A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects
Abstract
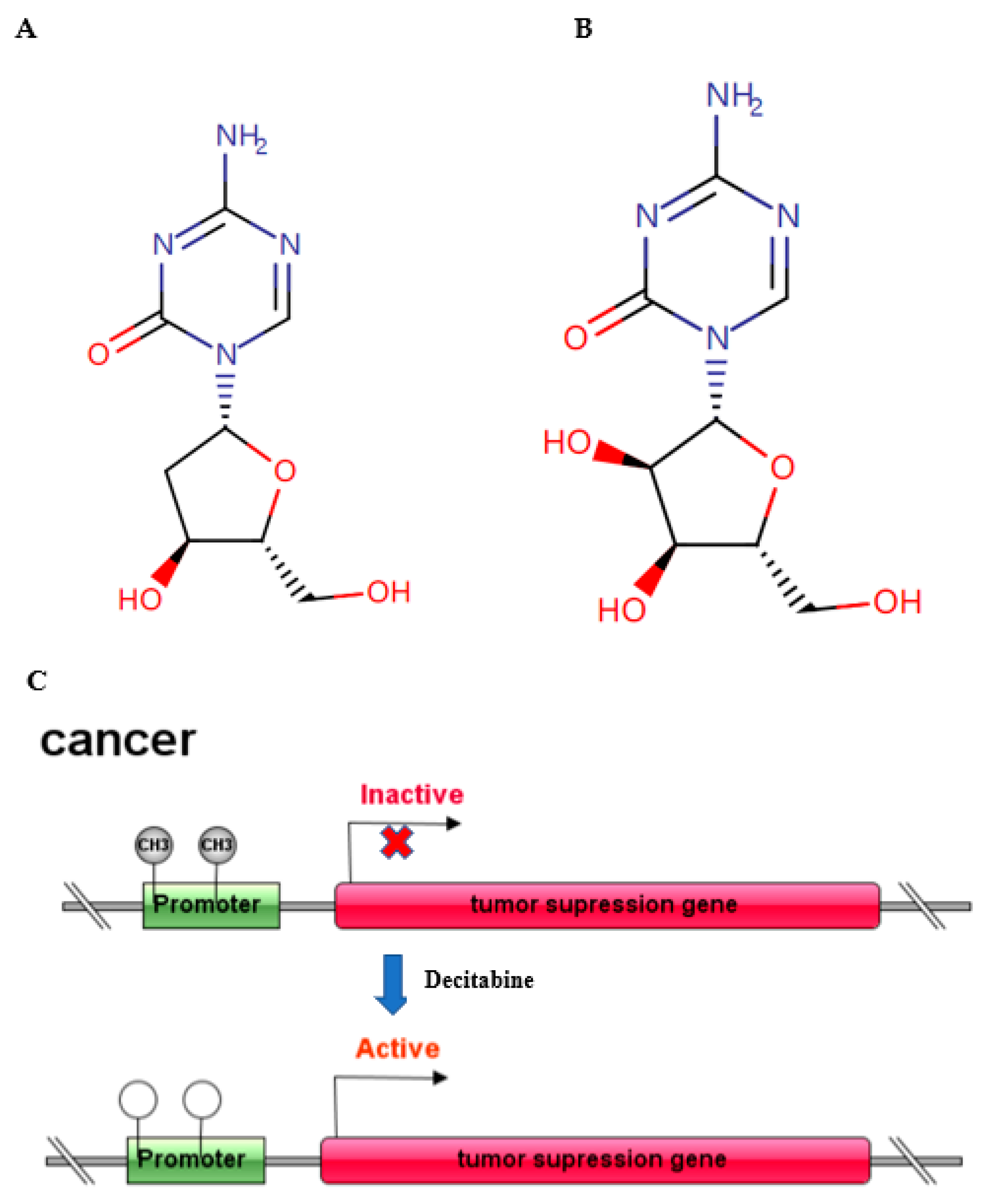
1. Background
2. DNA Methylation in Antiviral Innate Immune Response
3. Decitabine and Its Role in the Immune Modulation of Viral Diseases
4. Antiviral Effects of Decitabine
4.1. HIV
4.2. Hepatitis Viruses
4.3. Other Viruses
5. Conclusions and Future Prospects
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAC | 5-aza-2′-deoxycytidine, decitabine |
| DNMTs | DNA methyltransferases |
| MDS | myelodysplastic syndrome |
| HSV-1 | Herpes simplex virus type 1 |
| RIG-I | retinoic acid-inducible gene I |
| IRF-7 | interferon regulatory factor-7 |
| IRF-5 | interferon regulatory factor 5 |
| IFN | interferon |
| TLRs | Toll-like receptors |
| sPTL | spontaneous preterm labour |
| TNL | term not in labour |
| TL | term in labour |
| AZA | 5-azacytidine, azacytidine |
| DNMTis | DNMT inhibitors |
| ERVs | endogenous retroviruses |
| EOC | epithelial ovarian cancer |
| CLP | caecal ligation and puncture |
| GO | gene ontology |
| LTA | lipoteichoic acid |
| hOBs | stimulated human odontoblast-like cells |
| ERK | extracellular signal-regulated kinase |
| HIV | human immunodeficiency virus |
| AIDS | acquired immunodeficiency syndrome |
| MuLV | murine leukaemia virus |
| dNTP | deoxyribonucleoside triphosphate |
| HDACIs | histone deacetylase inhibitors |
| HBV | hepatitis B virus |
| ApoA1 | apolipoprotein A1 |
| CHB | chronic hepatitis B |
| HCVcc | HCV cell culture |
| E6AP | E6-associated protein |
| AcMNPV | Autographa californica nuclear polyhedrosis virus |
| B19V | human parvovirus B19 |
| EHV-1 | Equid herpesvirus-1 |
| FeLV | feline leukaemia virus |
| ACV | acyclovir |
| GCV | ganciclovir |
| AML | acute myelocytic leukaemia |
| EBV | Ebola virus |
| KSHV | herpesvirus |
References
- Mehdi, A.; Rabbani, S. Role of Methylation in Pro- and Anti-Cancer Immunity. Cancers 2021, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Au, W.-C.; Yeow, W.-S.; Hageman, N.; Pitha, P.M. Regulation of the Promoter Activity of Interferon Regulatory Factor-7 Gene. Activation by interferon snd silencing by hypermethylation. J. Biol. Chem. 2000, 275, 31805–31812. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, G.; Hagemann, S.; Aran, D.; Söhle, J.; Kulkarni, P.P.; Kaderali, L.; Hellman, A.; Winnefeld, M.; Lyko, F. Aging is associated with highly defined epigenetic changes in the human epidermis. Epigenet. Chromatin 2013, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.D.; Conneely, K.N. The role of DNA methylation and hydroxymethylation in immunosenescence. Ageing Res. Rev. 2019, 51, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef]
- Mackin, S.-J.; O’Neill, K.M.; Walsh, C.P. Comparison of DNMT1 inhibitors by methylome profiling identifies unique signature of 5-aza-2′deoxycytidine. Epigenomics 2018, 10, 1085–1101. [Google Scholar] [CrossRef]
