptomycin and against B. subtilis, K. pneumoniae and E. coli and showed lower MIC values for the chloro, fluoro and para substituents (Table 2). Moreover, lower values were also recorded for the above-mentioned derivatives and for the derivative with the chlorine atom in the “ortho” position (Table 3) against fluconazole and against A. flavus, A. niger, C. albicans and Candida6 [136].
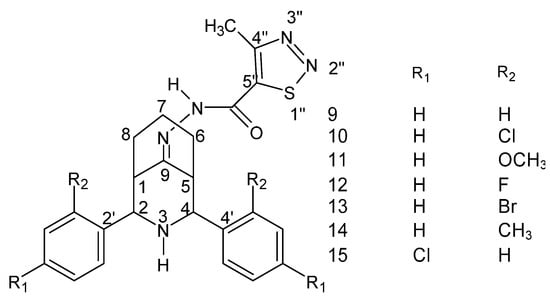
Figure 31.
Structure of the 2r, 4c-diaryl-3-azabicyclo [3.3.1] nonan-9-one-4-methyl-1,2,3-thiadiazole-5-carbonyl hydrazide–hydrazone [136].

Table 2.
Summary of MIC values (μg/mL) of selected hydrazide–hydrazone derivatives against selected bacterial strains [136].

Table 3.
Summary of MIC values (μg/mL) of selected hydrazide–hydrazone derivatives against selected fungal strains [136].
There have also been reports in the literature on the anti-cancer properties of hydrazide–hydrazones, e.g., in the case of colorectal cancer acting by increasing the permeability of the outer mitochondrial membranes of neoplastic cells. The cytotoxic activity of the hydrazide–hydrazone derivative of 2,6-difluorobenzoic acid and 6-bromoindole (Figure 32) was confirmed against the HTC-116, DLD-1 and SW-620 cell lines, while its lack—against healthy fibroblasts of the L929 cell line [77].
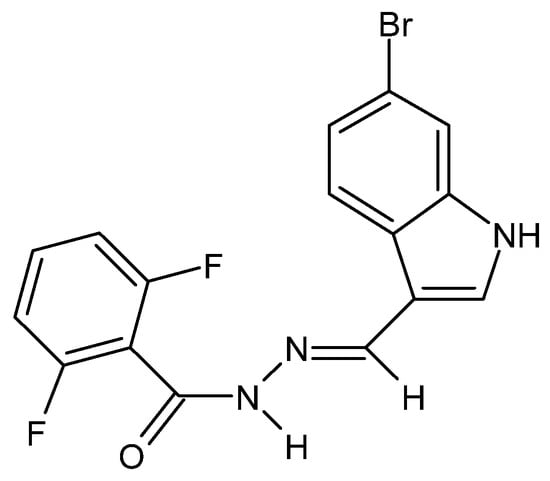
Figure 32.
Derivative of 2,6-difluorobenzoic acid and 6-bromoindole—hydrazide–hydrazone with anti-cancer properties [77].
For the HTC-116 cell line, the pathway of programmed cell death has been established, starting with the inhibition of Bcl2 proteins, which regulate this process and act in an anti-apoptotic manner, and, consequently, regulate the release of pro-apoptotic cytochrome c. Caspases-9 and -3, which regulate the production of reactive oxygen species, take over further control of the process [77]. Cytochrome c binds Apaf-1, which is the first factor activating the apoptotic protease-activating factor 1. The formation of this complex (apoptosome) leads to the activation of procaspase-9 to the initiator caspase-9, which in turn activates the executive caspases, caspase-3 and caspase-7, of which caspase-3 is the master caspase and caspase-7 is the helper caspase. Caspase-9 begins the secretion of reactive oxygen species that have a proteolytic effect on the cell subjected to apoptosis, and caspase-3 acts as an inhibitor, ending the process [137].
Hydrazide–hydrazones are also used as antidiabetic agents in type II diabetes [138,139]. They play the role of non-competitive antagonists of the glucagon receptor—thus, they inhibit the glucagon-induced processes: glycogenolysis and gluconeogenesis, which in turn leads to a reduction in blood sugar levels.
A very interesting biological activity of the hydrazide–hydrazone of lactic acid was described by Noshiranzadeh et al. [140]. They synthesized a number of this type of lactic acid derivatives, two of which (Figure 33) showed particular activity against the selected bacterial strains (Minimum Inhibitory Concentration MIC = 64–128 µg/mL), which turned out to be lower than the reference gentamicin.
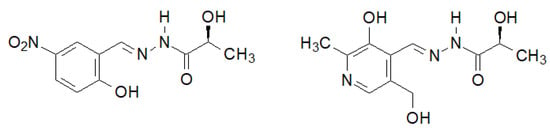
Figure 33.
New hydrazide–hydrazones of lactic acid with antibacterial activity.
Olayinka et al. synthesized several 2-propylquinoline-4-carboxylic acid hydrazide–hydrazones [141]. The compound shown in the Figure 34 showed the highest activity against six strains of bacteria.
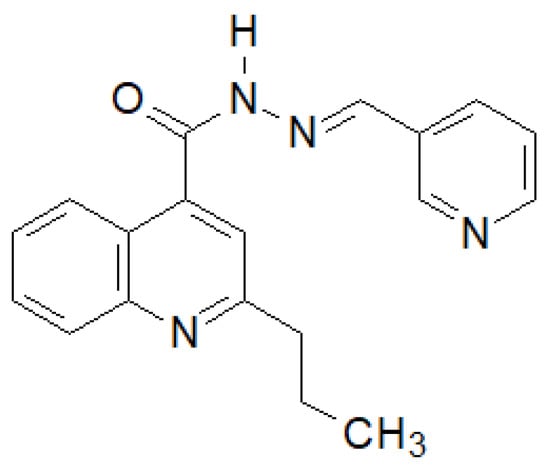
Figure 34.
Quinoline derivative with significant antibacterial properties.
The authors showed that the presence of an electron-donating substituent in position 4 and an electron-withdrawing substituent in position 2 is of key importance for the activity of the derivatives obtained.
Indole-2-one was used by Salem et al. to obtain a series of hydrazide–hydrazones, of which the compound in Figure 35 had the most interesting properties [142].
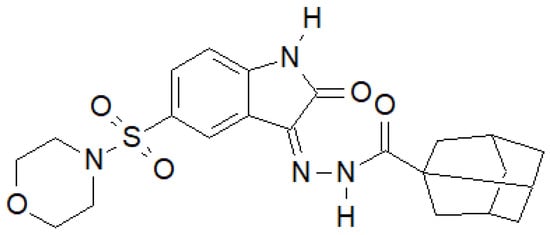
Figure 35.
