Multi-Elemental Analysis as a Tool to Ascertain the Safety and the Origin of Beehive Products: Development, Validation, and Application of an ICP-MS Method on Four Unifloral Honeys Produced in Sardinia, Italy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Pre-Treatment
2.2. Validation of the ICP-MS Method
2.3. Honey Analysis
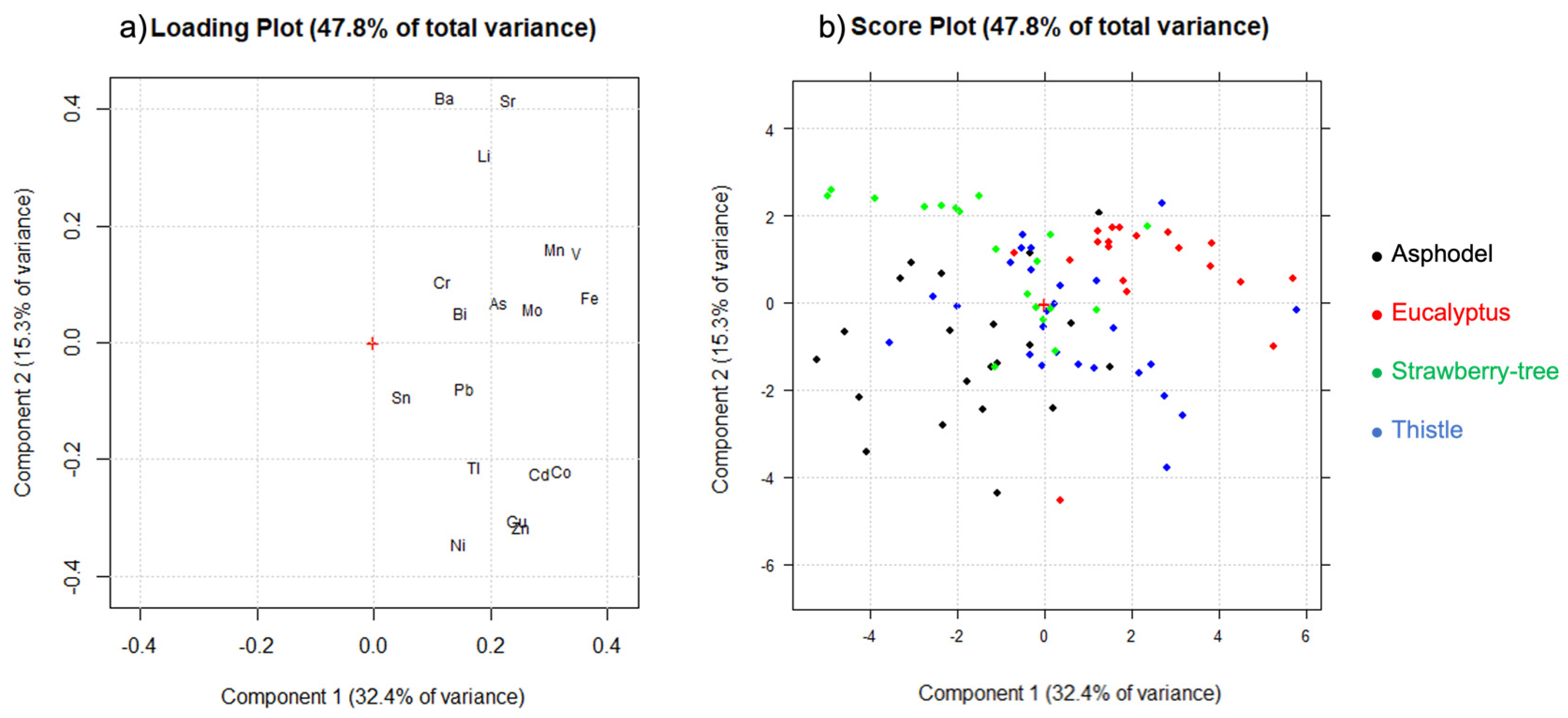
2.4. Chemometric Analysis
3. Materials and Methods
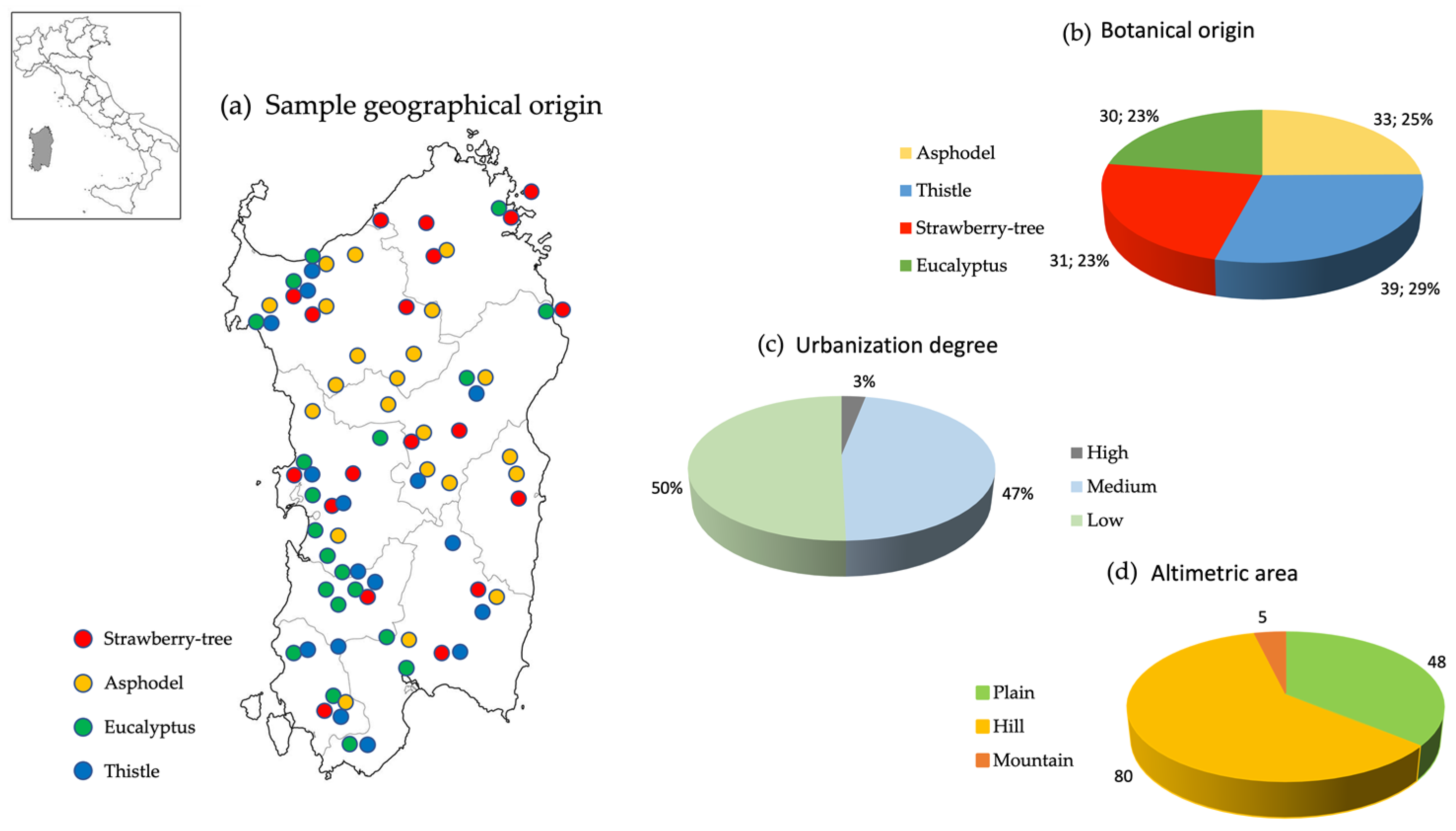
3.1. Honey Samples
3.2. Reagents and Standard Solutions
3.3. Instrumentation
3.4. ICP-MS Method Assessment, Quality Control and Assurance
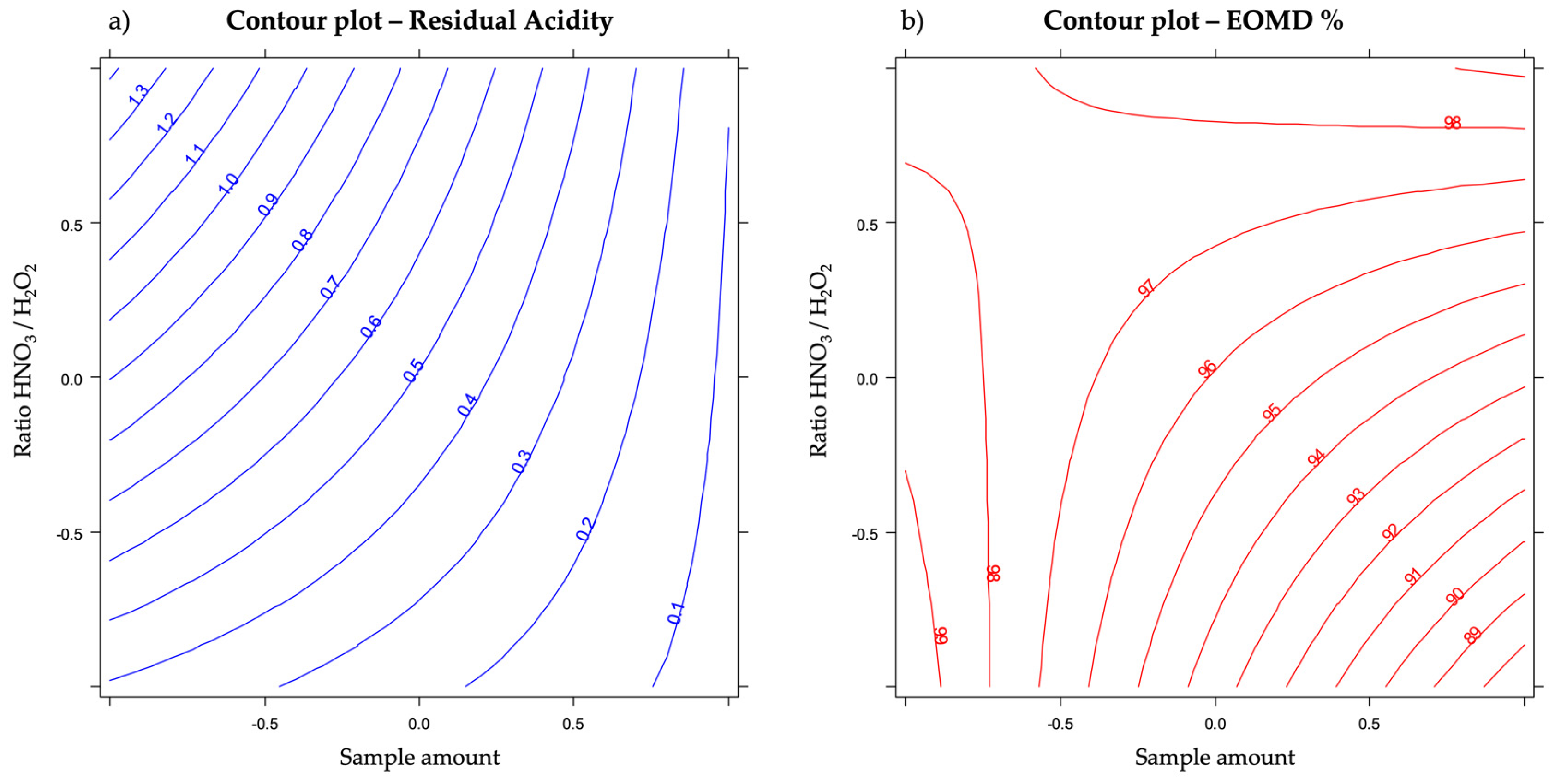
3.5. Optimization of the Composition of the Acidic/Oxidizing Mixture
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Melo, A.A.M.; De Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2017, 57, 5–37. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, N.; Fatima, N.; Shahjalal, H.M.; Gan, S.H.; Khalil, I. Chemical composition and biological properties of aromatic compounds in honey: An overview. J. Food Biochem. 2017, 41, e12405. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Istituto di Servizi per il Mercato Agricolo Alimentare (Ismea). Il settore apistico: Analisi di mercato. Roma. 2019. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/9685 (accessed on 16 February 2022).
- Floris, I.; Satta, A.; Ruiu, L. Honeys of Sardinia (Italy). J. Apic. Res. 2007, 46, 198–209. [Google Scholar] [CrossRef]
- Floris, I.; Pusceddu, M.; Satta, A. The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings. Antioxidants 2021, 10, 506. [Google Scholar] [CrossRef]
