Abstract
Lipid-based nanoparticles (LBNPs) are biocompatible and biodegradable vesicles that are considered to be one of the most efficient drug delivery platforms. Due to the prominent advantages, such as long circulation time, slow drug release, reduced toxicity, high transfection efficiency, and endosomal escape capacity, such synthetic nanoparticles have been widely used for carrying genetic therapeutics, particularly nucleic acids that can be applied in the treatment for various diseases, including congenital diseases, cancers, virus infections, and chronic inflammations. Despite great merits and multiple successful applications, many extracellular and intracellular barriers remain and greatly impair delivery efficacy and therapeutic outcomes. As such, the current state of knowledge and pitfalls regarding the gene delivery and construction of LBNPs will be initially summarized. In order to develop a new generation of LBNPs for improved delivery profiles and therapeutic effects, the modification strategies of LBNPs will be reviewed. On the basis of these developed modifications, the performance of LBNPs as therapeutic nanoplatforms have been greatly improved and extensively applied in immunotherapies, including infectious diseases and cancers. However, the therapeutic applications of LBNPs systems are still limited due to the undesirable endosomal escape, potential aggregation, and the inefficient encapsulation of therapeutics. Herein, we will review and discuss recent advances and remaining challenges in the development of LBNPs for nucleic acid-based immunotherapy.
1. Introduction
In recent decades, gene therapy that involves either replacing a mutated gene that functions abnormally or regulating the expression of one or more specific genes has been widely explored for the treatment of various diseases, such as cancers, inherited diseases, infections, and immunodeficiencies [1]. At present, the strategy of gene therapy is to deliver genetic agents, such as antisense oligonucleotides (ASOs), small interfering RNA (siRNA), messenger RNA, micro-RNA (miRNA), and plasmid-DNA, into cells and target the nucleus or cytosol [2,3]. ASOs, as a therapeutic constituent, are generally short, synthetically modified DNAs or RNAs, comprising 8–50 nucleotides in length, that can target mRNA or pre-mRNA through complementary base pairing [4]. ASOs can regulate the expression of target mRNA via RNase H-mediated gene translation reduction, a direct steric hindrance to mRNA, or exon-modulated splicing alteration [4,5,6,7,8,9]. Compared to other small molecules, the advantage of ASOs is that their sequence can be modified to precisely target specific mRNA directly, which can enhance the targeting specificity and is less likely to cause off-target effects or activate the host immune system [10]. With the development of ASO modifications over the years, more druggable targets of ASOs have been identified and, therefore, contribute to potential clinical applications of antisense therapy [8].
RNA interference (RNAi) was discovered in 1998 by Andrew Fire and Craig C. Mello, who won the Nobel Prize in physiology or medicine in 2006 for their contribution to the discovery of RNAi [11,12]. In general, RNAi exists in eukaryotes and is mediated by RNA-induced silencing complex (RISC), which is formed from the guide strand of siRNA cleaved by Dicer [3,13,14]. RNAi includes two types of RNA materials, small interfering RNA (siRNA) and microRNA (miRNA). siRNA is a double-stranded non-coding RNA with a length of 20–30 base pairs [15]. It targets mRNA and silences target gene to prevent the subsequent translation process [16]. In the past decade, siRNAs have been widely used for the treatment of various cancers and inherited diseases; the most famous example is patisiran [17]. miRNA is another main type of RNAi molecule, which functions as a gene mediator to regulate specific gene expression in the post-transcription process. The biological structure of miRNAs is similar to siRNAs, and they share the same RNAi downstream pathway. The difference is that miRNA derives from its own short hairpin precursor molecule while a siRNA derives from long double-stranded RNA. Generally, siRNAs silence the same loci they derive from, whereas miRNAs silence heterogeneous genes [18]. miR34a is used as an inhibitor of non-small cell lung cancer (NSCLC) [19]. Moreover, RNAi is also of great importance in immunotherapy. For instance, MN-siPDL1 is known as a programmed death-ligand 1 (PD-L1) inhibitor, which downregulates PD-L1 to initiate cell apoptosis in the treatment of pancreatic cancer [20]. miR34a and miR29b also function as tumor suppressors in immunotherapy [21,22,23].
With the development of a non-viral gene delivery system, messenger RNAs (mRNAs) have become popular and promising agents in gene therapy. mRNAs are single-strand RNA molecules that regulate the synthesis of proteins in the cytoplasm via a process called translation. Precursor mRNAs (pre-mRNAs) are formed through transcription and then become mature mRNA via RNA splicing. During this process, introns are removed while exons are joined to form a contiguous coding sequence. Since the target of mRNA is the cytoplasm, the nuclear membrane is not a barrier for mRNA, which makes it advantageous compared to some other gene molecules, such as plasmid DNA [24]. mRNA has extensive applications in vaccine development [25,26,27,28,29,30], the innate immune response [24,31], and the treatment of cancers [32,33,34], virus infections [26], heart failure [35], and HIV [36,37]. In 2020, BNT162b2 and mRNA-1273, which are mRNA-based cationic lipid nanoparticles, have been approved for the COVID-19 vaccine. In addition, mRNA has been applied for gene editing. Remarkably, the progress of gene engineering technology has made more and more genomic disorders curable. Particularly, CRISPR (clustered regularly interspaced short palindromic repeats)-based genome editing, such as with CRISPR-Cas9 and CRISPR-Cpf1, contributes to a revolutionary breakthrough in this field [38,39,40,41,42,43,44]. Jennifer Doudna and Emmanuelle Charpentier won the Nobel Prize in chemistry in 2020 because of the discovery of the CRISPR-Cas9 gene technology tool [45]. Apart from those mentioned above, mRNA molecules still have breathtaking potentials in other therapeutic applications (Table 1).

Table 1.
Representative of FDA-Approved RNA Agents for Clinical Use.
Though genetic agents have shown their clinical practice, delivering these agents as part of gene therapy faces many challenges. Apart from production costs, nucleic acids can be degraded easily in plasma by nucleases or other components, which reduces the efficiency of gene delivery [46]. As the first barrier, the extracellular matrix (ECM), including the interstitial matrix and basement membrane, plays a significant role in gene transport [47,48,49]. The deposition of ECM makes tumor tissues denser than normal tissues [50,51], which leads to an increase in interstitial tissue pressure and subsequently causes difficulties to gene transport [52,53]. Moreover, nucleic acids without any protections can be degraded by ribonucleases (RNases) in plasma [54,55]. Exogenous vesicles are also easily cleared by macrophages and dendritic cells [56,57,58,59,60,61]. Actually, it is mentionable that the mononuclear phagocytic system (MPS) plays an important role in the non-specific clearance of foreign nucleic acids [56,62]. MPS consists of macrophages, neutrophils, dendritic cells, blood monocytes, and granulocytes. It exists in many tissues, such as the liver, spleen, lymph nodes, and lung [63,64]. MPS can eliminate recognized exogenous materials from biological environments. Other extracellular barriers include excessive superfluous vasculature, vascular endothelial cells, and high interstitial fluid pressure [49,65,66]. Another challenge is the intracellular barrier, affecting mainly the cellular uptake, which is a process of interaction between extracellular materials and cell membrane. There are various pathways involved in cellular uptake, including macropinocytosis [67,68], clathrin-dependent endocytosis [69,70,71,72], caveolin-dependent endocytosis [73,74], and other clathrin/caveolin-independent endocytoses [75]. Once vesicles are endocytosed by the cells, they will be trapped by the endosome (Figure 1).
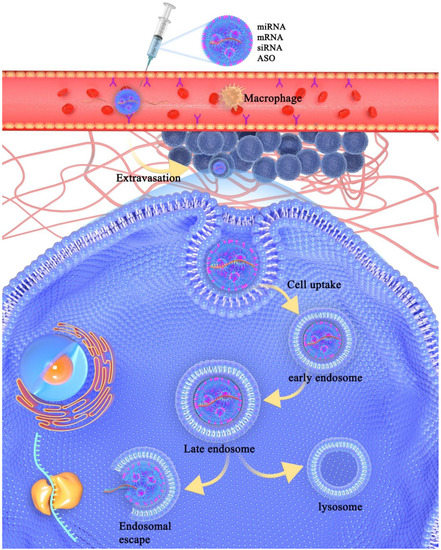
Figure 1.
Schematic demonstration of extracellular and intracellular barriers during the process of nucleic acids delivery to the cytosol. Once nucleic acids are injected into the vein, the LBNP will protect them from being degraded by RNase in plasma and eliminated by macrophage. LBNP can carry nucleic acids go through the cell membrane via receptor-mediated endocytosis. The components such as cationic lipids and ionizable lipids can fuse with endosomal membrane and help nucleic acids release from endosome and deliver to target site.
Lipid-based nanoparticles (LBNP) have been developed as a mature delivery platform for gene therapy during recent decades. They are tiny vesicles with a diameter range of 10–500 nm that is composed of biocompatible and biodegradable lipids. The suitable particle size enables LNP to escape mononuclear phagocyte system (MPS) uptake, subsequently prolonging the circulation time of LBNP and making LBNPs passively target tumor sites efficiently through an enhanced permeability and retention effect (EPR). Therefore, the off-target side effects and unnecessary harm to normal tissues can be minimized [76,77,78]. LBNPs can also promote endosomal escape after cellular uptake. Some LBNPs containing protonatable tertiary amine head groups with pKa values ranging from 6 to 7 can cause osmotically induced endosomal swelling and, subsequently, the burst of endosomes via the proton sponge effect [79,80,81]. LBNPs with highly fusogenic lipids can induce lipid fusion between the membranes of LBNPs and cells during structure phase transitions [3,79,82,83,84]. With the development of LBNP, it has become a mature and complicated drug delivery system that can encapsulate either small molecule components or nucleic acids, thus protecting molecules from clearance and overcoming intracellular and extracellular barriers, subsequently helping nucleic acids escape from the endosome and travel to target genes. For different purposes, various types of LBNP are designed, including for liposomes, lipid nanoparticles, solid lipid nanoparticles, lipid nanoemulsions, and nanostructured lipid carriers [85,86,87]. Apart from different kinds of natural or synthetic lipids, oils, wax, polymer conjugates, and steroids are also included. The advantages of these nano-sized formulations are not limited to enhancing drug stability, prolonging circulation, reducing toxicity, and controlling the release rate. They are also able to increase the local drug level at tumor sites, strengthen the drug bioavailability and selectivity, and decrease drug resistance [85].
Although the development of LBNP is promising, the pitfalls remain and need to be addressed. The low encapsulation efficiency of small molecules [88], cytotoxicity caused by cationic lipids [89,90], and systemic toxicity due to liver penetration are the primary causes that impair the further application of LBNP [91,92]. Due to its enhanced transfection efficiency and advantageous ability to go through extracellular and intracellular barriers as well as protect nucleic acids from degradation and elimination, LBNPs have still been explored and applied in gene delivery and gene editing. This review will introduce the influence of different components on the characteristics of LBNP, the modification strategies to overcome the existing pitfalls of LBNPs as nanoplatforms, and applications of LBNPs used for the treatment of different types of diseases, as well as some instances of gene therapy related to LBNP delivery system.
2. Modifications of Lipid-Based Nanoparticles
2.1. Types of Lipid-Based Nanoparticles
2.1.1. Liposomes
Liposomes are spherical vesicles with the lipid bilayer, of which the external structure is like a cell, and are composed of multiple phospholipids. A phospholipid used to form liposomes has a hydrophilic head, which consists of a phosphate group, and two long hydrophobic tails consisting of a long hydrocarbon chain [93,94,95,96]. The characteristics of phospholipids mean that they can easily form a w/o/w lipid bilayer structure in an aqueous solution. Therefore, liposomes are able to encapsulate hydrophilic components stably in their internal aqueous phase, in which case those encapsulated components can hardly release from liposomes through the membrane. Some components with low aqueous solubility can also be entrapped in the aqueous core of the liposome by gradient-driven remote loading. The most famous instance is doxorubicin. In this strategy, there is an ammonium sulfate gradient between the internal phase of the liposome and the external solution, and the doxorubicin tends to go through the membrane and exchange ammonium with ammonium sulfate to form salt precipitation. Once the precipitation is formed inside the liposome, it will be stably trapped [97,98]. Doxil is the first drug loaded using the lipid-based nanoparticle that was approved by FDA in 1995. It shows great merits in minimizing the toxicity of doxorubicin when compared to the traditional formulation [99]. Another extensively applied drug is paclitaxel, and its clinical application was limited due to its low solubility and toxicity. Similarly, liposomal formulation encapsulating paclitaxel can enhance its aqueous solubility and reduce the toxicity, subsequently improving its clinical performance [100,101].
