Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods
Abstract
:1. Introduction
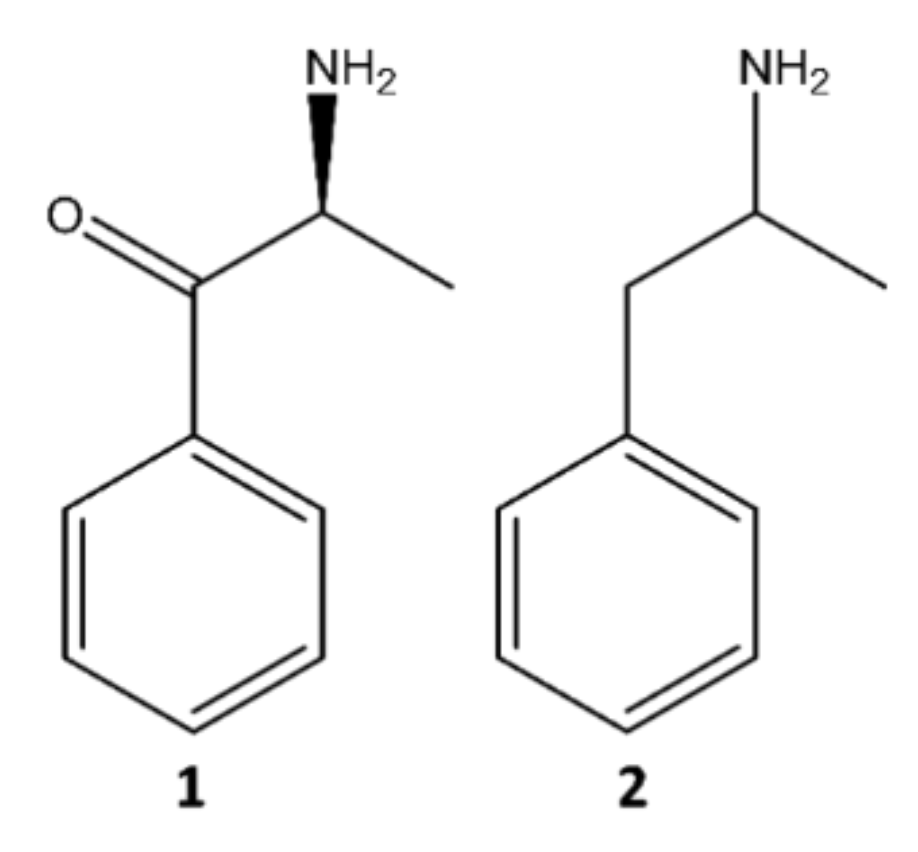
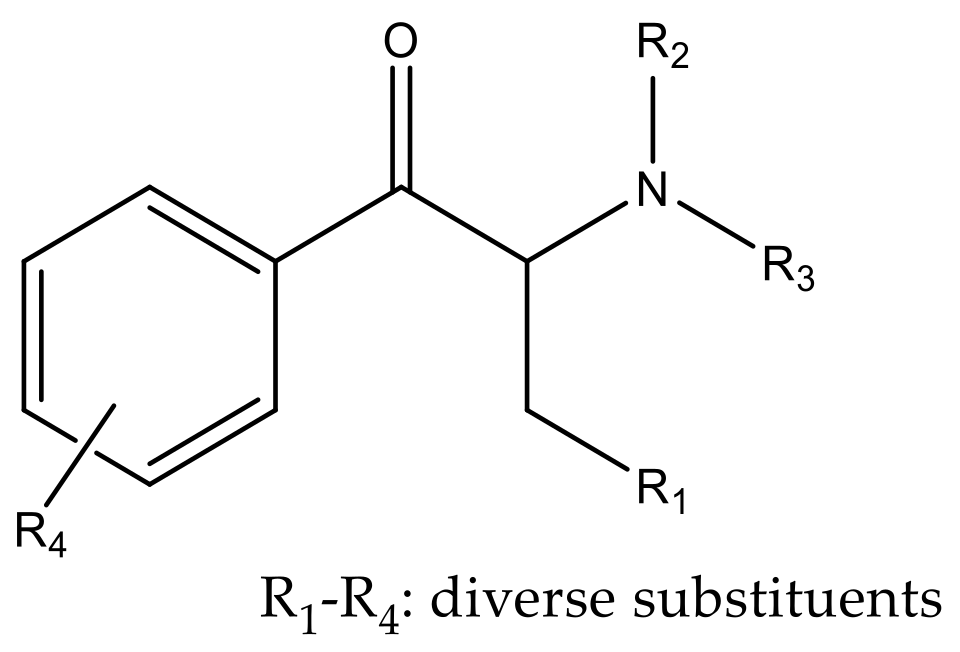
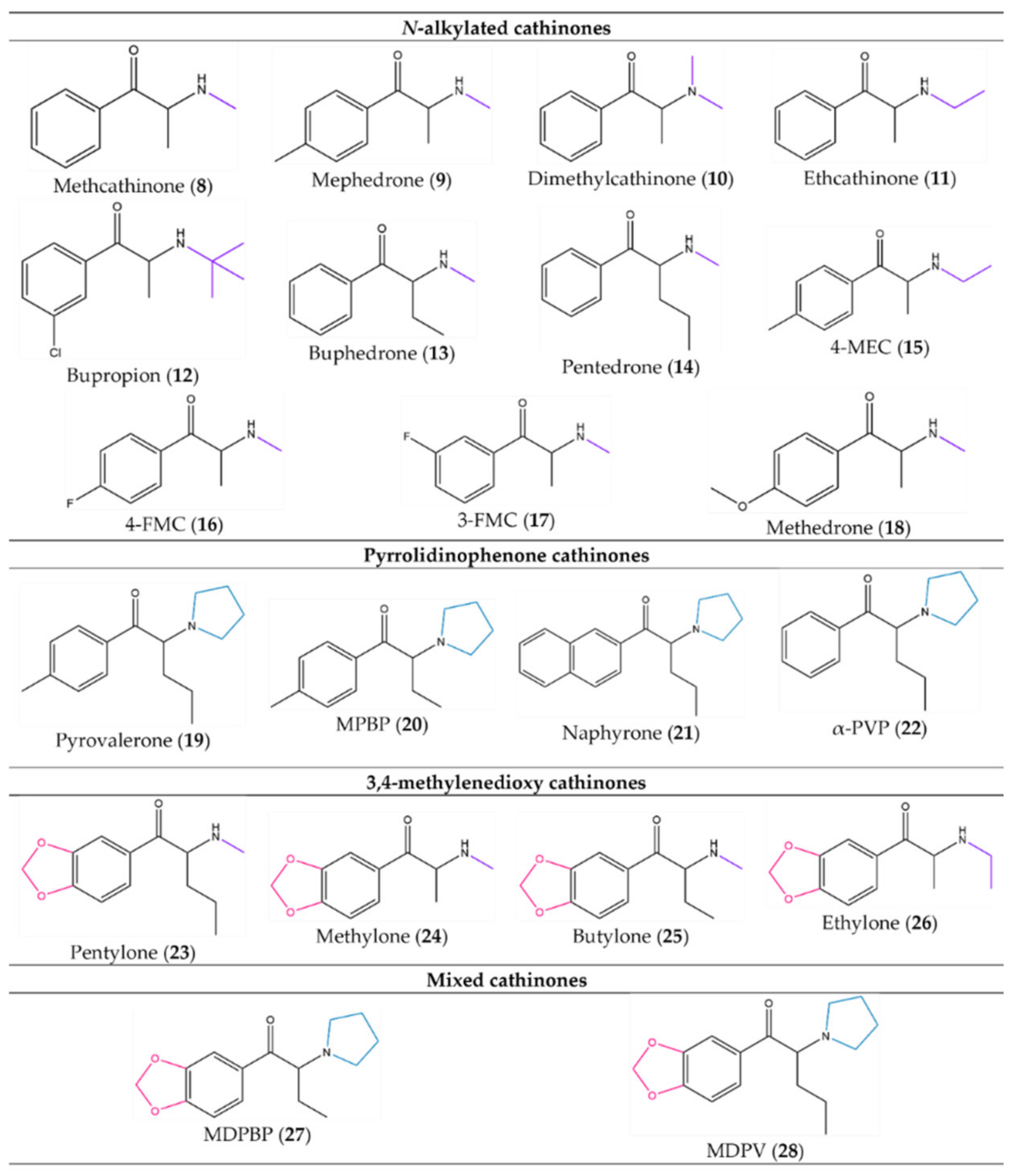
2. Classification of Synthetic Cathinones
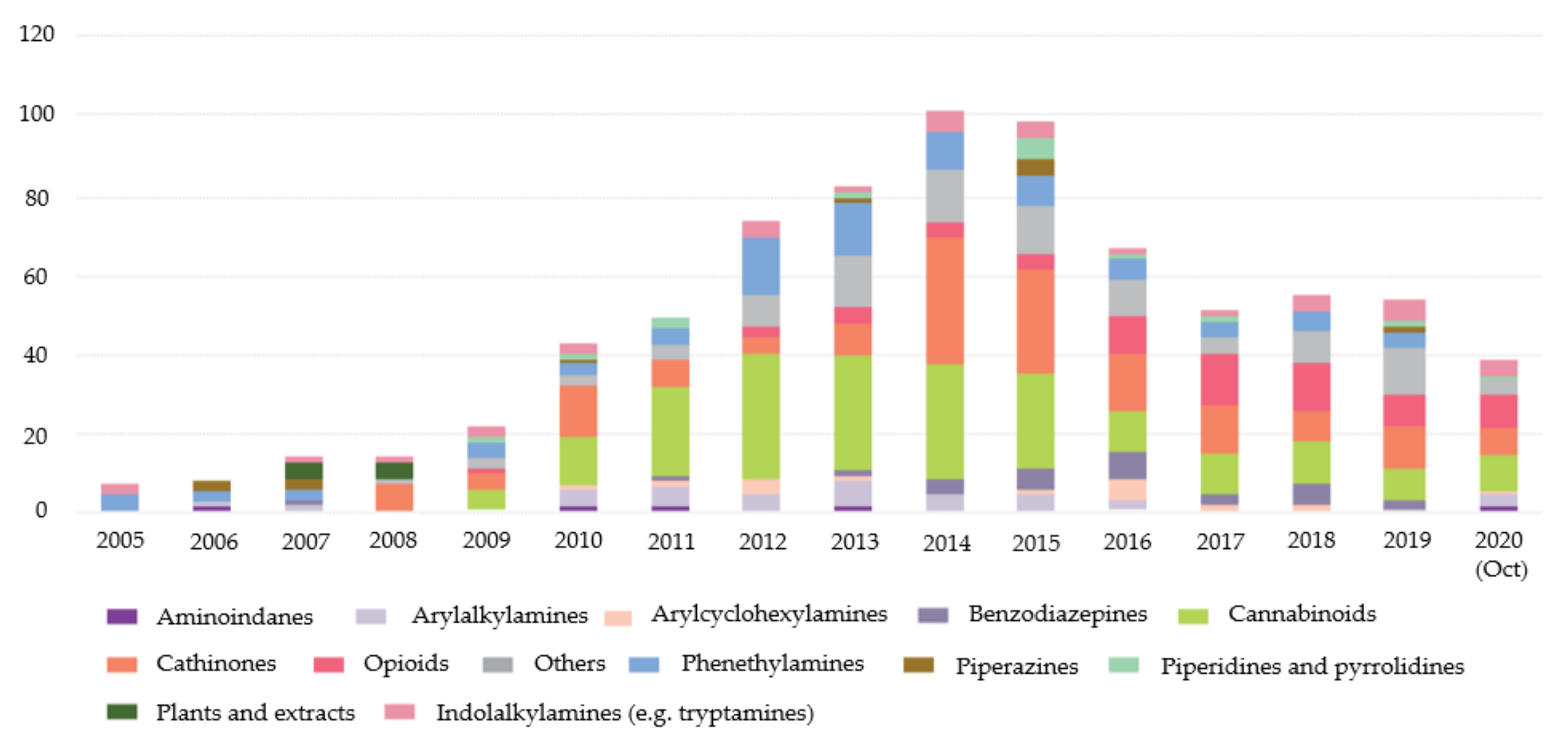
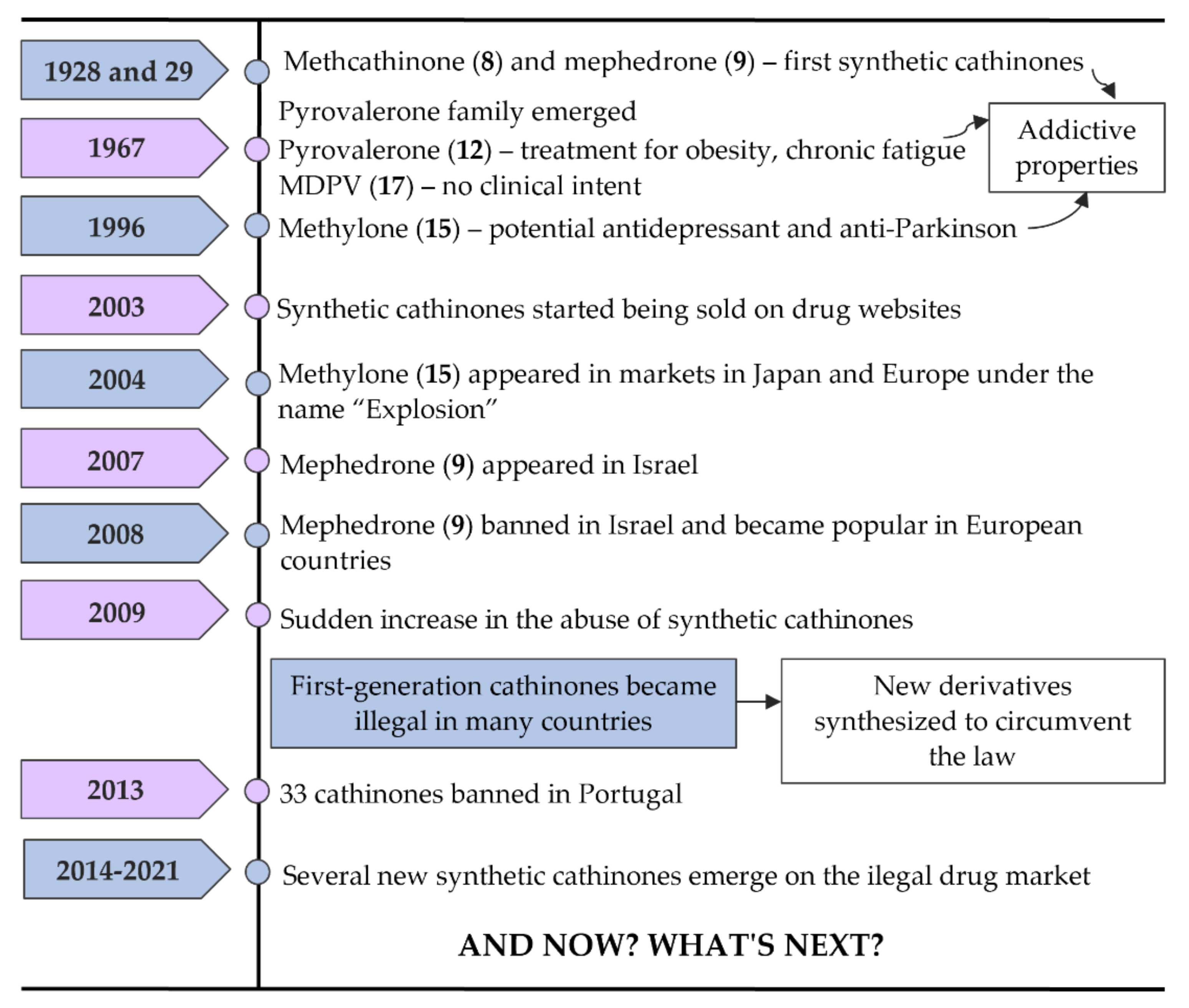
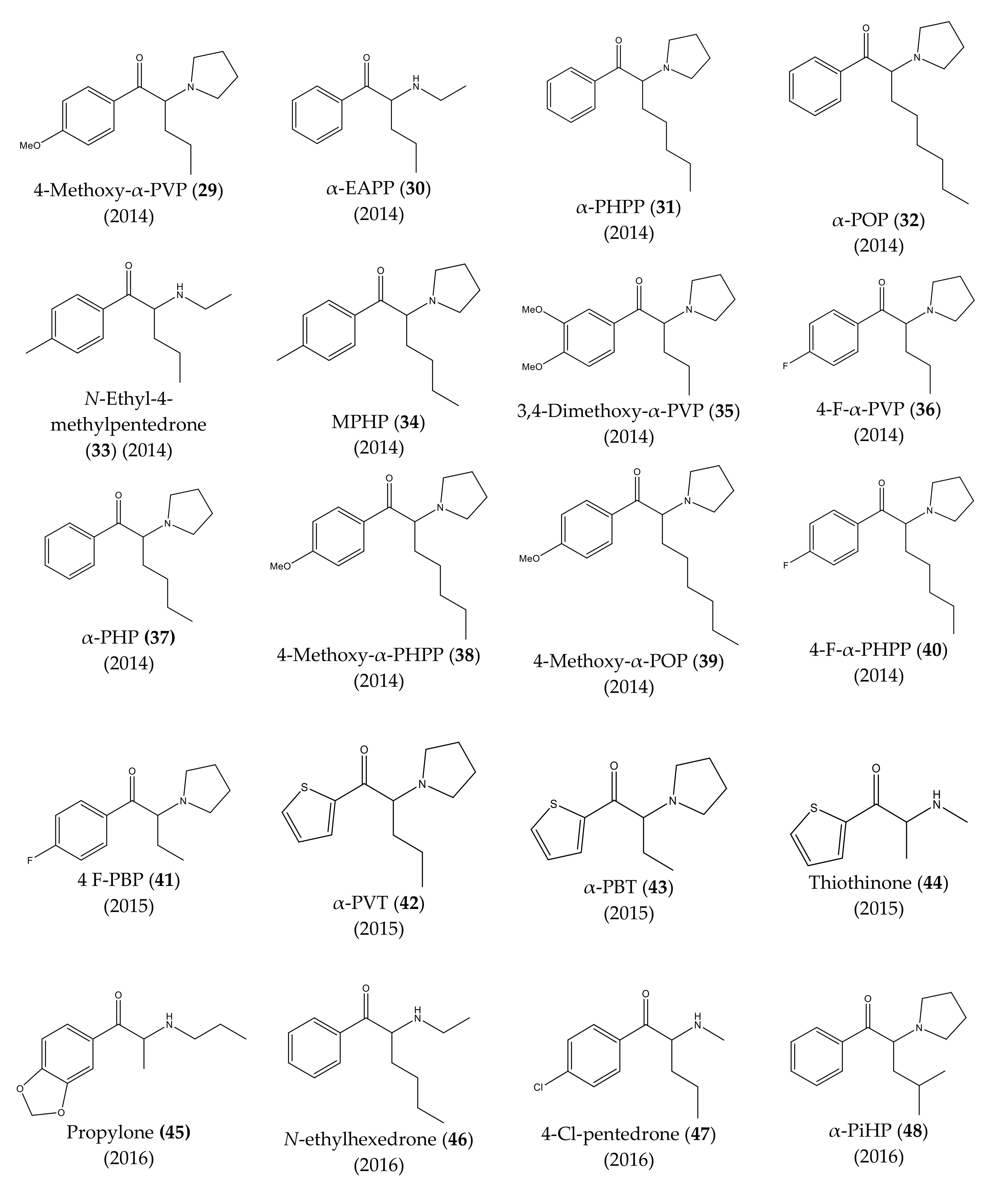
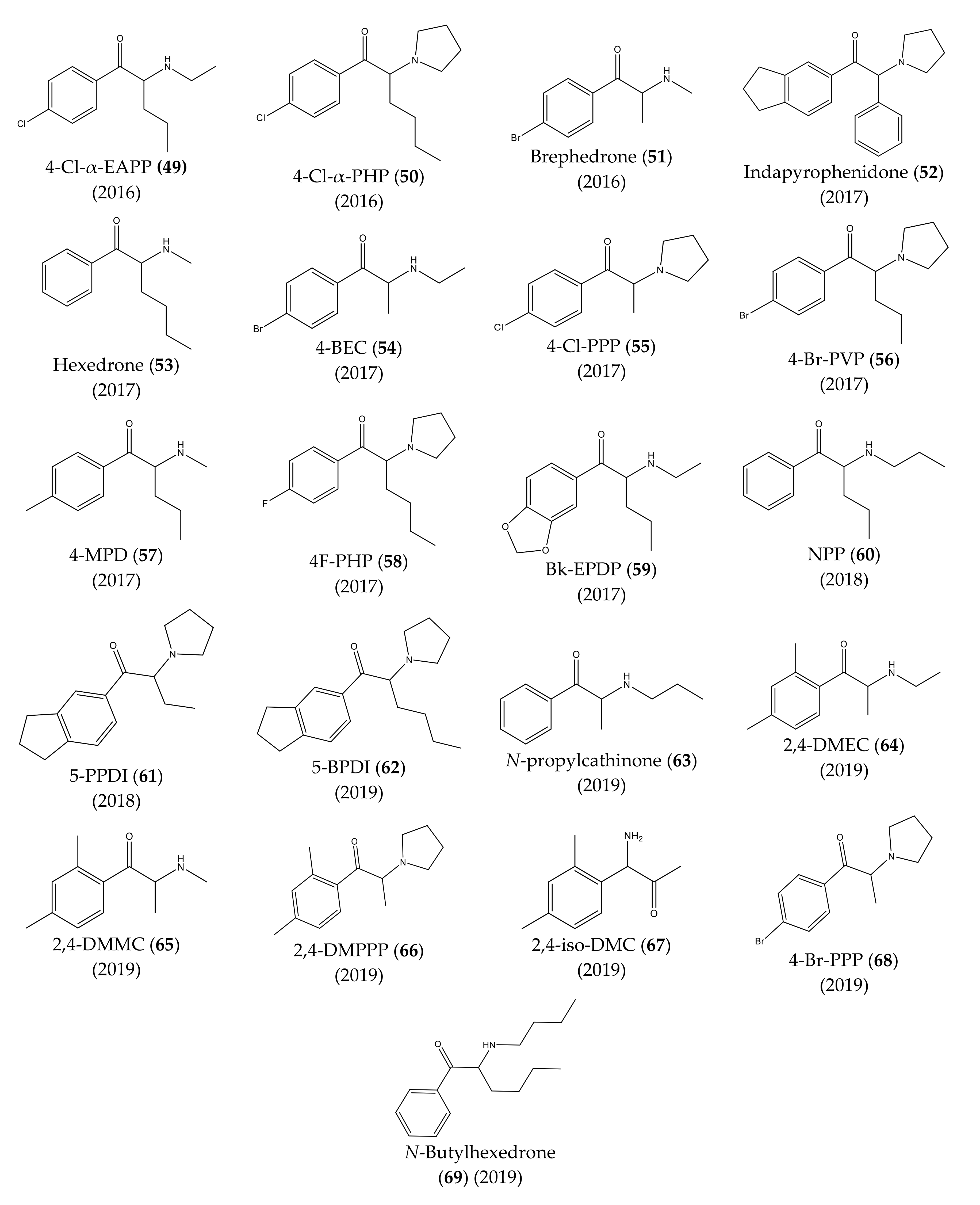
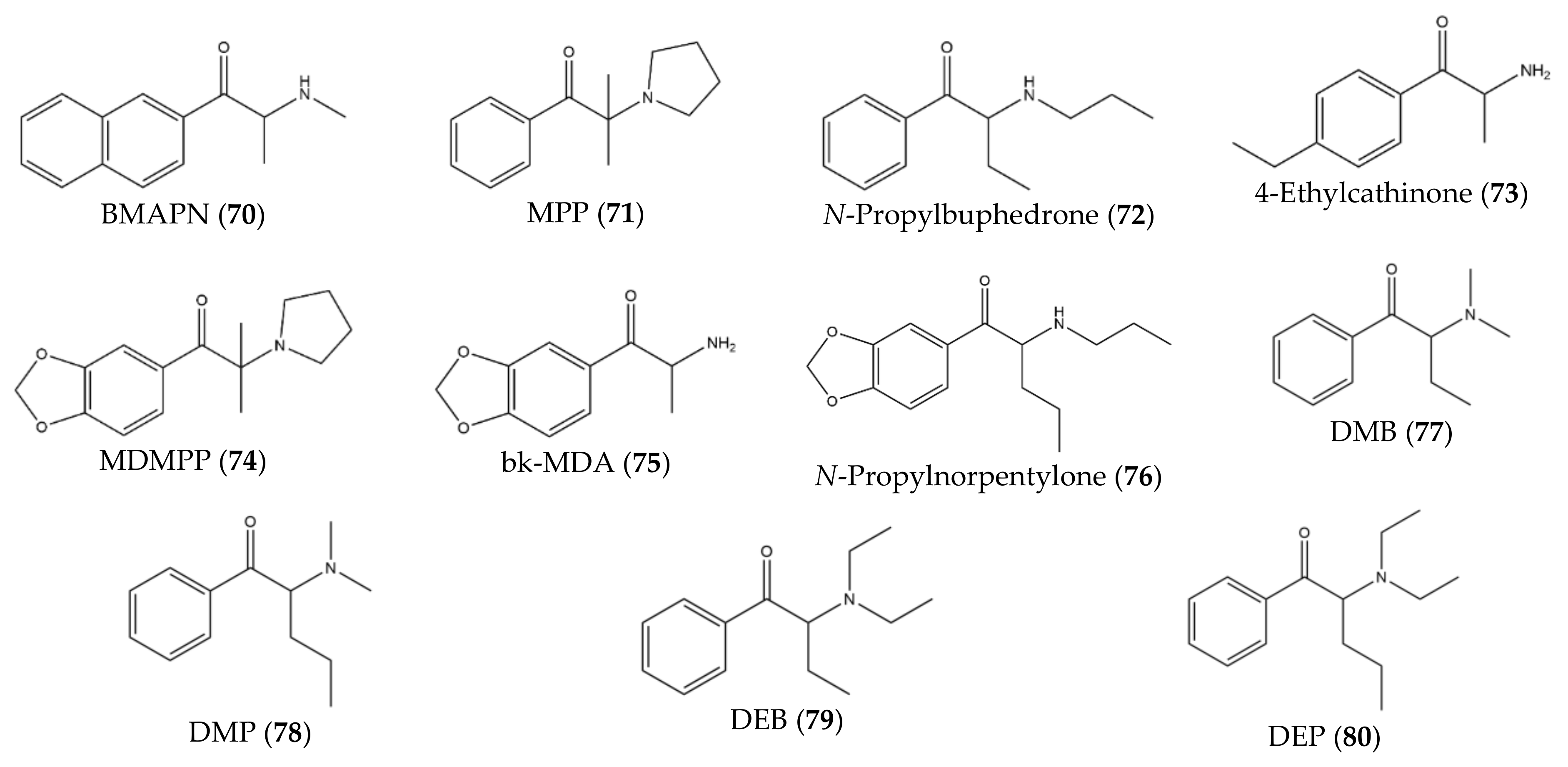
3. Chronological Evolution and Recent Developments of Cathinone Derivatives
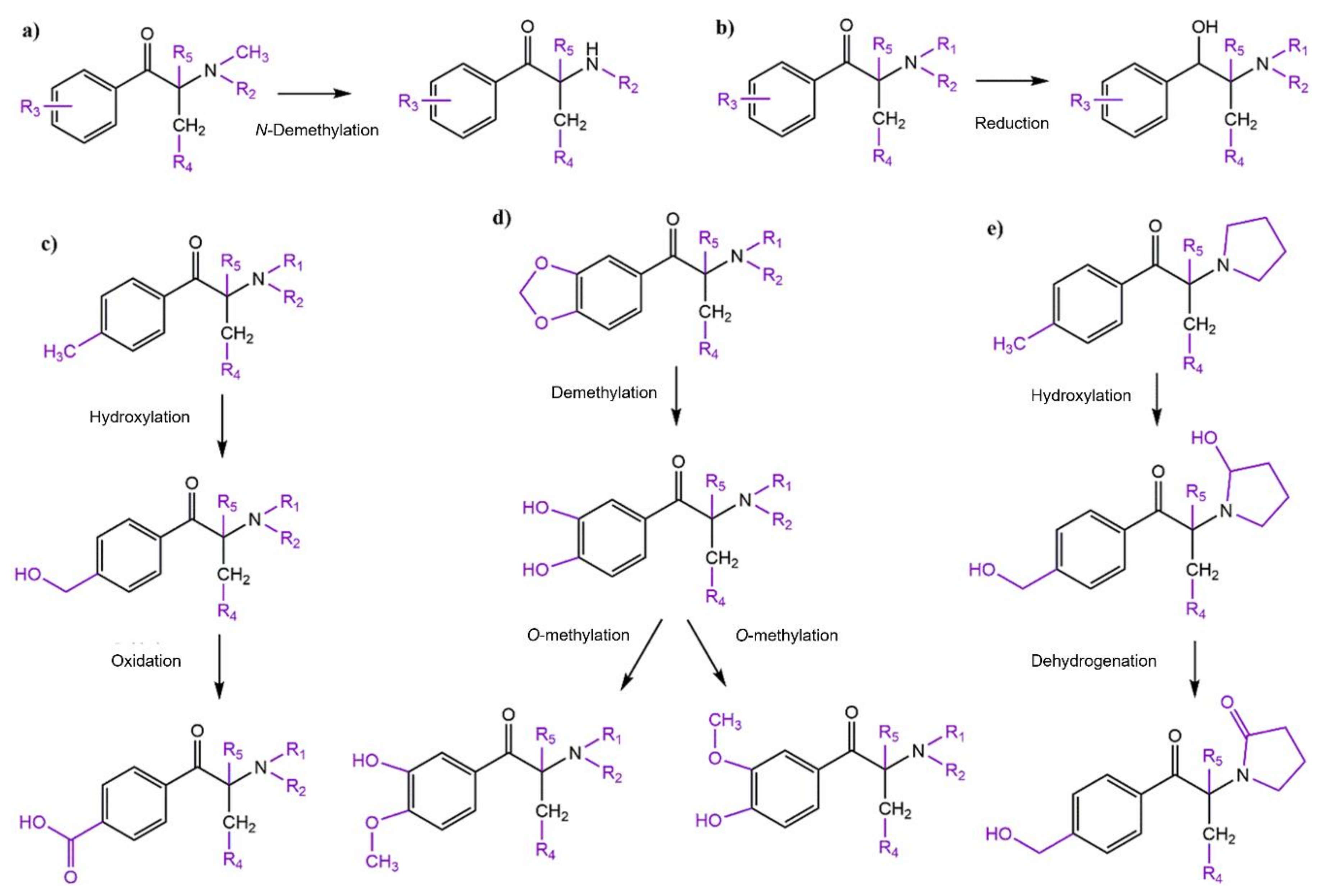
4. Toxicokinetic Properties
5. Mechanism of Action and Effects
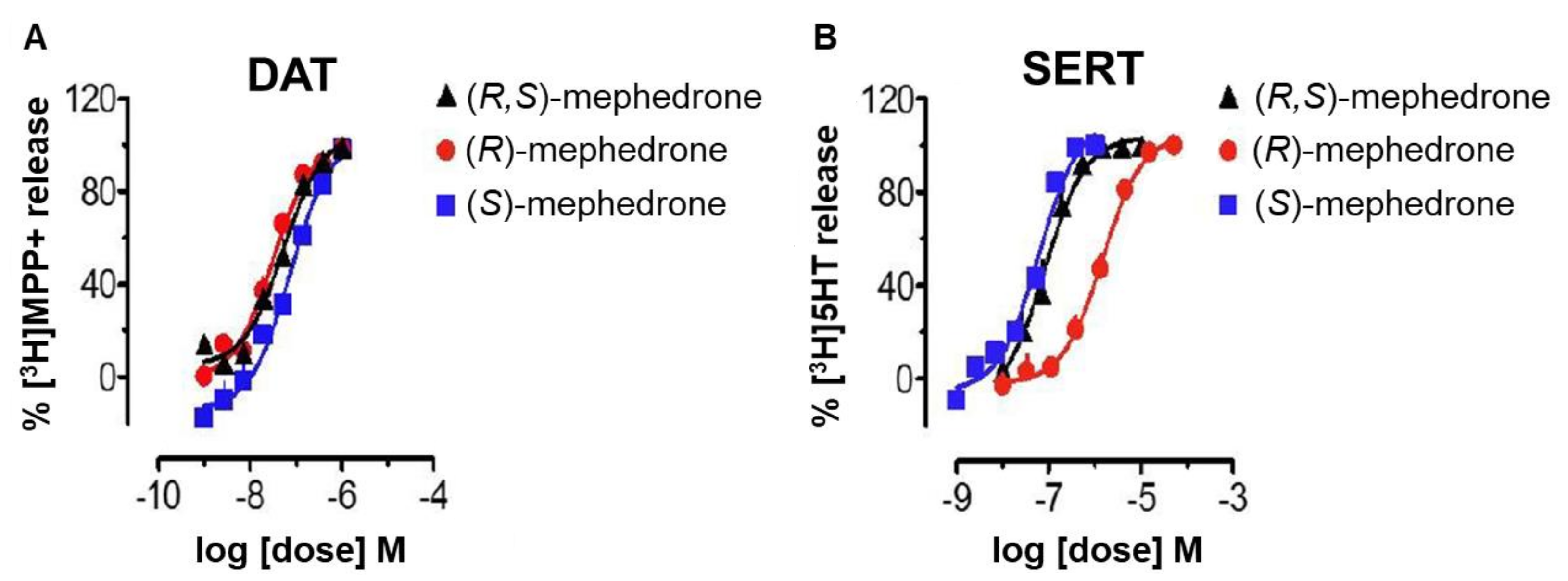
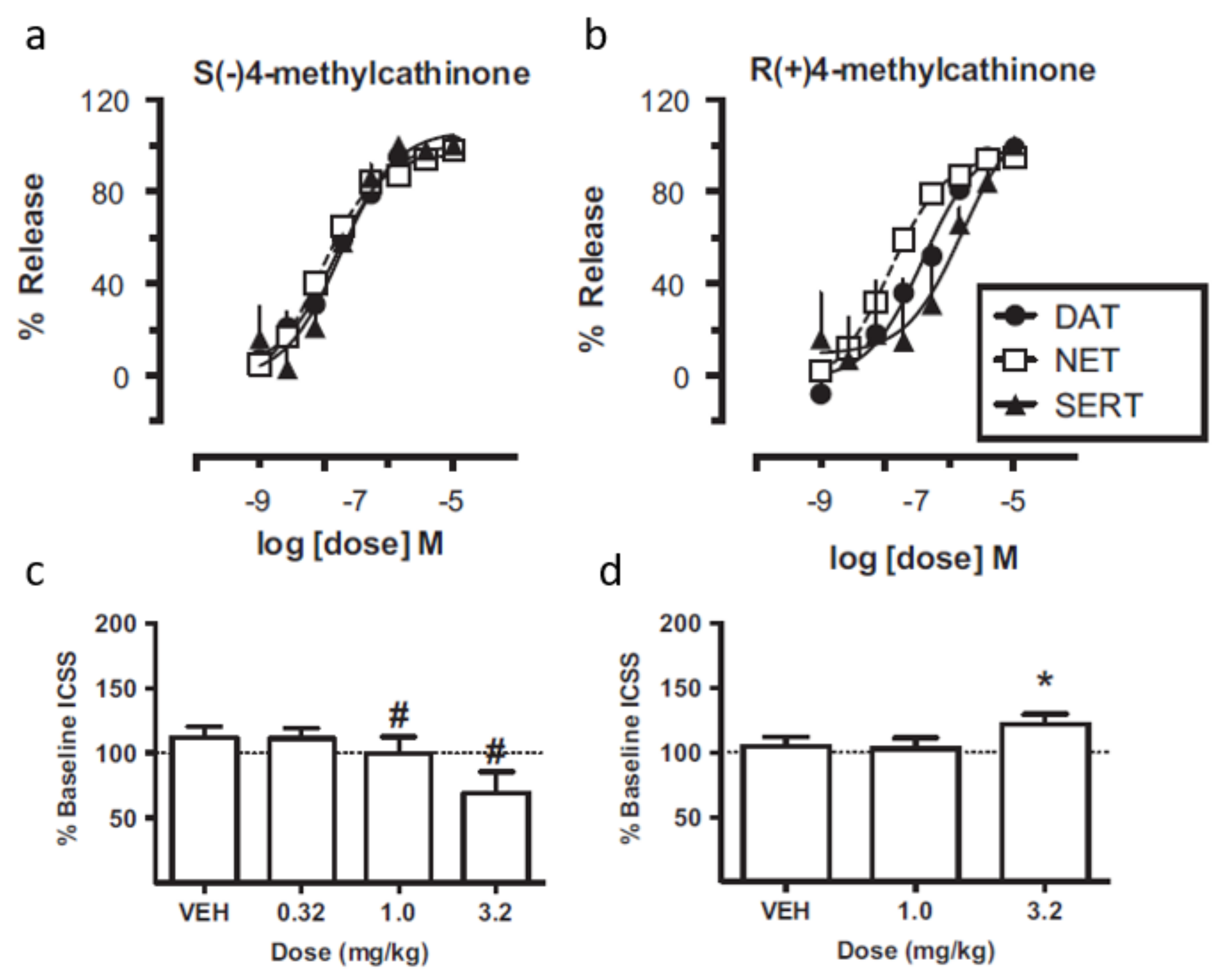
6. Enantioselectivity Studies
7. Enantiomeric Resolution
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2:4-DMEC | 2,4-Dimethylethcathinone |
| 2,4-DMMC | 2,4-Dimethylmethcathinone |
| 2,4-DMPPP | 2,4-Dimethyl-α-pyrrolidinopropiophenone |
| 2,4-iso-DMC | 2,4-Dimethylisocathinone |
| 3,4-Dimethoxy-α-PVP | 3,4-Dimethoxy-α-pyrrolidinopentiophenone |
| 3-FMC | 3-Fluoromethcathinone |
| 4 F-PBP | 4′-Fluoro-α-pyrrolidinobutyrophenone |
| 4-BEC | 4-Bromoethcathinone |
| 4-Br-PPP | 4-Bromo-α-pyrrolidinopropiophenone |
| 4-Br-PVP | 1-(4-Bromophenyl)-2-(pyrrolidin-1-yl)pentan-1-one |
| 4-Cl-PPP | 1-(4-Chlorophenyl)-2-(pyrrolidin-1-yl)propan-1-one |
| 4-Cl-α-EAPP | 1-(4-Chlorophenyl)-2-(ethylamino)pentan-1-one |
| 4-Cl-α-PHP | 1-(4-Chlorophenyl)-2-(pyrrolidin-1-yl)hexan-1-one] |
| 4-FMC | 4-Fluoromethcathinone |
| 4F-PHP | 1-(4-Fluorophenyl)-2-(pyrrolidin-1-yl)hexanone |
| 4-F-α-PHPP | 4-Fluoro-α-pyrrolidinoheptanophenone |
| 4-F-α-PVP | 4-Fluoro-α-pyrrolidinopentiophenone |