- Zhang, P.; Georgiou, C.A.; Brusic, V. Elemental metabolomics. Brief. Bioinform. 2017, 19, 524–536. [Google Scholar] [CrossRef]
- Danezis, G.P.; Georgiou, C.A. Elemental metabolomics: Food elemental assessment could reveal geographical origin. Curr. Opin. Food Sci. 2022, 44, 100812. [Google Scholar] [CrossRef]
- Voyslavov, T.; Mladenova, E.; Balkanska, R. A New Approach for Determination of the Botanical Origin of Monofloral Bee Honey, Combining Mineral Content, Physicochemical Parameters, and Self-Organizing Maps. Molecules 2021, 26, 7219. [Google Scholar] [CrossRef] [PubMed]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2019, 33, 50–76. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Satta, A.; Verdinelli, M.; Ruiu, L.; Buffa, F.; Salis, S.; Sassu, A.; Floris, I. Combination of beehive matrices analysis and ant biodiversity to study heavy metal pollution impact in a post-mining area (Sardinia, Italy). Environ. Sci. Pollut. Res. 2012, 19, 3977–3988. [Google Scholar] [CrossRef]
- Goretti, E.; Pallottini, M.; Rossi, R.; La Porta, G.; Gardi, T.; Goga, B.C.; Elia, A.C.; Galletti, M.; Moroni, B.; Petroselli, C.; et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ. Pollut. 2019, 256, 113388. [Google Scholar] [CrossRef]
- Kastrati, G.; Paçarizi, M.; Sopaj, F.; Tašev, K.; Stafilov, T.; Mustafa, M. Investigation of Concentration and Distribution of Elements in Three Environmental Compartments in the Region of Mitrovica, Kosovo: Soil, Honey and Bee Pollen. Int. J. Environ. Res. Public Health 2021, 18, 2269. [Google Scholar] [CrossRef]