2.1.2. Lipid Nanoparticles
In comparison to the traditional liposomes, lipid nanoparticles (LNP) are advanced non-viral vectors used for the delivery of genetic medicines. They are sphere-shaped, nano-sized vesicles composed of one or more ionizable lipids. These lipids are positively charged, which enables the encapsulation of negatively charged nucleic acids in their internal aqueous phase [102,103,104]. The head of cationic lipids can be either quaternary amine or tertiary amine [105,106,107]. Cationic lipids containing quaternary amine are positively charged at both acidic pH and physiological pH, and it can trap nucleic acids such as messenger RNA (mRNA), small interfering RNA (siRNA), an antisense oligonucleotide (AS ON) to avoid the degradation of nucleic acids in vivo and enable endosomal escape after cellular uptake [87,103,108,109,110,111]. To reduce the cytotoxicity caused by ionizability, pH-sensitive cationic lipids containing tertiary amine are often used. These lipids are neutral at physiological pH, in which lipid nanoparticles will not cause cytotoxicity and process extracellular drug release, while they still enable endosomal escape in late endosome where the pH ranges from 5.5 to 6.5, so nucleic acids can target either the nucleus or cytosol [105,112,113,114,115,116]. LNPs have become a mature drug delivery system for gene therapy, with a series of advantages, such as high encapsulation efficiency, reduced toxicity, release control, enhanced cellular uptake, prolonged circulation time, and stability. Since 2012, the FDA has approved several ASOs carried by LNP for clinical use. In 2018, patisiran, the first mRNA drug for treating hereditary transthyretin-mediated amyloidosis, was encapsulated by cationic lipid nanoparticles and achieved significant success. It is a milestone for an RNAi-based lipid nanoparticle delivery system.
2.1.3. Lipid Nanoemulsions
Lipid nanoemulsions (LNE) are colloidal droplet systems composed of oil, phospholipids, and an emulsifier, such as medium-chain triglycerides, which can reduce the interfacial tension on the droplet surface [117]. LNEs are normally used as carriers for lipophilic components. Like liposomes, LNEs are fully fledged drug delivery systems. However, the structure between LNE and liposome is different. An oil-in-water (O/W) LNE system consists of an interior oil core and a monolayer of phospholipids and emulsifiers. Drugs with poorly water solubility can dissolve in the interior liquid oil phase and be trapped, surrounded by a lipid monolayer. The hydrophilic head of phospholipids faces to exterior water phase so that LNEs can stably carry lipophilic drugs in an aqueous solution, thereby protecting the chemical stability of entrapped drugs [118,119,120,121]. Other types of lipid nanoemulsions, such as w/o or w/o/w, are also put into use for different purposes [121]. The compositions of LNE are biocompatible and biodegradable, so it will hardly induce toxicity or irritation [122]. LNEs can protect drugs from being trapped by the mononuclear phagocytic system, therefore improving the bioavailability of drugs [119,123,124]. The large surface area due to small particle size strengthens the absorption in vivo [118]. The lipophilic fusogenic properties promote cellular uptake as well as endosomal escape [125].
2.1.4. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLN) are tiny spherical vesicles with a core, which can solubilize lipophilic components, surrounded by a layer of surfactants in the aqueous dispersion acting as stabilizers. To form a biological membrane, phospholipids are also utilized. The core of SLN, composed of glycerides, steroids, fatty acids, or waxes, remains solid at room temperature. The particle size of SLN can range from 50 to 1000 nm after encapsulation [126,127]. For the delivery of genetic drugs, cationic lipids are used to build a positive charge on the membrane surface. SLNs have a stronger ability to protect nucleic acids from degradation and leakage during storage compared with lipid nanoparticles [126,128,129]. They can encapsulate both hydrophilic and hydrophobic components because of their physical properties, which is helpful to lower the toxicity and improve the PK character [130].
2.1.5. Nanostructured Lipid Carriers
Nowadays, much research suggests that most of the encapsulated drugs may be attached or stranded on the outside layer surface instead of the solid core, in which the loading ability and stability of SLNs remain doubtful. As such, nanostructured lipid carriers are developed to improve drug loading efficiency and release characteristics based on SLNs [131,132,133]. NLCs are modified on the basis of SLNs and are different from SLNs because of the core, which is composed of both solid and liquid lipids at room temperature. Accordingly, NLCs can encapsulate components in both the solid phase and liquid phase simultaneously and subsequently control the drug release rate [134,135]. The above introduced lipid carriers are shown in (Figure 2).
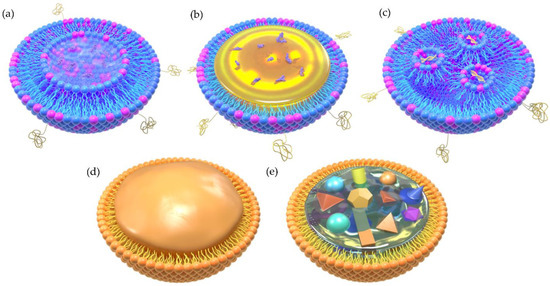
Figure 2.
Schematic illumination of the two-dimensional structure of different types of LBNPs. (a) Liposome, (b) lipid nanoemulsion (LNE), (c) lipid nanoparticle (LNP), (d) solid lipid nanoparticle (SLN), (e) nanostructured lipid carrier (NLC).
2.1.6. Other Nanostructured Lipid Carriers
Exosomes are nano-sized lipid vesicles secreted from living cells, ranging from 30 to 200 nm, present in cell culture medium and other biological fluids [136,137]. Exosomes are promising candidates for drug delivery platforms because of their high biocompatibility, blood–brain barrier (BBB) crossing capability, and low immunogenicity [138,139]. Exosomes carry various proteins and nucleic acids, reflecting their cell of origin; thus, the choice of donor cell type for exosomes is important if utilizing exosomes in drug delivery and diagnosis systems [140,141]. Avoiding immunomodulating activity and possible inflammation is one of the important criteria. Exosomes present in biofluids that contain other contaminations, thus requiring isolation, ultracentrifugation, sucrose density gradients, tangential flow filtration, size-exclusion chromatography, polymer-based precipitation, and immunoaffinity magnetic beads have been successfully used to isolate and purify exosomes [142,143]. Besides the natural cargos exosomes carry, engineering exosomes generated by cell transfection that contain enriched therapeutic biomaterials are interesting due to their potential high efficacy. Mainly, exosome-loaded nucleic acids (e.g., miRNA-155 and PTEN mRNA) have been widely used in cancer immunotherapy for tumor suppression by immune response regulation [144,145]. The summary of current drug delivery systems is listed in Table 2.

Table 2.
Current Drug Delivery Systems.
2.2. Development and Modification Strategies
2.2.1. Component Modification
For different purposes, LBNP can be designed using various strategies. Generally, one LBNP used for gene delivery usually consists of phospholipids, ionizable lipids, lipopolymers, and sterols.
Phospholipids are one of the most important components of the cell membrane, which consists of a hydrophilic phosphate head group, a glycerol bridge, and two hydrophobic fatty acid tails. Due to its amphiphilic characteristics, a lipid bilayer can be readily formed in the aqueous phase. On the basis of the modification of the head group, phospholipids can be divided into different subtypes, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidic acid (PA), phosphatidylinositol (PI), phosphatidylglycerol (PG), and cardiolipin (CL) [146]. Many researchers have reported that the saturation degree of lipids has an influence on the structure phase transition temperature (Tc). The more unsaturated lipids have a lower phase transition temperature that makes lipids transform from a lamellar phase to an inverted hexagonal phase, which contributes to an enhanced fusogenicity [147,148,149]. The highly saturated lipid chain causes a high Tc; for example, DSPC that contains two saturated fatty acid chains can form a cylindrical shape at room temperature, which enhances the stability and prolongs the circulation time. However, in some cases, it is hard to process drug release and endosomal escape due to less fusion with an anionic lipid membrane [150]. On the other hand, phospholipids such as 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) with unsaturated fatty acid chains usually form inverted conical shapes at room temperature, and in this way the lipid nanoparticles containing these lipids are more likely to escape from the endosome. The alkyl chain length also has an effect on the physicochemical properties of LBNP. With the increase in the alkyl chain length, fewer drugs can be entrapped [151]. A long chain length can also enhance the stability of LBNP [152]. It is also mentioned that lipids with branched tails can strengthen the delivery efficiency of nucleic acids [153]. The head group is responsible for the pKa value, the charge, and the amphiphilicity of phospholipids. Lipids with head groups such as phosphatidylcholine and phosphatidylethanolamine show a neutral charge, while lipids with phosphatidylserine and phosphatidylglycerol are negatively charged at physiological pH conditions [154]. The structure of head groups and alkyl tails determine the geometry of lipids, including phospholipids, cationic lipids, and ionizable lipids together. Lipids can form different structural phases in aqueous solution, such as hexagonal phases, lamellar phases, or inverted hexagonal phases, which can be divided by a packing parameter (P) decided by the ratio of hydrocarbon volume (V), the area of the head group (a), and the critical length of lipid tails (lc). The equation is P = V/a*lc (Figure 3). Lipid-based nanoparticles containing lipids that adopt inverted hexagonal phases, such as DOPE, have better delivery efficiency since they can promote the lipid transition process from lamellar phase to non-lamellar phase and destabilize the structure of the endosomal membrane, which subsequently facilitates endosomal escape, and nucleic acids can be delivered to the cytosol [150,155,156].
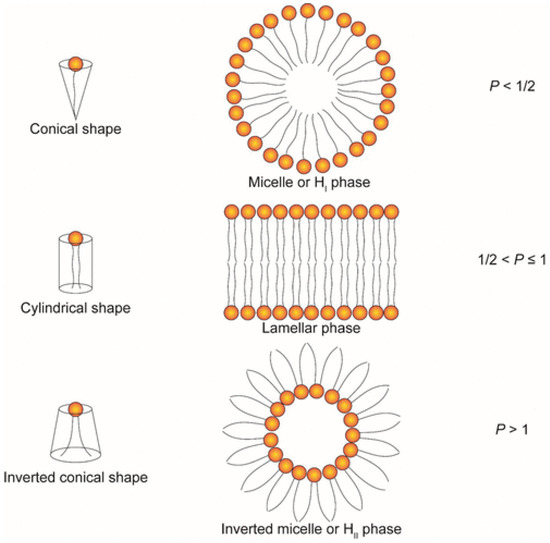
Figure 3.
The schematic demonstrates different structural phases under different conditions [3]. p = v/a*lc. If p < ½, lipids with conical shape are more likely to adopt hexagonal phase. If ½ < p < 1, lipids with cylindrical shape tend to adopt a lamellar phase. If p > 1, inverted conical-shaped lipids will adopt an inverted hexagonal phase [3,155]. Copyright 2022 American Chemical Society.
In addition to phospholipids, cationic lipids are another important component used specifically for the delivery of nucleic acids due to their permanent positively charged head groups, which can encapsulate negatively charged nucleic acids in the internal aqueous phase through electrostatic self-assembly [157,158]. The cationic lipid nanoparticle carries nucleic acids through the cell membrane before being trapped in the endosome [159]. Then, the cationic lipids interact with the anionic phospholipids of the endosomal membrane to induce the disruption of the endosomal membrane and subsequently release the nucleic acids from the endosome. N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) is the first cationic lipid used for gene delivery by Felgner et al. in 1987. They used liposomes consisting of DOTMA to encapsulate DNA. The DOTMA fuses with the negatively charged cell membrane and promotes endosomal escape [160]. N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium (DOTAP) is a quaternary ammonium lipid in which the linker is two ester bonds, instead of the ether bonds in DOTMA. In 1997, Templeton et al. applied liposome complexes containing DOTAP to improve DNA delivery [161]. Later, many studies have proven that DOTAP is a feasible and efficient cationic lipid in gene delivery [162,163,164,165]. Other cationic lipids apart from quaternary ammonium lipids have also been developed through the delocalization of cationic head groups, such as guanidinium lipids, imidazolium lipids, pyridinium lipids, piperidinium lipids, pyrrolidinium lipids, and phosphonium lipids [166,167,168,169,170,171,172].
