HPLC-FLD-Based Method for the Detection of Sulfonamides in Organic Fertilizers Collected from Poland
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Procedure
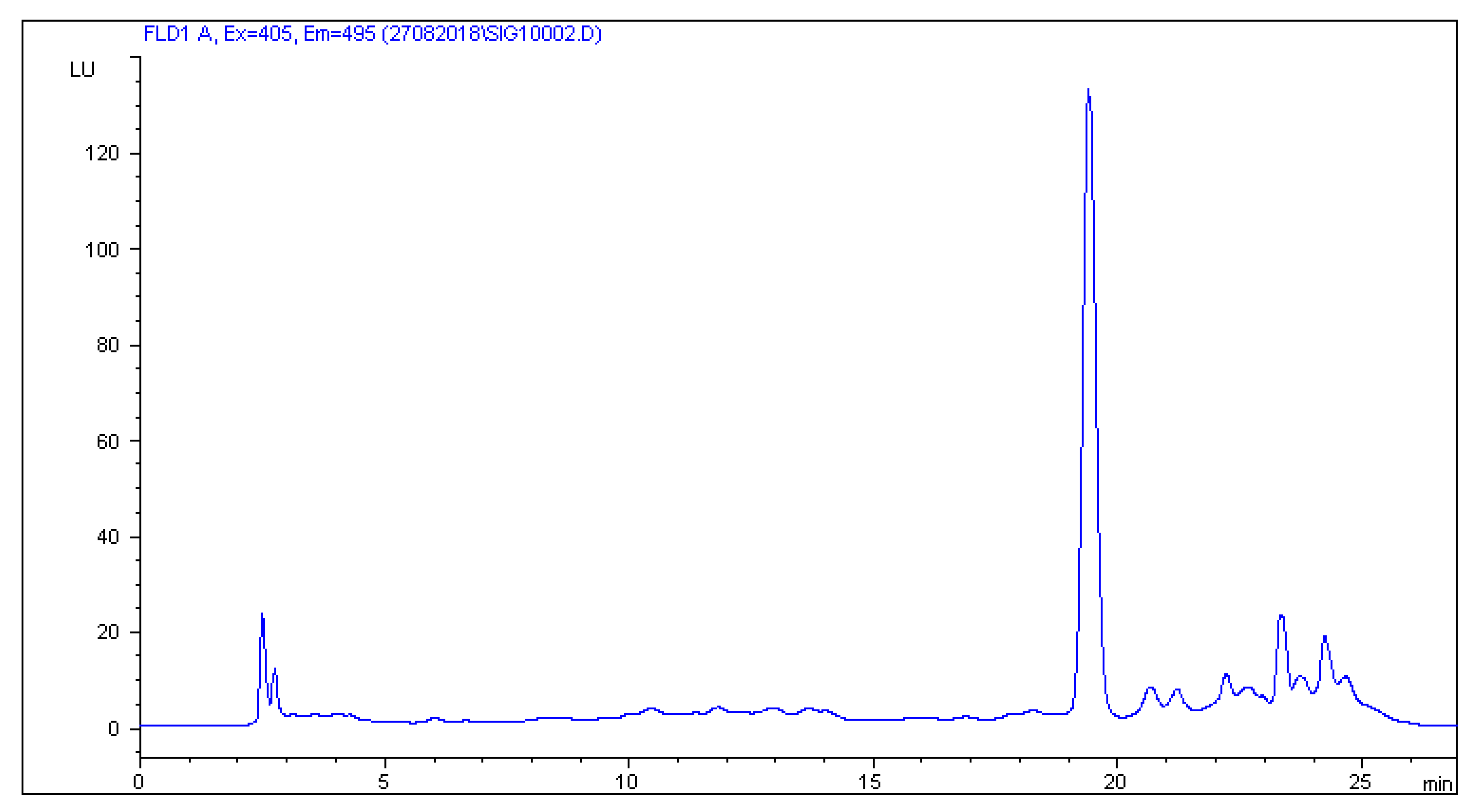
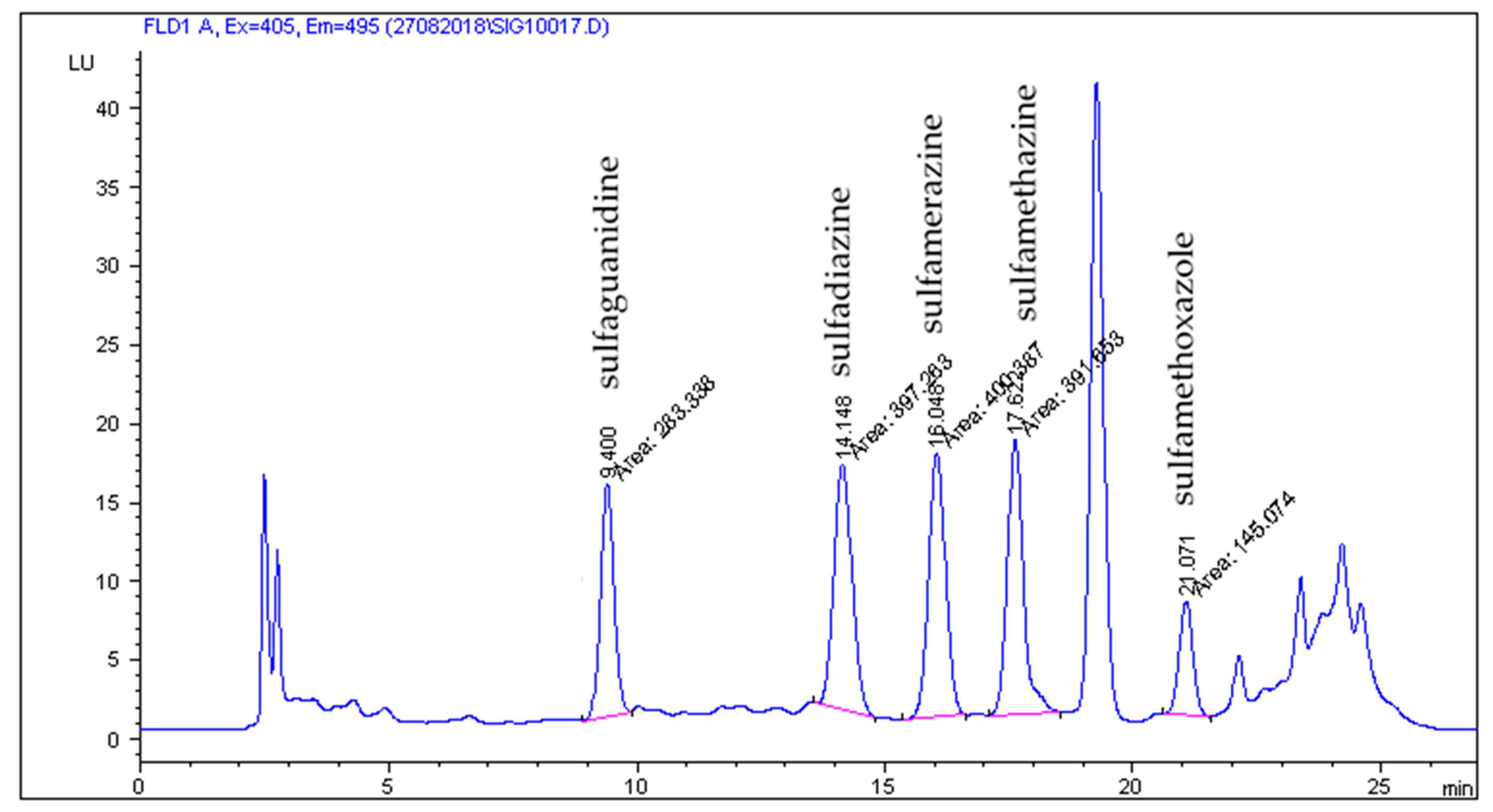
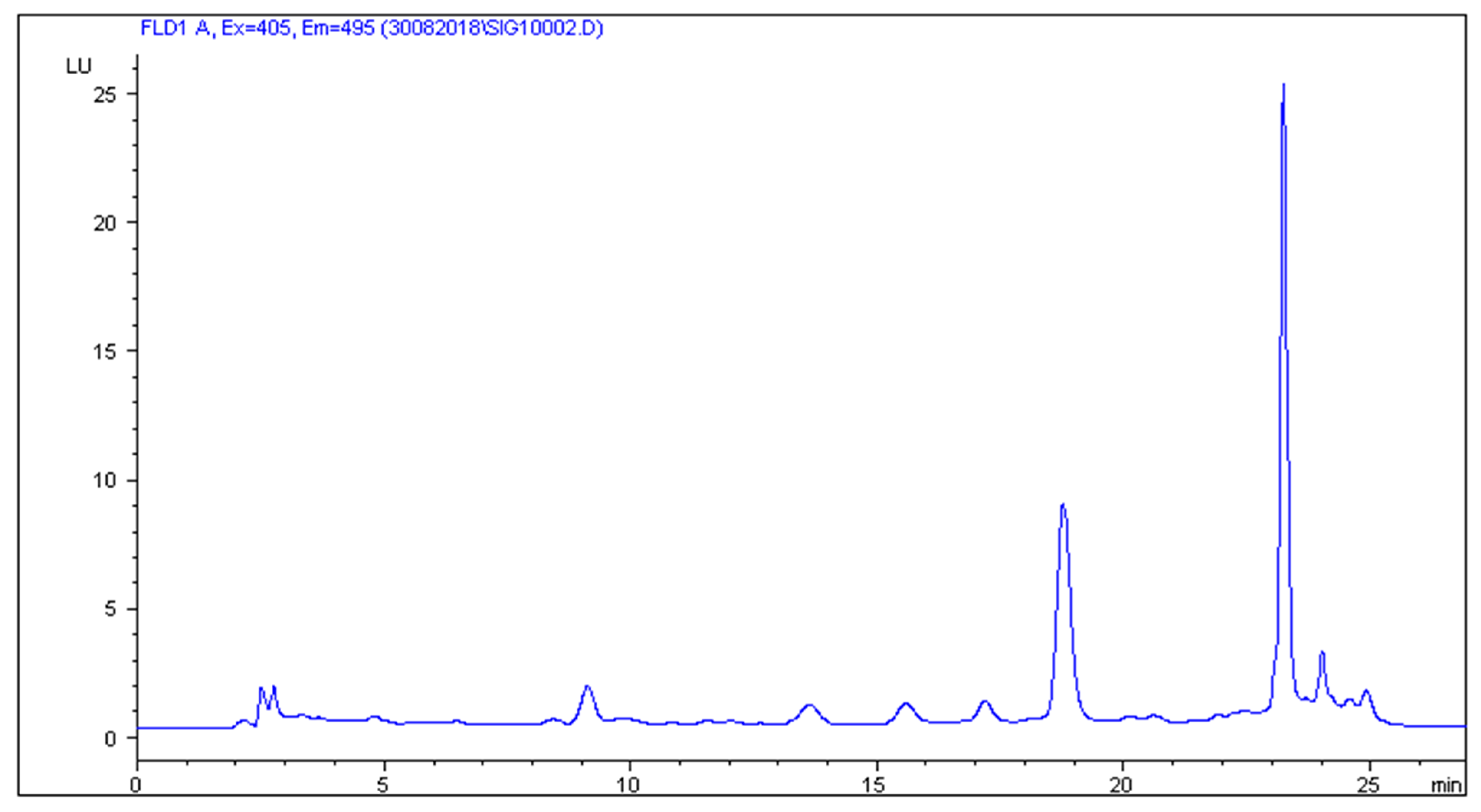
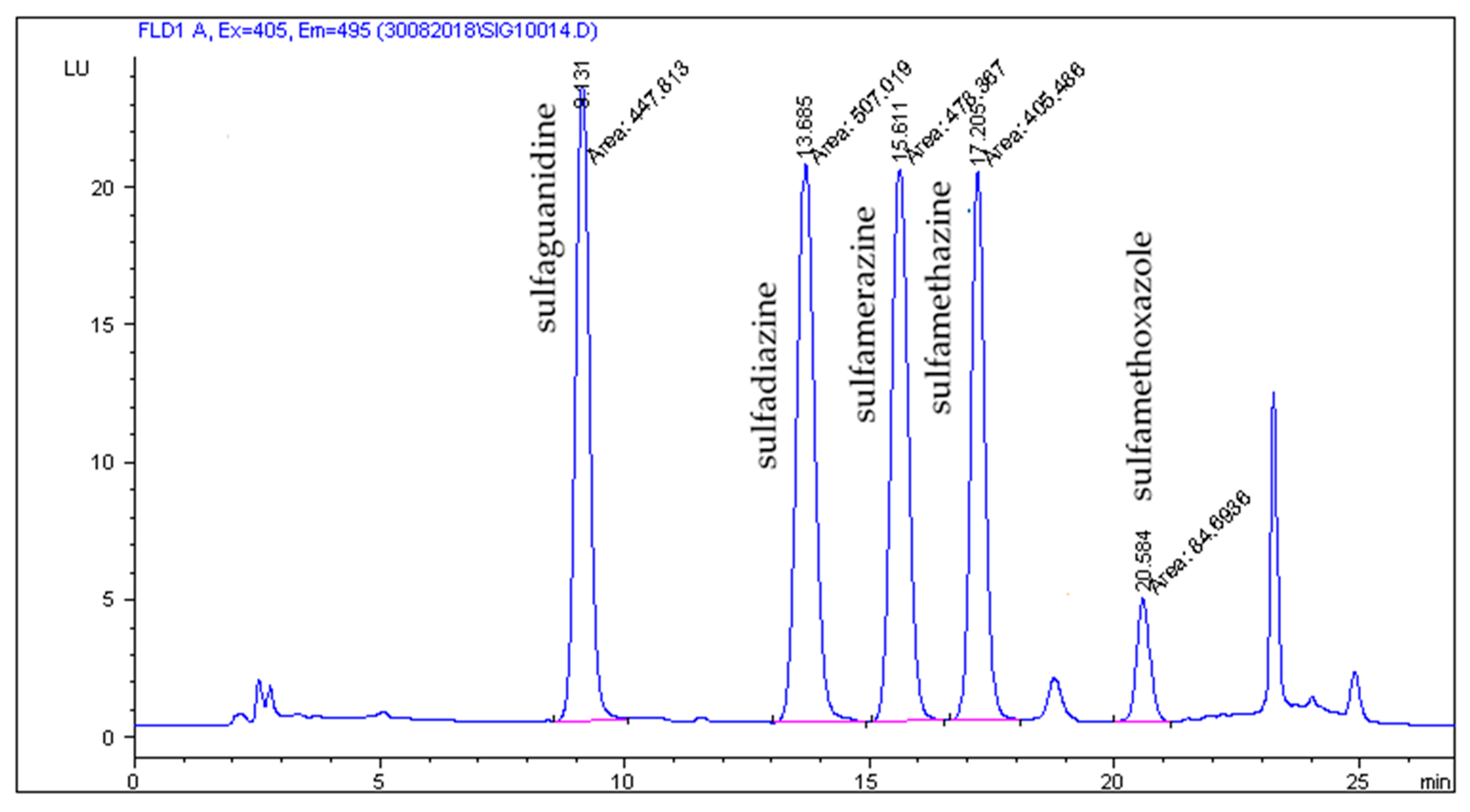