- Rashed, M.N.; El-Haty, M.T.A.; Mohamed, S.M. Bee honey as environmental indicator for pollution with heavy metals. Toxicol. Environ. Chem. 2009, 91, 389–403. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Ślebioda, M.; Namiesnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Ćirić, J.; Spirić, D.; Baltić, T.; Lazić, I.B.; Trbović, D.; Parunović, N.; Petronijević, R.; Đorđević, V. Honey Bees and Their Products as Indicators of Environmental Element Deposition. Biol. Trace Elem. Res. 2020, 199, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Yayinie, M.; Atlabachew, M. Multi-element Analysis of Honey from Amhara Region-Ethiopia for Quality, Bioindicator of Environmental Pollution, and Geographical Origin Discrimination. Biol. Trace Elem. Res. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fodor, P.; Molnar, E. Honey as an environmental indicator: Effect of sample preparation on trace element determination by ICP-AES. Mikrochim. Acta 1993, 112, 113–118. [Google Scholar] [CrossRef]
- Tuzen, M.; Silici, S.; Mendil, D.; Soylak, M. Trace element levels in honeys from different regions of Turkey. Food Chem. 2007, 103, 325–330. [Google Scholar] [CrossRef]
- Perna, A.M.; Grassi, G.; Gambacorta, E.; Simonetti, A. Minerals content in Basilicata region (southern Italy) honeys from areas with different anthropic impact. Int. J. Food Sci. Technol. 2021, 56, 4465–4472. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/73 of 16 January 2018 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Mercury Compounds in or on Certain Products. Available online: https://eur-lex.europa.eu/eli/reg/2018/73/oj (accessed on 16 February 2022).
