Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. In Vivo Studies
Effect on Carrageenan-Induced Rat Paw Edema
2.3. In Vitro Studies
2.3.1. Inhibition of Cyclooxygenase (COX) Activity (Isoforms 1 and 2)
2.3.2. Inhibition of Lipoxygenase (LOX) Activity
2.3.3. Effect on Lipid Peroxidation
2.3.4. Preliminary Stability Study
2.4. In Silico Study
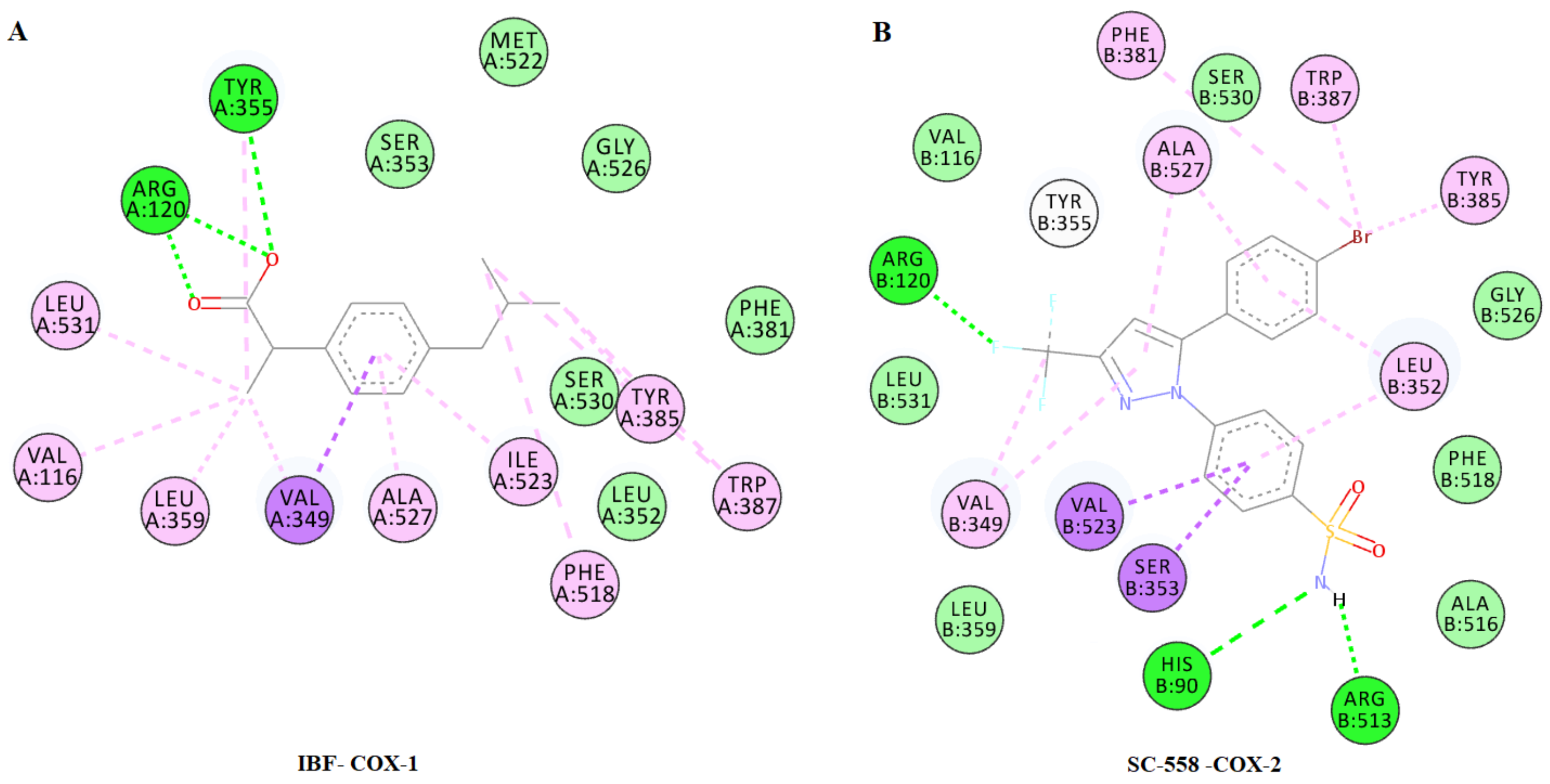
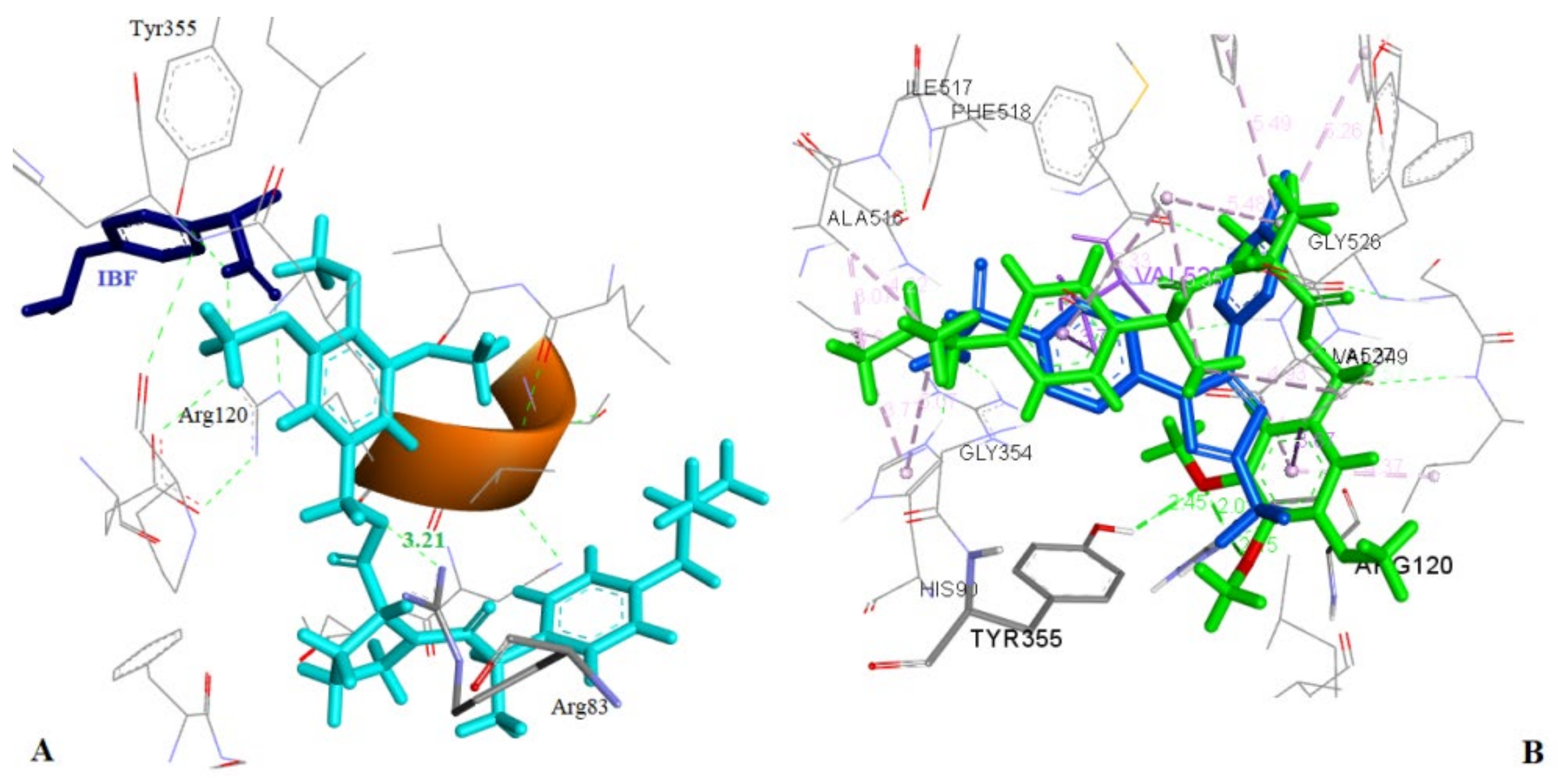
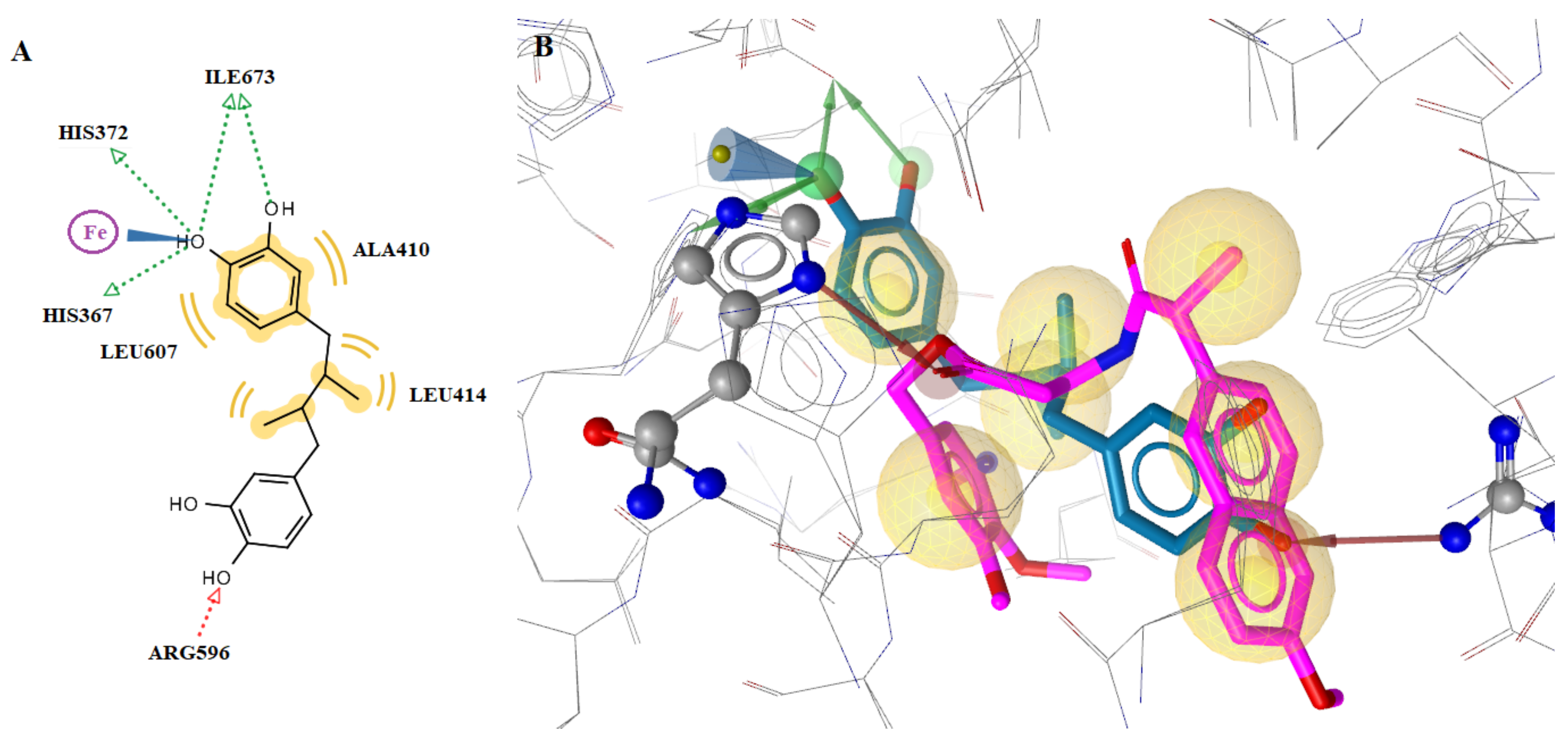
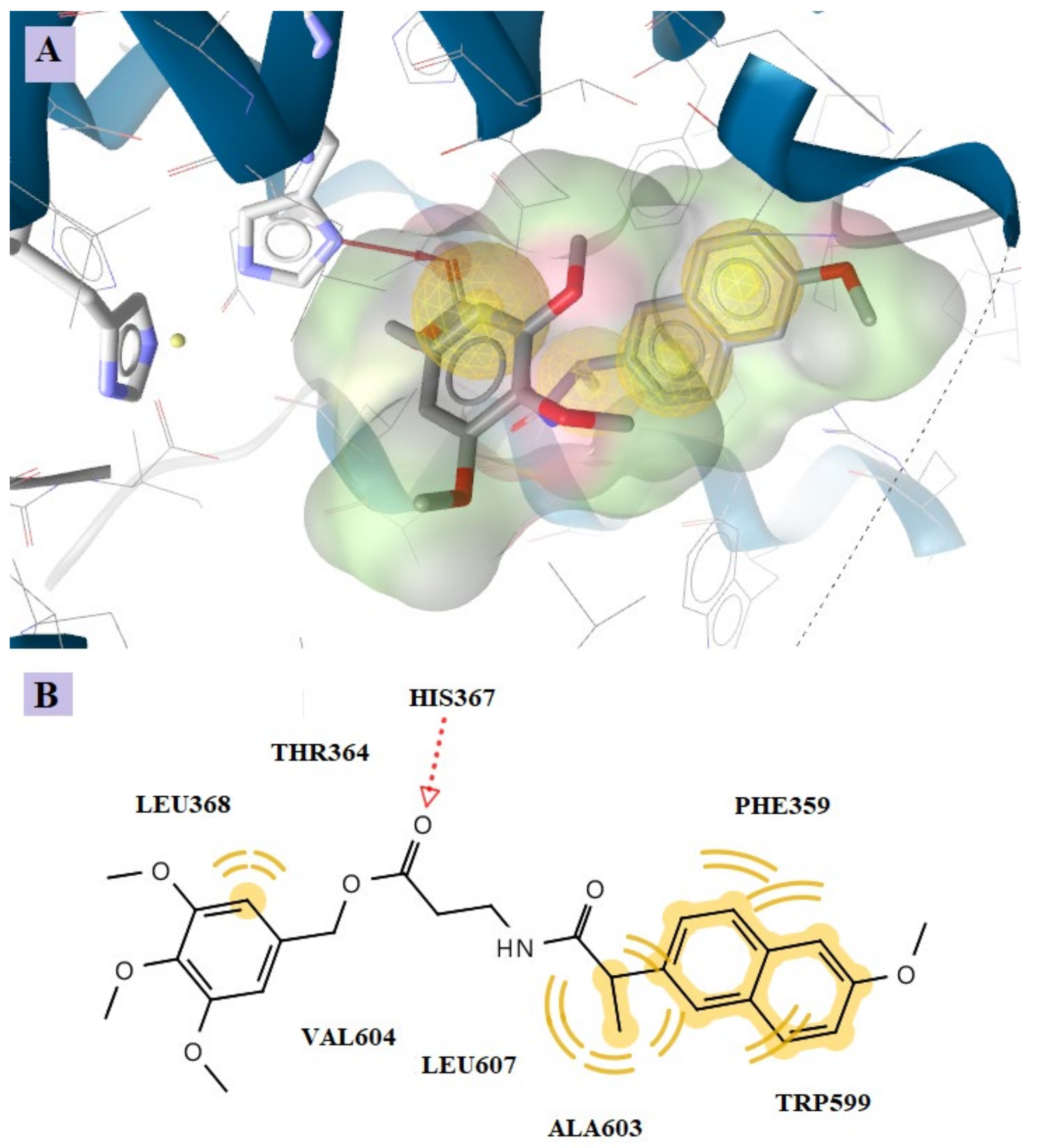
2.4.1. Docking to COX-1 and COX-2
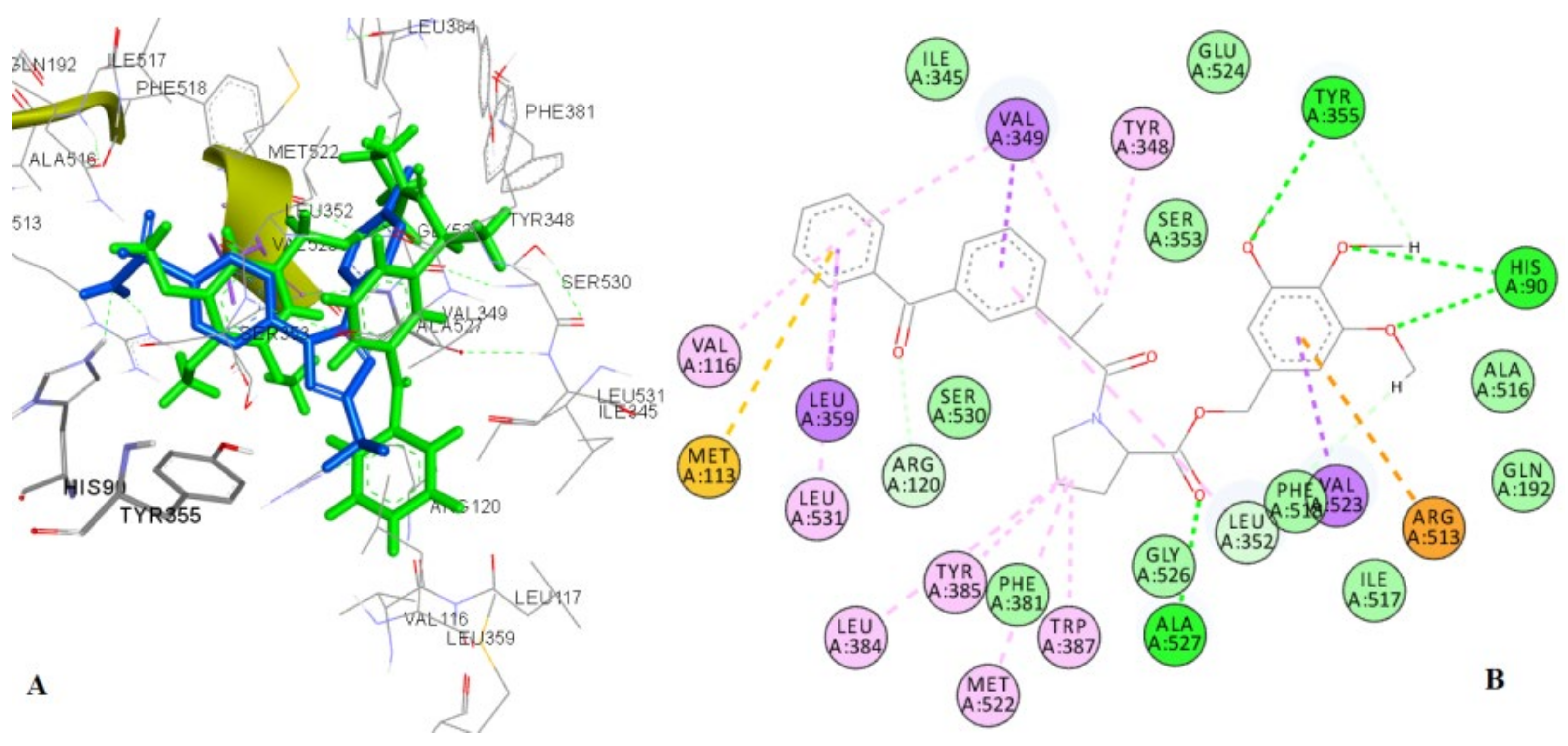
2.4.2. Docking to the Active Site of 5-LOX
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Synthesis of Amino Acid Methyl Esters (as Hydrochlorides) 1, 2, 3 and 4
- (a)
- The corresponding amino acid (alpha-L-alanine, beta-alanine, 0.1 mol) was dissolved in 100 mL methanol and 0.2 mol of freshly distilled trimethylchlorosilane was added with stirring at room temperature. After the completion of the reaction (about 12 h), the solvent was evaporated under reduced pressure to give the esters 1 ((S)-1-methoxy-1-oxopropan-2-aminium chloride (S-alanine methyl ester hydrochloride)) and 2 (3-methoxy-3-oxopropan-1-aminium chloride (beta-alanine methyl ester hydrochloride)).
- (b)
- 0.1 mol of amino acid (L-proline, L-tyrosine) was mixed with freshly distilled SOCl2 (0.3 mol) and Et3N (0.1 mol) in 100 mL methanol at room temperature. After the completion of the reaction (about 10 h), the mixture was neutralized with Et3N, the volatiles were removed under reduced