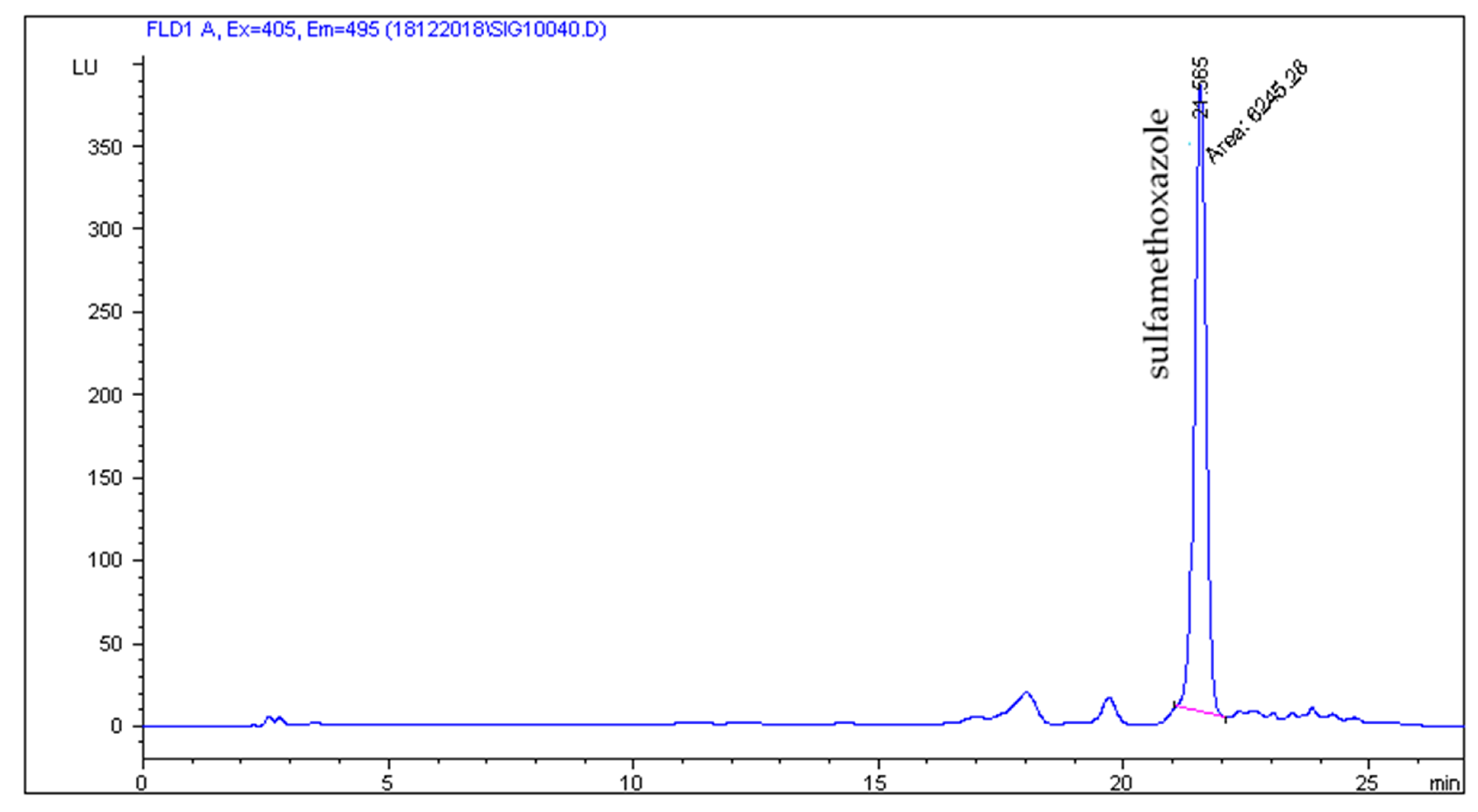
2.2. HPLC-FLD Analysis
2.3. Method Validation Results
2.4. Presence of SAs in Animal Feces, Liquid Manure, and Digestate
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Reagents
3.3. Extraction
3.4. Clean-Up
3.5. HPLC-FLD Analysis
3.6. Validation Studies
3.7. Fertilizer Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
Sample Availability
References and Note
- Zuccato, E.; Bagnati, R.; Fioretti, F.; Natangelo, M.; Calamari, D.; Fanelli, R. Environmental loads and detection of pharmaceuticals in Italy. In Pharmaceuticals in the Environment—Sources, Fate, Effects and Risks; Kummerer, K., Ed.; Springer: Berlin, Germany, 2001; pp. 19–27. [Google Scholar]
- Berendsen, B.J.A.; Wegh, R.S.; Memolink, J.; Zuidema, T.; Stolker, L.A.M. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils—A review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Gou, M.; Hu, H.W.; Zhang, Y.J.; Wang, J.T.; Hayden, H.; Tang, Y.Q.; Heet, J.Z. Aerobic composting reduces antibiotic resistance genes in cattle manure and the resistome dissemination in agricultural soils. Sci. Total Environ. 2018, 612, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://urpl.gov.pl/pl/produkty-lecznicze-weterynaryjne-0 (accessed on 12 February 2022).