| 4-MEC | 4-Methyl-N-ethylcathinone |
| 4-Methoxy-α-PHPP | 4-methoxy-α-pyrrolidinoheptanophenone |
| 4-Methoxy-α-POP | 4-Methoxy-α-pyrrolidinooctanophenone |
| 4-Methoxy-α-PVP | 4-Methoxy-α-pyrrolidinovalerophenone) |
| 4-MPD | 4-Methylpentedrone |
| 5-BPDI | 1-(2,3-Dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)hexan-1-one |
| 5-HT | 5-Hydroxytryptamine |
| 5-PPDI | 1-(2,3-Dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)butan-1-one |
| ACN | Acetonitrile |
| AGP | α1-Acid glycoprotein |
| BGE | Background electrolyte |
| bk-MDA | 2-Amino-1-(benzo[d][1,3]dioxol-5-yl)propan-1-one |
| bk-PDP | 1-(1,3-Benzodioxol-5-yl)-2-(ethylamino)-1-pentanone |
| BMAPN | 2-(Methylamino)-1-(naphthalen-2-yl) propan-1-one |
| BTA | 2′-Bromotartranilic acid |
| ButOH | Butanol |
| CBH | Cellobiohydrolase I |
| CD | Cyclodextrin |
| CE | Capillary electrophoresis |
| CEC | Capillary electrochromatography |
| COMT | Catechol O-methyltransferase |
| CSP | Chiral stationary phase |
| DAD | Diode array detection |
| DAT | Dopamine transporters |
| DEA | Diethylamine |
| DEB | N,N-Diethylbuphedrone |
| DEP | N,N: Diethylpentedrone |
| DMB | N,N-Dimethylbuphedrone |
| DMP | N,N-Dimethylpentedrone |
| DNFP-L-V | Nα-(2,4-Dinitro-5-fluorophenyl)-L-valinamide |
| EKS | Electrokinetic supercharging |
| EMCDDA | European Monitoring Centre for Drugs and Drug Addiction |
| EPH | Ephedrone or Methcathinone |
| EtOH | Ethanol |
| FA | Formic acid |
| GC | Gas chromatography |
| Hex | Hexane |
| Hexedrone | α-Methylaminohexanophenone |
| HPLC | High performance liquid chromatography |
| HRMS | High resolution mass spectrometry |
| HSA | Human serum albumin |
| ICSS | Intracranial self-stimulation |