pressure to give the amino acid methyl esters 3 ((S)-methyl pyrrolidine-2-carboxylate hydrochloride (L-proline methyl ester hydrochloride)) and 4 ((S)-methyl 2-amino-3-(4-hydroxyphenyl)propanoate hydrochloride (L-tyrosine methyl ester hydrochloride)).
3.2.2. General Method for the Synthesis of Amides 5–14
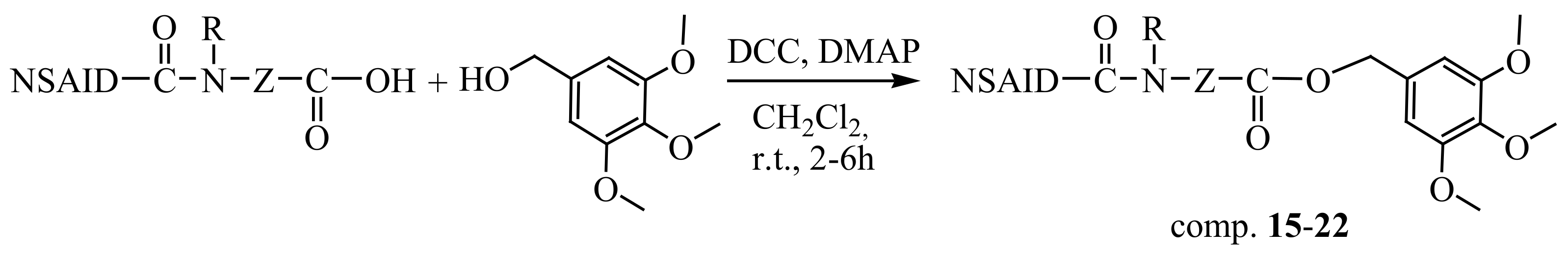
3.2.3. General Method for the Synthesis of Compounds 15–24
3.3. Biological Evaluation
3.3.1. Effect on Carrageenan-Induced Rat Paw Edema
3.3.2. Inhibition of COX-1 and COX-2 Activity
3.3.3. Inhibition of LOX Activity
3.3.4. Effect on Lipid Peroxidation
3.3.5. Preliminary Stability Study
3.4. In Silico Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Meirow, Y.; Baniyash, M. Immune biomarkers for chronic inflammation related complications in non-cancerous and cancerous diseases. Cancer Immunol. Immunother. 2017, 66, 1089–1101. [Google Scholar]
- Theodosis-Nobelos, P.; Tziona, P.; Poptsis, A.; Athanasekou, C.; Kourounakis, P.N.; Rekka, E.A. Novel polyfunctional esters of ibuprofen and ketoprofen with hypolipidemic, lipoxygenase inhibitory and enhanced anti-inflammatory activity. Med. Chem. Res. 2017, 26, 461–472. [Google Scholar]
- Cai, J.; Hosmane, N.S.; Takagaki, M.; Zhu, Y. Synthesis, molecular docking, and in vitro boron neutron capture therapy assay of carboranyl sinomenine. Molecules 2020, 25, 4697. [Google Scholar]
- Tziona, P.; Theodosis-Nobelos, P.; Rekka, E.A. Medicinal Chemistry approaches of controlling gastrointestinal side effects of non-steroidal anti-inflammatory drugs. Endogenous protective mechanisms and drug design. Med. Chem. 2017, 13, 408–420. [Google Scholar]