Indol-2-one derivative with antibacterial activity.
For the above compound, they performed an activity assay against DNA gyrase isolated from S. aureus, which showed that its half-maximal inhibitory concentration was IC50 = 19.32 ± 0.99 µM, while for the reference ciprofloxacin, IC50 = 26.43 ± 0.64 µM.
Of the N-substituted indole derivatives synthesized by Tiwari et al., the compound shown in Figure 36 showed the highest activity against Gram-positive bacteria [143]. In the case of E. coli MTCC 433 and B. subtilis MTCC 1427, it showed higher activity than the reference Chloramphenicol.
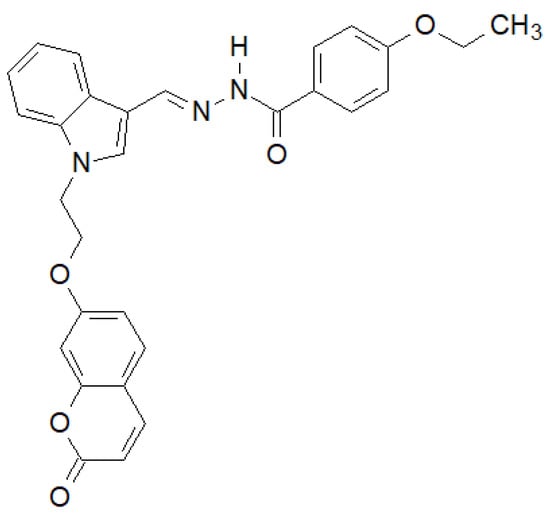
Figure 36.
N-substituted indole derivative with antibacterial properties.
El-Etrawa et al. prepared a number of 2-thiouracil derivatives, of which the following compound (Figure 37) turned out to be the most active against E. coli, P. aeruginosa and S. aureus [144].
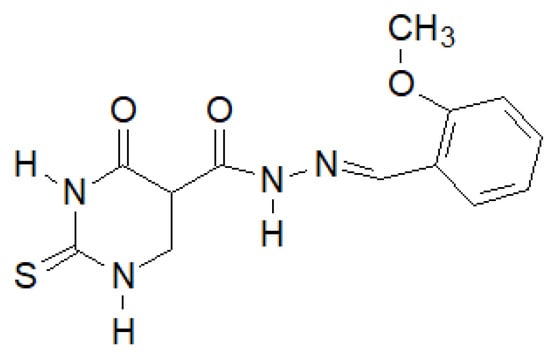
Figure 37.
N-(2-Thiouracil-5-oyl)hydrazone derivative with antibacterial activity.
Recently, Paruch et al. synthesized 1,2,3-thiadiazole hydrazide–hydrazones, of which the compound shown in Figure 38 showed the highest activity [145].
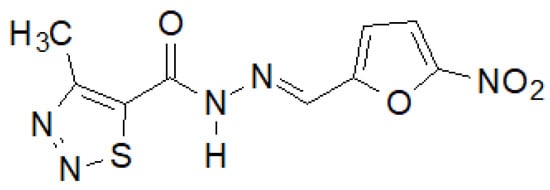
Figure 38.
4-Methyl-1,2,3-thiadiazole-carboxylic acid hydrazide derivative active against a panel of bacterial strains.
This compound showed activity against almost all tested bacterial strains, and in the case of S. epidermidis ATCC 12228 and M. luteus ATCC 10240, even two and eight times higher, respectively, than the reference nitrofurantoin.
In 2020, the results of the synthesis and biological tests of eugenol hydrazide–hydrazones were published [146]. The compound in the Figure 39 showed the highest activity against M. tuberculosis H37Rv.
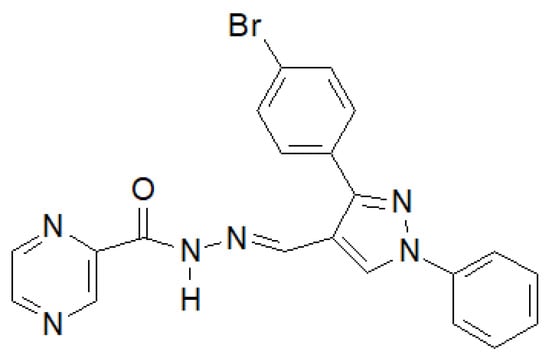
Figure 39.
Pyrazine derivative with antitubercular properties.
6. Conclusions
The group of compounds presented in this article seems to be exceptional due to the already known and predicted biological activity. New groups of organic compounds are still being described, the combinations of which may form a group of extremely desirable compounds with antibiotic properties, suitable for an attempt to challenge the problem of antimicrobial resistance. In the instance of the results described in the article by R. C. N. Reis et al. from 2008 [147], in which the activity of the coupling products of D-ribonic acid hydrazide and long-chain aldehydes and the hydrazinolysis products of D-ribo-1,4-lactone with N,N-disubstituted hydrazines was tested against M. tuberculosis, S. aureus, E. coli and C. Albicans, versus i.o. rifampicin, penicillin-G, chloramphenicol and nystatin, the authors noted that the greater the activity of the coupling products against M. tuberculosis and S. aureus, the longer the attached hydrocarbon chain was. Moreover, the results of examining the biological activity of monosaccharide hydrazide derivatives and their complexes with transition metal ions in relation to selected microbial strains may turn out to be remarkable, and may be used to create a complete description of their significance and enable the comparison of properties with hydrazide derivatives already described in the literature.