Although the permanent positive charges bring cationic lipids high transfection efficiency and endosomal escape ability, they may cause severe cytotoxicity and short systemic circulation time [149]. To solve this problem, researchers proposed adjusting the charge ratio of lipid to nucleic acids [173,174,175]. However, the consequences of those attempts are not satisfying. To overcome the challenge, ionizable lipids have been developed for gene delivery. Unlike the cationic lipids with pH-independent positive charges, ionizable lipids normally are neutrally charged in a physiological pH (7.4) environment, while they become positively charged in an acidic pH environment. This is because the acid dissociation constant (pKa) of the tertiary amino head groups usually ranges from 6.0 to 7.0 [176]. They can electrostatically interact with nucleic acids in acidic aqueous solutions. Lipid nanoparticles containing pH-dependent ionizable lipids are neutral in plasma and normal tissues, and therefore they will hardly interact with anionic lipid membranes. The pH range in the endosome, as mentioned above, is 5.5 to 6.5, in which condition the ionizable lipids become positively charged and are able to interact with the endosomal membrane, helping nucleic acids escape from the endosome [110,177]. For example, 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane (DODMA) is a pH sensitive ionizable lipid that has been well applied in the delivery of nucleic acids [113]. Hsu et al. used DODMA-based lipid nanoparticles and successfully delivered miRNA to a murine liver tumor [165]. Afterwards, 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane (DLinDMA) and 1,2-dilinolenyloxy-N,N-dimethyl-3-aminopropane (DLenDMA) were developed to further improve the transfection efficiency. The difference between 1,2-distearyloxy-N,N-dimethyl-3-aminopropane (DSDMA), DODMA, DLinDMA, and DLenDMA is the saturation degree of their alkyl chains, with 0, 1, 2, and 3 double bonds on each chain, respectively [178]. Koynova R. et al. mentioned that LNPs containing DLinDMA or DLenDMA to deliver siRNA show better transfection efficiency than DODMA, while LNPs containing DSDMA show no gene silencing [179]. Apart from the saturation degree of the lipid tails, it is also remarked that lipids containing branched tails can promote endosomal escape better than those with linear tails [180,181]. Fenton et al. mentioned that longer unsaturated alkyl tails could enhance mRNA delivery efficiency. It is reported by Miao et al. that the incorporation of ester or alkyne groups rather than double bonds in the lipid tails can enhance the fusogenicity of the ionizable lipids [182]. The pKa value of ionizable lipids is also important for gene delivery efficiency. This can be determined through the modification of both head groups and hydrophobic tails. DLin-KC2-DMA, of which the pKa value is 6.7, is proven to be tenfold more potent than DLinDMA [150,183]. According to Jayaraman M. et al., the optimum pKa value ranges from 6.2 to 6.5 [184]. The lipid nanoparticle used to encapsulate Onpattro contains DLin-MC3-DMA, which is one of the most effective ionizable lipids and is even more potent than KC2 [184,185]. DLin-MC3-DMA, of which the pKa is 6.44, is shown to be the most active ionizable lipid among 56 amino lipid candidates [184]. Two COVID-19 vaccines named mRNA-1273 and BNT162b are encapsulated by LNPs containing SM-102 and Alc-0315, respectively (Figure 4). To balance the transfection efficiency and cytotoxicity, the researcher also utilizes both cationic lipids and ionizable lipids in one LNP system simultaneously. Yung et al. applied a combination of quaternary amine and tertiary amine cationic lipids to form lipid nanoparticles for the delivery of antimir-21, and it is successful for lung cancer treatment in vivo [105].
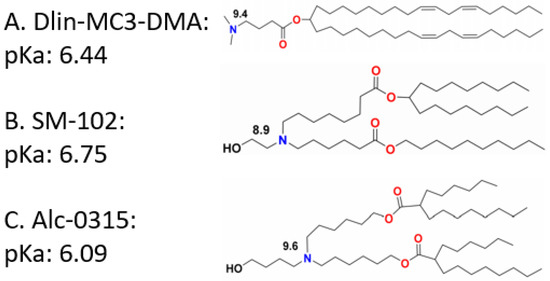
Figure 4.
Structure and pKa value of DLin-MC3-DMA (A), SM-102 (B), and Alc-0315 (C). Copyright is reserved by 1996–2022 MDPI (Basel, Switzerland).
Sterols are a subtype of steroids and are important for stabilizing the structure of LBNP. Cholesterol is a representative that can fill the gaps between lipids to support the stable spherical structure, reduce the particle size of LBNP, and enhance the fluidity of the nanoparticle surface, and subsequently promote the fusion between LBNP and the cell membrane [111,186,187]. Nanoparticles containing cholesterol can also be taken up by the cell via low-density lipoprotein receptor (LDLR)-mediated endocytosis [188,189]. Zhigaltsev et al. used liposomes containing cholesterol to encapsulate doxorubicin and mentioned that high-ratio cholesterol triggers faster drug release [190]. Briuglia et al. also claimed that 2:1 is an optimum lipid–cholesterol ratio in order to achieve a suitable drug release rate [191]. Cholesterol can be modified via esterification and oxidation. It is reported that cholesterol variants, such as esterified cholesterol, can enhance RNA delivery efficiency [192,193]. Another cholesterol analogue used in lipid nanoparticles is β-sitosterol, which is a naturally occurring phytosterol that has similar structures to cholesterol, only with a modified C24 position [194]. LNP containing β-sitosterol has a polymorphic shape which enhances the fusogenicity of LNP, meaning that the liposomal membranes are more fluid than those containing cholesterol [194,195]. β-Sitosterol, as a composition in LNP, has been evaluated to have enhanced capability to escape from the endosome and deliver mRNA efficiently [196]. Other naturally occurring phytosterols include ergosterol, fucosterol, campesterol, and stigmastanol, all of which can be a composition of lipid nanoparticles for the improvement of RNA delivery performance [197,198].
2.2.2. Surface Modification
LBNP surface modifications can increase the circulation time, enhance specific distribution and targeting ability, avoid particle aggregation, and promote cellular uptake. Lipopolymers are commonly used materials in nanoparticle surface modification that can increase the stability of nanoparticles in aqueous solutions [199,200]. Poly(ethylene glycol) (PEG) is a gold standard polymer used to avoid nanoparticle aggregation and increase the hydrophilic nature of nanoparticles in suspensions [201,202]. Nanoparticles coated with PEG are protected from phagocytosis and interaction with plasma proteins [203], and will not be recognized by MPS [204], which prolongs the circulation time and improves nanoparticle distribution [205]. PEG products have been investigated and widely used. Researchers usually bind PEG to an alkyl chain, of which the chain length and branches can be modified [3]. DSPE-PEG2000 was firstly used as one of the components of Doxil, which is the first FDA-approved liposomal drug in 1995 [206,207]. It was recently used as a component of Atu027 [208]. In 2020, Moderna applied DMG-PEG2000 in the lipid nanoparticle of the mRNA-1273 vaccine formulation [209,210]. To enhance the targeted drug delivery, Sun et al. conjugated PEG with folic acid in 2006 [211]. Folate receptors are reported to be overexpressed in cancer tissues [212,213], and nanoparticles conjugated with folic acid can be easily delivered [214]. PEG can be conjugated with proteins, which can specifically bind to transport receptors on the cell membrane to improve targeted delivery. For example, transferrin-conjugated PEG can improve cellular uptake by transferrin receptors [215,216]. However, PEG also faces several challenges, such as decreased drug activity and immunogenicity induction [217].
In addition to PEG, polyethylenimine (PEI) is another lipopolymer that has high transfection efficiency. The molecular weights and the amount of branches can be designed to possess different physical properties [218]. PEI can condense with nucleic acids through electrostatic self-assembly to form stable nano-sized particles [219]. PEI has a high buffering capacity and promotes cellular uptake and endosomal escape through the proton sponge effect. It functions like a sponge and pumps a large number of protons through the biological membrane, which, as a result, leads to an influx of chloride ions and water molecules and subsequently leads to an osmotic swelling and the bursting of the endosome [220,221]. Due to its properties, such as large molecule weight and high buffering capacity, PEI is not biocompatible enough and may also cause cytotoxicity [222,223]. To overcome the deficiencies of PEI, researchers conjugated PEG with PEI via covalent coupling to improve its performance as well [224,225,226]. Ligand modifications are also able to increase its transfection efficiency and biocompatibility. In 2016, Hu et al. modified PEI with trifunctional peptide R18 to enhance gene transfection efficiency [227]. Zheng et al. used transferrin-conjugated PEG-based LNP to deliver ASO LOR-2501 [228]. Conjugations with ionizable or neutral phospholipid cholesterols are frequently used as well [229,230,231].
Other surface modifications of lipids, including surface charge modification, targeting ligand modification, protein modification, and peptide modification strategies, are similar to surface modification of PEG and PEI mentioned above.
3. Applications of Lipid-Based Nanoparticles
3.1. LBNP in Treatment of Inherited Disease
The LBNP delivery system is a revolutionary breakthrough in gene therapy that can enable antisense oligonucleotides (ASOs), siRNA, mRNA, DNA, or gene-editing complexes, which may degrade easily in vivo, being delivered to their target to process anti-tumor activity via gene silencing, relevant protein expression, or genetic defect fixing [232]. Although LBNP can protect genetic drugs from degradation and elimination quickly, the main barrier that genetic drugs can hardly be delivered efficiently to target tissues still exists. Cytotoxicity is another issue that needs to be fixed before enabling clinical use. However, these barriers have been overcome recently. Antisense therapy has a long history. Since 1998, the first antisense oligonucleotide drug, called fomivirsen, which is an antiviral ASO that is used for the treatment of cytomegalovirus retinitis (CMV) in AIDS patients, was approved by the FDA. An important contribution to the development of ASOs has been made during the recent decades. To improve the therapeutic effect of ASOs and enhance their stability, various chemical modifications of ASOs have been processed for improved nuclease resistance and specifically target-binding affinity [233]. However, due to low solubility, weak cellular uptake, and unsatisfactory RNA–target affinity, the clinical use of ASOs was limited. Mipomersen is an apolipoprotein B-100 (ApoB-100) inhibitor used for the treatment of familial hypercholesterolemia (FH) [234]. FH is a genetic disease that is caused by mutations of apolipoprotein B [235]. This ASO can bind to mRNA coding for ApoB-100 and prevent the translation of ApoB-100 [236]. Afterward, other ASOs, such as golodirsen and volanesorsen, were approved by the FDA successively. However, the bioavailability of these ASOs without protection is not promising [237,238]. The most recent FDA-approved ASO drug is casimersen, which is used for the treatment of Duchenne muscular dystrophy (DMD) and works by inducing the exon 45 skipping of dystrophin so functional dystrophin can be translated [239,240,241].
The first FDA-approved application of LBNP for the RNAi-related therapy is patisiran, which is an LNP-based siRNA drug used for the treatment of polyneuropathy in people with hereditary transthyretin-mediated amyloidosis (ATTRm), which is a lethal disease caused by the mutation of transthyretin (TTR) gene expression [242]. Transthyretin is a transport protein found in serum and cerebrospinal fluid and predominantly synthesized in the liver [243,244]. TTR is normally a tetramer protein, which dissociates into monomers and leads to the formation of amyloid fibril and, subsequently, the accumulation of amyloid. Those insoluble fibrils aggregate at various tissues and finally result in ATTRm [244,245]. Patisiran is the first FDA-approved gene-silencing drug that can bind to the mRNA sequence of TTR and promote the degradation of target mRNA [246]. To target to mRNA specifically, patisiran is encapsulated into an LNP composed of DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), DLin-MC3-DMA (dilinoleylmethyl-4-dimethylaminobutyrate), mPEG2000-DMG (1,2-dimyristoyl-rac-glycero-3-methylpolyoxyethylene-2000), and cholesterol. This LNP formulation can avoid siRNA from degradation by RNase and help patisiran target hepatocytes. mPEG2000-DMG stabilizes the LNP structure and prevents the interaction with proteins and uptake via MPS clearance, subsequently prolonging the circulation time. DLin-MC3-DMA can enhance the fusogenicity of patisiran LNP. It can become positively charged in the endosome and interact with the endosomal membrane to facilitate siRNA to realize endosomal escape and to target the cytoplasm [164]. Afterward, other RNAi genetic drugs, such as givosiran, lumasiran, and inclisiran, have also been approved for clinical use. They are all siRNA drugs used for the treatment of acute hepatic porphyria, primary hyperoxaluria type 1 (PH1), and hypercholesterolemia, respectively [247,248,249,250]. Subsequently, as the second FDA-approved siRNA drug, givosiran, which targets aminolevulinate synthase 1 (ALAS1) mRNA, was used for the treatment of acute hepatic porphyria [251]. EPHARNA is a siRNA directed against ephrin type-A receptor 2. It is used for the treatment of advanced solid tumors. It is encapsulated by DOPC, which is a neutral lipid with 10 times greater efficiency than cationic liposomes [252].