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of inflammation: Mechanisms and opportunity for drug development. Pharmacol. Ther. 2013, 139, 189–212. [Google Scholar]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar]
- Bertolini, A.; Ottani, A.; Sandrini, M. Dual acting anti-inflammatory drugs: A reappraisal. Pharmacol. Res. 2001, 44, 437–450. [Google Scholar]
- Abdellatif, K.R.A.; Abdelall, E.K.A.; Elshemy, H.A.H.; El-Nahass, E.S.; Abdel-Fattah, M.M.; Abdelgawad, Y.Y.M. New indomethacin analogs as selective COX-2 inhibitors: Synthesis, COX-1/2 inhibitory activity, anti-inflammatory, ulcerogenicity, histopathological, and docking studies. Arch. Pharm. 2021, 354, e2000328. [Google Scholar]
- Bosquesi, P.L.; Melo, T.R.; Vizioli, E.O.; Santos, J.L.; Chung, M.C. Anti-inflammatory drug design using a molecular hybridization approach. Pharmaceuticals 2011, 4, 1450–1474. [Google Scholar]
- Galanakis, D.; Kourounakis, A.P.; Tsiakitzis, K.C.; Doulgkeris, C.; Rekka, E.A.; Gavalas, A.; Kravaritou, C.; Charitos, C.; Kourounakis, P.N. Synthesis and pharmacological evaluation of amide conjugates of NSAIDs with L-cysteine ethyl ester, combining potent antiinflammatory and antioxidant properties with significantly reduced gastrointestinal toxicity. Bioorg. Med. Chem. Lett. 2004, 14, 3639–3643. [Google Scholar]