Author Contributions
Writing—original draft preparation, review and editing, E.R., J.M. and B.D.; literature search and partial draft preparation, B.D. and J.S.-F.; further management and supervision, J.M. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education DS 531-T100-D 501-21.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schiff, H. Mittheilungen aus dem Universitäts-laboratorium in Pisa: 2. Eine neue Reihe organischer Basen [Communications from the university laboratory in Pisa: 2. A new series of organic bases]. Ann. Der Chem. Und Pharm. 1864, 131, 118–119. (In German) [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Breith, E.; Llibbe, E.; Tsumaki, T. Tricyclische orthokondensierte Nebenvalenzringe. Justus Liebigs Ann. Chem. 1933, 503, 84–130. [Google Scholar] [CrossRef]
- Hunter, L.; Marriott, J.A. Co-ordinated copper and nickel compounds of salicylidene derivatives. J. Chem. Soc. 1937, 422, 2000–2003. [Google Scholar] [CrossRef]
- Sacconi, L.; Ciampolini, M.; Maggio, F.; Cavasini, F.P. Studies in Coordination Chemistry. IX.1Investigation of the Stereochemistry of Some Complex Compounds of Cobalt(II) with N-Substituted Salicylaldimines. J. Am. Chem. Soc. 1962, 84, 3246–3248. [Google Scholar] [CrossRef]
- Holm, R.H.; Swaminathan, K. Studies on Nickel(II) Complexes. III. Bis-(N-arylsalicylaldimine) Complexes. Inorg. Chem. 1962, 1, 599–607. [Google Scholar] [CrossRef]
- Percy, G.C.; Thornton, D.A. N-aryl salicylaldimine complexes: Infrared and PMR spectra of the ligands and vibrational frequencies of their metal (II) chelates. J. Inorg. Nucl. Chem. 1972, 34, 3357–3367. [Google Scholar] [CrossRef]
- Lundgren, R.L.; Stradiotto, M. Ligand Design in Metal Chemistry: Reactivity and Catalysis; Key Concepts in Ligand Design: An Introduction; John Wiley & Sons, Ltd.: Chichester, UK; Hoboken, NJ, USA, 2016; pp. 1–13. [Google Scholar]
- Fryzuk, M.D.; Haddad, T.S.; Berg, D.J.; Rettig, S.J. Phosphine complexes of the early metals and the lanthanoids. Pure Appl. Chem. 1991, 63, 845–850. [Google Scholar] [CrossRef]
- Dilli, S.; Maitra, A.M.; Patsalides, E. Oxidative transformations in nickel(II) chelates of tetradentate Schiff bases. Inorg. Chem. 1982, 21, 2832–2838. [Google Scholar] [CrossRef]
- Camp, C.; Chatelain, L.; Mougel, V.; Pecaut, J.; Mazzanti, M. Ferrocene-Based Tetradentate Schiff Bases as Supporting Ligands in Uranium Chemistry. Inorg. Chem. 2015, 54, 5774–5783. [Google Scholar] [CrossRef]
- Zoubi, W.A. Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int. J. Org. Chem. 2013, 3, 73–95. [Google Scholar] [CrossRef]
- Donzelli, A.; Metushi, I.; Potvin, P.G. Titanium(IV) Complexes of Disulfide-Linked Schiff Bases. Inorg. Chem. 2012, 51, 5138–5145. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Mishra, P. Metal complexes of a novel Schiff base based on penicillin: Characterization, molecular modeling, and antibacterial activity study. Bioinorg. Chem. Appl. 2017, 2017, 6927675. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Mishra, P. Bioactivity of some divalent M(II) complexes of penicillin based Schiff base ligand: Synthesis, spectroscopic characterization, and thermal study. J. Saudi Chem. Soc. 2018, 22, 601–613. [Google Scholar] [CrossRef]
- Md Yusof, E.N.; Ravoof, T.B.S.A.; Tiekink, E.R.T.; Veerakumarasivam, A.; Crouse, K.A.; Tahir, M.I.M.; Ahmad, H. Synthesis, characterization and biological evaluation of transition metal complexes derived from N, S bidentate ligands. Int. J. Mol. Sci. 2015, 16, 11034–11054. [Google Scholar] [CrossRef]
- Sridhar, G.; Bilal, M.; Easwaramoorthy, D.; Rani, K.; Kumar, S.; Manohar, C.S. Synthesis, Characterization and Antimicrobial Activities of Copper, Nickel, Cobalt, Chromium Complexes Derived from (Z)-4-Fluoro-N-(2,7-dimethylhept-6-enylidene) benzenamine. J. Braz. Chem. Soc. 2017, 28, 756–767. [Google Scholar] [CrossRef]
- Moustafa, S.A.; Ali, M.M.; El-rashedy, A.A. Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety. J. Basic Appl. Sci. 2016, 5, 85–96. [Google Scholar] [CrossRef]
- El-Boraey, H.A.; EL-Gammal, O.A. Novel (N4) Macrocyclic Metal Complexes: Synthesis, Characterization, Spectral Studies and Anticancer Activity. Open Chem. J. 2018, 5, 51–63. [Google Scholar] [CrossRef]
- Hu, K.; Liu, C.; Li, J.; Liang, F. Copper(II) complexes based on quinoline-derived Schiff-base ligands: Synthesis, characterization, HSA/DNA binding ability, and anticancer activity. MedChemComm 2018, 9, 1663–1672. [Google Scholar] [CrossRef]
- Chioma, F.; Ekennia, A.C.; Osowole, A.A.; Okafor, S.N.; Ibeji, C.U.; Onwudiwe, D.C.; Ujam, O.T. Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes. Open Chem. 2018, 16, 184–200. [Google Scholar] [CrossRef]
- Kuate, M.; Conde, M.A.; Nchimi, K.N.; Paboudam, A.G.; Ntum, S.-J.E.; Ndifon, P.T. Synthesis, characterization and antimicrobial studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes of (E)-2-(4-dimethylbenzydimino)-Glycylglycine, (Glygly-DAB) a Schiff Base Derived from 4-Dimethylaminobenzaldehyde and glycylglycine. Int. J. Org. Chem. 2018, 8, 298–308. [Google Scholar] [CrossRef]