| IPA | Isopropyl alcohol |
| LC | Liquid chromatography |
| L-TPC | Trifluoroacetyl-L-prolyl chloride |
| MCF | (1R)-(–)-Menthylchloroformate |
| MDMA | 3,4-Methylenedioxymethamphetamine |
| MDPV | 3,4-Methylenedioxypyrovalerone |
| MeOH | Methanol |
| MEPH | Mephedrone |
| MPHP | 4-Methyl-α-pyrrolidinohexanophenone |
| MPP | 2-Methyl-1-phenyl-2-(pyrrolidin-1-yl)propan-1-one |
| MPP+ | 1-Methyl-4-phenylpyridinium |
| MRP1 | Multidrug resistance associated protein 1 |
| MS | Mass spectrometry |
| NAT | Noradrenaline transporters |
| NCI | Negative ion chemical ionization |
| NET | Norepinephrine transporters |
| NPLC | Normal-phase liquid chromatography |
| N-PP | α-Propyloaminopentiophenone |
| NPS | New psychoactive substances |
| P-gp | P-glycoprotein |
| POSC | Polar organic solvent chromatography |
| RPLC | Reversed-phase liquid chromatography |
| SERT | Serotonin transporters |
| SFC | Super critical fluid chromatography |
| SPE | Solid-phase extraction |
| TEA | Triethylamine |
| TFA | Trifluoroacetic acid |
| UHPLC | Ultra-high-performance liquid chromatography |
| UV | Ultra-violet |
| Vis | visible |
| VMAT2 | Vesicular monoamine transporter-2 |
| α-EAPP | α-Ethylaminopentiophenone |
| α-PBT | α-Pyrrolidinobutiothiophenone |
| α-PHP | α-Pyrrolidinohexanophenone |
| α-PHPP | α-Pyrrolidinoheptanophenone |
| α-PiHP | 4-Methyl-1-phenyl-2-(pyrrolidin-1-yl)pentan-1-one |
| α-POP | α-Pyrrolidinooctanophenone |
| α-PVP | α-Pyrrolidinopentiophenone |
| α-PVT | α-Pyrrolidinopentiothiophenone |
References
- UNODC. World Drugs Report. 2014. Available online: https://www.unodc.org/documents/data-and-analysis/WDR2014/World_Drug_Report_2014_web.pdf (accessed on 18 March 2021).