- Anon: Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin.
- Karci, A.; Balcioglu, I.A. Investigation of the tetracycline, sulfonamide and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef] [PubMed]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Klaus, K. Antibiotics in the aquatic environment: A review—Part II. Chemosphere 2009, 75, 417–434. [Google Scholar]
- Martinez-Carballo, E.; Gonzalez-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 162, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces in China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhao, T.; Liu, Q.; He, J. Residual veterinary antibiotics in pig excreta after oral administration of sulfonamides. Environ. Geochem. Health 2016, 38, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qian, S.; Luo, Y. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soil of Northern China. Environ. Sci. Poll. Res. 2015, 22, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Xian-Gang, H.; Yi, L.; Qi-Xing, Z.; Lin, X. Determination of thirteen antibiotics residues in manure by solid phase extraction and high performance liquid chromatography. Chin. J. Anal. Chem. 2008, 36, 1162–1166. [Google Scholar]
- Jansen, L.J.M.; van de Schans, M.G.M.; de Boer, D.; Bongers, I.E.A.; Schmitt, G.; Hoeksma, P.; Berendsen, B.J.A. A new extraction procedure to abate the burden of non-extractable antibiotic residues in manure. Chemosphere 2019, 224, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wu, H.; Wang, J.; Zhang, H.; Zhang, Z.; Zhang, Y.; Lin, H.; Ma, J. Occurrence of trace elements and antibiotics in amnure-based fertilizers from the Zhejiang Province of China. Sci. Total Environ. 2016, 559, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.S.; Aga, D.S. Enhancing extraction and detection of veterinary antibiotics in solid and liquid fractions of manure. J. Environ. Qual. 2016, 45, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Müller, S.R.; McArdell, C.S.; Alder, A.C.; Sute, M.J.-F. Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography-mass spectrometry. J. Chromatogr. A 2002, 952, 111–120. [Google Scholar] [CrossRef]
- Patyra, E.; Przeniosło-Siwczyńska, M.; Kwiatek, K. Determination of sulfonamides in feeds by high-performance liquid chromatogarphy after fluorescamine precolumn derivatization. Molecules 2019, 24, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anon: Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32021R0808 (accessed on 12 February 2022).
- VICH GL49: Studies to Evaluate the Metabolism and Residues Kinetics of Veterinary Drugs in Human Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies. 2016. Available online: https://www.ema.europa.eu/en/vich-gl49-studies-evaluate-metabolism-residue-kinetics-veterinary-drugs-food-producing-animals (accessed on 12 February 2022).
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How Safe is Chicken Litter for Land Application as an Organic Fertilizer? A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Fan, L.; Dong, Y.; Li, D.; Zhao, L.; Yuan, X.; Wang, L. Determination of sulfonamide residues in livestock and poultry manure using carbon nanotube extraction combined with UPLC-MS/MS. Food Anal. Methods 2021, 14, 641–652. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Recovery (%) | Repeatability (%) | Within-Laboratory Reproducibility (%) | Limit of Detection (µg/kg) | Limit of Quantification (µg/kg) | Decision Limit (µg/kg) | Detection Capability (µg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration Levels (µg/kg) | Concentration Levels (µg/kg) | Concentration Levels (µg/kg) | |||||||||||