- Doulgkeris, C.M.; Galanakis, D.; Kourounakis, A.P.; Tsiakitzis, K.C.; Gavalas, A.M.; Eleftheriou, P.T.; Victoratos, P.; Rekka, E.A.; Kourounakis, P.N. Synthesis and pharmacochemical study of novel polyfunctional molecules combining anti-inflammatory, antioxidant, and hypocholesterolemic properties. Bioorg. Med. Chem. Lett. 2006, 16, 825–829. [Google Scholar]
- Kaur, A.; Pathak, D.P.; Sharma, V.; Narasimhan, B.; Sharma, P.; Mathur, R.; Wakode, S. Synthesis, biological evaluation and docking study of N-(2-(3,4,5-trimethoxybenzyl)benzoxazole-5-yl) benzamide derivatives as selective COX-2 inhibitor and anti-inflammatory agents. Bioorg. Chem. 2018, 81, 191–202. [Google Scholar]
- Chang, C.S.; Liu, J.F.; Lin, H.J.; Lin, C.D.; Tang, C.H.; Lu, D.Y.; Sing, Y.T.; Chen, L.Y.; Kao, M.C.; Kuo, S.C.; et al. Synthesis and bioevaluation of novel 3,4,5-trimethoxybenzylbenzimidazole derivatives that inhibit Helicobacter pylori-induced pathogenesis in human gastric epithelial cells. Eur. J. Med. Chem. 2012, 48, 244–254. [Google Scholar]
- Abdel-Salam, O.M.; El-Batran, S. Pharmacological investigation of trimetazidine in models of inflammation, pain and gastric injury in rodents. Pharmacology 2005, 75, 122–132. [Google Scholar]
- Li, J.; Sha, Y. A convenient synthesis of amino acid methyl esters. Molecules 2008, 13, 1111–1119. [Google Scholar]
- Hosangadi, B.D.; Dave, R.H. An efficient general method for esterification of aromatic carboxylic acids. Tetrahedron Lett. 1996, 37, 6375–6378. [Google Scholar]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Kourounakis, P.N.; Rekka, E.A. Active anti-inflammatory and hypolipidemic derivatives of lorazepam. Molecules 2019, 24, 3277. [Google Scholar]