- Abu-khadra, A.S.; Afify, A.S.; Mohamed, A.; Farag, R.S.; Hassan, Y. Preparation, characterization and antimicrobial activity of Schiff base of (E)-N-(4-(Thiophen-2ylmethyleneamino) Phenylsulfonyl) Acetamide metal complexes. Open Bioact. Compd. J. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Festus, C.; Okafor, S.N.; Ekennia, A.C. Heteroleptic metal complexes of a Pyrimidinyl based Schiff base ligand incorporating 2,2′-Bipyridine moiety: Synthesis, characterization, and biological studies. Front. Chem. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Al-zaidi, B.H.; Hasson, M.M.; Ismail, A.H. New complexes of chelating Schiff base: Synthesis, spectral investigation, antimicrobial, and thermal behavior studies. J. Appl. Pharm. Sci. 2019, 9, 45–57. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Hansda, A.; Chandra, A.; Kumar, A.; Kumar, M.; Sithambaresan, M.; Faizi, M.S.H.; Kumar, V. Co(II), Ni(II), Cu(II) and Zn(II) complexes of acenaphthoquinone 3-(4-benzylpiperidyl) thiosemicarbazone: Synthesis, structural, electrochemical and antibacterial studies. Polyhedron 2017, 134, 11–21. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Mihorianu, M.; Franz, M.H.; Jones, P.G.; Freytag, M.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. N-Heterocyclic carbenes derived from imidazo-[1,5-a]pyridines related to natural products: Synthesis, structure and potential biological activity of some corresponding gold(I) and silver(I) complexes. Appl. Organometal. Chem. 2016, 30, 581–589. [Google Scholar] [CrossRef]
- Al-aghbari, S.A.; Al-shuja, O.M.; Al-badani, R.; Japir, A.A.M. Synthesis, characterization and anticancer activity studies of new Schiff base Pt (II) complex. J. Mater. Sci. Chem. Eng. 2019, 7, 94137. [Google Scholar] [CrossRef]
- Deng, J.; Yu, P.; Zhang, Z.; Zhang, J.; Sun, Z.; Cai, M.; Yuan, H.; Liang, H.; Yang, F. Novel Pt(II) complexes with modified aroyl-hydrazone Schiff- base ligands: Synthesis, cytotoxicity and action mechanism. Metallomics 2019, 11, 1847–1863. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Zamisa, S.J.; Islam, M.S.; Olofinsan, K.; Salau, V.F.; Mocktar, C.; Omondi, B. Quinoline Functionalized Schiff Base Silver (I) Complexes: Interactions with Biomolecules and In Vitro Cytotoxicity, Antioxidant and Antimicrobial Activities. Molecules 2021, 26, 1205. [Google Scholar] [CrossRef]
- Al-Masoudi, N.A.; Aziz, N.; Mohammed, A. Synthesis and In vitro anti-HIV activity of some new Schiff base ligands derived from 5-Amino-4-phenyl-4H-1,2,4-triazole-3- thiol and their metal complexes. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2891–2901. [Google Scholar] [CrossRef]
- Westheimer, F.H.; Taguchi, K. Catalysis by molecular sieves in the preparation of ketimines and enamines. J. Org. Chem. 1971, 36, 1570–1572. [Google Scholar] [CrossRef]
- Król-Starzomska, I.; Filarowski, A.; Rospenk, M.; Koll, A.; Melikova, S. Proton Transfer Equilibria in Schiff Bases with Steric Repulsion. J. Phys. Chem. A 2004, 108, 2131–2138. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Bhagat, S.; Rudrawar, S. Magnesium perchlorate as an efficient catalyst for the synthesis of imines and phenylhydrazones. Tetrahdron Lett. 2004, 45, 7641–7644. [Google Scholar] [CrossRef]
- Dalpozzo, R.; de Nino, A.; Nardi, M.; Russo, B.; Procopio, A. Erbium(III) triflate: A valuable catalyst for the synthesis of aldimines, ketimines and enaminones. Synthesis 2006, 7, 1127–1132. [Google Scholar] [CrossRef]
- Naeimi, H.; Salimi, F.; Rabiei, K. Mild and convenient one pot synthesis of Schiff bases in the presence of P2O5/Al2O3 as new catalyst under solvent-free conditions. J. Mol. Catal. A Chem. 2006, 260, 100–104. [Google Scholar] [CrossRef]
- Barluenga, J.; Aznar, F.; Valdes, C. N-trialkylsilylimines as coupling partners for Pd-catalyzed C-N bond-forming reactions: One-step synthesis of imines and azadienes from aryl and alkenyl bromides. Angew. Chem. Int. Ed. 2004, 116, 347–349. [Google Scholar] [CrossRef]
- Arluenga, J.B.; Jimenez-Aquino, A.; Fernandez, M.A.; Aznar, F.; Valdes, C. Multicomponent and one-pot synthesis of trisubstituted pyridines through a Pd catalyzed cross-coupling/cross-coupling/cycloaddition sequence. Tetrahedron Lett. 2007, 64, 778–786. [Google Scholar] [CrossRef]
- Jiang, L.; Jin, L.; Tian, H.; Yuan, X.; Yu, X.; Xu, Q. Direct and mild palladium-catalyzed aerobic oxidative synthesis of imines from alcohols and amines under ambient conditions. Chem. Commun. 2011, 47, 10833–10835. [Google Scholar] [CrossRef]
- Huang, B.; Tian, H.; Lin, S.; Xie, M.; Yu, X.; Xu, Q. Cu(I)/TEMPO-catalyzed aerobic oxidative synthesis of imines directly from primary and secondary amines under ambient and neat conditions. Tetrahedron Lett. 2013, 54, 2861–2864. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Ikeda, M.; Tsukamoto, D.; Tanaka, S.; Hirai, T. One-pot synthesis of imines from alcohols and amines with TiO2 loading Pt nanoparticles under UV irradiation. Chem. Commun. 2011, 47, 4811–4813. [Google Scholar] [CrossRef] [PubMed]
- Largeron, M.; Fleury, M.B. Bioinspired oxidation catalysts. Science 2013, 339, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.S.; Liao, B.S.; Liu, Y.H.; Peng, S.M.; Liu, S.T. Preparation of imines by oxidative coupling of benzyl alcohols with amines catalysed by dicopper complexes. Eur. J. Org. Chem. 2013, 2013, 5160–5164. [Google Scholar] [CrossRef]
- Largeron, M. Protocols for the catalytic oxidation of primary amines to imines. Eur. J. Org. Chem. 2013, 2013, 5225–5235. [Google Scholar] [CrossRef]