Codon-optimized human frataxin (FXN) mRNAs were encapsulated in LNPs to treat Friedreich’s ataxia caused by the downregulation of FXN in 2016 [253]. In this study, adequate cellular uptake in hepatocytes and a remarkable expression of FXN protein was observed in adult mice after intravenous administration, and a recombinant human FXN protein was detected in the dorsal root ganglia 24 hours after 0.2 mg/kg intrathecal administration. Truong et al. developed LNPs encapsulating an mRNA encoding arginase 1 (ARG1) to treat inherited metabolic liver disorder arginase deficiency caused by biallelic mutations in ARG1 [254]. All the mice were ARG1-knockout mice and survived more than 11 weeks without hyperammonemia or body weight loss after continuous treatment of human codon-optimized ARG1 mRNA LNP, which indicates a potential therapeutic strategy for the treatment of arginase deficiency [254]. In 2019, another group used LNPs to deliver CYP7B1 mRNA as a treatment for hereditary spastic paraplegia type 5 (SPG5), a neurodegenerative disease caused by CYP7B1 gene loss-of-function mutations, and this resulted in noteworthy oxysterol degradation in liver and serum after two days of treatment, as well as the restoration of functional human CYP7B1 protein and the elimination of abnormal cholesterol metabolites [255]. These instances have proven that LBNPs serve as a good delivery system for the nucleic acid-based treatment of inherited diseases.
3.2. LBNP in the Treatment of Infectious Diseases
mRNA vaccines have potent potentials to guard against infectious diseases caused by a virus-like influenza virus, Zika virus, rabies virus, human cytomegalovirus (CMV), and hepatitis C virus (HCV) [256]. They can cause significant and rapid immune response along with T cell induction. From previous liposome and solid lipid nanoparticles to ionizable lipid-based LNP, LBNPs have been developed and applied to be an advanced delivery system for RNA-based vaccines, with enhanced RNA loading capability and transfection efficiency. It has been well recognized that LBNP can protect mRNA from degradation and help mRNA go through the cell membrane via endocytosis and escape from the endosome by fusing with the endosomal membrane.
Influenza is a viral infection that leads to damage to the respiratory system. An mRNA encoding influenza virus antigens encapsulated in LNPs can induce remarkable immune responses, including the expansions of central memory and effector memory CD4 and CD8 T cells against the influenza virus [257]. In 2013, Hekele et al. claimed that a self-amplifying mRNA (SAM) vaccine platform, which can encode the H1 hemagglutinin (HA) antigen from the H1N1 virus and the H7 HA antigen from the H7N9 virus, delivered by LNP could induce more rapid responses [258]. Mice receiving the first immunization showed considerable hemagglutinin inhibition and neutralizing antibody titers against the virus, and all mice had HI titers which are considered to be protective after the second immunization [258]. Afterward, Brazzoli et al. used cationic nanoemulsion to encapsulate SAM encoding influenza virus HA from H1N1 and induced strong immune responses, subsequently protecting mice immunized with this vaccine [259]. A modified non-replicating mRNA encoding influenza H10 was developed recently, which is encapsulated by LNP and can effectively induce an immune response [260]. It is also reported that circulating H10-specific memory B cells expanded after immunization [260]. SAMs encoding influenza antigens encapsulated by LNPs containing chitosan were delivered to DC and can also elicit the immune response after subcutaneous injection [261].
The rabies virus is a neurotropic virus that can cause irreversible damage to the central nervous system (CNS) in humans and animals. Due to the high occurrence of the rabies virus around the world, the development of nucleic acid-based vaccines has been driven over several years. Lutz et al. developed a sequence-optimized, chemically unmodified mRNA encoding rabies virus antigens encapsulated in LNPs that can induce protective antibody titers and remain stable for up to 1 year [262]. It is demonstrated that the innate immune response was activated remarkably at the injection site and in the draining lymph nodes (dLNs), and the functional antibody and T cell responses were stronger compared with the licensed vaccine [262]. Researchers designed a CV7201 and CV7202 (NCT03713086) mRNA-LNP formulation, consisting of cholesterol, DSPC, PEGylated lipid, and a cationic lipid, encoding the rabies virus glycoprotein (RABV-G) from a Pasteurized strain [263,264]. All participants showed measurable rabies neutralizing antibody responses after two 1 ug or 2 ug doses of CV7202 LNP [263].
The Zika virus (ZIKV) is a mosquito-borne arbovirus that emerged in 2015 as a severe pandemic around the world [265]. Pardi et al. designed an LNP-encapsulated nucleoside-modified mRNA encoding the pre-membrane and envelope glycoproteins derived from ZIKV that can induce potent and durable immune responses in mice and macaques after single low-dose intradermal immunization in 2017 [266]. Soon afterward, Richner et al. designed a modified mRNA vaccine LNP formulation encoding prM-E genes, which contributed to high neutralizing antibody titers that promote the immune response against ZIKV infection after single IM immunization in mice, while a second dose can elicit a more potent immune response [267]. In 2018, a replicating viral RNA encoding ZIKV antigens encapsulated by physico-chemically modified NLC was designed and induced a strong protective immune response after a single 10 ng IM dose in mice [268].
Since microbial infectious diseases show resistance to conventional antibiotics in many cases, LBNPs have been applied to improve the performance of antimicrobials. Vancomycin encapsulated by liposomes consisting of tetraether lipid can enhance the bioavailability of vancomycin 1 hour after oral administration on mice by about three fold, compared to free vancomycin [269]. Alhariri et al. successfully enhanced the efficacy of gentamicin by using anionic liposomes, which has better performance against planktonic P. aeruginosa and K. oxytoca, and which is better able to inhibit the formation of the biofilm of these strain than free gentamicin [270]. NLC has also been used to improve the biodistribution and affinity of rifampicin two fold against M. tuberculosis compared to free rifampicin [271].
LBNP can also deliver nucleic acid-based vaccines against other infectious diseases. Scientists used cationic nanoemulsion to deliver the human immunodeficiency virus (HIV) SAM vaccine encoding a clade C envelope glycoprotein, which induced remarkable cellular immune responses in rhesus macaques [272]. Cationic nanoemulsion containing DOTAP and squalene was also used to deliver nucleic acids encoding HCV, respiratory syncytial virus (RSV), and CMV, respectively [273]. The Ebola virus (EBOV) was firstly identified in 1976 and is a virus that can cause blood clots. During the past few years, many nucleic acid-based vaccine candidates against EBOV have been developed, one of which is an mRNA encoding EBOV glycoprotein (GP) encapsulated by LNP that induces EBOV-specific immunoglobulin G (IgG) and neutralizing antibody responses successfully [274]. Another vaccine candidate encapsulated by LNP against EBOV is siVP35-LNP, which can directly reduce viral load and promote an adaptive immune response to the virus [275]. Some nucleotide-modified mRNA-LNP vaccines (prME-mRNA, E80-mRNA, and NS1-mRNA) were also developed against the dengue virus-2 (DENV-2), and they successfully induce high levels of neutralizing antibodies and antigen-specific T cell responses [276].
3.3. LBNP in the Treatment of COVID-19
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) brought the coronavirus disease-19 (COVID-19) pandemic, which has caused 265 million infectious cases and over 5 million deaths. Consequently, the health care, business, and psychology of the entire world face big challenges. Since directly killing the virus is far from clinically possible, producing vaccines against SARS-CoV-2 is, thus, central in the race among the worldwide pharmaceutical companies and research institutes. mRNA vaccines are the most promising vaccines with the shortest manufacturing time, the most economical effectiveness, and clinically proven capability, and they have been developed to overcome the pandemic [277]. Both Moderna Biotechnology and Pfizer/BioNTech have developed mRNA vaccines in an unparalleled rapid manner and took less than one year to finish the manufacturing, efficacy testing, safety examination, and U.S. FDA Emergency Use Authorization approval [278].
The Moderna vaccine, mRNA-1273, demonstrated immunogenicity without safety concerns within trial limitations and a 94.1% efficacy for blocking COVID-19 among 30,420 clinical trial participants [279,280,281,282]. At the same time, BNT162b2, the Pfizer/BioNTech vaccine, has passed the clinical trial with 43,548 individuals with a 95% clinical efficacy in preventing COVID-19 illness, and the safety and anti-SARS-CoV-2 immune responses were also demonstrated [283,284]. Both mRNA vaccines were delivered using LNPs, and the compositions of LNPS are similar in that they both include an ionizable lipid, SM-102 (9-heptadecanyl 8 ((2 hydroxyethyl) (6 oxo 6—(undecyloxy) hexyl) amino) octanoate) for Moderna and ALC-0315 ((4-hydroxybutyl) azanediyl) bis (hexane-6,1-diyl) bis (2-hexyldecanoate) for Pfizer/BioNTech. The ionizable lipids are positive at the RNA complexation stage with a low pH because the lipids are tertiary amines that are protonated, and they are neutral at physiological pH, allowing efficient payload release and toxicity mitigation. Both vaccines have the 1,2-distearoyl-snglycero-3 phosphocholine (DSPC) as a helper lipid and cholesterol to facilitate packing cargos into the LNPs. PEG-DMG (1 monomethoxypolyethyleneglycol-2,3-dimyristylglycerol with polyethylene glycol) for Moderna and PEG-DMA (2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide) for Pfizer/BioNTech are also applied to increase the circulation time by eliminating the clearance of LNPs by phagocytes and reducing the association of antibody and serum proteins [285]. Briefly, mRNAs in water and lipid mixtures in ethanol are mixed thoroughly in microfluidic devices. Subsequently, these two components form nanoparticles at pH 4.0 with an average size of 80 to 100 nm that encapsulates 100 negatively charged mRNA molecules per nanoparticle [110,186,286,287]. After two separate doses, intramuscularly administered, the host generates immune responses because of the presence of the SARS-CoV-2 spike glycoprotein that is encoded by the mRNA [281,288].
Compared with other vaccine platforms, mRNA vaccines have various advantages. First, the manufacturing is rapid, versatile, and flexible because it only requires the sequence encoding of the immunogen when generating different vaccines. Additionally, they are much safer compared with DNA-based vaccines because there is less possibility of mRNAs being integrated into the genome, since the translation happens in the cytoplasm, not the nucleus. In addition, the expression of target antigen after translation is much faster than other platforms [256,289,290]. Though promising progress has been made of mRNA vaccines in combating COVID-19, long-term effects are still unknown, and the long-term storage and delivery of mRNA vaccines are still an issue since mRNAs are not stable at room temperature [291].
3.4. LBNP in the Treatment of Cancer Immunotherapy
In addition to inherited diseases, infectious diseases, and COVID-19, cancer immunotherapy is another area where LBNP-based therapies have been extensively applied in both preclinical and early clinical trials. Currently, LBNPs are often designed to carry multiple nucleic acids as cancer immunotherapeutic agents that target antigen-presenting cells (APCs) for the priming and activation of antigen-specific T cell-relative immune responses. Among a series of LBNPs, cationic LBNPs show great advantages in drug encapsulation and delivery when considering the intrinsic molecular charge of most involved nucleic acids. Chaudhuri et al. introduced lysinylated cationic amphiphiles covalently grafted with mannose-mimicking shikimoyl and quinoyl groups in the head group region for stable and efficient DNA loading and long-term immune responses [292]. In another study, Sayour’s group developed an RNA–liposomal (RNA-NPs) cancer vaccine with personalized tumor-derived mRNA (representing a tumor-specific transcriptome) encapsulated in DOTAP nanoparticles [293]. Despite the great merits of efficient encapsulation, the surface charge of cationic LBNP has impaired their further use. This is because nanoparticles with a positive charge tend to affect lymphatic transport and permeation through tissues after injection, as they will immobilize within the negatively charged extracellular matrix, thus inducing hemolysis and platelet aggregation [294]. Accordingly, many studies began to localize cationic LBNP in vivo. For example, Kranz et al. significantly decreased the cumulative charge of mRNA and lipid-based nanoparticles (composed of DOTMA and DOPE) by regulating the ratio of lipid:mRNA [295]. According to their results, near-neutral mRNA–liposomes systems with slightly positive charging are able to localize within the spleen, instead of aggregating in regions such as the heart and lung. The interaction between loaded mRNA and different LBNP delivery platforms was systematically evaluated to determine the best combination for the efficient uptake and expression of the encoded antigen by DC populations. Consequently, the mRNA incorporated in this study can activate Toll-like receptor 7 (TLR7) on DCs to induce the secretion of IFN and desired anti-cancer responses. An initial phase I trial based on this study demonstrated that IFNα and strong antigen-specific T cell responses could be induced at low-dose levels in patients with advanced malignant melanoma (NCT02410733) (Table 3).