- Commission Regulation (EU) 2015/1005 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2015/1005/oj (accessed on 16 February 2022).
- Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gallmann, P. Minerals in honey: Environmental, geographical and botanical aspects. J. Apic. Res. 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical origin and botanical type honey authentication through elemental metabolomics via chemometrics. Food Chem. 2020, 338, 127936. [Google Scholar] [CrossRef]
- Quinto, M.; Miedico, O.; Spadaccino, G.; Paglia, G.; Mangiacotti, M.; Li, D.; Centonze, D.; Chiaravalle, A.E. Characterization, chemometric evaluation, and human health-related aspects of essential and toxic elements in Italian honey samples by inductively coupled plasma mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 25374–25384. [Google Scholar] [CrossRef]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grainger, M.N.C.; Klaus, H.; Hewitt, N.; French, A.D. Investigation of inorganic elemental content of honey from regions of North Island, New Zealand. Food Chem. 2021, 361, 130110. [Google Scholar] [CrossRef]
- Di Bella, G.; Potortì, A.G.; Beltifa, A.; Ben Mansour, H.; Nava, V.; Turco, V.L. Discrimination of Tunisian Honey by Mineral and Trace Element Chemometrics Profiling. Foods 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.E.; Finoia, M.G.; Fontana, L.; Mele, G.; Botrè, F.; Iavicoli, I. Characterization of Argentine honeys on the basis of their mineral content and some typical quality parameters. Chem. Cent. J. 2014, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; D’Ilio, S.; Caroli, S. Honey as a Candidate Reference Material for Trace Elements. J. AOAC Int. 2001, 84, 1972–1975. [Google Scholar] [CrossRef] [Green Version]
- Caroli, S.; Forte, G.; Alessandrelli, M.; Cresti, R.; Spagnoli, M.; D’Ilio, S.; Pauwels, J.; Kramer, G. A pilot study for the production of a certified reference material for trace elements in honey. Microchem. J. 2000, 67, 227–233. [Google Scholar] [CrossRef]
- Lovaković, B.T.; Lazarus, M.; Karačonji, I.B.; Jurica, K.; Semren, T.Ž.; Lušić, D.; Brajenović, N.; Pelaić, Z.; Pizent, A. Multi-elemental composition and antioxidant properties of strawberry tree (Arbutus unedo L.) honey from the coastal region of Croatia: Risk-benefit analysis. J. Trace Elem. Med. Biol. 2018, 45, 85–92. [Google Scholar] [CrossRef]
- Pohl, P.; Bielawska-Pohl, A.; Dzimitrowicz, A.; Jamroz, P.; Welna, M.; Lesniewicz, A.; Szymczycha-Madeja, A. Recent achievements in element analysis of bee honeys by atomic and mass spectrometry methods. Trends Anal. Chem. 2017, 93, 67–77. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Conti, M.E.; Marconi, E.; Massimi, L.; Canepari, S. Effectiveness of Different Sample Treatments for the Elemental Characterization of Bees and Beehive Products. Molecules 2020, 25, 4263. [Google Scholar] [CrossRef]
- Caroli, S. Determination of essential and potentially toxic trace elements in honey by inductively coupled plasma-based techniques. Talanta 1999, 50, 327–336. [Google Scholar] [CrossRef]
- Sadowska, M.; Hyk, W.; Ruszczyńska, A.; Roszak, A.; Mycka, A.; Krasnodębska-Ostręga, B. Statistical evaluation of the effect of sample preparation procedure on the results of determinations of selected elements in environmental samples. Honey bees as a case study. Chemosphere 2021, 279, 130572. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.I.; Müller, C.C.; de Souza, J.P.; Muller, A.L.; Enders, M.S.; Doneda, M.; Frohlich, A.C.; Iop, G.D.; Anschau, K.F. Green microwave-assisted wet digestion method of carbohydrate-rich foods with hydrogen peroxide using single reaction chamber and further elemental determination using ICP-OES and ICP-MS. Microchem. J. 2017, 134, 257–261. [Google Scholar] [CrossRef]
- Leme, A.B.P.; Bianchi, S.; Carneiro, R.L.; Nogueira, A.R.A. Optimization of Sample Preparation in the Determination of Minerals and Trace Elements in Honey by ICP-MS. Food Anal. Methods 2013, 7, 1009–1015. [Google Scholar] [CrossRef]
- Oliveira, S.; Alves, C.N.; Morte, E.S.B.; Junior, A.D.F.S.; Araujo, R.G.O.; Santos, D.C.M.B. Determination of essential and potentially toxic elements and their estimation of bioaccessibility in honeys. Microchem. J. 2019, 151, 104221. [Google Scholar] [CrossRef]