- Joshi, H.H.; Kamounah, F.S.; Gooijer, C.; Zwan, G.; Antonov, L. Excited state intramolecular proton transfer in some tautomeric azo dyesand schiff bases containing an intramolecular hydrogen bond. J. Photochem. Photobiol. B 2002, 152, 183–191. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; Salem, A.N.M.; Sayed, M.A.; Aboaly, M.M. Synthesis, structural characterization, thermal studies, catalytic efficiency and antimicrobial activity of some M(II) complexes with ONO tridentate Schiff base N-salicylidene Oaminophenol (saphH2). J. Mol. Struct. 2012, 1010, 130–138. [Google Scholar] [CrossRef]
- Saravanan, G.; Pannerselvam, P.; Prakash, C.R. Synthesis and anti-microbial screening of novel Schiff bases of 3-amino-2-methyl quinazolin 4-(3H)-one. J. Adv. Pharm. Technol. Res. 2010, 1, 320–325. [Google Scholar] [CrossRef]
- Gungor, O.; Gurkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ganguly, S.; Veerasamy, R.; De Clercq, E. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4 (3) H-ones. Eur. J. Med. Chem. 2010, 45, 5474–5479. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeswari, P.; Myneedu, N.S.; Saraswat, V. Abacavir prodrugs: Microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg. Med. Chem. Lett. 2006, 16, 2127–2129. [Google Scholar] [CrossRef]
- Hu, G.; Wang, G.; Duan, N.; Wen, X.; Cao, T.; Xie, S.; Huang, W. Design, synthesis and antitumor activities of fluoroquinolone C-3 heterocycles (IV): S-triazole Schiff–Mannich bases derived from ofloxacin. Acta Pharm. Sin. B 2012, 2, 312–317. [Google Scholar] [CrossRef]
- El-wakiel, N.; El-keiy, M.; Gaber, M. Synthesis, spectral, antitumor, antioxidant and antimicrobial studies on Cu (II), Ni (II) and Co (II) complexes of 4-[(1HBenzoimidazol-2-ylimino)-methyl]-benzene-1, 3-diol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 147, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Chaviara, A. Evaluation of anti-inflammatory and antioxidant activities of copper (II) Schiff mono-base and copper (II) Schiff base coordination compounds of dien with heterocyclic aldehydes and 2-amino-5-methylthiazole. J. Enzym. Inhib. Med. Chem. 2008, 23, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Rathelot, P.; Vanelle, P.; Gasquet, M.; Delmas, F.; Crozet, M.P.; Timon-David, P.J. Maldonado, Synthesis of novel functionalized 5-nitroisoquinolines and evaluation of in vitro Antimalarial activity. Eur. J. Med. Chem. 1995, 30, 503–508. [Google Scholar] [CrossRef]
- Bensaber, S.M.; Allafe, H.; Ermeli, N.B.; Mohamed, S.B.; Zetrini, A.A.; Alsabri, S.G.; Erhuma, M.; Hermann, A.; Jaeda, M.I.; Gbaj, A.M. Chemical synthesis, molecular modelling, and evaluation of anticancer activity of some pyrazol-3-one Schiff base derivatives. Med. Chem. Res. 2014, 23, 5120–5134. [Google Scholar] [CrossRef]
- Desai, S.B.; Desai, P.B.; Desai, K.R. Synthesis of some Schiff bases, thiazolidinones and azetidinones derived from 2,6-diaminobenzo1,2-d: 4,5-d’ bisthiazole and their anticancer activities. Heterocycl. Commun. 2001, 7, 83–90. [Google Scholar] [CrossRef]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of Schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Bluhm, M.E.; Ciesielski, M.; Gorls, H.; Walter, O.; Doring, M. Complexes of Schiff Bases and Intermediates in the Copper-Catalyzed Oxidative Heterocyclization by Atmospheric Oxygen. Inorg. Chem. 2003, 42, 8878–8885. [Google Scholar] [CrossRef]
- Wang, L.; Qin, W.; Tang, X.; Dou, W.; Liu, W.; Teng, Q.; Yao, X. A selective, cell-permeable fluorescent probe for Al3+ in living cells. Org. Biomol. Chem. 2010, 8, 3751–3757. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Kandii, F.; Chebani, K. Active transport of metal ions by using Schiff bases. Phys. Sci. Res. Int. 2014, 2, 12–23. [Google Scholar]
- Velezheva, V.; Brennan, P.; Ivanov, P.; Kornienko, A.; Lyubimov, S.; Kazarian, K.; Nikonenko, B.; Majorov, K.; Apt, A. Synthesis, Spectroscopic, Molecular Modeling and Anti-Fungal Studies of Some Divalent Metal Complexes of 4-Hydroxyacetophenone Isonicotinoyl Hydrazone. Bioorg. Med. Chemi. Lett. 2016, 26, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Gutowsky, H.S.; Holm, C.H. Rate Processes and Nuclear Magnetic Resonance Spectra II. Hindered Internal Rotation of Amides. J. Chem. Phys. 1956, 25, 1228–1234. [Google Scholar] [CrossRef]
- Grel, P.L.; Salaün, A.; Mocquet, C.; Grel, B.L.; Roisnel, T.; Potel, M. Postsynthetic Modification of C3-Symmetric Aza-β3-Cyclohexapeptides. J. Org. Chem. 2011, 76, 8756–8767. [Google Scholar] [CrossRef]
- Gloaguen, E.; Brenner, V.; Alauddin, M.; Tardivel, B.; Mons, M.; Zehnacker-Rentien, A.; Aitken, D.J. Direct Spectroscopic Evidence of Hyperconjugation Unveils the Conformational Landscape of Hydrazides. Angew. Chem. Int. Ed. 2014, 53, 13756–13759. [Google Scholar] [CrossRef]
- Takahashi, O.; Kirikoshi, R. Intramolecular cyclization of aspartic acid residues assisted by three water molecules: A density functional theory study. Comput. Sci. Discov. 2014, 7, 015005. [Google Scholar] [CrossRef]
- Knapp, S.; Toby, B.H.; Sebastian, M.; Krogh-Jespersen, K.; Potenza, J.A. Relative reactivity and structures of benzoyltrimethylhydrazine and 1-benzoyl-2-methylpyrazolidine. J. Org. Chem. 1981, 46, 2490–2497. [Google Scholar] [CrossRef]
- Stackhouse, J.; Baechler, R.D.; Mislow, K. Pyramidal inversion barriers: The significance of ground state geometry. Tetrahedron Lett. 1971, 12, 3437–3440. [Google Scholar] [CrossRef]
- Andrade, L.A.F.; Silla, J.M.; Cormanich, R.A.; Freitas, M.P. Infrared Fingerprints of nN → σ*NH Hyperconjugation in Hydrazides. J. Org. Chem. 2017, 82, 12181–12187. [Google Scholar] [CrossRef]
- Lii, J.-H.; Chen, K.-H.; Allinger, N.L. Alcohols, Ethers, Carbohydrates, and Related Compounds Part V.2 The Bohlmann Torsional Effect. J. Phys. Chem. A 2004, 108, 3006–3015. [Google Scholar] [CrossRef]