Table 3.
FDA-Approved Drugs Encapsulated by Lipid-Based Nanoparticles.
These promising pre-clinical and early clinical outcomes suggest the effective regulation of the physicochemical properties of LBNPs and optimized interaction with delivered cargos. Like previous applications we discussed, challenges in LBNPs-based cancer immunotherapy also include uncontrollable release profiles, which could lead to low bioavailability and efficiency of the delivery system. As such, corresponding solutions to solve the uncontrollable releasing behaviors and associated mechanisms are necessary for better LBNPs for cancer immunotherapy in future clinical stages.
4. Conclusions and Future Perspectives
In recent decades, the importance of LBNP in gene delivery has been realized, and multiple strategies to improve these vehicles have been applied. In this review, we have discussed multiple kinds of LBNPs in various applications, particularly gene therapy and immunotherapy, with the roles of LBNP highlighted. Because of the significant advantages of gene delivery, research has been carried out for the purpose of improving vesicle stability, cellular uptake, and endosomal escape. In recent years, modification strategies rendered LBNP a mature and outstanding gene delivery system with feasible production procedures and acceptable costs. With the wide use of LBNP, not only gene therapies, but also conventional therapies, such as chemotherapy and immunotherapy to treat cancers, inherited diseases, infections, and immunodeficiencies, have been advanced as well. Despite the success in many preclinical studies and clinical cases, many barriers remain. The main challenge is that the ratio of contents such as helper lipids, ionizable lipids, and cholesterol that can process endosomal escape and be delivered to the target is far from being enough. The therapeutic effect could be further enhanced if this barrier is overcome. Additionally, although LBNP can protect drugs from degradation and excretion, the accumulation of the LBNP in the desired sites is limited, which leads to undesirable therapeutic effects and potential side effects. Moreover, there is a safety issue; for instance, some components in LBNP may cause irritation and inflammation and activate host immune responses. In addition, cationic lipids can cause cytotoxicity, and the short circulation time of the delivered LBNPs; these are other challenges that need much attention. In these cases, more fundamental studies are required. Once the understanding of LBNP in gene delivery is strengthened, the LBNP-based drug delivery platform will become more promising and advance in future clinical applications.
Author Contributions
J.Z. (Jingjing Zhang) and T.L., coordinated this project. C.Z., wrote this manuscript and compiled writings from other authors. Y.M., edited the whole manuscript and wrote the LBNP in the Treatment of Cancer Immunotherapy. J.Z. (Jing Zhu) wrote the LBNP in the Treatment of COVID-19. J.C.-T.K. and Z.Z. collected and summarized the literatures. C.Z. and H.X., drew the figures in the manuscript. J.Z. (Jingjing Zhang) and T.L., revised the manuscript obtained funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81773758) and the Natural Science Foundation of Guangdong Province (No. 2019A1515011934).
Institutional Review Board Statement
This review was completed with no requirement for Institutional Review Board Statement.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was generated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81773758) and the Natural Science Foundation of Guangdong Province (No. 2019A1515011934). All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gonçalves, G.A.R.; Paiva, R.d.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M. Non Viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Evers, M.M.; Toonen, L.J.A.; van Roon-Mom, W.M.C. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015, 87, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Muntoni, F.; Wood, M.J.A. Targeting RNA to treat neuromuscular disease. Nat. Rev. Drug Discov. 2011, 10, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Opalinska, J.B.; Gewirtz, A.M. Nucleic-acid therapeutics: Basic principles and recent applications. Nat. Rev. Drug Discov. 2002, 1, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, D.; Dinallo, V.; Marafini, I.; Figliuzzi, M.M.; Romano, B.; Monteleone, G. Antisense Oligonucleotide: Basic Concepts and Therapeutic Application in Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10, 305. [Google Scholar] [CrossRef]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense oligonucleotides. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef]
- Sharma, V.K.; Watts, J.K. Oligonucleotide therapeutics: Chemistry, delivery and clinical progress. Future Med. Chem. 2015, 7, 2221–2242. [Google Scholar] [CrossRef]
- Heidersbach, A.; Gaspar-Maia, A.; McManus, M.T.; Ramalho-Santos, M. RNA interference in embryonic stem cells and the prospects for future therapies. Gene Ther. 2006, 13, 478–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Levanova, A.; Poranen, M.M. RNA Interference as a Prospective Tool for the Control of Human Viral Infections. Front. Microbiol. 2018, 9, 2151. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Speiser, J.J.; Erşahin, Ç.; Osipo, C. The Functional Role of Notch Signaling in Triple-Negative Breast Cancer. Vitnam Horm. 2013, 93, 277–306. [Google Scholar] [CrossRef]
- Laganà, A.; Veneziano, D.; Russo, F.; Pulvirenti, A.; Giugno, R.; Croce, C.M.; Ferro, A. Computational Design of Artificial RNA Molecules for Gene Regulation. In RNA Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2015; pp. 393–412. [Google Scholar] [CrossRef]
- Kristen, A.V.; Ajroud-Driss, S.; Conceição, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener. Dis. Manag. 2019, 9, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Das, G.M.; Shi, Y.; Liu, C.; Liu, X.; Tang, D.G.; Wang, J. The microRNA miR-34a Inhibits Non-Small Cell Lung Cancer (NSCLC) Growth and the CD44hi Stem-Like NSCLC Cells. PLoS ONE 2014, 9, e90022. [Google Scholar] [CrossRef]
- Yoo, B.; Jordan, V.C.; Sheedy, P.; Billig, A.-M.; Ross, A.; Pantazopoulos, P.; Medarova, Z. RNAi-Mediated PD-L1 Inhibition for Pancreatic Cancer Immunotherapy. Sci. Rep. 2019, 9, 4712. [Google Scholar] [CrossRef]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a Lung Cancer Therapeutic Based on the Tumor Suppressor MicroRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, L.; Quek, C.; Bellingham, S.A.; Hill, A.F. Novel miR-29b target regulation patterns are revealed in two different cell lines. Sci. Rep. 2019, 9, 17449. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-R.; Yan, B.; Guo, Q.; Fu, F.-J.; Wang, Z.; Yin, Z.; Wei, Y.-B. The role of miR-29b in cancer: Regulation, function, and signaling. OncoTargets Ther. 2015, 2015, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kormann, M.; Rosenecker, J.; Rudolph, C. Current prospects for mRNA gene delivery. Eur. J. Pharm. Biopharm. 2009, 71, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-Based Vaccines in Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 794528. [Google Scholar] [CrossRef]
- Abu Abed, O.S. Gene therapy avenues and COVID-19 vaccines. Genes Immun. 2021, 22, 120–124. [Google Scholar] [CrossRef]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA Is an Endogenous Ligand for Toll-like Receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, S.; Fu, R.; Zhang, L.; Huang, K.; Peng, H.; Dai, L.; Chen, Q. Therapeutic Prospects of mRNA-Based Gene Therapy for Glioblastoma. Front. Oncol. 2019, 9, 1208. [Google Scholar] [CrossRef]
- Liang, X.; Li, D.; Leng, S.; Zhu, X. RNA-based pharmacotherapy for tumors: From bench to clinic and back. Biomed. Pharmacother. 2020, 125, 109997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, H.-H.; Yang, Y.; Hu, Y.; Zhang, L.; Blancafort, P.; Huang, L. Systemic Delivery of Modified mRNA Encoding Herpes Simplex Virus 1 Thymidine Kinase for Targeted Cancer Gene Therapy. Mol. Ther. 2013, 21, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Hadas, Y.; Sultana, N.; Youssef, E.; Sharkar, M.T.K.; Kaur, K.; Chepurko, E.; Zangi, L. Optimizing Modified mRNA In Vitro Synthesis Protocol for Heart Gene Therapy. Mol. Ther. Methods Clin. Dev. 2019, 14, 300–305. [Google Scholar] [CrossRef]
- Pardi, N.; LaBranche, C.C.; Ferrari, G.; Cain, D.W.; Tombácz, I.; Parks, R.J.; Muramatsu, H.; Mui, B.L.; Tam, Y.K.; Karikó, K.; et al. Characterization of HIV-1 Nucleoside-Modified mRNA Vaccines in Rabbits and Rhesus Macaques. Mol. Ther. Nucleic Acids 2019, 15, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Amorim, R.; Niu, M.; Breton, Y.; Tremblay, M.J.; Mouland, A.J. Host mRNA decay proteins influence HIV-1 replication and viral gene expression in primary monocyte-derived macrophages. Retrovirology 2019, 16, 3. [Google Scholar] [CrossRef]
- Jiang, C.; Mei, M.; Li, B.; Zhu, X.; Zu, W.; Tian, Y.; Wang, Q.; Guo, Y.; Dong, Y.; Tan, X. A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 2017, 27, 440–443. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, D.; Wang, Y.; Bai, M.; Tang, W.; Bao, S.; Yan, Z.; Li, D.; Li, J. Correction of a Genetic Disease in Mouse via Use of CRISPR-Cas9. Cell Stem Cell 2013, 13, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Bin Moon, S.; Lee, J.M.; Kang, J.G.; Lee, N.-E.; Ha, D.-I.; Kim, D.Y.; Kim, S.H.; Yoo, K.; Kim, D.; Ko, J.-H.; et al. Highly efficient genome editing by CRISPR-Cpf1 using CRISPR RNA with a uridinylate-rich 3′-overhang. Nat. Commun. 2018, 9, 3651. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Niu, Y.; Ji, W.; Dong, Y. Strategies for the CRISPR-Based Therapeutics. Trends Pharmacol. Sci. 2020, 41, 55–65. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.; Luo, X.; Zhang, X.; Li, C.; Zeng, C.; Dong, Y. Engineering CRISPR–Cpf1 crRNAs and mRNAs to maximize genome editing efficiency. Nat. Biomed. Eng. 2017, 1, 0066. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zeng, C.; Dong, Y. Design and assessment of engineered CRISPR–Cpf1 and its use for genome editing. Nat. Protoc. 2018, 13, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Glass, Z.; Chen, J.; Haas, M.; Jin, X.; Zhao, X.; Rui, X.; Ye, Z.; Li, Y.; Zhang, F.; et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. USA 2021, 118, e2020401118. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E.; Doudna, J.A. Rewriting a genome. Nature 2013, 495, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, Y.; Li, L.; Zhang, P.; Guo, H.; Liu, N.; Yang, X.; Xu, F. Engineering extracellular matrix to improve drug delivery for cancer therapy. Drug Discov. Today 2020, 25, 1727–1734. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Chonn, A.; Cullis, P.R. Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behaviour in vivo. Adv. Drug Deliv. Rev. 1998, 32, 3–17. [Google Scholar] [CrossRef]
- Sorrentino, S. Human extracellular ribonucleases: Multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell. Mol. Life Sci. 1998, 54, 785–794. [Google Scholar] [CrossRef]
- Harrison, E.B.; Azam, S.H.; Pecot, C.V. Targeting Accessories to the Crime: Nanoparticle Nucleic Acid Delivery to the Tumor Microenvironment. Front. Pharmacol. 2018, 9, 307. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 3151. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Zamboni, B.A.; Gabizon, A.; Schmeeda, H.; Amantea, M.; Gehrig, P.A.; Zamboni, W.C. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother. Pharmacol. 2011, 69, 43–50. [Google Scholar] [CrossRef]