- Vinegar, R.; Schreiber, W.; Hugo, R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969, 166, 96–103. [Google Scholar]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar]
- Cryer, B.; Feldman, M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 413–421. [Google Scholar]
- Biringer, R.G. The enzymology of human eicosanoid pathways: The lipoxygenase branches. Mol. Biol. Rep. 2020, 47, 7189–7207. [Google Scholar] [PubMed]
- Somvanshi, R.K.; Singh, A.K.; Saxena, M.; Mishra, B.; Dey, S. Development of novel peptide inhibitor of lipoxygenase based on biochemical and BIAcore evidences. Biochim. Biophys. Acta 2008, 1784, 1812–1817. [Google Scholar] [PubMed]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [PubMed]
- Xu, S.; Mueser, T.C.; Marnett, L.J.; Funk, M.O., Jr. Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure 2012, 20, 1490–1497. [Google Scholar]
- Qian, H.; Fu, Z.; Huang, W.; Zhang, H.; Zhou, J.; Ge, L.; Lin, R.; Lin, H.; Hu, X. Synthesis and preliminary biological evaluation of capsaicin derivatives as potential analgesic drugs. Med. Chem. 2010, 6, 205–210. [Google Scholar]
- Dubey, N.; Jain, D.K.; Bhadoria, U.S.; Sinha, S. Synthesis, characterization and pharmacological evaluation of amino acid conjugates of ketoprofen. Asian J. Chem. 2012, 24, 1170–1174. [Google Scholar]
- Rodriguez-Muñiz, G.M.; Gomez-Mendoza, M.; Nuin, E.; Andreu, I.; Marin, M.L.; Miranda, M.A. “Snorkelling” vs. “diving” in mixed micelles probed by means of a molecular bathymeter. Org. Biomol. Chem. 2017, 15, 10281–10288. [Google Scholar]