- Zanda, M.T.; Fattore, L. Chapter 29—Novel Psychoactive Substances: A New Behavioral and Mental Health Threat. In Addictive Substances and Neurological Disease; Watson, R.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 341–353. [Google Scholar]
- Coppola, M.; Mondola, R.; Oliva, F.; Picci, R.L.; Ascheri, D.; Trivelli, F. Chapter 63—Treating the Phenomenon of New Psychoactive Substances: Synthetic Cannabinoids and Synthetic Cathinones. In Neuropathology of Drug Addictions and Substance Misuse; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 679–686. [Google Scholar]
- Zawilska, J.B. Chapter Thirteen—“Legal Highs”—An Emerging Epidemic of Novel Psychoactive Substances. In International Review of Neurobiology; Taba, P., Lees, A., Sikk, K., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 273–300. [Google Scholar]
- EMCDDA. New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic-An Update from the EU Early Warning System. 2020. Available online: https://www.emcdda.europa.eu/publications/rapid-communication/new-psychoactive-substances-global-markets-glocal-threats-and-covid-19-pandemic_en (accessed on 18 March 2021).
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. Life Sci. 2014, 97, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Getasetegn, M. Chemical composition of Catha edulis (khat): A review. Phytochem. Rev. 2016, 15, 907–920. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Drug Dependence: Thirty-Fourth Report; Technical Report Series No. 942; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Bretteville-Jensen, A.L.; Tuv, S.S.; Bilgrei, O.R.; Fjeld, B.; Bachs, L. Synthetic cannabinoids and cathinones: Prevalence and markets. Forensic Sci. Rev. 2013, 25, 7–26. [Google Scholar] [PubMed]
- EMCDDA. European Drug Report 2015: Trends and Developments; Publications Office of the European Union: Luxembourg, 2015; p. 86. [Google Scholar]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Schindler, C.W. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacol 2013, 38, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. Synthetic cathinones: An evolving class of new psychoactive substances. Crit. Rev. Toxicol. 2019, 49, 549–566. [Google Scholar] [CrossRef]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.; Costa, V.M.; Bastos, M.d.L.; Carvalho, F.; Capela, J.P. An updated review on synthetic cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Zuba, D.; Byrska, B. Prevalence and co-existence of active components of ‘legal highs’. Drug Test. Anal. 2013, 5, 420–429. [Google Scholar] [CrossRef]
- Paillet-Loilier, M.; Cesbron, A.; Boisselier, R.; Bourgine, J.; Debruyne, D. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abus. Rehabil. 2014, 5, 37–52. [Google Scholar]
- Young, R.; Glennon, R.A. Cocaine-stimulus generalization to two new designer drugs: Methcathinone and 4-methylaminorex. Pharmacol. Biochem. Behav. 1993, 45, 229–231. [Google Scholar] [CrossRef]
- Dal Cason, T.A.; Young, R.; Glennon, R.A. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 1997, 58, 1109–1116. [Google Scholar] [CrossRef]
- Dasgupta, A. 3-Designer drugs including bath salts and spices. In Alcohol, Drugs, Genes and the Clinical Laboratory; Dasgupta, A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 53–73. [Google Scholar]
- Government Decreto-Lei n.º 54/2013 Diário da República nº75/2013. 2013, pp. 2250–2254. Available online: https://dre.pt/dre/detalhe/decreto-lei/54-2013-260418 (accessed on 18 March 2021).
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Identification of two new-type designer drugs, piperazine derivative MT-45 (I-C6) and synthetic peptide Noopept (GVS-111), with synthetic cannabinoid A-834735, cathinone derivative 4-methoxy-α-PVP, and phenethylamine derivative 4-methylbuphedrine from illegal products. Forensic Toxicol. 2014, 32, 9–18. [Google Scholar]
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Shimokawa, Y.; Kikura-Hanajiri, R.; Aritake, K.; Goda, Y. Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci. Int. 2014, 243, 1–13. [Google Scholar] [CrossRef]
- Uchiyama, N.; Shimokawa, Y.; Kawamura, M.; Kikura-Hanajiri, R.; Hakamatsuka, T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014, 32, 266–281. [Google Scholar] [CrossRef]
- Gaspar, H.; Bronze, S.; Ciríaco, S.; Queirós, C.R.; Matias, A.; Rodrigues, J.; Santos, S. 4F-PBP (4′-fluoro-α-pyrrolidinobutyrophenone), a new substance of abuse: Structural characterization and purity NMR profiling. Forensic Sci. Int. 2015, 252, 168–176. [Google Scholar] [CrossRef]
- Doi, T.; Asada, A.; Takeda, A.; Tagami, T.; Katagi, M.; Matsuta, S.; Kamata, H.; Obana, H. Identification and characterization of α-PVT, α-PBT, and their bromothienyl analogs found in illicit drug products. Forensic Toxicol. 2016, 34, 76–93. [Google Scholar] [CrossRef]
- Gambaro, V.; Casagni, E.; Dell’Acqua, L.; Roda, G.; Tamborini, L.; Visconti, G.L.; Demartin, F. Identification and characterization of a new designer drug thiothinone in seized products. Forensic Toxicol. 2016, 34, 174–178. [Google Scholar] [CrossRef]
- Liu, C.; Jia, W.; Li, T.; Hua, Z.; Qian, Z. Identification and analytical characterization of nine synthetic cathinone derivatives N-ethylhexedrone, 4-Cl-pentedrone, 4-Cl-α-EAPP, propylone, N-ethylnorpentylone, 6-MeO-bk-MDMA, α-PiHP, 4-Cl-α-PHP, and 4-F-α-PHP. Drug Test. Anal. 2017, 9, 1162–1171. [Google Scholar] [CrossRef]
- Machado, Y.; Coelho Neto, J.; Barbosa, P.E.N.; Lordeiro, R.A.; Alves, R.B. Brephedrone: A new psychoactive substance seized in Brazil. Forensic Sci. Int. 2017, 275, 302–307. [Google Scholar] [CrossRef]
- Bijlsma, L.; Miserez, B.; Ibáñez, M.; Vicent, C.; Guillamón, E.; Hernán, F. Identification and characterization of a novel cathinone derivative 1-(2,3-dihydro-1H-inden-5-yl)-2-phenyl-2-(pyrrolidin-1-yl)-ethanone seized by customs in Jersey. Forensic Toxicol. 2016, 34, 144–150. [Google Scholar] [CrossRef]
- Błażewicz, A.; Bednarek, E.; Sitkowski, J.; Popławska, M.; Stypułkowska, K.; Bocian, W.; Kozerski, L. Identification and structural characterization of four novel synthetic cathinones: α-methylaminohexanophenone (hexedrone, HEX), 4-bromoethcathinone (4-BEC), 4-chloro-α-pyrrolidinopropiophenone (4-Cl-PPP), and 4-bromo-α-pyrrolidinopentiophenone (4-Br-PVP) after their seizures. Forensic Toxicol. 2017, 35, 317–332. [Google Scholar]
- Apirakkan, O.; Frinculescu, A.; Shine, T.; Parkin, M.C.; Cilibrizzi, A.; Frascione, N.; Abbate, V. Analytical characterization of three cathinone derivatives, 4-MPD, 4F-PHP and bk-EPDP, purchased as bulk powder from online vendors. Drug Test. Anal. 2018, 10, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Fabregat-Safont, D.; Carbón, X.; Gil, C.; Ventura, M.; Sancho, J.V.; Hernández, F.; Ibáñez, M. Reporting the novel synthetic cathinone 5-PPDI through its analytical characterization by mass spectrometry and nuclear magnetic resonance. Forensic Toxicol. 2018, 36, 447–457. [Google Scholar] [CrossRef]
- Blazewicz, A.; Bednarek, E.; Poplawska, M.; Olech, N.; Sitkowski, J.; Kozerski, L. Identification and structural characterization of synthetic cathinones: N-propylcathinone, 2,4-dimethylmethcathinone, 2,4-dimethylethcathinone, 2,4-dimethyl-α-pyrrolidinopropiophenone, 4-bromo-α-pyrrolidinopropiophenone, 1-(2,3-dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)hexan-1-one and 2,4-dimethylisocathinone. Forensic Toxicol. 2019, 37, 288–307. [Google Scholar]
- Shevyrin, V.; Eltsov, O.; Shafran, Y. Identification and analytical characterization of the synthetic cathinone N-butylhexedrone. Drug Test. Anal. 2020, 12, 159–163. [Google Scholar] [CrossRef]