- Halma, C.; Daha, M.R.; Van Es, L.A. In vivo clearance by the mononuclear phagocyte system in humans: An overview of methods and their interpretation. Clin. Exp. Immunol. 1992, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Monks, J.; Rosner, D.; Jon Geske, F.; Lehman, L.; Hanson, L.; Neville, M.C.; Fadok, V.A. Epithelial cells as phagocytes: Apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005, 12, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Veiga, E.; Cossart, P. The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol. 2006, 16, 499–504. [Google Scholar] [CrossRef]
- Robert, S.; Kleine-Vehn, J.; Barbez, E.; Sauer, M.; Paciorek, T.; Baster, P.; Vanneste, S.; Zhang, J.; Simon, S.; Čovanová, M.; et al. ABP1 Mediates Auxin Inhibition of Clathrin-Dependent Endocytosis in Arabidopsis. Cell 2010, 143, 111–121. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S.W.; Maher, M.; Tiwari, B.; Byrne, H.J.; Bourke, P.; et al. Cold Atmospheric Plasma Stimulates Clathrin-Dependent Endocytosis to Repair Oxidised Membrane and Enhance Uptake of Nanomaterial in Glioblastoma Multiforme Cells. Sci. Rep. 2020, 10, 6985. [Google Scholar] [CrossRef]
- Xu, B.-y.; Tang, X.-d.; Chen, J.; Wu, H.-b.; Chen, W.-s.; Chen, L. Rifampicin induces clathrin-dependent endocytosis and ubiquitin–proteasome degradation of MRP2 via oxidative stress-activated PKC-ERK/JNK/p38 and PI3K signaling pathways in HepG2 cells. Acta Pharmacol. Sin. 2019, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Kim, S.-W.; Park, J.-Y.; Kang, W.C.; Kang, Y.-J.; Khang, D. Suppression of human arthritis synovial fibroblasts inflammation using dexamethasone-carbon nanotubes via increasing caveolin-dependent endocytosis and recovering mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 5761–5779. [Google Scholar] [CrossRef] [PubMed]
- Puzik, K.; Tonnier, V.; Opper, I.; Eckert, A.; Zhou, L.; Kratzer, M.-C.; Noble, F.l.; Nienhaus, G.U.; Gradl, D. Lef1 regulates caveolin expression and caveolin dependent endocytosis, a process necessary for Wnt5a/Ror2 signaling during Xenopus gastrulation. Sci. Rep. 2019, 9, 15645. [Google Scholar] [CrossRef] [PubMed]
- Vocelle, D.; Chan, C.; Walton, S.P. Endocytosis Controls siRNA Efficiency: Implications for siRNA Delivery Vehicle Design and Cell-Specific Targeting. Nucleic Acid Ther. 2020, 30, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.; Nayak, P.; Mishra, V.; Sharma, M.; Saraogi, G.K. Treating blood cancer with nanotechnology: A paradigm shift. In Nano Drug Delivery Strategies for the Treatment of Cancers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 225–243. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Szoka, F.C. Lipid-based Nanoparticles for Nucleic Acid Delivery. Pharm. Res. 2007, 24, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Zuhorn, I.S.; Hoekstra, D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: Recent advances. J. Control. Release 2013, 166, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P.; Demeneix, B.; Loeffler, J.P.; Perez-Mutul, J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc. Natl. Acad. Sci. USA 1989, 86, 6982–6986. [Google Scholar] [CrossRef]
- Kunitake, T.; Okahata, Y. A totally synthetic bilayer membrane. J. Am. Chem. Soc. 2002, 99, 3860–3861. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Hoekstra, D. On the Mechanism of Cationic Amphiphile-mediated Transfection. To Fuse or not to Fuse: Is that the Question? J. Membr. Biol. 2002, 189, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Feng, J.; Liu, Y.; Che, J.; Bai, G.; Dong, X.; Wu, F.; Jin, T. A Synthetic Carrier of Nucleic Acids Structured as a Neutral Phospholipid Envelope Tightly Assembled on Polyplex Surface. Adv. Healthc. Mater. 2020, 9, 1901705. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Moss, K.H.; Popova, P.; Hadrup, S.R.; Astakhova, K.; Taskova, M. Lipid Nanoparticles for Delivery of Therapeutic RNA Oligonucleotides. Mol. Pharm. 2019, 16, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Gomez, A.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal Nanovesicles for Efficient Encapsulation of Staphylococcal Antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, A.; Sanna, V.; Duchemin, A.; Evrard, B.; Mottet, D.; Piel, G. Cationic Liposomes Carrying siRNA: Impact of Lipid Composition on Physicochemical Properties, Cytotoxicity and Endosomal Escape. Nanomaterials 2018, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamada, K.; Fujiwara, Y.; Sato, Y.; Harashima, H. Reducing the Cytotoxicity of Lipid Nanoparticles Associated with a Fusogenic Cationic Lipid in a Natural Killer Cell Line by Introducing a Polycation-Based siRNA Core. Mol. Pharm. 2018, 15, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.J.; Alishetty, S.; Alameh, M.-G.; Said, H.; Wright, L.; Paige, M.; Soliman, O.; Weissman, D.; Cleveland, T.E.; Grishaev, A.; et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun. Biol. 2021, 4, 956. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.-F.; Cai, J.-P.; Wong, W.-M.; Yip, C.C.-Y.; et al. Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model. Clin. Infect. Dis. 2021, 73, 2372–2373. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for Drug Delivery. J. Biotechnol. Biomater. 2017, 7, 276. [Google Scholar] [CrossRef]
- Fritze, A.; Hens, F.; Kimpfler, A.; Schubert, R.; Peschka-Süss, R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1633–1640. [Google Scholar] [CrossRef]
- Bolotin, E.M.; Cohen, R.; Bar, L.K.; Emanuel, N.; Ninio, S.; Barenholz, Y.; Lasic, D.D. Ammonium Sulfate Gradients for Efficient and Stable Remote Loading of Amphipathic Weak Bases into Liposomes and Ligandoliposomes. J. Liposome Res. 2008, 4, 455–479. [Google Scholar] [CrossRef]
- Alyane, M.; Barratt, G.; Lahouel, M. Remote loading of doxorubicin into liposomes by transmembrane pH gradient to reduce toxicity toward H9c2 cells. Saudi Pharm. J. 2016, 24, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Cui, F.-D.; Choi, M.-K.; Lin, H.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Liposome Formulation of Paclitaxel with Enhanced Solubility and Stability. Drug Deliv. 2008, 14, 301–308. [Google Scholar] [CrossRef]
- Lim, S.-J.; Hong, S.-S.; Choi, J.Y.; Kim, J.O.; Lee, M.-K.; Kim, S.H. Development of paclitaxel-loaded liposomal nanocarrier stabilized by triglyceride incorporation. Int. J. Nanomed. 2016, 11, 4465–4477. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, L. Chapter Two—Lipid Nanoparticles for Gene Delivery. Adv. Genet. 2014, 88, 13–36. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Myhre, J.L.; Chen, S.; Tam, Y.Y.C.; Danescu, A.; Richman, J.M.; Cullis, P.R. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Yung, B.C.; Li, J.; Zhang, M.; Cheng, X.; Li, H.; Yung, E.M.; Kang, C.; Cosby, L.E.; Liu, Y.; Teng, L.; et al. Lipid Nanoparticles Composed of Quaternary Amine–Tertiary Amine Cationic Lipid Combination (QTsome) for Therapeutic Delivery of AntimiR-21 for Lung Cancer. Mol. Pharm. 2016, 13, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, C.-C. Cationic solid lipid nanoparticles with primary and quaternary amines for release of saquinavir and biocompatibility with endothelia. Colloids Surf. B Biointerfaces 2013, 101, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Hsu, S.-H.; Zhou, C.; Wang, X.; Terp, M.C.; Wu, Y.; Teng, L.; Mao, Y.; Wang, F.; Xue, W.; et al. Lipid nanoparticles for hepatic delivery of small interfering RNA. Biomaterials 2012, 33, 5924–5934. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Zhou, C.; Teng, L.; Ren, W.; Yang, Z.; Shih, C.-H.; Wang, T.; Lee, R.J.; Tang, S.; et al. Insight into Mechanisms of Cellular Uptake of Lipid Nanoparticles and Intracellular Release of Small RNAs. Pharm. Res. 2014, 31, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, A.; Yang, Z.; Wang, X.; Chang, L.; Chen, Z.; James Lee, L. Application of DODMA and Derivatives in Cationic Nanocarriers for Gene Delivery. Curr. Org. Chem. 2016, 20, 1813–1819. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Dammer, E.B.; Ren, R.-J.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Wang, S.; Low, P.S. Measurement of endosome pH following folate receptor-mediated endocytosis. Biochim. Biophys. Acta Mol. Cell Res. 1996, 1312, 237–242. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.-L.; Lu, Z.-R. New Amphiphilic Carriers Forming pH-Sensitive Nanoparticles for Nucleic Acid Delivery. Langmuir 2010, 26, 13874–13882. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2014, 5, 123–127. [Google Scholar] [CrossRef]
- Desai, J.; Thakkar, H. Enhanced oral bioavailability and brain uptake of Darunavir using lipid nanoemulsion formulation. Colloids Surf. B Biointerfaces 2019, 175, 143–149. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J. Nanotechnol. 2012, 2012, 270383. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Chen, L.; Chen, B.; Deng, L.; Gao, B.; Zhang, Y.; Wu, C.; Yu, N.; Zhou, Q.; Yao, J.; Chen, J. An optimized two-vial formulation lipid nanoemulsion of paclitaxel for targeted delivery to tumor. Int. J. Pharm. 2017, 534, 308–315. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, Y.; Gan, L.; Nie, S.; Pan, W. PEGylated nanostructured lipid carriers loaded with 10-hydroxycamptothecin: An efficient carrier with enhanced anti-tumour effects against lung cancer. J. Pharm. Pharmacol. 2008, 60, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-P.; He, S.-N.; Li, Y.-L.; Feng, D.-L.; Lu, X.-Y.; Du, Y.-Z.; Yu, H.-Y.; Hu, F.-Q.; Yuan, H. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int. J. Nanomed. 2013, 8, 3141–3150. [Google Scholar] [CrossRef]
- Xue, H.; Guo, P.; Wen, W.-C.; Wong, H. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef]
- Del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Sánchez-Hernández, N.; García-Montoya, E.; Pérez-Lozano, P.; Suñé-Negre, J.M.; Ticó, J.R.; Suñé, C.; Miñarro, M. DNA delivery via cationic solid lipid nanoparticles (SLNs). Eur. J. Pharm. Sci. 2013, 49, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pink, D.L.; Loruthai, O.; Ziolek, R.M.; Wasutrasawat, P.; Terry, A.E.; Lawrence, M.J.; Lorenz, C.D. On the Structure of Solid Lipid Nanoparticles. Small 2019, 15, 1903156. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.M.; Mata, J.P.; Bryant, G.; Campo, L.; Ife, A.; Karpe, A.V.; Jadhav, S.R.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Structure Analysis of Solid Lipid Nanoparticles for Drug Delivery: A Combined USANS/SANS Study. Part. Part. Syst. Charact. 2018, 36, 1800359. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int. J. Pharm. 2006, 314, 83–89. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Zhang, J.; Yuan, Y.; Wang, J. Exosomes: A Novel Therapeutic Agent for Cartilage and Bone Tissue Regeneration. Dose Response 2019, 17, 1559325819892702. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, S.; Li, X.; Kim, B.Y.S.; Yang, Z.; Jiang, W. Extracellular Vesicles: An Emerging Nanoplatform for Cancer Therapy. Front. Oncol. 2020, 10, 606906. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Z.; Huntoon, K.; Jiang, W.; Kim, B.Y.S. Advanced Immunotherapy Approaches for Glioblastoma. Adv. Ther. 2021, 4, 2100046. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Xie, H.; Dong, S.; Rao, G.; Yang, Z.; Zhang, J.; Wu, Q. Extracellular Vesicles in the Treatment of Parkinson’s Disease: A Review. Curr. Med. Chem. 2021, 28, 6375–6394. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Y.; Zhao, H.; Yuan, Y.; Kim, B.Y.S. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1590. [Google Scholar] [CrossRef]