- Spano, N.; Ciulu, M.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Scanu, R.; Sanna, G. Chemical characterization of a traditional honey-based Sardinian product: Abbamele. Food Chem. 2008, 108, 81–85. [Google Scholar] [CrossRef]
- Spano, N.; Casula, L.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Scanu, R.; Tapparo, A.; Sanna, G. An RP-HPLC determination of 5-hydroxymethylfurfural in honey: The case of strawberry tree honey. Talanta 2006, 68, 1390–1395. [Google Scholar] [CrossRef]
- Salis, S.; Spano, N.; Ciulu, M.; Floris, I.; Pilo, M.; Sanna, G. Electrochemical Determination of the “Furanic Index” in Honey. Molecules 2021, 26, 4115. [Google Scholar] [CrossRef]
- Sanna, G.; Pilo, M.I.; Piu, P.C.; Tapparo, A.; Seeber, R. Determination of heavy metals in honey by anodic stripping voltammetry at microelectrodes. Anal. Chim. Acta 2000, 415, 165–173. [Google Scholar] [CrossRef]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC determination of water-soluble vitamins in honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef]
- Spano, N.; Piras, I.; Ciulu, M.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Sanna, G. Reversed-Phase Liquid Chromatographic Profile of Free Amino Acids in Strawberry-tree (Arbutus unedo L.) Honey. J. AOAC Int. 2009, 92, S73–S84. [Google Scholar] [CrossRef] [Green Version]
- Ciulu, M.; Floris, I.; Nurchi, V.M.; Panzanelli, A.; Pilo, M.I.; Spano, N.; Sanna, G. A Possible Freshness Marker for Royal Jelly: Formation of 5-Hydroxymethyl-2-furaldehyde as a Function of Storage Temperature and Time. J. Agric. Food Chem. 2015, 63, 4190–4195. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Floris, I.; Nurchi, V.M.; Panzanelli, A.; Pilo, M.I.; Spano, N.; Sanna, G. HPLC determination of pantothenic acid in royal jelly. Anal. Methods 2013, 5, 6682–6685. [Google Scholar] [CrossRef]
- Ciulu, M.; Serra, R.; Caredda, M.; Salis, S.; Floris, I.; Pilo, M.I.; Spano, N.; Panzanelli, A.; Sanna, G. Chemometric treatment of simple physical and chemical data for the discrimination of unifloral honeys. Talanta 2018, 190, 382–390. [Google Scholar] [CrossRef]
- Ciulu, M.; Oertel, E.; Serra, R.; Farre, R.; Spano, N.; Caredda, M.; Malfatti, L.; Sanna, G. Classification of Unifloral Honeys from SARDINIA (Italy) by ATR-FTIR Spectroscopy and Random Forest. Molecules 2020, 26, 88. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.A. Detection and quantification limits: Origins and historical overview. Anal. Chim. Acta 1999, 391, 127–134. [Google Scholar] [CrossRef]
- Horwitz, W. Evaluation of analytical methods used for regulation of foods and drugs. Anal. Chem. 1982, 54, 67–76. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists (AOAC). Guidelines for Standard Method Performance Requirements, AOAC International. 2016. Available online: https://www.aoac.org/wp-content/uploads/2019/08/app_f.pdf (accessed on 16 February 2022).
- Oliveri, P.; Malegori, C.; Simonetti, R.; Casale, M. The impact of signal pre-processing on the final interpretation of analytical outcomes—A tutorial. Anal. Chim. Acta 2018, 1058, 9–17. [Google Scholar] [CrossRef]
- Oliveri, P.; Malegori, C.; Mustorgi, E.; Casale, M. Qualitative pattern recognition in chemistry: Theoretical background and practical guidelines. Microchem. J. 2020, 162, 105725. [Google Scholar] [CrossRef]
- Aru, A.; Baldaccini, P.; Vacca, A.; Delogu, G.; Dessena, M.A.; Melis, R.T.; Vacca, S.; Madrau, S. Carta dei Suoli Della Sardegna. 1991, Cagliari. Available online: http://www.sardegnaportalesuolo.it/cartografia/carte-dei-suoli/carta-dei-suoli-della-sardegna-scala-1250000.html (accessed on 16 February 2022).
- Istituto Nazionale di Statistica (Istat). Principali Statistiche Geografiche sui Comuni. Roma. 2022. Available online: https://www.istat.it/it/archivio/156224 (accessed on 16 February 2022).
- Food and Agriculture Organization of the United Nations (FAO). Standard Operating Procedure for Soil Organic Carbon. Walkley-Black Method: Titration and Colorimetric Method. 2020, Rome. Available online: https://www.fao.org/publications/card/en/c/CA7471EN/ (accessed on 16 February 2022).