- Rojkiewicz, M.; Kuś, P.; Kusz, J.; Książek, M.; Sochanik, A. Spectroscopic and crystallographic characterization of a new cathinone derivative: 1-phenyl-2-(butylamino)hexan-1-one hydrochloride (N-butylhexedrone). Forensic Toxicol. 2020, 38, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Botanas, C.J.; Yoon, S.S.; de la Peña, J.B.; de la Peña, I.J.; Kim, M.; Woo, T.; Cheong, J.H. A novel synthetic cathinone, 2-(methylamino)-1-(naphthalen-2-yl) propan-1-one (BMAPN), produced rewarding effects and altered striatal dopamine-related gene expression in mice. Behav. Brain Res. 2017, 317, 494–501. [Google Scholar] [CrossRef]
- Carlsson, A.; Sandgren, V.; Svensson, S.; Konradsson, P.; Dunne, S.; Josefsson, M.; Dahlén, J. Prediction of designer drugs: Synthesis and spectroscopic analysis of synthetic cathinone analogs that may appear on the Swedish drug market. Drug Test. Anal. 2018, 10, 1076–1098. [Google Scholar] [CrossRef]
- Gaspar, H.; Bronze, S.; Oliveira, C.; Victor, B.; Machuqueiro, M.; Pacheco, R.; Caldeira, M.J.; Santos, S. Proactive response to tackle the threat of emerging drugs: Synthesis and toxicity evaluation of new cathinones. Forensic Sci. Int. 2018, 290, 146–156. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I. Identification and quantitation of a new cathinone designer drug PV9 in an “aroma liquid” product, antemortem whole blood and urine specimens, and a postmortem whole blood specimen in a fatal poisoning case. Forensic Toxicol. 2014, 32, 243–250. [Google Scholar] [CrossRef]
- Majchrzak, M.; Celiński, R.; Kowalska, T.; Sajewicz, M. Fatal case of poisoning with a new cathinone derivative: α-propylaminopentiophenone (N-PP). Forensic Toxicol. 2018, 36, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieprzyca, E.; Skowronek, R.; Korczyńska, M.; Kulikowska, J.; Chowaniec, M. A two fatal cases of poisoning involving new cathinone derivative PV8. Leg. Med. 2018, 33, 42–47. [Google Scholar] [CrossRef]
- Adamowicz, P.; Jurczyk, A.; Gil, D.; Szustowski, S. A case of intoxication with a new cathinone derivative α-PiHP–A presentation of concentrations in biological specimens. Leg. Med. 2020, 42, 101626. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karila, L.; Megarbane, B.; Cottencin, O.; Lejoyeux, M. Synthetic cathinones: A new public health problem. Curr. Neuropharmacol. 2015, 13, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Coppola, M.; Mondola, R. Synthetic cathinones: Chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicol. Lett. 2012, 211, 144–149. [Google Scholar] [CrossRef]
- Zaitsu, K. Metabolism of Synthetic Cathinones; Springer: Cham, Switzerland, 2018; pp. 71–96. [Google Scholar]
- Kelly, J.P. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef]
- Meyer, M.R.; Maurer, H.H. Metabolism of designer drugs of abuse: An updated review. Curr. Drug Metab. 2010, 11, 468–482. [Google Scholar] [CrossRef] [Green Version]
- Tyrkkö, E.; Andersson, M.; Kronstrand, R. The Toxicology of New Psychoactive Substances: Synthetic Cathinones and Phenylethylamines. Ther. Drug Monit. 2016, 38, 190–216. [Google Scholar] [CrossRef]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of Synthetic Cathinones. Handb. Exp. Pharmacol. 2018, 252, 113–142. [Google Scholar]
- Altun, B.; Çok, İ. Psychoactive Bath Salts and Neurotoxicity Risk. Turk. J. Pharma. Sci. 2020, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.M.; Nelson, L.S. The toxicology of bath salts: A review of synthetic cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schifano, F.; Corkery, J.; Ghodse, A.H. Suspected and Confirmed Fatalities Associated With Mephedrone (4-Methylmethcathinone, “Meow Meow”) in the United Kingdom. J. Clin. Psychopharmacol. 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Herrmann, E.S.; Johnson, P.S.; Johnson, M.; Vandrey, R. Novel Drugs of Abuse: Cannabinoids, Stimulants, and Hallucinogens. In General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 893–902. [Google Scholar]
- Silva, B.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. Chiral Resolution and Enantioselectivity of Synthetic Cathinones: A Brief Review. J. Anal. Toxicol. 2018, 42, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Martin, B.R.; Dal Cason, T.A.; Young, R. Methcathinone (“cat”): An enantiomeric potency comparison. Pharmacol. Biochem. Behav. 1995, 50, 601–606. [Google Scholar] [CrossRef]
- Gregg, R.A.; Baumann, M.; Partilla, J.S.; Bonano, J.S.; Vouga, A.; Tallarida, C.S.; Velvadapu, V.; Smith, G.R.; Peet, M.M.; Reitz, A.B.; et al. Stereochemistry of mephedrone neuropharmacology: Enantiomer-specific behavioural and neurochemical effects in rats. Br. J. Pharmacol. 2015, 172, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Hutsell, B.A.; Baumann, M.H.; Partilla, J.S.; Banks, M.L.; Vekariya, R.; Glennon, R.A.; Negus, S.S. Abuse-related neurochemical and behavioral effects of cathinone and 4-methylcathinone stereoisomers in rats. Eur. Neuropsychopharmacol. 2016, 26, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Kolanos, R.; Partilla, J.S.; Baumann, M.H.; Hutsell, B.A.; Banks, M.L.; Negus, S.S.; Glennon, R.A. Stereoselective Actions of Methylenedioxypyrovalerone (MDPV) To Inhibit Dopamine and Norepinephrine Transporters and Facilitate Intracranial Self-Stimulation in Rats. ACS Chem. Neurosci. 2015, 6, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Gannon, B.M.; Williamson, A.; Suzuki, M.; Rice, K.C.; Fantegrossi, W.E. Stereoselective Effects of Abused “Bath Salt” Constituent 3,4-Methylenedioxypyrovalerone in Mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J. Pharmacol. Exp. Ther. 2016, 356, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.; Fernandes, C.; Tiritan, M.E.; Pinto, M.M.; Valente, M.J.; Carvalho, M.; Remião, F. Chiral enantioresolution of cathinone derivatives present in “legal highs”, and enantioselectivity evaluation on cytotoxicity of 3,4-methylenedioxypyrovalerone (MDPV). Forensic Toxicol. 2016, 34, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, B.M.; Rice, K.C.; Collins, G.T. Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone and their enantiomers. Behav. Pharmacol. 2017, 28, 578–581. [Google Scholar] [CrossRef]
- Philogene-Khalid, H.L.; Hicks, C.; Reitz, A.B.; Liu-Chen, L.Y.; Rawls, S.M. Synthetic cathinones and stereochemistry: S enantiomer of mephedrone reduces anxiety- and depressant-like effects in cocaine- or MDPV-abstinent rats. Drug Alcohol Depend. 2017, 178, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Philogene-Khalid, H.L.; Simmons, S.J.; Nayak, S.; Martorana, R.M.; Su, S.H.; Caro, Y.; Rawls, S.M. Stereoselective Differences between the Reinforcing and Motivational Effects of Cathinone-Derived 4-Methylmethcathinone (Mephedrone) In Self-Administering Rats. ACS Chem. Neurosci. 2017, 8, 2648–2654. [Google Scholar] [CrossRef]
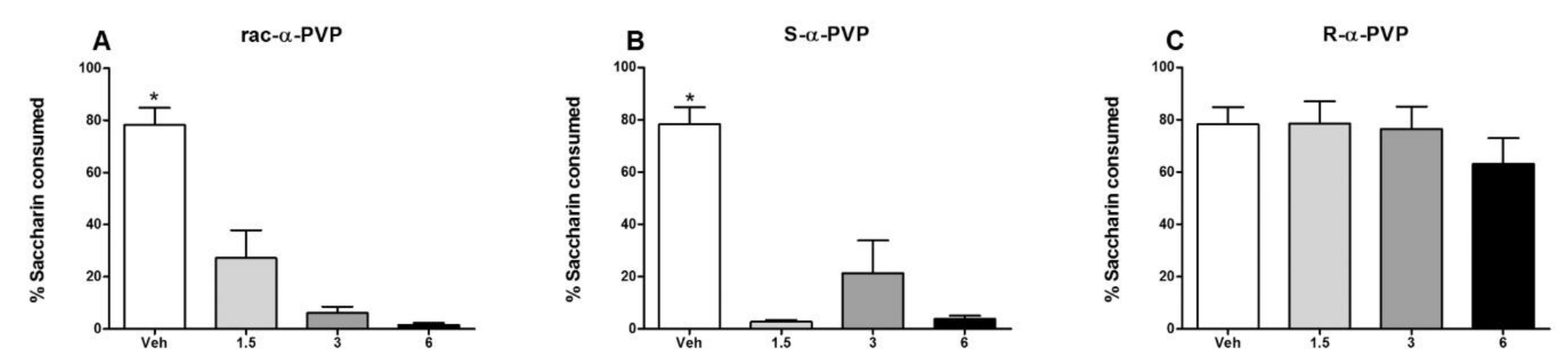
- Nelson, K.H.; López-Arnau, R.; Hempel, B.J.; To, P.; Manke, H.N.; Crissman, M.E.; Riley, A.L. Stereoselective effects of the second-generation synthetic cathinone α-pyrrolidinopentiophenone (α-PVP): Assessments of conditioned taste avoidance in rats. Psychopharmacol 2019, 236, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Cintulova, D.; Pittrich, D.A.; Wimmer, L.; Luethi, D.; Holy, M.; Jaentsch, K.; Tischberger, S.; Gmeiner, G.; Hoener, M.C.; et al. Stereochemistry of phase-1 metabolites of mephedrone determines their effectiveness as releasers at the serotonin transporter. Neuropharmacology 2019, 148, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Schindler, C.W.; Thorndike, E.B.; Walters, H.M.; Walther, D.; Rice, K.C.; Baumann, M.H. Stereoselective neurochemical, behavioral, and cardiovascular effects of α-pyrrolidinovalerophenone enantiomers in male rats. Addict. Biol. 2020, 25, e12842. [Google Scholar] [CrossRef] [PubMed]