- Saito, Y.; Nakaoka, T.; Sakai, K.; Muramatsu, T.; Toshimitsu, K.; Kimura, M.; Kanai, T.; Sato, T.; Saito, H. Inhibition of DNA Methylation Suppresses Intestinal Tumor Organoids by Inducing an Anti-Viral Response. Sci. Rep. 2016, 6, 25311. [Google Scholar] [CrossRef]
- Kaminskas, E.; Farrell, A.; Abraham, S.; Baird, A.; Hsieh, L.; Lee, S.; Leighton, J.K.; Patel, H.; Rahman, A.; Sridhara, R.; et al. Approval summary: Azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. 2005, 11, 3604–3608. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- Kimbrell, D.A.; Beutler, B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2001, 2, 256–267. [Google Scholar] [CrossRef]
- Morales-Nebreda, L.; McLafferty, F.; Singer, B. DNA methylation as a transcriptional regulator of the immune system. Transl. Res. 2019, 204, 1–18. [Google Scholar] [CrossRef]
- Gao, Z.-J.; Li, W.-P.; Mao, X.-T.; Huang, T.; Wang, H.-L.; Li, Y.-N.; Liu, B.-Q.; Zhong, J.-Y.; Renjie, C.; Jin, J. Single-nucleotide methylation specifically represses type I interferon in antiviral innate immunity. J. Exp. Med. 2021, 218, e20201798. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chuang, J.-H.; Wang, P.-W.; Lin, T.-K.; Wu, M.-T.; Hsu, W.-M.; Chuang, H.-C. 5-aza-2′-Deoxycytidine Induces a RIG-I-Related Innate Immune Response by Modulating Mitochondria Stress in Neuroblastoma. Cells 2020, 9, 1920. [Google Scholar] [CrossRef]
- Varanasi, S.K.; Reddy, P.B.J.; Bhela, S.; Jaggi, U.; Gimenez, F.; Rouse, B.T. Azacytidine Treatment Inhibits the Progression of Herpes Stromal Keratitis by Enhancing Regulatory T Cell Function. J. Virol. 2017, 91, e02367-16. [Google Scholar] [CrossRef]
- de Cubas, A.; Dunker, W.; Zaninovich, A.; Hongo, R.; Bhatia, A.; Panda, A.; Beckermann, K.E.; Bhanot, G.; Ganesan, S.; Karijolich, J.; et al. DNA hypomethylation promotes transposable element expression and activation of immune signaling in renal cell cancer. JCI Insight 2020, 5, e137569. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, T.; Lang, X.; Jia, S.; Yang, Y.; Zhu, C.; Wang, Y.; Feng, S.; Wang, C.; Zhang, P.; et al. Inhibiting DNA Methylation Improves Survival in Severe Sepsis by Regulating NF-κB Pathway. Front. Immunol. 2020, 11, 1360. [Google Scholar] [CrossRef]
- Rowles, D.L.; Tsai, Y.-C.; Greco, T.M.; Lin, A.; Li, M.; Yeh, J.; Cristea, I.M. DNA methyltransferase DNMT3A associates with viral proteins and impacts HSV-1 infection. Proteomics 2015, 15, 1968–1982. [Google Scholar] [CrossRef]
- Roulois, D.; Yau, H.L.; De Carvalho, D.D. Pharmacological DNA demethylation: Implications for cancer immunotherapy. OncoImmunology 2016, 5, e1090077. [Google Scholar] [CrossRef]
- Benakanakere, M.; Abdolhosseini, M.; Hosur, K.; Finoti, L.; Kinane, D. TLR2 Promoter Hypermethylation Creates Innate Immune Dysbiosis. J. Dent. Res. 2015, 94, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Chumble, A.A.; Washington, S.L.; Archer, K.J.; Sahingur, S.E.; Strauss, J.F. Increased expression of toll-like receptors 2 and 9 is associated with reduced DNA methylation in spontaneous preterm labor. J. Reprod. Immunol. 2017, 121, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Šorm, F.; Pískala, A.; Čihák, A.; Veselý, J. 5-Azacytidine, a new, highly effective cancerostatic. Experientia 1964, 20, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hackanson, B.; Daskalakis, M. Decitabine. Recent Results Cancer Res. 2014, 201, 269–297. [Google Scholar] [PubMed]