- Spanu, A.; Langasco, I.; Valente, M.; Deroma, M.A.; Spano, N.; Barracu, F.; Pilo, M.I.; Sanna, G. Tuning of the Amount of Se in Rice (Oryza sativa) Grain by Varying the Nature of the Irrigation Method: Development of an ICP-MS Analytical Protocol, Validation and Application to 26 Different Rice Genotypes. Molecules 2020, 25, 1861. [Google Scholar] [CrossRef] [Green Version]
- Spanu, A.; Valente, M.; Langasco, I.; Leardi, R.; Orlandoni, A.M.; Ciulu, M.; Deroma, M.A.; Spano, N.; Barracu, F.; Pilo, M.I.; et al. Effect of the irrigation method and genotype on the bioaccumulation of toxic and trace elements in rice. Sci. Total Environ. 2020, 748, 142484. [Google Scholar] [CrossRef]
- Mara, A.; Langasco, I.; Deidda, S.; Caredda, M.; Meloni, P.; Deroma, M.; Pilo, M.I.; Spano, N.; Sanna, G. ICP-MS Determination of 23 Elements of Potential Health Concern in Liquids of e-Cigarettes. Method Development, Validation, and Application to 37 Real Samples. Molecules 2021, 26, 6680. [Google Scholar] [CrossRef] [PubMed]
- Langasco, I.; Barracu, F.; Deroma, M.A.; López-Sánchez, J.F.; Mara, A.; Meloni, P.; Pilo, M.I.; Estrugo, S.; Sanna, G.; Spano, N.; et al. Assessment and validation of ICP-MS and IC-ICP-MS methods for the determination of total, extracted and speciated arsenic. Application to samples from a soil-rice system at varying the irrigation method. J. Environ. Manag. 2021, 302, 114105. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.A.; de Abreu, A.T.; Nascimento, N.D.O.; Froes, R.; Antonini, Y.; Nalini, H.A.; de Lena, J.C. Evaluation of matrix effect on the determination of rare earth elements and As, Bi, Cd, Pb, Se and In in honey and pollen of native Brazilian bees (Tetragonisca angustula—Jataí) by Q-ICP-MS. Talanta 2017, 162, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, C.A.; Pedrotti, M.F.; Silva, J.S.; Barin, J.S.; Nóbrega, J.A.; Flores, E.M.M. Microwave-assisted digestion methods: Towards greener approaches for plasma-based analytical techniques. J. Anal. At. Spectrom. 2017, 32, 1448–1466. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Software). Available online: http://gruppochemiometria.it/index.php/software (accessed on 16 February 2022).

| Step | Time (min) | Temperature (°C) | ||
|---|---|---|---|---|
| 1st | Heating | 25 | 240 | Initial pressure: 40 bar Final temperature: <40 °C Pressure release rate: 8 bar/min Rack: 15 positions Vessel: volume 15 cm3, PTFE Sample amount: 0.7 g of honey Reagents: 0.5 cm3 HNO3, 3 cm3 of H2O2, and 4 cm3 H2O |
| 2nd | Holding | 10 | 240 | |
| 3rd | Cooling | ~30 | <40 | |
| Element | LoD a | LoQ a | Calibration Range b | Repeatability c | Intermediate Precision d | Trueness, |
|---|---|---|---|---|---|---|
| (μg kg−1) | (μg kg−1) | (μg dm−3) | CV (%) | CV (%) | Recovery (% ± s e) | |
| Ag | 5 | 17 | 0.1–50 | 4 | 8 | 106 ± 5 |
| As | 2 | 7 | 0.1–50 | 4 | 6 | 92 ± 1 |
| Ba | 20 | 70 | 1–250 | 1 | 3 | 90 ± 20 |
| Be | 0.4 | 1.3 | 0.02–50 | 4 | 13 | 103 ± 1 |
| Bi | 0.1 | 0.3 | 0.005–50 | 4 | 12 | 85 ± 7 |
| Cd | 0.3 | 1.0 | 0.01–50 | 4 | 8 | 117 ± 1 |
| Co | 0.3 | 1.0 | 0.01–50 | 8 | 9 | 99 ± 1 |
| Cr | 7 | 23 | 0.1–50 | 4 | 5 | 97 ± 1 |
| Cu | 20 | 70 | 1–100 | 4 | 11 | 107 ± 4 |
| Fe | 30 | 100 | 1–100 | 4 | 17 | 105 ± 15 |