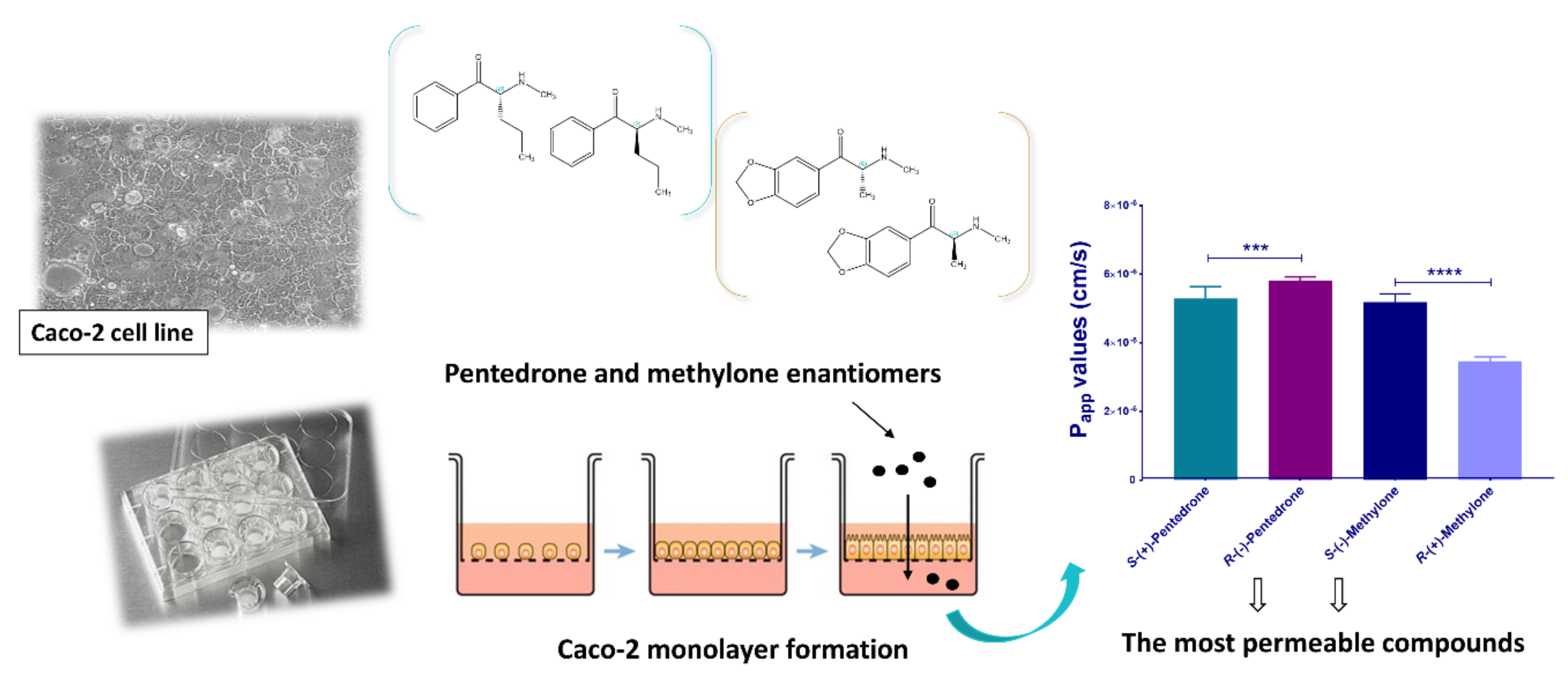
- Silva, B.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. Enantioselectivity on the absorption of methylone and pentedrone using Caco-2 cell line: Development and validation of an UHPLC method for cathinones quantification. Toxicol. Appl. Pharmacol. 2020, 395, 114970. [Google Scholar] [CrossRef]
- Davies, R.A.; Baird, T.R.; Nguyen, V.T.; Ruiz, B.; Sakloth, F.; Eltit, J.M.; Negus, S.S.; Glennon, R.A. Investigation of the Optical Isomers of Methcathinone, and Two Achiral Analogs, at Monoamine Transporters and in Intracranial Self-Stimulation Studies in Rats. ACS Chem. Neurosci. 2020, 11, 1762–1769. [Google Scholar] [CrossRef]
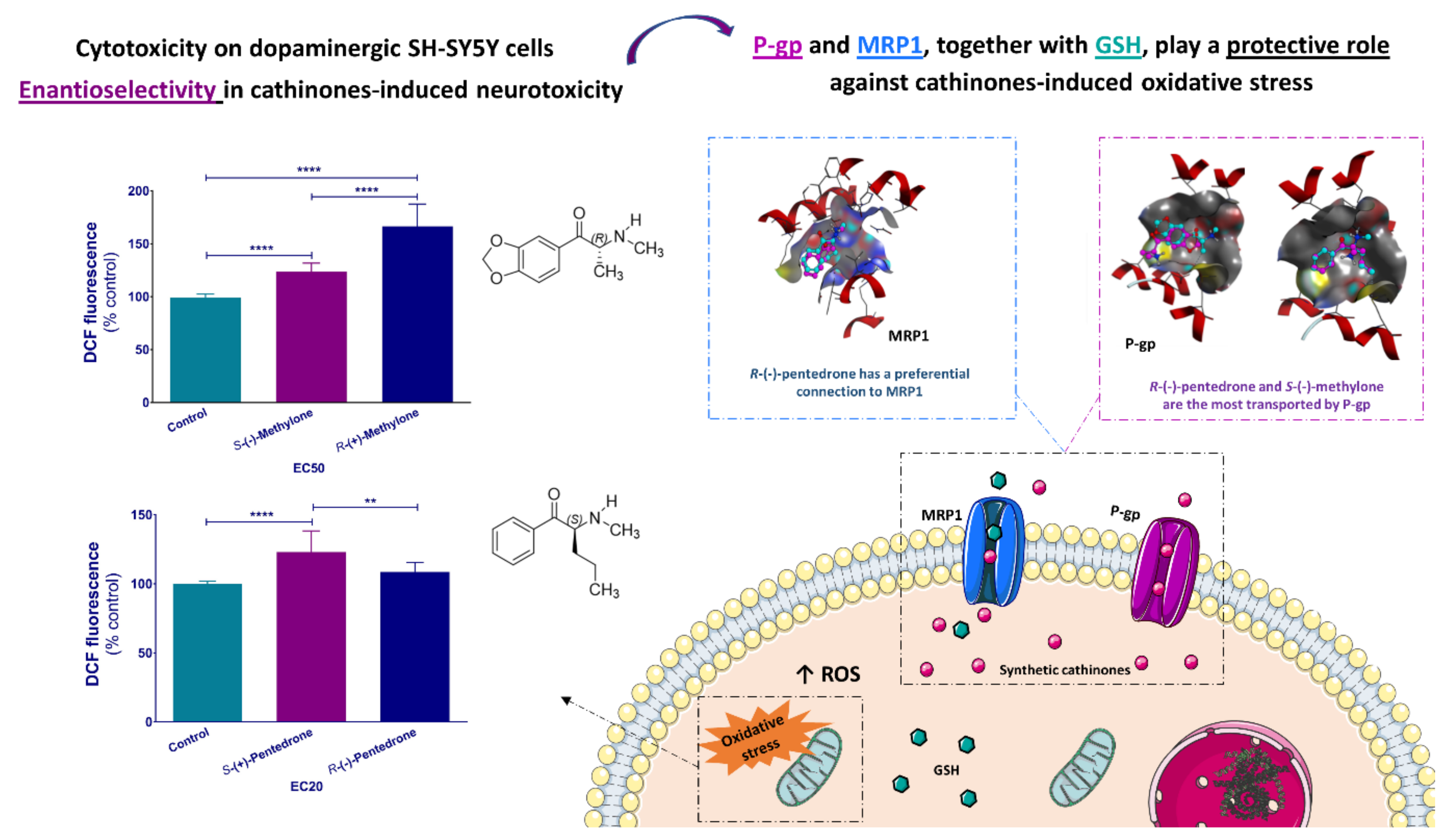
- Silva, B.; Palmeira, A.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. S-(+)-Pentedrone and R-(+)-methylone as the most oxidative and cytotoxic enantiomers to dopaminergic SH-SY5Y cells: Role of MRP1 and P-gp in cathinones enantioselectivity. Toxicol. Appl. Pharmacol. 2021, 416, 115442. [Google Scholar] [CrossRef]
- Francotte, E.R. Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Albals, D.; Heyden, Y.V.; Schmid, M.G.; Chankvetadze, B.; Mangelings, D. Chiral separations of cathinone and amphetamine-derivatives: Comparative study between capillary electrochromatography, supercritical fluid chromatography and three liquid chromatographic modes. J. Pharm. Biomed. Anal. 2016, 121, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Taschwer, M.; Weiß, J.A.; Kunert, O.; Schmid, M.G. Analysis and characterization of the novel psychoactive drug 4-chloromethcathinone (clephedrone). Forensic Sci. Int. 2014, 244, e56–e59. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Weiß, J.A.; Spreitz, J.; Schmid, M.G. Chiral separation of new cathinone- and amphetamine-related designer drugs by gas chromatography-mass spectrometry using trifluoroacetyl-l-prolyl chloride as chiral derivatization reagent. J. Chromatogr. A 2012, 1269, 352–359. [Google Scholar] [CrossRef]
- Weiß, J.A.; Mohr, S.; Schmid, M.G. Indirect chiral separation of new recreational drugs by gas chromatography-mass spectrometry using trifluoroacetyl-L-prolyl chloride as chiral derivatization reagent. Chirality 2015, 27, 211–215. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small Molecules as Chromatographic Tools for HPLC Enantiomeric Resolution: Pirkle-Type Chiral Stationary Phases Evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Chankvetadze, B. Application of enantioselective separation techniques to bioanalysis of chiral drugs and their metabolites. Trends Anal. Chem. 2021, 143, 116332. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M.M.M. Chiral Pharmaceuticals. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–28. [Google Scholar]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral pharmaceuticals in the environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, O.H.; Ciogli, A.; Villani, C.; De Martino, M.; Pierini, M.; Cavazzini, A.; Gasparrini, F. Ultra-fast high-efficiency enantioseparations by means of a teicoplanin-based chiral stationary phase made on sub-2 μm totally porous silica particles of narrow size distribution. J. Chromatogr. A 2016, 1427, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Krait, S.; Konjaria, M.L.; Scriba, G.K.E. Advances of capillary electrophoresis enantioseparations in pharmaceutical analysis (2017–2020). Electrophoresis 2021, 42, 1709–1725. [Google Scholar] [CrossRef]
- Salido-Fortuna, S.; Castro-Puyana, M.; Marina, M.L. Chiral Micellar Electrokinetic Chromatography. J. Chromatogr. A 2020, 1626, 461383. [Google Scholar] [CrossRef]
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M. Chiral Capillary Electrophoresis. Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Fanali, S.; Chankvetadze, B. Some thoughts about enantioseparations in capillary electrophoresis. Electrophoresis 2019, 40, 2420–2437. [Google Scholar] [CrossRef]
- Chankvetadze, B. Contemporary theory of enantioseparations in capillary electrophoresis. J. Chromatogr. A 2018, 1567, 2–25. [Google Scholar] [CrossRef]
- Scriba, G. Chiral Recognition in Separation Sciences. Part I: Polysaccharide and Cyclodextrin Selectors. Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Rezanka, P.; Navrátilová, K.; Rezanka, M.; Král, V.; Sýkora, D. Application of cyclodextrins in chiral capillary electrophoresis. Electrophoresis 2014, 35, 2701–2721. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral Separations: A Review of Current Topics and Trends. Anal. Chem. 2012, 84, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Fanali, S.; Chankvetadze, B. History, advancement, bottlenecks, and future of chiral capillary electrochromatography. J. Chromatogr. A 2021, 1637, 461832. [Google Scholar] [CrossRef]
- Schmid, M.G.; Hägele, J.S. Separation of enantiomers and positional isomers of novel psychoactive substances in solid samples by chromatographic and electrophoretic techniques–A selective review. J. Chromatogr. A 2020, 1624, 461256. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Serignese, V. Direct enantiomeric separation of cathinone and one major metabolite on cellobiohydrolase (CBH-I) chiral stationary phase. Biomed. Chromatogr. 1997, 11, 47–49. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Serignese, V. Direct chiral resolution of phenylalkylamines using a crown ether chiral stationary phase. Biomed. Chromatogr. 1997, 11, 7–10. [Google Scholar] [CrossRef]
- Wolrab, D.; Frühauf, P.; Moulisová, A.; Kuchař, M.; Gerner, C.; Lindner, W.; Kohout, M. Chiral separation of new designer drugs (Cathinones) on chiral ion-exchange type stationary phases. J. Pharm. Biomed. Anal. 2016, 120, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.W.H.; Abraham, I.; Gupta, S.; Kowalska, P.; Lightsey, D.; Marathaki, C.; Singh, N.S.; Lough, W.J. Screening approach, optimisation and scale-up for chiral liquid chromatography of cathinones. J. Chromatogr. A 2012, 1269, 189–197. [Google Scholar] [CrossRef]
- Taschwer, M.; Seidl, Y.; Mohr, S.; Schmid, M.G. Chiral separation of cathinone and amphetamine derivatives by HPLC/UV using sulfated ß-cyclodextrin as chiral mobile phase additive. Chirality 2014, 26, 411–418. [Google Scholar] [CrossRef]
- Weiß, J.A.; Taschwer, M.; Kunert, O.; Schmid, M.G. Analysis of a new drug of abuse: Cathinone derivative 1-(3,4-dimethoxyphenyl)-2-(ethylamino)pentan-1-one. J. Sep. Sci. 2015, 38, 825–828. [Google Scholar] [CrossRef]
- Merola, G.; Fu, H.; Tagliaro, F.; Macchia, T.; McCord, B.R. Chiral separation of 12 cathinone analogs by cyclodextrin-assisted capillary electrophoresis with UV and mass spectrometry detection. Electrophoresis 2014, 35, 3231–3241. [Google Scholar] [CrossRef]