- Siskou, I.C.; Rekka, E.A.; Kourounakis, A.P.; Chrysselis, M.C.; Tsiakitzis, K.; Kourounakis, P.N. Design and study of some novel ibuprofen derivatives with potential nootropic and neuroprotective properties. Bioorg. Med. Chem. 2007, 15, 951–961. [Google Scholar]
- Doulgkeris, C.M.; Siskou, I.C.; Xanthopoulou, N.; Lagouri, V.; Kravaritou, C.; Eleftheriou, P.; Kourounakis, P.N.; Rekka, E.A. Compounds against inflammation and oxidative insult as potential agents for neurodegenerative disorders. Med. Chem. Res. 2012, 21, 2280–2291. [Google Scholar]
- Rasheed, A.; Kumar, C.K.; Mishra, A. Synthesis, hydrolysis studies and phamacodynamic profiles of amide prodrugs of dexibuprofen with amino acids. J. Enzyme Inhib. Med. Chem. 2011, 26, 688–695. [Google Scholar]
- Katritzky, A.R.; Khelashvili, L.; Munawar Ali, M. Syntheses of IAA- and IPA-amino acid conjugates. J. Org. Chem. 2008, 73, 9171–9173. [Google Scholar] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Pantelidou, M.; Kourounakis, P.N.; Athanasekou, C.; Rekka, E.A. Design, synthesis and study of nitrogen monoxide donors as potent hypolipidaemic and anti-inflammatory agents. Molecules 2020, 25, 19. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar]
- Selinsky, B.S.; Gupta, K.; Sharkey, C.T.; Loll, P.J. Structural analysis of NSAID binding byprostaglandin H2 synthase: Time-dependent and time independent inhibitors elicit identical enzyme conformations. Biochemistry 2001, 40, 5172–5180. [Google Scholar]