| Hg | 6 | 20 | 0.1–50 | 4 | 13 | 130 ± 10 |
| Li | 2 | 7 | 0.1–500 | 4 | 14 | 96 ± 1 |
| Mn | 8 | 27 | 0.1–500 | 3 | 3 | 107 ± 4 |
| Mo | 0.7 | 2.3 | 0.04–50 | 4 | 8 | 94 ± 1 |
| Ni | 3 | 10 | 0.1–100 | 6 | 18 | 95 ± 2 |
| Pb | 3 | 10 | 0.1–250 | 4 | 6 | 92 ± 1 |
| Sb | 0.7 | 2.3 | 0.04–50 | 4 | 9 | 115 ± 1 |
| Sn | 2.1 | 6.9 | 0.1–50 | 5 | 5 | 103 ± 2 |
| Sr | 3 | 10 | 0.1–100 | 3 | 7 | 103 ± 1 |
| Te | 1.2 | 3.9 | 0.04–50 | 4 | 21 | 108 ± 3 |
| Tl | 0.04 | 0.13 | 0.005–50 | 7 | 5 | 96 ± 1 |
| V | 0.2 | 0.7 | 0.01–50 | 4 | 6 | 94 ± 2 |
| Zn | 40 | 130 | 1–500 | 12 | 16 | 101 ± 1 |
| Element | Asphodel (n = 33) Asphodel spp. | Eucalyptus (n = 30) Eucalyptus spp. | Strawberry Tree (n = 31) Arbutus unedo L. | Thistle (n = 39) Galactites tomentosa | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Ag | <5 | <5–<17 | <5 | <5–<5 | <5 | <5–<17 | <5 | <5–<5 |
| As | <3 | <2–9 | <6 | <2–19 | <2 | <2–<7 | <4 | <2–<7 |
| Ba | 180 | <20–1600 | 340 | 80–690 | 550 | <70–2600 | 300 | <70–1800 |
| Be | <0.4 | <0.4–<0.4 | <0.4 | <0.4–<0.4 | <0.4 | <0.4–<0.4 | <0.4 | <0.4–<0.4 |
| Bi | <0.2 | <0.1–1.6 | <0.2 | <0.1–1.2 | <0.2 | <0.1–0.8 | <0.6 | <0.1–10.7 |
| Cd | <0.7 | <0.3–2.5 | 1.7 | <0.3–9.2 | <0.3 | <0.3–<1 | 1.4 | <0.3–5.2 |
| Co | 2.5 | <1–10.2 | 7.6 | <1–109 | 1.8 | <0.3–9.7 | 5.1 | <1–15.5 |
| Cr | <11 | <7–24 | <17 | <7–26 | <15 | <7–24 | <8 | <7–24 |
| Cu | 90 | <20–250 | 170 | <70–630 | 90 | <20–250 | 180 | <70–1030 |
| Fe | 160 | <100–660 | 570 | <100–1600 | 180 | <100–630 | 340 | 120–820 |
| Hg | <6 | <6–<6 | <6 | <6–<6 | <6 | <6–<10 | <6 | <6–<20 |
| Li | <2 | <2–14 | 11 | <2–30 | 280 | <2–8500 | <5 | <2–22 |
| Mn | 190 | 40–770 | 2600 | 140–5100 | 330 | <27–4900 | 330 | 40–3300 |
| Mo | <1.8 | <0.7–3.6 | <2.3 | <0.7–3.9 | 1.8 | <0.7–3.8 | <2.1 | <0.7–3.7 |
| Ni | 19 | <3–170 | 22 | <3–122 | 12 | <3–33 | 24 | <10–220 |
| Pb | 23 | <3–400 | 16 | <3–95 | <10 | <3–90 | <9 | <3–30 |
| Sb | <0.8 | <0.7–<2.3 | <1.3 | <0.7–4.7 | <0.9 | <0.7–<2.3 | <0.8 | <0.7–2.8 |
| Sn | 44 | <2.1–210 | 30 | <6.9–110 | 43 | <2.1–200 | 46 | <7.1–240 |
| Sr | 38 | <3–174 | 180 | 20–290 | 140 | 22–350 | 98 | 18–420 |
| Te | <1.5 | <1.2–6.8 | <1.2 | <1.2–<1.2 | <1.2 | <1.2–<1.2 | <1.2 | <1.2–<3.9 |
| Tl | <0.13 | <0.04–0.4 | 0.3 | <0.04–2.2 | <0.13 | <0.04–1.4 | 0.18 | <0.04–1.3 |
| V | <0.4 | <0.2–<0.7 | 4.1 | <0.7–12.8 | <0.6 | <0.2–1.8 | 1.3 | <0.7–5.6 |
| Zn | 550 | <130–1400 | 660 | 330–1400 | 400 | <40–1200 | 800 | 300–2000 |
| Total (mg kg−1) | 1.30 | 0.56–2.70 | 2.20 | 0.74–6.60 | 2.10 | 0.76–10.30 | 4.70 | 1.00–8.40 |
| Element | Tunisia (n = 3) [36] | Argentina (n = 1) [37] | Italy (n = 1) [38] | Unknown (n = 1) [39] | Sardinia (n = 29) (This Work) |
|---|---|---|---|---|---|
| Ag | <5; <5–<5 | ||||
| As | 19.08 | <10 | 5.99 | 3.33 | <6; <2–19 |
| Ba | 340; 80–690 | ||||
| Be | <10 | <0.4; <0.4–<0.4 | |||
| Bi | <0.2; <0.1–1.2 | ||||
| Cd | <0.01 | <10 | 0.592 | 0.70 | 1.7; <0.3–9.2 |
| Co | 10 | 7.6; <1–109 | |||
| Cr | 130 | <10 | 1.50 | 2.73 | <17; <7–26 |
| Cu | 800 | 120 | 219 | 140 | 170; <70–630 |
| Fe | 7100 | 3380 | 1008 | 914 | 570; <100–1600 |
| Hg | <0.75 | <6; <6–<6 | |||
| Li | 11; <2–30 | ||||
| Mn | 1250 | 8840 | 1009 | 1976 | 2600; 140–5100 |
| Mo | <2.3; <0.7–3.9 | ||||