- Bohlmann, F. Zur Konfigurationsbestimmung von Chinolizin-Derivaten. Agnew. Chem. 1957, 69, 641–642. [Google Scholar] [CrossRef]
- Gribble, G.W.; Nelson, R.B. Conformational Requirements for the Existence of Bohlmann Bands in the Infrared Spectra of Indolo[2,3-a]quinolizidines. I. cis- and trans-2-tert-Butyl Derivatives. J. Org. Chem. 1973, 38, 2831–2834. [Google Scholar] [CrossRef]
- Skolik, J.; Krueger, P.J.; Wiewiorowski, M. Correlation between the stereochemistry of quinolizidine alkaloids and their infrared spectra from 2840-2600 CM−1. Tetrahedron 1968, 24, 5439–5456. [Google Scholar] [CrossRef]
- Wolfe, S.; Schlegel, H.B.; Whangbo, M.-H.; Bernardi, F. On the origin of the Bohlmann bands. Can. J. Chem. 1974, 52, 3787–3792. [Google Scholar] [CrossRef]
- Ernstbrunner, E.E.; Hudec, J. Bohlmann bands—A reassessment. J. Mol. Struct. 1973, 17, 249–256. [Google Scholar] [CrossRef]
- Moss, G.P. Basic terminology of stereochemistry (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing Powerhouse in Colon Cancer Cells by Hydrazide−Hydrazone-Based Small Molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef]
- Hastings, J.; Owen, G.; Dekker, A.; Ennis, M.; Kale, N.; Muthukrishnan, V.; Turner, S.; Swainston, N.; Mendes, P.; Steinbeck, C. Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016, 44, D1214–D1219. [Google Scholar] [CrossRef]
- Tiec, C.L.; Barrail, A.; Goujard, C.; Taburet, A.-M. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharmacokinet. 2005, 44, 1035–1050. [Google Scholar] [CrossRef]
- Ventura, C.; Martins, F. Application of Quantitative Structure−Activity Relationships to the Modeling of Antitubercular Compounds. 1. The Hydrazide Family. J. Med. Chem. 2008, 51, 612–624. [Google Scholar] [CrossRef]
- Nagy, J.M.; Cass, A.E.G.; Brown, K.A. Purification and Characterization of Recombinant Catalase-Peroxidase, Which Confers Isoniazid Sensitivity in Mycobacterium tuberculosis. J. Biol. Chem. 1997, 272, 31265–31271. [Google Scholar] [CrossRef]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, J.; Wang, C.; Zhang, Y.; Wei, S.; Jiang, F.; Wu, Y. Protonated N′-benzyl-N′-prolyl proline hydrazide as highly enantioselective catalyst for direct asymmetric aldol reaction. Chem. Commun. 2006, 37, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Jiang, Y.; Ma, D. Assembly of N,N-Disubstituted Hydrazines and 1-Aryl-1H-indazoles via Copper-Catalyzed Coupling Reactions. Org. Lett. 2012, 14, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Liu, X.-L.; He, Z.-Y.; Li, X.-M.; Kang, H.-J.; Tian, S.-K. Palladium/Copper-Catalyzed Oxidative Arylation of Terminal Alkenes with Aroyl Hydrazides. Chem. Eur. J. 2014, 20, 2765–2769. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-L.; Ma, X.-T.; Tian, S.-K. Oxidative Mizoroki-Heck-Type Reaction of Arylsulfonyl Hydrazides for a Highly Regio- and Stereoselective Synthesis of Polysubstituted Alkenes. Chem. Eur. J. 2012, 18, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Yang, F.-L.; Tian, S.-K. Copper-Catalyzed Sulfenylationof Boronic Acids with Sulfonyl Hydrazides. Adv. Synth. Catal. 2015, 357, 928–932. [Google Scholar] [CrossRef]
- Eldridge, G.M.; Weiss, G.A. Hydrazide Reactive Peptide Tags for Site-Specific Protein Labeling. Bioconjugate Chem. 2011, 22, 2143–2153. [Google Scholar] [CrossRef]
- Huang, J.-P.; Bian, X.-Z.; Chang, K.; Hou, L.-J.; Feng, H.-T. Capture and Analysis of Cell Surface N-Glycans by Hydrazide-Modified Magnetic Beads and CE-LIF. Chromatographia 2019, 82, 1079–1088. [Google Scholar] [CrossRef]
- Wang, L.; Aryal, U.K.; Dai, Z.; Mason, A.C.; Monroe, M.E.; Tian, Z.-X.; Zhou, J.-Y.; Su, D.; Weitz, K.K.; Liu, T.; et al. Mapping N-Linked Glycosylation Sites in the Secretome and Whole Cells of Aspergillus niger Using Hydrazide Chemistry and Mass Spectrometry. J. Proteome Res. 2012, 11, 143–156. [Google Scholar] [CrossRef]
- Roveda, J.-G.; Clavette, C.; Hunt, A.D.; Gorelsky, S.I.; Whipp, C.J.; Beauchemin, A.M. Hydrazides as Tunable Reagents for Alkene Hydroamination and Aminocarbonylation. J. Am. Chem. Soc. 2009, 131, 8740–8741. [Google Scholar] [CrossRef]
- White, E.H.; Roswell, D.F. Chemiluminescence of organic hydrazides. Acc. Chem. Res. 1970, 3, 54–62. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, Y.; Li, C.; Yang, C. A highly selective chemiluminescent probe for the detection of chromium(VI). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Maeda, M.; Tsuji, A.; Takahashi, T. Highly Sensitive Biotin-Labelled Hybridization Probe. Chem. Pharm. Bull 1989, 37, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.; Sujatha, D.; Prasad, K.V.S.R.G.; Bharathi, K. A Review on Azapeptides: The Promising Peptidomimetics. Asian J. Chem. 2017, 29, 1879–1887. [Google Scholar] [CrossRef]
- Verhelst, S.H.L.; Witte, M.D.; Arastu-Kapur, S.; Fonovic, M.; Bogyo, M. Novel Aza Peptide Inhibitors and Active-Site Probes of Papain-Family Cysteine Proteases. ChemBioChem 2006, 7, 943–950. [Google Scholar] [CrossRef]
- Lee, H.-J.; Ahn, I.-A.; Ro, S.; Choi, K.-H.; Choi, Y.-S.; Lee, K.-B. Role of azaamino acid residue in β-turn formation stability in designed peptide. J. Peptide Res. 2000, 56, 35–46. [Google Scholar] [CrossRef]
- Słomiak, K.; Łazarenkow, A.; Checinska, L.; Kusz, J.; Ochocki, J.; Nawrot-Modranka, J. Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone. Molecules 2018, 23, 2067. [Google Scholar] [CrossRef]