- He, X.; Ma, Y.; Xie, H.; Rao, G.; Yang, Z.; Zhang, J.; Feng, Z. Biomimetic Nanostructure Platform for Cancer Diagnosis Based on Tumor Biomarkers. Front. Bioeng. Biotechnol. 2021, 9, 687664. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, F.; Ma, Y.; Wang, J.; Li, H.; Zhang, J. Isolation and Detection Technologies of Extracellular Vesicles and Application on Cancer Diagnostic. Dose Response 2019, 17, 1559325819891004. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K.; et al. MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Mudgil, P.; Borchman, D.; Yappert, M.C.; Duran, D.; Cox, G.W.; Smith, R.J.; Bhola, R.; Dennis, G.R.; Whitehall, J.S. Lipid order, saturation and surface property relationships: A study of human meibum saturation. Exp. Eye Res. 2013, 116, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Jendrasiak, G.L.; Mendible, J.C. The effect of the phase transition on the hydration and electrical conductivity of phospholipids. Biochim. Biophys. Acta Lipids Lipid Metab. 1976, 424, 133–148. [Google Scholar] [CrossRef]
- Stanton, M.G.; Colletti, S.L. Medicinal Chemistry of siRNA Delivery. J. Med. Chem. 2010, 53, 7887–7901. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Iwao, Y.; Noguchi, S.; Itai, S. Effect of Alkyl Chain Length and Unsaturation of the Phospholipid on the Physicochemical Properties of Lipid Nanoparticles. Chem. Pharm. Bull. 2015, 63, 731–736. [Google Scholar] [CrossRef]
- Hajj, K.A.; Melamed, J.R.; Chaudhary, N.; Lamson, N.G.; Ball, R.L.; Yerneni, S.S.; Whitehead, K.A. A Potent Branched-Tail Lipid Nanoparticle Enables Multiplexed mRNA Delivery and Gene Editing In Vivo. Nano Lett. 2020, 20, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J.Y. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef]
- Wasungu, L.; Hoekstra, D. Cationic lipids, lipoplexes and intracellular delivery of genes. J. Control. Release 2006, 116, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Chen, H.-L.; Liou, W.; Lin, H.-K.; Liu, W.-L. Mesomorphic Complexes of DNA with the Mixtures of a Cationic Surfactant and a Neutral Lipid. Langmuir 2005, 21, 9426–9431. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.E.; Drummond, D.C.; Kirpotin, D.B.; Zheng, W.W.; Noble, C.O.; Park, J.W.; Marks, J.D.; Benz, C.C.; Hong, K. Genospheres: Self-assembling nucleic acid-lipid nanoparticles suitable for targeted gene delivery. Gene Ther. 2005, 13, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Colombani, T.; Peuziat, P.; Dallet, L.; Haudebourg, T.; Mével, M.; Berchel, M.; Lambert, O.; Habrant, D.; Pitard, B. Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery. J. Control. Release 2017, 249, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Templeton, N.S.; Lasic, D.D.; Frederik, P.M.; Strey, H.H.; Roberts, D.D.; Pavlakis, G.N. Improved DNA: Liposome complexes for increased systemic delivery and gene expression. Nat. Biotechnol. 1997, 15, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Zon, G.; Rait, A.; Zhou, Q.; Yu, W.; Hogrefe, R.; Chang, E.H. Tumor-Targeting Nanoimmunoliposome Complex for Short Interfering RNA Delivery. Hum. Gene Ther. 2006, 17, 117–124. [Google Scholar] [CrossRef]
- Ciani, L.; Ristori, S.; Salvati, A.; Calamai, L.; Martini, G. DOTAP/DOPE and DC-Chol/DOPE lipoplexes for gene delivery: Zeta potential measurements and electron spin resonance spectra. Biochim. Biophys. Acta Biomembr. 2004, 1664, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, J.; He, F.; Wilson, A.; Pitt, B.; Li, S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem. Biophys. Res. Commun. 2005, 330, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Yu, B.; Wang, X.; Lu, Y.; Schmidt, C.R.; Lee, R.J.; Lee, L.J.; Jacob, S.T.; Ghoshal, K. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2017, 50, 33–44. [Google Scholar] [CrossRef]
- Wang, D.; Galla, H.-J.; Drücker, P. Membrane interactions of ionic liquids and imidazolium salts. Biophys. Rev. 2018, 10, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Richter, C.; Rühling, A.; Drücker, P.; Siegmund, D.; Metzler-Nolte, N.; Glorius, F.; Galla, H.-J. A Remarkably Simple Class of Imidazolium-Based Lipids and Their Biological Properties. Chem. Eur. J. 2015, 21, 15123–15126. [Google Scholar] [CrossRef]
- Ilies, M.A.; Seitz, W.A.; Ghiviriga, I.; Johnson, B.H.; Miller, A.; Thompson, E.B.; Balaban, A.T. Pyridinium Cationic Lipids in Gene Delivery: A Structure-Activity Correlation Study. J. Med. Chem. 2004, 47, 3744–3754. [Google Scholar] [CrossRef]
- Guénin, E.; Hervé, A.-C.; Floch, V.; Loisel, S.; Yaouanc, J.-J.; Clément, J.-C.; Férec, C.; des Abbayes, H. Cationic Phosphonolipids Containing Quaternary Phosphonium and Arsonium Groups for DNA Transfection with Good Efficiency and Low Cellular Toxicity. Angew. Chem. Int. Ed. 2000, 39, 629–631. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Oudrhiri, N.; Fauquet, M.; Vergely, L.; Bradley, J.C.; Basseville, M.; Lehn, P.; Lehn, J.M. Guanidinium-cholesterol cationic lipids: Efficient vectors for the transfection of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 9682–9686. [Google Scholar] [CrossRef]
- Meka, R.R.; Godeshala, S.; Marepally, S.; Thorat, K.; Reddy Rachamalla, H.K.; Dhayani, A.; Hiwale, A.; Banerjee, R.; Chaudhuri, A.; Vemula, P.K. Asymmetric cationic lipid based non-viral vectors for an efficient nucleic acid delivery. RSC Adv. 2016, 6, 77841–77848. [Google Scholar] [CrossRef]
- Khazanov, E.; Simberg, D.; Barenholz, Y. Lipoplexes prepared from cationic liposomes and mammalian DNA induce CpG-independent, direct cytotoxic effects in cell cultures and in mice. J. Gene Med. 2006, 8, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Dass, C.R. Cytotoxicity issues pertinent to lipoplex-mediated gene therapy in-vivo. J. Pharm. Pharmacol. 2002, 54, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.L.; Nguyen, J.; Tiffany, M.R.; Szoka, F.C. Synthesis, Characterization, and Evaluation of Ionizable Lysine-Based Lipids for siRNA Delivery. Bioconjug. Chem. 2012, 24, 36–43. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control. Release 2005, 107, 276–287. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Cationic Lipids: Molecular Structure/Transfection Activity Relationships and Interactions with Biomembranes. In Nucleic Acid Transfection; Springer: Berlin/Heidelberg, Germany, 2010; pp. 51–93. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Hajj, K.A.; Ball, R.L.; Deluty, S.B.; Singh, S.R.; Strelkova, D.; Knapp, C.M.; Whitehead, K.A. Branched-Tail Lipid Nanoparticles Potently Deliver mRNA In Vivo due to Enhanced Ionization at Endosomal pH. Small 2019, 15, 1805097. [Google Scholar] [CrossRef]
- Miao, L.; Lin, J.; Huang, Y.; Li, L.; Delcassian, D.; Ge, Y.; Shi, Y.; Anderson, D.G. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 2020, 11, 2424. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Schmidt, M.L.; Bodnariuc, I.; Kulkarni, J.A.; Leung, S.S.W.; Cullis, P.R.; Thewalt, J.L.; Tieleman, D.P. Ionizable amino lipid interactions with POPC: Implications for lipid nanoparticle function. Nanoscale 2019, 11, 14141–14146. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the Potency of siRNA Lipid Nanoparticles for Hepatic Gene Silencing In Vivo. Angew. Chem. 2012, 124, 8657–8661. [Google Scholar] [CrossRef]
- Zukancic, D.; Suys, E.J.A.; Pilkington, E.H.; Algarni, A.; Al-Wassiti, H.; Truong, N.P. The Importance of Poly(ethylene glycol) and Lipid Structure in Targeted Gene Delivery to Lymph Nodes by Lipid Nanoparticles. Pharmaceutics 2020, 12, 1068. [Google Scholar] [CrossRef] [PubMed]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef] [PubMed]
- Viger-Gravel, J.; Schantz, A.; Pinon, A.C.; Rossini, A.J.; Schantz, S.; Emsley, L. Structure of Lipid Nanoparticles Containing siRNA or mRNA by Dynamic Nuclear Polarization-Enhanced NMR Spectroscopy. J. Phys. Chem. B 2018, 122, 2073–2081. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Gil, S.; Andrieux, K.; Nicolas, V.; Appel, M.; Chacun, H.; Desmaële, D.; Taran, F.; Georgin, D.; Couvreur, P. Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells. Cell. Mol. Life Sci. 2007, 64, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Zhigaltsev, I.V.; Maurer, N.; Wong, K.F.; Cullis, P.R. Triggered release of doxorubicin following mixing of cationic and anionic liposomes. Biochim. Biophys. Acta Biomembr. 2002, 1565, 129–135. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Paunovska, K.; Gil, C.J.; Lokugamage, M.P.; Sago, C.D.; Sato, M.; Lando, G.N.; Gamboa Castro, M.; Bryksin, A.V.; Dahlman, J.E. Analyzing 2000 in Vivo Drug Delivery Data Points Reveals Cholesterol Structure Impacts Nanoparticle Delivery. ACS Nano 2018, 12, 8341–8349. [Google Scholar] [CrossRef]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative Effects of Cholesterol and β-Sitosterol on the Liposome Membrane Characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Bernsdorff, C.; Winter, R. Differential Properties of the Sterols Cholesterol, Ergosterol, β-Sitosterol, trans-7-Dehydrocholesterol, Stigmasterol and Lanosterol on DPPC Bilayer Order. J. Phys. Chem. B 2003, 107, 10658–10664. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Morla-Folch, J.; Guerrini, L.; Pazos-Perez, N.; Arenal, R.; Alvarez-Puebla, R.A. Synthesis and Optical Properties of Homogeneous Nanoshurikens. ACS Photonics 2014, 1, 1237–1244. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified Nanocarriers for Tumor-targeted and Intracellular Delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Suzuki, Y.; Hihara, T.; Kubara, K.; Kondo, K.; Hyodo, K.; Yamazaki, K.; Ishida, T.; Ishihara, H. PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: Faster PEG shedding attenuates anti-PEG IgM production. Int. J. Pharm. 2020, 588, 119792. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Ishida, E.; Hashimoto, K.; Kobayashi, H.; Aoki, T.; Yasuda, J.; Obata, K.; Kikuchi, H.; Ishida, T.; et al. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int. J. Pharm. 2007, 342, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Baranyi, L.; Savay, S.; Milosevits, J.; Bunger, R.; Laverman, P.; Metselaar, J.M.; Storm, G.; Chanan-Khan, A.; Liebes, L.; et al. Role of Complement Activation in Hypersensitivity Reactions to Doxil and Hynic Peg Liposomes: Experimental and Clinical Studies. J. Liposome Res. 2002, 12, 165–172. [Google Scholar] [CrossRef]
- Huntington, J.; Pachauri, M.; Ali, H.; Giacca, M. RNA interference therapeutics for cardiac regeneration. Curr. Opin. Genet. Dev. 2021, 70, 48–53. [Google Scholar] [CrossRef]
- Smola, A.; Samadzadeh, S.; Müller, L.; Adams, O.; Homey, B.; Albrecht, P.; Meller, S. Omalizumab prevents anaphylactoid reactions to mRNA COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e743–e745. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Maltezou, H.C.; Tsakris, A.; Poland, G.A.; Anastassopoulou, C. Anaphylactic reactions to mRNA COVID-19 vaccines: A call for further study. Vaccine 2021, 39, 2605–2607. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sze, R.; Zhang, M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J. Biomed. Mater. Res. Part A 2006, 78A, 550–557. [Google Scholar] [CrossRef]