- Tratrat, C.; Haroun, M.; Tsolaki, E.; Petrou, A.; Gavalas, A.; Geronikaki, A. Thiazole-based chalcone derivatives as potential anti-inflammatory agents: Biological evaluation and molecular modelling. Curr. Top. Med. Chem. 2021, 11, 257–268. [Google Scholar]



| Compound | % Edema Inhibition | Compound | % Edema Inhibition |
|---|---|---|---|
| Ibuprofen | 36 ** | 22 | 59 *** |
| 15 | 35 * | Naproxen | 11 * |
| 18 | 49 *** | 17 | 23 * |
| 21 | 66.5 *** | 20 | 37 * |
| Ketoprofen | 47 ** | Indole-3-acetic acid | 0 |
| 16 | 91 *** | 23 | 40.5 * |
| 19 | 51 ** | 24 | 0 |
| No | Compound | % Edema Inhibition | No | Compound | % Edema Inhibition |
|---|---|---|---|---|---|
| 5a | 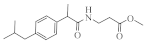 | 14 * | 13a | 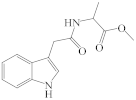 | 18 * |
| 8a |  | 20 * | 14a | 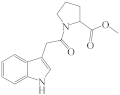 | 0 |
| Compound | COX-1 % Inhibition | COX-2 % Inhibition | ΜV * (Å3) |
|---|---|---|---|
| Ibuprofen | 68 | 46 | 211.2 |
| 18 | - | 57 | 465.2 |
| 21 | 51 | 67 | 521.4 |
| Ketoprofen | 94 | 49 | 234.8 |
| 16 | 28 | 56 | 465.6 |
| 19 | 57 | 94 | 488.8 |
| 22 | - | - | 545.1 |
| Naproxen | 34 | 17 | 214.0 |
| 17 | - | - | 444.8 |
| 20 | - | 14 | 467.9 |
| Compound | IC50 (μM) * | Compound | IC50 (μM) * |
|---|---|---|---|
| Ibuprofen | 200 | 22 | 85 |
| 15 | 160 | Naproxen | 218 |
| 18 | 83 | 17 | 41 |
| 21 | 64 | 20 | b |
| Ketoprofen | 220 | 23 | c |
| 16 | a | 24 | 128 |
| 19 | 140 | NDGA | 1.3 |
| Compound | Ovine COX-1 (PDB:1EQG) | Mus musculus COX-2 (PDB: 1CX2) | ||
|---|---|---|---|---|
| Binding Free Energy (kcal/mol) | Hydrogen Bonds | Binding Free Energy (kcal/mol) | Hydrogen Bonds | |