- Zhang, X.; Breslav, M.; Grimm, J.; Guan, K.; Huang, A.; Liu, F.; Maryanoff, C.A.; Palmer, D.; Patel, M.; Qian, Y.; et al. A new procedure for preparation of carboxylic acid hydrazides. J. Org. Chem. 2002, 67, 9471–9474. [Google Scholar] [CrossRef]
- Curtius, T.; Schöfer, G.; Schwan, N. Hydrazide und Azide organischer Säuren. IV. Abhandlung. 26. Ueber einige Hydrazide einbasischer und zweibasischer Säuren der Fettreihe. J. Prakt. Chem. 1895, 51, 180–196. [Google Scholar] [CrossRef]
- Slagel, R.C. Aminimides VI. Synthesis of aminimides from carboxylic acid esters, unsymmetrically disubstituted hydrazines, and epoxides. J. Org. Chem. 1968, 33, 1374–1378. [Google Scholar] [CrossRef]
- Han, H.; Janda, K.D. Azatides: Solution and Liquid Phase Syntheses of a New Peptidomimetic. J. Am. Chem. Soc. 1996, 118, 2539–2544. [Google Scholar] [CrossRef]
- Grel, P.L.; Salaün, A.; Potel, M.; Grel, B.L.; Lassagne, F. Aza-β3-Cyclohexapeptides: Pseudopeptidic Macrocycles with Interesting Conformational and Configurational Properties Slow Pyramidal Nitrogen Inversion in 24-Membered Rings! J. Org. Chem. 2006, 71, 5638–5645. [Google Scholar] [CrossRef] [PubMed]
- Samdal, S.; Møllendal, H. The Structural and Conformational Properties of Formic Hydrazide (Formylhydrazine) Studied by Microwave Spectroscopy and Quantum Chemical Calculations. J. Phys. Chem. A 2003, 107, 8845–8850. [Google Scholar] [CrossRef]
- Elian, M.; Hoffmann, R. Bonding Capabilities of Transition Metal Carbonyl Fragment. Inorg. Chem. 1975, 14, 1058–1076. [Google Scholar] [CrossRef]
- Hoffmann, R. Building Bridges Between Inorganic and Organic Chemistry (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1982, 21, 711–724. [Google Scholar] [CrossRef]
- Licandro, E.; Perdicchia, D. N-Acylhydrazines: Future Perspectives Offered by New Syntheses and Chemistry. Eur. J. Org. Chem. 2004, 4, 665–675. [Google Scholar] [CrossRef]
- Pradier, C.M.; Salmain, M.; Boujday, S. Biointerface Characterization by Advanced IR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2011; Chapter 7; pp. 167–216. [Google Scholar]
- Bhatt, V. Essentials of Coordination Chemistry; Academic Press: Cambridge, MA, USA, 2016; Chapter 8; pp. 191–236. [Google Scholar]
- Shriver, D.F. Encyclopædia Britannica. 2018. Available online: https://www.britannica.com/science/organometallic-compound (accessed on 5 December 2020).
- Licandro, E.; Maiorana, S.; Vandoni, B.; Perdicchia, D.; Paravidino, P.; Baldoli, C. Synthesis of Medium-Sized N-Heterocycles through RCM of Fisher-type Hydrazino Carbene Complexes. Synlett 2001, 6, 757–760. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Buchardt, O. Peptide nucleic acid (PNA). A DNA mimic with a peptide backbone. Bioconjugate Chem. 1994, 5, 3–7. [Google Scholar] [CrossRef]
- Cauteruccio, S.; Licandro, E.; Panigati, M.; D’Alfonso, G.; Maiorana, S. Modifying the properties of organic molecules by conjugation with metal complexes: The case of peptide nucleic acids and of the intrinsically chiral thiahelicenes. Coord. Chem. Rev. 2019, 386, 119–137. [Google Scholar] [CrossRef]
- Cavier, R.; Rips, R. Dihydrazides, a New Class of Anthelmintics. J. Med. Chem. 1965, 8, 706–708. [Google Scholar] [CrossRef]
- Zadykowicz, B.; Romanowska, A.; Pieńkos, M. Photophyysical basis of chemiluminescent labeling—A modern medical diagnostic tool. Wiadomości Chem. 2018, 72, 887–906. [Google Scholar]
- Albrecht, H.O. Uber die Chemiluminescenz der Aminophthalhydrazid. Z. Für Phys. Chem. 1928, 136, 321–330. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Song, H.; Zhang, W. A fluorescent probe of N′-formyl-rhodamine B hydrazide: Structure and spectral properties of protonation behaviour. Org. Biomol. Chem. 2012, 10, 7677–7680. [Google Scholar] [CrossRef] [PubMed]
- Kricka, L.J. Chemiluminescent and bioluminescent techniques. Clin. Chem. 1991, 37, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Bedia, K.-K.; Elçin, O.; Seda, U.; Fatma, K.; Nathaly, S.; Sevim, R.; Dimoglo, A. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef]
- Enders, D.; Schubert, H.; Nübling, C. Enantioselective Synthesis of α-Substituted Primary Amines by Nucleophilic Addition to Aldehyde-SAMP Hydrazones. Angew. Chem. Int. Ed. Engl. 1986, 25, 1109–1110. [Google Scholar] [CrossRef]
- Perdicchia, D.; Licandro, E.; Maiorana, S.; Baldoli, C.; Giannini, C. A new ‘one-pot’ synthesis of hydrazidesby reduction of hydrazones. Tetrahedron 2003, 59, 7733–7742. [Google Scholar] [CrossRef]
- Lazny, R.; Nodzewska, A. N,N-dialkylhydrazones in organic synthesis. From simple N,N-dimethylhydrazones to supported chiral auxiliaries. Chem. Rev. 2010, 110, 1386–1434. [Google Scholar] [CrossRef]
- Curti, C.; Battistini, L.; Sartori, A.; Zanardi, F. New Developments of the Principle of Vinylogy as Applied to π-Extended Enolate-Type Donor Systems. Chem. Rev. 2020, 120, 2448–2612. [Google Scholar] [CrossRef]
- Lei, Y.; Li, T.-Z.; Fu, C.; Guan, X.-L.; Tan, Y. Synthesis, crystal structures, and antibacterial activity of a series of hydrazone compounds derived from 4-methylbenzohydrazide. J. Chil. Chem. Soc. 2015, 60, 2961–2965. [Google Scholar] [CrossRef]
- Navakoski de Oliveira, K.; Chiaradia, L.D.; Alves Martins, P.G.; Mascarello, A.; Sechini Cordeiro, M.N.; Carvalho Guido, R.V.; Andricopulo, A.D.; Yunes, R.A.; Nunes, R.J.; Vernalb, J.; et al. Sulfonyl-hydrazones of cyclic imides derivatives as potent inhibitors of the Mycobacterium tuberculosisprotein tyrosine phosphatase B (PtpB). Med. Chem. Commun. 2011, 2, 500–504. [Google Scholar] [CrossRef]