- Baciu, T.; Borrull, F.; Calull, M.; Aguilar, C. Enantioselective determination of cathinone derivatives in human hair by capillary electrophoresis combined in-line with solid-phase extraction. Electrophoresis 2016, 37, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Aturki, Z.; Schmid, M.G.; Chankvetadze, B.; Fanali, S. Enantiomeric separation of new cathinone derivatives designer drugs by capillary electrochromatography using a chiral stationary phase, based on amylose tris(5-chloro-2-methylphenylcarbamate). Electrophoresis 2014, 35, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Deschamps, J.R.; Jacobson, A.E.; Rice, K.C. Chiral Resolution and Absolute Configuration of the Enantiomers of the Psychoactive “Designer Drug” 3,4-Methylenedioxypyrovalerone. Chirality 2015, 27, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alremeithi, R.H.; Meetani, M.A.; Khalil, S.A. A validated gas chromatography mass spectrometry method for simultaneous determination of cathinone related drug enantiomers in urine and plasma. RSC Adv. 2016, 6, 80576–80584. [Google Scholar] [CrossRef]
- Taschwer, M.; Grascher, J.; Schmid, M.G. Development of an enantioseparation method for novel psychoactive drugs by HPLC using a Lux(®) Cellulose-2 column in polar organic phase mode. Forensic Sci. Int. 2017, 270, 232–240. [Google Scholar] [CrossRef]
- Alremeithi, R.; Meetani, M.A.; Alaidaros, A.A.; Lanjawi, A.; Alsumaiti, K. Simultaneous Quantitative Determination of Synthetic Cathinone Enantiomers in Urine and Plasma Using GC-NCI-MS. Anal. Methods Chem. 2018, 2018, 4396043. [Google Scholar] [CrossRef] [Green Version]
- Kadkhodaei, K.; Forcher, L.; Schmid, M.G. Separation of enantiomers of new psychoactive substances by high-performance liquid chromatography. J. Sep. Sci. 2018, 41, 1274–1286. [Google Scholar] [CrossRef]
- Nowak, P.M.; Olesek, K.; Woźniakiewicz, M.; Kościelniak, P. Simultaneous enantioseparation of methcathinone and two isomeric methylmethcathinones using capillary electrophoresis assisted by 2-hydroxyethyl-β-cyclodextrin. Electrophoresis 2018, 39, 2406–2409. [Google Scholar] [CrossRef]
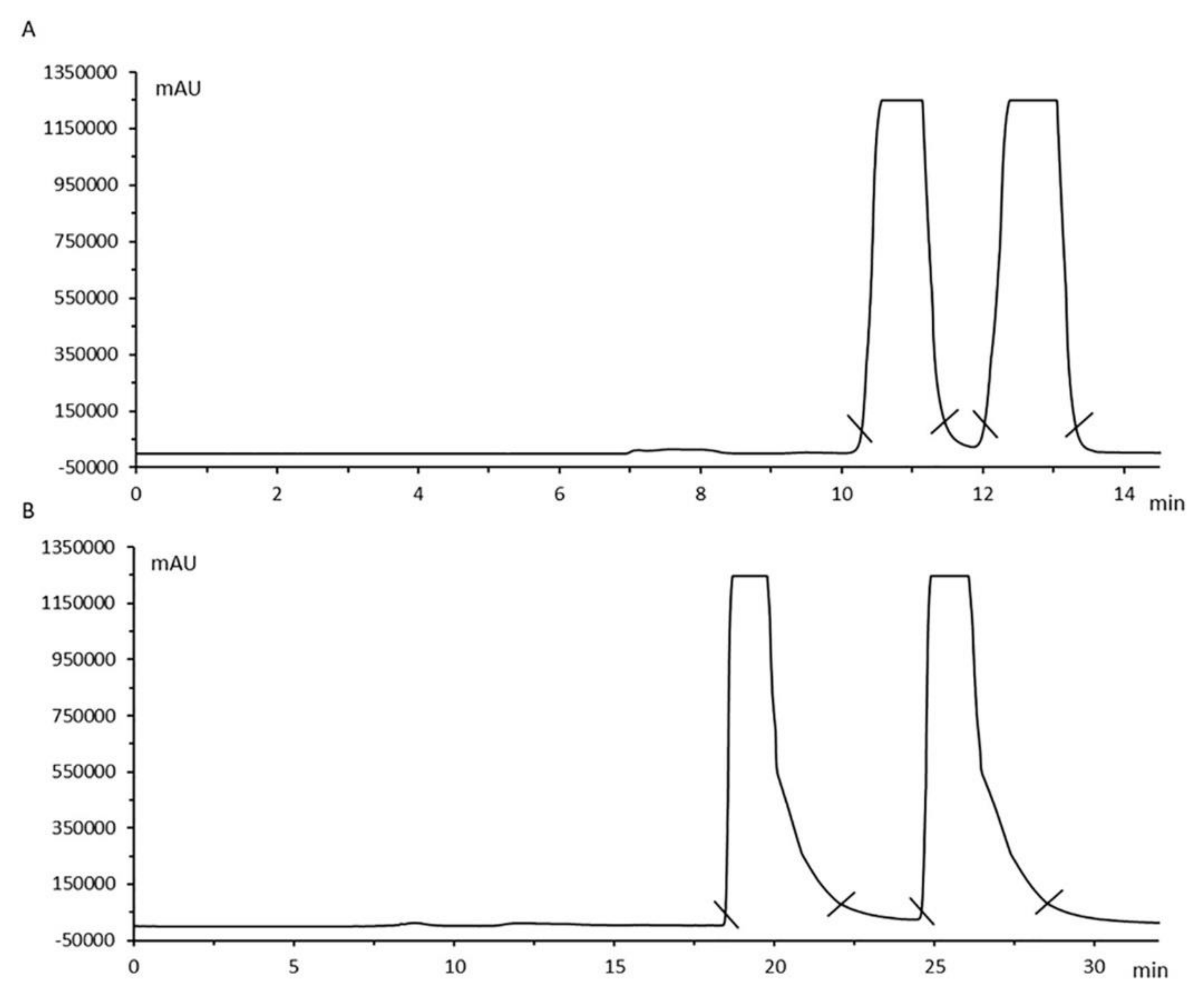
- Silva, B.; Pereira, J.A.; Cravo, S.; Araújo, A.M.; Fernandes, C.; Pinto, M.M.M.; Remião, F. Multi-milligram resolution and determination of absolute configuration of pentedrone and methylone enantiomers. J. Chromatogr. B 2018, 1100–1101, 158–164. [Google Scholar] [CrossRef]
- Hägele, J.S.; Hubner, E.M.; Schmid, M.G. Chiral separation of cathinone derivatives using β-cyclodextrin-assisted capillary electrophoresis-Comparison of four different β-cyclodextrin derivatives used as chiral selectors. Electrophoresis 2019, 40, 1787–1794. [Google Scholar] [CrossRef] [Green Version]
- Meetani, M.A.; Alremeithi, R.H.; Mousa, M.K. Enantioseparation of Synthetic Cathinones Enantiomers with Tertiary Amine Structure in Urine and Plasma. J. Chromatogr. Sci. 2019, 57, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M. Enantioselective determination of cathinones in urine by high pressure in-line SPE-CE. Electrophoresis 2019, 40, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Dhabbah, A.M. Determination of chiral cathinone in fresh samples of Catha edulis. Forensic Sci. Int. 2020, 307, 110105. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Borrull, F.; Marcé, R.M.; Fontanals, N. Enantiomeric determination of cathinones in environmental water samples by liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2020, 1626, 461359. [Google Scholar] [CrossRef] [PubMed]
- Hägele, J.S.; Basrak, M.; Schmid, M.G. Enantioselective separation of Novel Psychoactive Substances using a Lux® AMP 3 μm column and HPLC-UV. J. Pharm. Biomed. Anal. 2020, 179, 112967. [Google Scholar] [CrossRef]
- Hägele, J.S.; Seibert, E.; Schmid, M.G. A Simple HPLC–UV Approach for Rapid Enantioseparation of Cathinones, Pyrovalerones and Other Novel Psychoactive Substances on a 2.5-µm Cellulose Tris-(3,5-dimethylphenyl-carbamate) Column. Chromatographia 2020, 83, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Kadkhodaei, K.; Kadisch, M.; Schmid, M.G. Successful use of a novel lux® i-Amylose-1 chiral column for enantioseparation of “legal highs” by HPLC. Chirality 2020, 32, 42–52. [Google Scholar] [CrossRef]
- Kolderová, N.; Jurásek, B.; Kuchař, M.; Lindner, W.; Kohout, M. Gradient supercritical fluid chromatography coupled to mass spectrometry with a gradient flow of make-up solvent for enantioseparation of cathinones. J. Chromatogr. A 2020, 1625, 461286. [Google Scholar] [CrossRef]
- Lin, H.R.; Kuo, F.W. Determination of the R- and S-enantiomers of methylone and ethylone in seized drugs by enantioselective liquid chromatography tandem mass spectrometry analysis. Forensic Sci. Int. 2020, 317, 110528. [Google Scholar] [CrossRef]
- Meetani, M.; Ja, A.; Mk, A. Simultaneous Quantitative Determination of Synthetic Cathinone Enantiomers in Urine by GC-NCI-MS/MS using Menthylchloroformate as Chiral Derivatization Reagent. J. Chromatogr. Sep. Tech. 2020, 11, 436. [Google Scholar]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M. An electrokinetic supercharging approach for the enantiodetermination of cathinones in urine samples by capillary electrophoresis. Microchemical 2020, 158, 105300. [Google Scholar] [CrossRef]
- Řezanka, P.; Macková, D.; Jurok, R.; Himl, M.; Kuchař, M. Enantioseparation and Determination of Mephedrone and Its Metabolites by Capillary Electrophoresis Using Cyclodextrins as Chiral Selectors. Molecules 2020, 25, 2879. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, D.; Yi, R.; Patel, B.; Zhang, J.; Kong, N. A sensitive HPLC-MS/MS method for the detection, resolution and quantitation of cathinone enantiomers in horse blood plasma and urine. Anal. Bioanal. Chem. 2021, 413, 2147–2161. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M.; Benavente, F. Enantiodetermination of R,S-3,4-methylenedioxypyrovalerone in urine samples by high pressure in-line solid-phase extraction capillary electrophoresis-mass spectrometry. Talanta 2021, 225, 121994. [Google Scholar] [CrossRef]
- Naegele, E. Making Your LC Method Compatible with Mass Spectrometry; Technical Overview; Agilent Technologies Inc.: Santa Clara, CA, USA, 2011; Available online: http://www.chem.agilent.com/Library/technicaloverviews/Public/5990--7413EN.pdf (accessed on 19 June 2021).


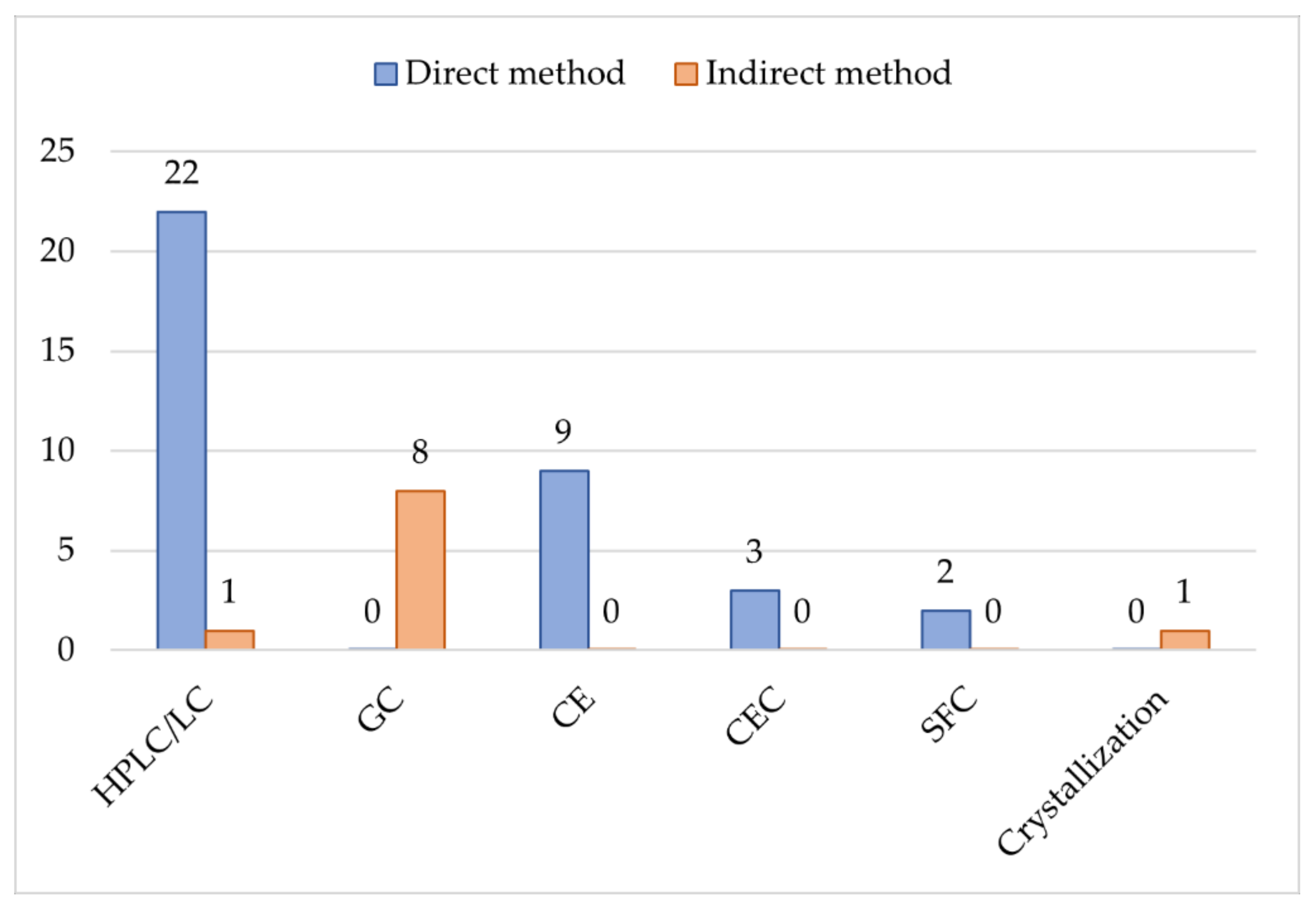
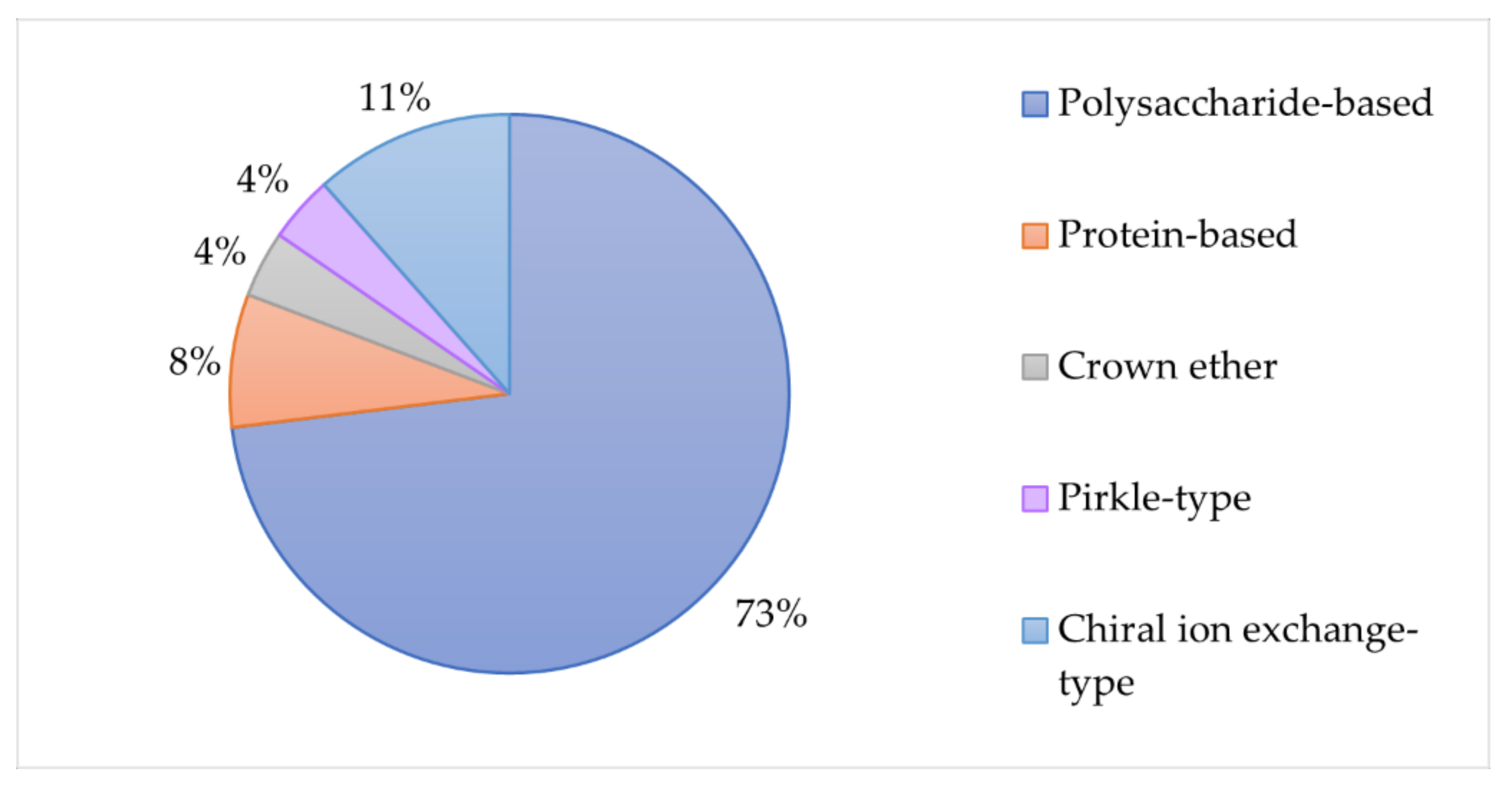
| Analyte | Sample | Method | Analytical conditions | Ref. |
|---|---|---|---|---|
| 3-FMC; 4-FEC; Ethcathinone; Buphedrone; 3-MMC; Pentedrone; 4-Methylbuphedrone; 3,4-DMMC; Methedrone; 2,3-MDMC; Eutylone; Pentylone | Urine and plasma | GC-MS (indirect method) | Achiral stationary phase: HP-5MS capillary column Derivatization with L-TPC | [106] |
| 2-AIMP; bk-iVP; 4-BMC; 4-CMC; 5-DBFPV; DL-4662; 4-FMC; 4F-PV8; Methedrone; 3-MeOMC; 3-MEC; 4-MEC; 2-MMC; 3-MMC; 4-MMC; 5-PPDi; α-PVP; 4-MeO-α-PVP; TH-PVP | Solid | HPLC-UV (direct method) | Polar organic mode CSP: Lux® Cellulose-2 column Mobile phase: ACN/IPA/DEA/FA (95:5:0.1:0.1) Flow rate: 1 mL/min UV detection: 254 nm | [107] |
| 4-FMC; 4-FEC; Nor-mephedrone; Buphedrone; 3-MMC; 3-Methylbuphedrone; 4-Methylbuphedrone; 3-EMC; 3-EEC; 4-EEC; 3,4-DMEC; 2,3-MDMC; Butylone; Pentylone | Urine and plasma | GC-MS (indirect method) | Achiral stationary phase: HP-5MS Ultra-Inert capillary column Derivatization with L-TPC Flow rate: 0.8 mL/min | [108] |
| 4-MMC; 3-MMC; 2-MMC; 3,4-DMMC; 4-MeOMC; 3-MeOMC; 3-CMC; 4-CMC; 4-EMC; Mexedrone; 4-FMC; 3-FMC; 2-FMC; 4-BMC; Buphedrone; 4-Methylbuphedrone; Pentedrone; 3-CEC; 4-CEC; N-Ethyl-Buphedrone; N-Ethyl-Hexedrone; Amfepramone; 4-MEC; 3-MEC; Methylone; Dimethylone; Butylone; N,N-Dimethylbutylone; Pentylone; Ethylone; 5-ME; N-Ethylpentylone; MDPV; MD-PHP; bk-IVP; 5-DBFPV; DOMC; 5-PPDI; TH-PVP; 4-MC; α-PPP; M-PPP; 4-MPrC; 4-MeO-α-PVP; 4-Cl-PVP; Naphyrone | Hydrochloride salts | HPLC-UV (direct method) | NPLC mode CSP: Lux® i-cellulose-5 column Mobile phase :Hex/IPA/DEA (95:5:0.1), Flow rate: 1 mL/min UV detection: 254 nm | [109] |