| 50 | 200 | 1000 | 50 | 200 | 1000 | 50 | 200 | 1000 | |||||
| Sulfaguanidine | 103.20 | 119.63 | 81.20 | 13.68 | 11.58 | 9.20 | 16.03 | 11.50 | 8.77 | 13.53 | 26.02 | 33.87 | 53.36 |
| Sulfadiazine | 99.53 | 117.66 | 82.96 | 14.08 | 11.70 | 9.88 | 16.84 | 10.79 | 8.51 | 14.14 | 29.27 | 38.78 | 60.15 |
| Sulfamerazine | 101.07 | 120.94 | 82.53 | 12.57 | 8.28 | 8.12 | 16.48 | 9.83 | 7.94 | 17.93 | 34.88 | 45.58 | 65.69 |
| Sulfamethazine | 100.93 | 121.16 | 81.76 | 14.75 | 4.36 | 9.24 | 13.42 | 9.73 | 8.80 | 15.11 | 31.33 | 38.44 | 60.28 |
| Sulfamethoxazole | 90.53 | 115.14 | 84.93 | 9.78 | 12.30 | 7.64 | 18.55 | 13.76 | 6.83 | 16.71 | 35.88 | 53.80 | 73.12 |
| Analyte | Recovery (%) | Repeatability (%) | Within-Laboratory Reproducibility (%) | Limit of Detection (µg/kg) | Limit of Quantification (µg/kg) | Decision Limit (µg/kg) | Detection Capability (µg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration Levels (µg/kg) | Concentration Levels (µg/kg) | Concentration Levels (µg/kg) | |||||||||||
| 50 | 200 | 1000 | 50 | 200 | 1000 | 50 | 200 | 1000 | |||||
| Sulfaguanidine | 77.00 | 86.16 | 80.42 | 14.59 | 11.26 | 15.41 | 13.52 | 15.29 | 11.48 | 17.11 | 30.21 | 53.61 | 71.37 |
| Sulfadiazine | 96.86 | 108.71 | 80.04 | 11.77 | 10.64 | 14.91 | 11.55 | 13.61 | 14.30 | 15.59 | 33.30 | 33.31 | 56.67 |
| Sulfamerazine | 78.80 | 101.43 | 79.14 | 12.59 | 11.89 | 16.11 | 18.82 | 14.48 | 14.46 | 20.75 | 40.38 | 34.04 | 54.02 |
| Sulfamethazine | 96.46 | 107.85 | 77.34 | 15.27 | 12.41 | 17.34 | 14.54 | 14.78 | 15.57 | 16.62 | 34.00 | 37.19 | 59.56 |
| Ssulfamethoxazole | 125.63 | 91.89 | 85.77 | 15.45 | 13.35 | 10.39 | 15.94 | 16.54 | 12.37 | 23.30 | 41.97 | 67.63 | 92.00 |
| Analyte (µg/kg) | ||||||
|---|---|---|---|---|---|---|
| Sulfaguanidine | Sulfadizine | Sulfamerazine | Sulfamethazine | Sulfamethoxazol | Standard Deviation | |
| Pig feces | ||||||
| S1 | - | - | - | - | 11,070 | 245 |
| S2 | - | - | - | - | 6877 | 313 |
| S3 | - | - | - | - | 158 | 18 |
| Poultry feces | ||||||
| S4 | - | - | - | - | 2000 | 90 |
| Time (min) | Phase A 0.08% Acetic Acid in Water (%) | Phase B Acetonitrile (%) | Phase C Methanol (%) |
|---|---|---|---|
| 0 | 48 | 10 | 42 |
| 10 | 48 | 10 | 42 |
| 15 | 41 | 10 | 49 |
| 17 | 41 | 10 | 49 |
| 20 | 18 | 40 | 42 |
| 22 | 48 | 10 | 42 |
| 27 | 48 | 10 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osiński, Z.; Patyra, E.; Kwiatek, K. HPLC-FLD-Based Method for the Detection of Sulfonamides in Organic Fertilizers Collected from Poland. Molecules 2022, 27, 2031. https://doi.org/10.3390/molecules27062031
Osiński Z, Patyra E, Kwiatek K. HPLC-FLD-Based Method for the Detection of Sulfonamides in Organic Fertilizers Collected from Poland. Molecules. 2022; 27(6):2031. https://doi.org/10.3390/molecules27062031
Chicago/Turabian StyleOsiński, Zbigniew, Ewelina Patyra, and Krzysztof Kwiatek. 2022. "HPLC-FLD-Based Method for the Detection of Sulfonamides in Organic Fertilizers Collected from Poland" Molecules 27, no. 6: 2031. https://doi.org/10.3390/molecules27062031
APA StyleOsiński, Z., Patyra, E., & Kwiatek, K. (2022). HPLC-FLD-Based Method for the Detection of Sulfonamides in Organic Fertilizers Collected from Poland. Molecules, 27(6), 2031. https://doi.org/10.3390/molecules27062031