- Cunha, M.R.; Tavares, M.T.; Carvalho, C.F.; Silva, N.A.T.; Souza, A.D.F.; Pereira, G.J.V.; Ferreira, F.F.; Parise-Filho, R. Environmentally Safe Condition for the Synthesis of Aryl and Alkyl Sulfonyl Hydrazones via One-Pot Reaction. Sustain. Chem. Eng. 2016, 4, 1899–1905. [Google Scholar] [CrossRef]
- La Regina, G.; Gatti, V.; Piscitelli, F.; Silvestri, R. Open Vessel and Cooling while Heating Microwave-Assisted Synthesis of Pyridinyl N-Aryl Hydrazones. ACS Comb. Sci. 2011, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, Y.; Zhang, T.; Jia, J. Synthesis, characterization, theoretical and antimicrobial studies of tridentate hydrazone metal complexes of Zn(II), Cd(II), Cu(II) and Co(III). Polyhedron 2018, 147, 62–68. [Google Scholar] [CrossRef]
- Anacona, J.R.; Rincones, M. Tridentate hydrazone metal complexes derived from cephalexin and 2-hydrazinopyridine: Synthesis, characterization and antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 169–175. [Google Scholar] [CrossRef]
- Aly, S.A.; Fathalla, S.K. Preparation, characterization of some transition metal complexes of hydrazone derivatives and their antibacterial and antioxidant activities. Arab. J. Chem. 2020, 13, 3735–3750. [Google Scholar] [CrossRef]
- Özmen, Ü.Ö.; Olgun, G. Synthesis, characterization and antibacterial activity of new sulfonyl hydrazone derivatives and their nickel(II) complexes. Spectrochim. Acta A 2008, 70, 641–645. [Google Scholar] [CrossRef]
- Friedman, L.; Litle, R.L.; Reichle, W.R. p-Toluenesulfonylhydrazide. Org. Synth. 1960, 40, 93. [Google Scholar] [CrossRef]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Öztürk, S.; Stables, J. Synthesis and Biological Activities of Diflunisal Hydrazide-Hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef]
- Deep, A.; Jain, S.; Sharma, P.C.; Verma, P.; Kumar, M.; Dora, C.P. Design and biological evaluation of biphenyl-4-carboxylic acid hydrazide-hydrazone for antimicrobial activity. Acta Pol. Pharm. 2010, 67, 255–259. [Google Scholar] [PubMed]
- Kodisundaram, P.; Amirthaganesan, S.; Balasankar, T. Antimicrobial evaluation of a set of heterobicyclic methylthiadiazole hydrazones: Synthesis, characterization, and SAR studies. J. Agric. Food Chem. 2013, 61, 11952–11956. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology 2013, 14, 32. [Google Scholar] [CrossRef]
- Ling, A.; Plewe, M.; Gonzalez, J.; Madsen, P.; Sams, C.K.; Lau, J.; Gregor, V.; Murphy, D.; Teston, K.; Kuki, A.; et al. Human Glucagon Receptor Antagonists Based on Alkylidene Hydrazides. Bioorg. Med. Chem. Lett. 2002, 12, 663–666. [Google Scholar] [CrossRef]
- Madsen, P.; Ling, A.; Plewe, M.; Sams, C.K.; Knudsen, L.B.; Sidelman, U.G.; Ynddal, L.; Brand, C.; Andersen, B.; Murphy, D.; et al. Optimization of alkylidene hydrazide based human glucagon receptor antagonists. Discovery of the highly potent and orally available 3-cyano-4-hydroxybenzoic acid [1-(2,3,5,6-tetramethylbenzyl)-1H-indol-4-ylmethylene]hydrazide. J. Med. Chem. 2002, 45, 5755–5775. [Google Scholar] [CrossRef]
- Noshiranzadeh, N.; Heidari, A.; Haghi, F.; Bikas, R.; Lis, T. Chiral lactic hydrazone derivatives as potential bioactive antibacterial agents: Synthesis, spectroscopic, structural and molecular docking studies. J. Mol. Struct. 2017, 1128, 391–399. [Google Scholar] [CrossRef]
- Ajani, O.O.; Iyaye, K.T.; Aderohunmu, D.V.; Olanrewaju, I.O.; Germann, M.W.; Olorunshola, S.J.; Bello, B.L. Microwaveassisted synthesis and antibacterial propensity of N0-s-benzylidene-2-propylquinoline-4-carbohydrazide and N0-((s-1H-pyrrol-2-yl)methylene)-2-propylquinoline-4-carbohydrazide motifs. Arab. J. Chem. 2020, 13, 1809–1820. [Google Scholar] [CrossRef]
- Salem, M.A.; Ragab, A.; El-Khalafawy, A.; Makhlouf, A.H.; Askar, A.A.; Ammar, Y.A. Design, synthesis, in vitro antimicrobial evaluation and molecular docking studies of indol-2-one tagged with morpholinosulfonyl moiety as DNA gyrase inhibitors. Bioorg. Chem. 2020, 96, 103619. [Google Scholar] [CrossRef]
- Tiwari, S.; Kirar, S.; Banerjee, U.C.; Neerupudi, K.B.; Singh, S.; Wani, A.A.; Bharatam, P.V.; Singh, I.P. Synthesis of N-substituted indole derivatives as potential antimicrobial and antileishmanial agents. Bioorg. Chem. 2020, 99, 103787. [Google Scholar] [CrossRef]
- El-Etrawy, A.-A.; Sherbiny, F.F. Design, synthesis, biological evaluation and molecular modeling investigation of new N0-(2-Thiouracil-5-oyl) hydrazone derivatives as potential anti-breast cancer and anti-bacterial agents. J. Mol. Struct. 2021, 1232, 129993. [Google Scholar] [CrossRef]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Berecka-Rycerz, A.; Malm, A.; Gumieniczek, A.; Wujec, M. Novel Derivatives of 4-Methyl-1,2,3-Thiadiazole-5-Carboxylic Acid Hydrazide: Synthesis, Lipophilicity, and In Vitro Antimicrobial Activity Screening. Appl. Sci. 2021, 11, 1180. [Google Scholar] [CrossRef]
- Rohane, S.H.; Chauhan, A.J.; Fuloria, N.K.; Fuloria, S. Synthesis and in vitro antimycobacterial potential of novel hydrazones of eugenol. Arab. J. Chem. 2020, 13, 4495–4504. [Google Scholar] [CrossRef]
- Reis, R.C.N.; Oda, S.C.; De Almeida, M.V.; Lourenco, M.C.S.; Vicente, F.R.C.; Barbosa, N.R.; Trevizani, R.; Santos, P.L.C.; Le Hyaric, M. Synthesis and Antimicrobial Activity of Amphiphilic Carbohydrate Derivatives. J. Braz. Chem. Soc. 2008, 19, 1065–1072. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).