| Ni | 220 | 50 | 11.3 | 8.04 | 22; <3–122 |
| Pb | 250 | 10 | 5.00 | 141 | 16; <3–95 |
| Se | 130 | 10 | 5.60 | ||
| Sb | 100 | <1.3; <0.7–4.7 | |||
| Sn | 7.85 | 30; <6.9–110 | |||
| Sr | 180; 20–290 | ||||
| Ti | 610 | ||||
| Te | <1.2; <1.2–<1.2 | ||||
| Tl | <10 | 0.3; <0.04–2.2 | |||
| U | <10 | ||||
| V | 50 | <10 | 3.36 | 4.1; <0.7–12.8 | |
| Zn | 2060 | 550 | 791 | 414 | 660; 330–1400 |
| % Correctly Classified | Asphodel | Eucalyptus | Strawberry Tree | Thistle | Total |
|---|---|---|---|---|---|
| Cross- validation | 61.9 | 84.2 | 78.9 | 76.9 | 75.5 |
| Prediction a | 91.7 | 81.5 | 75.0 | 100.0 | 87.1 |
| Asphodel | Eucalyptus | Strawberry Tree | Thistle | |
|---|---|---|---|---|
| Asphodel | 11 | 0 | 0 | 1 |
| Eucalyptus | 1 | 9 | 0 | 1 |
| Strawberry tree | 1 | 0 | 9 | 2 |
| Thistle | 0 | 0 | 0 | 13 |
| Experiment | Sample Amount (g) | Ratio HNO3/H2O2 | X1 a | X2 a | Residual Acidity (mol dm−3) | EOMD% |
|---|---|---|---|---|---|---|
| 1 | 0.50 | 0.17 | −1 | −1 | 0.39 | 99.7 |
| 2 | 1.00 | 0.17 | +1 | −1 | 0.06 | 87.2 |
| 3 | 0.50 | 1.33 | −1 | +1 | 1.41 | 97.7 |
| 4 | 1.00 | 1.33 | +1 | +1 | 0.16 | 99.1 |
| 5 | 0.75 | 0.56 | 0 | 0 | 0.48 | 95.8 |
| 6 | 0.75 | 0.56 | 0 | 0 | 0.39 | 96.4 |
| Residual Acidity | EOMD% | |||
|---|---|---|---|---|
| Coefficient | Coefficient Value | Significance a | Coefficient Value | Significance a |
| b0 | 0.49 | *** | 95.9 | *** |
| b1 | −0.41 | ** | −2.8 | * |
| b2 | 0.27 | * | 2.5 | * |
| b12 | −0.25 | * | 3.5 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mara, A.; Deidda, S.; Caredda, M.; Ciulu, M.; Deroma, M.; Farinini, E.; Floris, I.; Langasco, I.; Leardi, R.; Pilo, M.I.; et al. Multi-Elemental Analysis as a Tool to Ascertain the Safety and the Origin of Beehive Products: Development, Validation, and Application of an ICP-MS Method on Four Unifloral Honeys Produced in Sardinia, Italy. Molecules 2022, 27, 2009. https://doi.org/10.3390/molecules27062009
Mara A, Deidda S, Caredda M, Ciulu M, Deroma M, Farinini E, Floris I, Langasco I, Leardi R, Pilo MI, et al. Multi-Elemental Analysis as a Tool to Ascertain the Safety and the Origin of Beehive Products: Development, Validation, and Application of an ICP-MS Method on Four Unifloral Honeys Produced in Sardinia, Italy. Molecules. 2022; 27(6):2009. https://doi.org/10.3390/molecules27062009
Chicago/Turabian StyleMara, Andrea, Sara Deidda, Marco Caredda, Marco Ciulu, Mario Deroma, Emanuele Farinini, Ignazio Floris, Ilaria Langasco, Riccardo Leardi, Maria I. Pilo, and et al. 2022. "Multi-Elemental Analysis as a Tool to Ascertain the Safety and the Origin of Beehive Products: Development, Validation, and Application of an ICP-MS Method on Four Unifloral Honeys Produced in Sardinia, Italy" Molecules 27, no. 6: 2009. https://doi.org/10.3390/molecules27062009
APA StyleMara, A., Deidda, S., Caredda, M., Ciulu, M., Deroma, M., Farinini, E., Floris, I., Langasco, I., Leardi, R., Pilo, M. I., Spano, N., & Sanna, G. (2022). Multi-Elemental Analysis as a Tool to Ascertain the Safety and the Origin of Beehive Products: Development, Validation, and Application of an ICP-MS Method on Four Unifloral Honeys Produced in Sardinia, Italy. Molecules, 27(6), 2009. https://doi.org/10.3390/molecules27062009