- Laska, M.J.; Nissen, K.K.; Nexø, B.A. (Some) Cellular Mechanisms Influencing the Transcription of Human Endogenous Retrovirus, HERV-Fc1. PLoS ONE 2013, 8, e53895. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, L.; Liu, J.; Wang, C.; Zhang, Y.; Mei, Q.; Han, W.; Xie, P.; Nie, J. Low-Dose Decitabine Augments the Activation and Anti-Tumor Immune Response of IFN-γ CD4 T Cells Through Enhancing IκBα Degradation and NF-κB Activation. Front. Cell Dev. Biol. 2021, 9, 647713. [Google Scholar] [CrossRef]
- Feng, Z.; Meng, R.; Li, Q.; Li, D.; Xu, Q. 5-aza-2’-deoxycytidine may regulate the inflammatory response of human odontoblast-like cells through the NF-κB pathway. Int. Endod. J. 2021, 54, 1105–1117. [Google Scholar] [CrossRef]
- Guan, H.; Mi, B.; Li, Y.; Wu, W.; Tan, P.; Fang, Z.; Li, J.; Zhang, Y.; Li, F. Decitabine represses osteoclastogenesis through inhibition of RANK and NF-κB. Cell. Signal. 2015, 27, 969–977. [Google Scholar] [CrossRef]
- Kauder, S.E.; Bosque, A.; Lindqvist, A.; Planelles, V.; Verdin, E. Epigenetic Regulation of HIV-1 Latency by Cytosine Methylation. PLOS Pathog. 2009, 5, e1000495. [Google Scholar] [CrossRef]
- Bouchard, J.; Walker, M.C.; Leclerc, J.M.; Lapointe, N.; Beaulieu, R.; Thibodeau, L. 5-azacytidine and 5-azadeoxycytidine inhibit human immunodeficiency virus type 1 replication in vitro. Antimicrob. Agents Chemother. 1990, 34, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.M.O.; Daly, M.B.; Xie, J.; Clouser, C.L.; Landman, S.R.; Reilly, C.S.; Bonnac, L.; Kim, B.; Patterson, S.E.; Mansky, L.M. 5-Azacytidine Enhances the Mutagenesis of HIV-1 by Reduction to 5-Aza-2′-Deoxycytidine. Antimicrob. Agents Chemother. 2016, 60, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.M.O.; Landman, S.R.; Reilly, C.S.; Bonnac, L.; Patterson, S.E.; Mansky, L.M. Lack of Mutational Hot Spots during Decitabine-Mediated HIV-1 Mutagenesis. Antimicrob. Agents Chemother. 2015, 59, 6834–6843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorganic Med. Chem. Lett. 2012, 22, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Exploiting Drug Repositioning for Discovery of a Novel HIV Combination Therapy. J. Virol. 2010, 84, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Holtz, C.M.; Mullett, M.; Crankshaw, D.L.; Briggs, J.E.; O’Sullivan, M.G.; Patterson, S.E.; Mansky, L.M. Activity of a Novel Combined Antiretroviral Therapy of Gemcitabine and Decitabine in a Mouse Model for HIV-1. Antimicrob. Agents Chemother. 2012, 56, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Bonnac, L.; Mansky, L.M.; Patterson, S.E. Characterization of Permeability, Stability and Anti-HIV-1 Activity of Decitabine and Gemcitabine Divalerate Prodrugs. Antivir. Chem. Chemother. 2014, 23, 223–230. [Google Scholar] [CrossRef][Green Version]