| Methcathinone; 4-MMC and 3-MMC | Solid | CE (direct method) | BGE: phosphate buffer I (H3PO4/NaH2PO4, pH 3.0), acetic buffer (CH3COOH/CH3COONa, pH 5.0), and phosphate buffer II (NaH2PO4/Na2HPO4, pH 8.7), all of 50 mM ionic strength with different CD additives DAD: set at 200 nm | [110] |
| Pentedrone | Powder | HPLC-UV (direct method) | NPLC mode CSP: Chiralpak® AS-H column Mobile phase: Hex/IPA (97:3) Flow rate: 2 mL/min UV detection: 254 nm | [111] |
| Methylone | Powder | HPLC-UV (direct method) | NPLC mode CSP: Chiralpak® AS-H column Mobile phase: Hex/IPA (85:15, v/v) Flow rate: 2 mL/min UV detection: 254 nm | [111] |
| 4-MC; 2-MMC; 3-MMC; 4-MMC; 3,4-DMMC; 3-MeO-MC; Methedrone; 3-CMC; 4-CMC; 4-EMC; Mexedrone; 2-FMC; 3-FMC; 4-FMC; 4-BMC; Buphedrone; 4-Methylbuphedrone; Pentedrone; Amfepramone; 3-CEC; 4-CEC; DL-4662; N-Ethylhexedrone; 3-MEC; 4-MEC; Bupropione; 4-MPD; N-Ethylbuphedrone; N-Ethylpentedrone; Ethylone; N-Ethylpentylone; 5-ME; bk-Ivp; 5-DBFPV; DOMC; 5-PPDi; 4-MBC; Methylone; 2-AIMP; Dimethylone; Butylone; N-Benzylnorbutylone; N,N-Dimethylbutylone; Pentylone; PV8; 4-F-PV8; α-PVP; 4-Cl-PVP; 4F-PVP; 4-MeO-α-PVP; PV9; α-PPP; M-PPP; α-PIHP; 4F-PHP; Naphyrone; MDPV; MDPHP | Solid | CE (direct method) | BGE: 10 mM of a β-CD derivative, 10 mM sodium phosphate adjusted with diluted phosphoric acid (pH 2.5) DAD: set at 209 nm | [112] |
| Dimethylone; α-PPP; N,N-DMC; 2-Methyl-α-PPP; 4-Ethyl-N,N-DMC; 3-Methyl-α-PPP; 3,4-MD-α-PPP; 4′-MeO-α-PPP; 4′-Methyl-α-PHP; Diethylcathinone; 4-Methyl PBP; α-PVP; α-PBP; 4′-Methyl-α-PPP; 3-Methyl PBP; 3,4-MDPBP; N-Ethyl-N-Methylcathinone; 2-Methyl PBP; 4-Meo-N,N-DMC | Blood and urine | HPLC-UV (direct method) | CSP: Astec® Cellulose DMP column Mobile phase: Hex/IPA/TEA (99.0:1.0:0.1) Flow rate: 0.5 mL/min UV detection: 270 nm | [113] |
| Dimethylone; N,N-DMC; 2-Methyl-α-PPP; 4-Ethyl-N,N-DMC; 4′-MeO-α-PPP; 3,4-MDPBP; 2-Methyl PBP | Blood and urine | HPLC-UV (direct method) | Direct chiral separation: CSP: Amylose-based Chiralpak® AS-H Mobile phase: Hex/IPA/TEA (99.0:1.0:0.1) Flow rate: 0.5 mL/min UV detection: 270 nm | [113] |
| MDPV; Mephedrone; Methylephedrine | Urine | SPE-CE (direct method) | BGE: aqueous solution of 70 mM of monosodium phosphate, adjusted to pH 2.5 with concentrated phosphoric acid, containing 8 mM 2-hydroxypropil-β-CD and 5 mM β-CD DAD: set at 200 nm | [114] |
| Cathinone | Catha edulis | GC-MS (indirect method) | Achiral stationary phase: HP-5 MSI capillary column Derivatization with MCF Flow rate: 1 mL/min | [115] |
| Mephedrone; Butylone: Flephedrone; Methylone; Methedrone | River water | LC-HRMS (direct method) | RPLC mode CSP: Chiralpak® CBH column Mobile phase: 1 mM ammonium acetate buffer/MeOH (98:2) Flow rate: 0.4 mL/min | [116] |
| Nor-Mephedrone; 3-MMC; 4-MMC; 3,4-DMMC; 3-MeO-MC; Methedrone; 3-CMC; 4-CMC; 4-EMC; Mexedrone; 2-FMC; 3-FMC; 4-FMC; 4-BMC; Buphedrone; 4-Methylbuphedrone; Pentedrone; 3-CEC; 4-CEC; DL-4662; 3-MEC; 4-MEC; Ethcathinone; 4-MPD; N-ethylbuphedrone; N-ethylpentedrone; 4-ethylcathinone; Methylone; 2-AIMP; Dimethylone; Butylone; N,N-dimethylbutylone; Pentylone; Ethylone; 5-ME; bk-iVP; 5-DBFPV; DOMC; 5-PPDi | Hydrochloride salts | HPLC-UV (direct method) | CSP: Phenomenex Lux® AMP Mobile phase: ammonium bicarbonate (5 mM) adjusted to pH 11.3 with conc. ammonium hydroxide/ ACN (70:30) Flow rate: 0.5 mL/min UV detection: 230 nm | [117] |
| 4-MC; 4-MMC; 3-MMC; 3,4-DMMC; 3-CMC; 4-CMC; 4-EMC; 4-FMC; 4-BMC; Buphedrone; 4-Methylbuphedrone; Ethcathinone; 4-EEC; 3-CEC; 4-CEC; N-Ethylbuphedrone; N-Ethylpentedrone; DL-4662; 3-MEC; 4-MEC; N-Propcathinone; 4-MPC; 4-CPRC; Dimethylone; 2-AIMP; Butylone; Ethylone; 5-ME; N-Ethylpentylone; 4-MBC; bk-IVP; DOMC; 4-CDC | Hydrochloride salts | HPLC-UV (direct method) | NPLC mode CSP: Trefoil® CEL1 column with cellulose tris-(3,5-dimethylphenyl-carbamate) Mobile phase: Hex/ButOH/DEA (100:0.3:0.2) Flow rate: 1 mL/min UV detection: 230 nm | [118] |
| 4-MMC; 3-MMC; 2-MMC; Methedrone; 3-MeoMC; 4-CMC; 4-BMC; 4-FMC; 4-EMC; Mexedrone; Buphedrone; 4-Methylbuphedrone; Pentedrone; 3-CEC; 4-CEC; 4 MPD; N-Ethyl-pentedrone; DL-4662; 4-EEC; 4-MPC; 4-CPRC; 4-F-PVP; 4 M-PHP; N-Ethylpentylone; MDPV and TH-PVP | Solid | HPLC-UV (direct method) | NPLC mode CSP: Lux® i-Amylose-1 column Mobile phase: Hex/IPA/DEA (90:10:0.1) Flow rate: 1 mL/min UV detection: 254 nm | [119] |
| 4-MC; 3-CMC; 2-FMC; 3-FMC: 3,4-DMMC; N-ethyl-buphedrone; N-ethyl-hexedrone; Amfepramone; 3-MEC; 4-MEC; Ethcathinone; 4-ClC; 4-Chlorbutcathinone; α-PPP; M-PPP; α-PVP; 4-Cl-PVP; 4-MPrC; 4-MeO-α-PVP; Naphyrone; Methylone; Dimethylone; 2-AIMP; Butylone; N,N-Dimethylbutylone; Pentylone; 5-ME; Ethylone; MD-PHP; bk-iVP; 5-DBFPV; DOMC; 5-PPDI; 5-BPDI; 4-MBC | Solid | HPLC-UV (direct method) | NPLC mode CSP: Lux® i-Amylose-1 column Mobile phase: Hex/IPA/DEA (99:10:0.1) Flow rate: 1 mL/min UV detection: 254 nm | [119] |
| 4-MMC; 3,4-DMMC; 4-EMC; 4-MEC; 4-Methylbuphedrone; Buphedrone; N-Ethylbuphedrone; Pentedrone; Pyrovalerone; bk-PMA; bk-PMMA; Methylone; Ethylone; Butylone; Pentylone; MDPV; MDPBP; Naphyrone; 4F-NEB; 4F-MABP; 2-FMC; 4-FMC; 4-CMC; 4-BMC | Solid | SFC-MS (direct method) | CSP: Chiralpak® ZWIX (+) and Chiralpak® ZWIX (−) Mobile phase: MeOH/H2O/FA (90:10:1) using a gradient elution method Flow rate: 1 mL/min | [120] |
| Methylone and ethylone | Crystals | LC- MS/MS (direct method) | RPLC mode CSP: Lux® AMP polysaccharide-based chiral column Mobile phase: MeOH with a decreasing concentration gradient from 95% to 85% Flow rate: 0.48 mL/min | [121] |
| 2-FMC; 2-FEC; Buphedrone; 3-MMC; 4-MEC; 3-MethylBP; 2,4-DMMC; 4-Methyl-α-ethylaminobutiophenone; 3,4-DMEC; 4-BMC; Butylone | Urine | GC-NCI-MS/MS (indirect method) | Achiral stationary phase: Agilent Ultra Inert capillary column Derivatization with MCF Flow rate: 1 mL/min | [122] |
| Mephedrone; Methylone; 4-Methylephedrine; MDPV | Urine | EKS-CE (direct method) | BGE: 70 mM of monosodium phosphate, 8 mM of 2-hydroxypropyl β-CD and 5 mM of β-CD (adjusted to pH 2.5 with concentrated phosphoric acid) DAD: set at 220 nm | [123] |
| Mephedrone and its metabolites | Hydrochloride salts | CE (direct method) | BGE: 50 mmol/L Phosphate buffer; pH 2.75; 7.5 mmol/L CM-β-CD DAD: set at 258; 236 or 214 nm | [124] |
| Cathinone | Horse plasma and urine | HPLC-MS/MS (indirect method) | RPLC mode Achiral stationary phase: fused core HALO-C18 column Mobile phase: 5 mM ammonium formate/0.1 % FA in H2O/ACN, in linear gradient Derivatization with DNFP-L-V Flow rate: 0.3 mL/min | [125] |
| MDPV | Urine | SPE-CE-MS (direct method) | BGE: 10 mM ammonium acetate aqueous solution (pH 7) with 0.5% (m/v) of sulphated-α-CD Sheath liquid: IPA/H2O/FA 60:40:0.25 (v/v) Flow rate: 3.3 μL/min | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.S.; Silva, B.; Pinho, P.G.d.; Remião, F.; Fernandes, C. Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules 2022, 27, 2057. https://doi.org/10.3390/molecules27072057
Almeida AS, Silva B, Pinho PGd, Remião F, Fernandes C. Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules. 2022; 27(7):2057. https://doi.org/10.3390/molecules27072057
Chicago/Turabian StyleAlmeida, Ana Sofia, Bárbara Silva, Paula Guedes de Pinho, Fernando Remião, and Carla Fernandes. 2022. "Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods" Molecules 27, no. 7: 2057. https://doi.org/10.3390/molecules27072057
APA StyleAlmeida, A. S., Silva, B., Pinho, P. G. d., Remião, F., & Fernandes, C. (2022). Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules, 27(7), 2057. https://doi.org/10.3390/molecules27072057