| 15 | −6.36 | O-Arg83 | −8.70 | O-Arg120, O-Arg120, O-Tyr355 |
| 16 | −5.90 | O-Arg120 | −8.54 | O-Arg120, O-Tyr355 |
| 18 | −5.52 | - | −8.41 | O-Arg120, O-Tyr355 |
| 19 | −7.60 | O-Arg120, O-Tyr355 | −10.25 | O-His90, O-His90, O-Tyr355, O-Ala527 |
| 20 | −5.14 | - | −6.72 | O-Arg120 |
| 21 | −7.48 | O-Arg120, O-Arg120 | −8.02 | O-Arg120, O-Tyr355 |
| Ketoprofen | −9.53 | O-Arg120, O-Arg120, H-Tyr355 | −8.11 | O-Arg120, H-Tyr355 |
| Ibuprofen | −9.22 | O-Arg120, O-Arg120, O-Tyr355 | - | - |
| SC-558 | - | - | −9.17 | O-His90, F-Arg120, H-Arg513 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziona, P.; Theodosis-Nobelos, P.; Papagiouvannis, G.; Petrou, A.; Drouza, C.; Rekka, E.A. Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol. Molecules 2022, 27, 2104. https://doi.org/10.3390/molecules27072104
Tziona P, Theodosis-Nobelos P, Papagiouvannis G, Petrou A, Drouza C, Rekka EA. Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol. Molecules. 2022; 27(7):2104. https://doi.org/10.3390/molecules27072104
Chicago/Turabian StyleTziona, Paraskevi, Panagiotis Theodosis-Nobelos, Georgios Papagiouvannis, Anthi Petrou, Chryssoula Drouza, and Eleni A. Rekka. 2022. "Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol" Molecules 27, no. 7: 2104. https://doi.org/10.3390/molecules27072104
APA StyleTziona, P., Theodosis-Nobelos, P., Papagiouvannis, G., Petrou, A., Drouza, C., & Rekka, E. A. (2022). Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol. Molecules, 27(7), 2104. https://doi.org/10.3390/molecules27072104