- Fernandez, G.; Zeichner, S.L. Cell line-dependent variability in HIV activation employing DNMT inhibitors. Virol. J. 2010, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Bouchat, S.; Delacourt, N.; Kula, A.; Darcis, G.; Van Driessche, B.; Corazza, F.; Gatot, J.S.; Melard, A.; Vanhulle, C.; Kabeya, K.; et al. Sequential treatment with 5-aza-2’-deoxycytidine and deacetylase inhibitors reactivates HIV-1. EMBO Mol. Med. 2016, 8, 117–138. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Liu, X.; Wang, H.; Zeng, X.; Yang, J.; Li, L.; Kuang, X.; Zhang, T. The mechanism of apoliprotein A1 down-regulated by Hepatitis B virus. Lipids Health Dis. 2016, 15, 64. [Google Scholar] [CrossRef]
- Chen, C.; Pan, D.; Deng, A.-M.; Huang, F.; Sun, B.-L.; Yang, R.-G. DNA methyltransferases 1 and 3B are required for hepatitis C virus infection in cell culture. Virology 2013, 441, 57–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwak, J.; Shim, J.H.; Tiwari, I.; Jang, K.L. Hepatitis C virus core protein inhibits E6AP expression via DNA methylation to escape from ubiquitin-dependent proteasomal degradation. Cancer Lett. 2016, 380, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Cramer, A.; Scholtissek, C. Effect of methyltransferase inhibitors on the regulation of baculovirus protein synthesis. J. Gen. Virol. 1995, 76, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, J.; Huang, X.; Zhong, J. DNA methyltransferase inhibitors increase baculovirus-mediated gene expression in mammalian cells when applied before infection. Anal. Biochem. 2010, 396, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hao, S.; Zhang, J.; Chen, Z.; Wang, H.; Guan, W. The formation and modification of chromatin-like structure of human parvovirus B19 regulate viral genome replication and RNA processing. Virus Res. 2017, 232, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Thieulent, C.; Hue, E.S.; Sutton, G.; Fortier, C.; Dallemagne, P.; Zientara, S.; Munier-Lehmann, H.; Hans, A.; Paillot, R.; Vidalain, P.-O.; et al. Identification of antiviral compounds against equid herpesvirus-1 using real-time cell assay screening: Efficacy of decitabine and valganciclovir alone or in combination. Antivir. Res. 2020, 183, 104931. [Google Scholar] [CrossRef]
- Morel, A.; Baguet, A.; Perrard, J.; Demeret, C.; Jacquin, E.; Guenat, D.; Mougin, C.; Prétet, J.-L. 5azadC treatment upregulates miR-375 level and represses HPV16 E6 expression. Oncotarget 2017, 8, 46163–46176. [Google Scholar] [CrossRef]
- Greggs, W.M.; Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Discovery of drugs that possess activity against feline leukemia virus. J. Gen. Virol. 2012, 93, 900–905. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef]
- Sadowski, L.; Upadhyay, R.; Greeley, Z.; Margulies, B. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Christman, J.K.; Mendelsohn, N.; Herzog, D.; Schneiderman, N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983, 43, 763–769. [Google Scholar] [PubMed]
- Hattori, N.; Sako, M.; Kimura, K.; Iida, N.; Takeshima, H.; Nakata, Y.; Kono, Y.; Ushijima, T. Novel prodrugs of decitabine with greater metabolic stability and less toxicity. Clin. Epigenetics 2019, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Daher-Reyes, G.S.; Merchan, B.M.; Yee, K.W.L. Guadecitabine (SGI-110): An investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Expert Opin. Investig. Drugs 2019, 28, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Cano-Soldado, P.; Arcas, M.M.; Lostao, M.P.; Larráyoz, I.; Martínez-Picado, J.; Casado, F.J. Cell entry and export of nucleoside analogues. Virus Res. 2005, 107, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Hubeek, I.; Stam, R.W.; Peters, G.J.; Broekhuizen, R.; Meijerink, J.P.P.; Van Wering, E.R.; Gibson, B.E.S.; Creutzig, U.; Zwaan, C.M.; Cloos, J.; et al. The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. Br. J. Cancer 2005, 93, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Kuss-Duerkop, S.K.; Westrich, J.A.; Pyeon, D. DNA Tumor Virus Regulation of Host DNA Methylation and Its Implications for Immune Evasion and Oncogenesis. Viruses 2018, 10, 82. [Google Scholar] [CrossRef]
- Dong, S.M.; Lee, H.G.; Cho, S.-G.; Kwon, S.-H.; Yoon, H.; Kwon, H.-J.; Lee, J.H.; Kim, H.; Park, P.-G.; Kim, H.; et al. Hypermethylation of the interferon regulatory factor 5 promoter in Epstein-Barr virus-associated gastric carcinoma. J. Microbiol. 2015, 53, 70–76. [Google Scholar] [CrossRef]
| Virus | Nucleic Acid Type | Potential Mechanism | References |
|---|---|---|---|
| HIV | RNA | Regulated the methylation of viral DNA, leading to the instability of HIV provirus DNA | [31,32,33,34,35,36,37,39] |
| B19 V | DNA | Regulated the formation and modification of chromatin-like structure | [45] |
| HBV | DNA | Upregulated anti-inflammatory protein ApoA1 | [40] |
| EHV1 | DNA | Integrated into the EHV-1 DNA and/or jams the viral polymerase | [46] |
| HPV16 | DNA | Upregulated miR-375 level and represses HPV16 E6 expression | [47] |
| HCV | RNA | Induced E6AP to increase degradation of HCV viral protein | [41,42] |
| FeLV | RNA | - | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Liu, P.; Wang, Y.; Zhu, Y.; Zeng, Q.; Hu, X.; Ren, Z.; Wang, Y. A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects. Molecules 2022, 27, 1973. https://doi.org/10.3390/molecules27061973
Xiao J, Liu P, Wang Y, Zhu Y, Zeng Q, Hu X, Ren Z, Wang Y. A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects. Molecules. 2022; 27(6):1973. https://doi.org/10.3390/molecules27061973
Chicago/Turabian StyleXiao, Ji, Ping Liu, Yiliang Wang, Yexuan Zhu, Qiongzhen Zeng, Xiao Hu, Zhe Ren, and Yifei Wang. 2022. "A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects" Molecules 27, no. 6: 1973. https://doi.org/10.3390/molecules27061973
APA StyleXiao, J., Liu, P., Wang, Y., Zhu, Y., Zeng, Q., Hu, X., Ren, Z., & Wang, Y. (2022). A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects. Molecules, 27(6), 1973. https://doi.org/10.3390/molecules27061973