- Sega, E.I.; Low, P.S. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008, 27, 655–664. [Google Scholar] [CrossRef]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R., Jr.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Nag, M.; Gajbhiye, V.; Kesharwani, P.; Jain, N.K. Transferrin functionalized chitosan-PEG nanoparticles for targeted delivery of paclitaxel to cancer cells. Colloids Surf. B Biointerfaces 2016, 148, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Sun, Y.; Cai, C.; Fan, R.; Guo, R.; Xie, D. Targeted delivery of DOX by transferrin conjugated DSPE-PEG nanoparticles in leukemia therapy. Int. J. Polym. Mater. Polym. Biomater. 2019, 70, 27–36. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Wightman, L.; Wagner, E. Design and gene delivery activity of modified polyethylenimines. Adv. Drug Deliv. Rev. 2001, 53, 341–358. [Google Scholar] [CrossRef]
- Song, X.; Huang, Q.; Jin, B.; Peng, R. Fabrication and characterization of CL-20/PEI/GO composites with enhanced thermal stability and desensitization via electrostatic self-assembly. Appl. Surf. Sci. 2021, 558, 149933. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “Proton Sponge ” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Brunot, C.; Ponsonnet, L.; Lagneau, C.; Farge, P.; Picart, C.; Grosgogeat, B. Cytotoxicity of polyethyleneimine (PEI), precursor base layer of polyelectrolyte multilayer films. Biomaterials 2007, 28, 632–640. [Google Scholar] [CrossRef]
- Neu, M.; Germershaus, O.; Behe, M.; Kissel, T. Bioreversibly crosslinked polyplexes of PEI and high molecular weight PEG show extended circulation times in vivo. J. Control. Release 2007, 124, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Neu, M.; Germershaus, O.; Merkel, O.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Influence of Polyethylene Glycol Chain Length on the Physicochemical and Biological Properties of Poly(ethylene imine)-graft-Poly(ethylene glycol) Block Copolymer/SiRNA Polyplexes. Bioconjug. Chem. 2006, 17, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Merkel, O.M.; Beyerle, A.; Librizzi, D.; Pfestroff, A.; Behr, T.M.; Sproat, B.; Barth, P.J.; Kissel, T. Nonviral siRNA Delivery to the Lung: Investigation of PEG−PEI Polyplexes and Their In Vivo Performance. Mol. Pharm. 2009, 6, 1246–1260. [Google Scholar] [CrossRef] [PubMed]
- Gasset, M.; Hu, J.; Zhu, M.; Liu, K.; Fan, H.; Zhao, W.; Mao, Y.; Zhang, Y. A Biodegradable Polyethylenimine-Based Vector Modified by Trifunctional Peptide R18 for Enhancing Gene Transfection Efficiency In Vivo. PLoS ONE 2016, 11, e0166673. [Google Scholar] [CrossRef]
- Zheng, B.I.N.; Yang, S.; Tian, Q.; Xie, Y.I.N.; Zhang, S.; Lee, R.J. Delivery of Antisense Oligonucleotide LOR-2501 Using Transferrin-conjugated Polyethylenimine-based Lipid Nanoparticle. Anticancer Res. 2019, 39, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, G.; He, B.; Li, L.; Li, C.; Lai, Y.; Xu, X.; Gu, Z. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int. J. Nanomed. 2012, 2012, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Sriraman, S.K.; Navarro, G.; Biswas, S.; Dalvi, R.A.; Torchilin, V.P. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials 2012, 33, 3942–3951. [Google Scholar] [CrossRef]
- Wiseman, J.W.; Goddard, C.A.; McLelland, D.; Colledge, W.H. A comparison of linear and branched polyethylenimine (PEI) with DCChol/DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene Ther. 2003, 10, 1654–1662. [Google Scholar] [CrossRef]
- Witzigmann, D.; Kulkarni, J.A.; Leung, J.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv. Drug Deliv. Rev. 2020, 159, 344–363. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Passarelli, C.; Ferlini, A. Nanoparticle Delivery of Antisense Oligonucleotides and Their Application in the Exon Skipping Strategy for Duchenne Muscular Dystrophy. Nucleic Acid Ther. 2014, 24, 87–100. [Google Scholar] [CrossRef]
- Hair, P.; Cameron, F.; McKeage, K. Mipomersen Sodium: First Global Approval. Drugs 2013, 73, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A. Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1999, 1440, 1–31. [Google Scholar] [CrossRef]
- Gelsinger, C.; Steinhagen-Thiessen, E.; Kassner, U. Therapeutic Potential of Mipomersen in the Management of Familial Hypercholesterolaemia. Drugs 2012, 72, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Patel, N.J.; Bhowmik, T.; D’Souza, B.; Akalkotkar, A.; Etzlar, F.; Oettinger, C.W.; D’Souza, M. Enhanced bioavailability of orally administered antisense oligonucleotide to nuclear factor kappa B mRNA after microencapsulation with albumin. J. Drug Target. 2013, 21, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Yokota, T. An Overview of Recent Advances and Clinical Applications of Exon Skipping and Splice Modulation for Muscular Dystrophy and Various Genetic Diseases. In Exon Skipping and Inclusion Therapies; Springer: Berlin/Heidelberg, Germany, 2018; pp. 31–55. [Google Scholar] [CrossRef]
- Dzierlega, K.; Yokota, T. Optimization of antisense-mediated exon skipping for Duchenne muscular dystrophy. Gene Ther. 2020, 27, 407–416. [Google Scholar] [CrossRef]
- Kole, R.; Leppert, B.J. Targeting mRNA splicing as a potential treatment for Duchenne muscular dystrophy. Discov. Med. 2012, 14, 59–69. [Google Scholar]
- Let’s talk about lipid nanoparticles. Nat. Rev. Mater. 2021, 6, 99. [CrossRef]
- Ruberg, F.L.; Berk, J.L. Transthyretin (TTR) Cardiac Amyloidosis. Circulation 2012, 126, 1286–1300. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, S.; Rahman, S.; Singh, L.R. The Extracellular Protein, Transthyretin Is an Oxidative Stress Biomarker. Front. Physiol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Waddington-Cruz, M.; Ackermann, E.J.; Polydefkis, M.; Heitner, S.B.; Dyck, P.J.; Barroso, F.A.; Wang, A.K.; Berk, J.L.; Dyck, P.J.B.; Monia, B.P.; et al. Hereditary transthyretin amyloidosis: Baseline characteristics of patients in the NEURO-TTR trial. Amyloid 2018, 25, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Patisiran for the treatment of hereditary transthyretin-mediated amyloidosis. Expert Rev. Clin. Pharmacol. 2019, 12, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Keam, S.J. Lumasiran: First Approval. Drugs 2021, 81, 277–282. [Google Scholar] [CrossRef]
- Jaklevic, M.C. First Drug Approved for Rare Genetic Disorder Affecting Kidneys. JAMA 2021, 325, 214. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Landen, C.N.; Chavez-Reyes, A.; Bucana, C.; Schmandt, R.; Deavers, M.T.; Lopez-Berestein, G.; Sood, A.K. TherapeuticEphA2Gene TargetingIn vivoUsing Neutral Liposomal Small Interfering RNA Delivery. Cancer Res. 2005, 65, 6910–6918. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Wood, K.M.; Rao, V.P.; Morin, J.; Bhamidipaty, S.; LaBranche, T.P.; Gooch, R.L.; Bozal, F.; Bulawa, C.E.; Guild, B.C. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci. Rep. 2016, 6, 20019. [Google Scholar] [CrossRef]
- Truong, B.; Allegri, G.; Liu, X.-B.; Burke, K.E.; Zhu, X.; Cederbaum, S.D.; Häberle, J.; Martini, P.G.V.; Lipshutz, G.S. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 21150–21159. [Google Scholar] [CrossRef]
- Hauser, S.; Poenisch, M.; Schelling, Y.; Höflinger, P.; Schuster, S.; Teegler, A.; Betten, R.; Gustafsson, J.-Å.; Hübener-Schmid, J.; Schlake, T.; et al. mRNA as a Novel Treatment Strategy for Hereditary Spastic Paraplegia Type 5. Mol. Ther. Methods Clin. Dev. 2019, 15, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Stambas, J.; Magini, D.; Giovani, C.; Mangiavacchi, S.; Maccari, S.; Cecchi, R.; Ulmer, J.B.; De Gregorio, E.; Geall, A.J.; Brazzoli, M.; et al. Self-Amplifying mRNA Vaccines Expressing Multiple Conserved Influenza Antigens Confer Protection against Homologous and Heterosubtypic Viral Challenge. PLoS ONE 2016, 11, e0161193. [Google Scholar] [CrossRef]
- Hekele, A.; Bertholet, S.; Archer, J.; Gibson, D.G.; Palladino, G.; Brito, L.A.; Otten, G.R.; Brazzoli, M.; Buccato, S.; Bonci, A.; et al. Rapidly produced SAM® vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes Infect. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Brazzoli, M.; Magini, D.; Bonci, A.; Buccato, S.; Giovani, C.; Kratzer, R.; Zurli, V.; Mangiavacchi, S.; Casini, D.; Brito, L.M.; et al. Induction of Broad-Based Immunity and Protective Efficacy by Self-amplifying mRNA Vaccines Encoding Influenza Virus Hemagglutinin. J. Virol. 2016, 90, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, G.; Ols, S.; Liang, F.; Thompson, E.A.; Lin, A.; Hellgren, F.; Bahl, K.; John, S.; Yuzhakov, O.; Hassett, K.J.; et al. Induction of Robust B Cell Responses after Influenza mRNA Vaccination Is Accompanied by Circulating Hemagglutinin-Specific ICOS+ PD-1+ CXCR3+ T Follicular Helper Cells. Front. Immunol. 2017, 8, 1539. [Google Scholar] [CrossRef]
- McCullough, K.C.; Bassi, I.; Milona, P.; Suter, R.; Thomann-Harwood, L.; Englezou, P.; Démoulins, T.; Ruggli, N. Self-replicating Replicon-RNA Delivery to Dendritic Cells by Chitosan-nanoparticles for Translation In Vitro and In Vivo. Mol. Ther. Nucleic Acids USA 2014, 3, e173. [Google Scholar] [CrossRef]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Aldrich, C.; Leroux–Roels, I.; Huang, K.B.; Bica, M.A.; Loeliger, E.; Schoenborn-Kellenberger, O.; Walz, L.; Leroux-Roels, G.; von Sonnenburg, F.; Oostvogels, L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine 2021, 39, 1310–1318. [Google Scholar] [CrossRef]
- Armbruster, N.; Jasny, E.; Petsch, B. Advances in RNA Vaccines for Preventive Indications: A Case Study of A Vaccine Against Rabies. Vaccines 2019, 7, 132. [Google Scholar] [CrossRef]
- Mehrjardi, M.Z. Is Zika Virus an Emerging TORCH Agent? An Invited Commentary. Virol. Res. Treat. 2017, 8, 1178122X17708993. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, J.H.; Khandhar, A.P.; Guderian, J.; Granger, B.; Archer, J.; Archer, M.; Gage, E.; Fuerte-Stone, J.; Larson, E.; Lin, S.; et al. A Nanostructured Lipid Carrier for Delivery of a Replicating Viral RNA Provides Single, Low-Dose Protection against Zika. Mol. Ther. 2018, 26, 2507–2522. [Google Scholar] [CrossRef]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Alhariri, M.; Majrashi, M.A.; Bahkali, A.H.; Almajed, F.S.; Azghani, A.O.; Khiyami, M.; Alyamani, E.J.; Aljohani, S.M.; Halwani, M.A. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int. J. Nanomed. 2017, 12, 6949–6961. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.P.; Carvalho, K.V.; de Oliveira Aguiar Soares, R.D.; Carneiro, C.M.; de Andrade, M.H.G.; Duarte, R.S.; dos Santos, O.D.H. Functionalized rifampicin-loaded nanostructured lipid carriers enhance macrophages uptake and antimycobacterial activity. Colloids Surf. B Biointerfaces 2019, 175, 306–313. [Google Scholar] [CrossRef]
- Bogers, W.M.; Oostermeijer, H.; Mooij, P.; Koopman, G.; Verschoor, E.J.; Davis, D.; Ulmer, J.B.; Brito, L.A.; Cu, Y.; Banerjee, K.; et al. Potent Immune Responses in Rhesus Macaques Induced by Nonviral Delivery of a Self-amplifying RNA Vaccine Expressing HIV Type 1 Envelope with a Cationic Nanoemulsion. J. Infect. Dis. 2014, 211, 947–955. [Google Scholar] [CrossRef]
- Brito, L.A.; Kommareddy, S.; Maione, D.; Uematsu, Y.; Giovani, C.; Berlanda Scorza, F.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W.; et al. Self-Amplifying mRNA Vaccines. Adv. Genet. 2015, 89, 179–233. [Google Scholar] [CrossRef]
- Meyer, M.; Huang, E.; Yuzhakov, O.; Ramanathan, P.; Ciaramella, G.; Bukreyev, A. Modified mRNA-Based Vaccines Elicit Robust Immune Responses and Protect Guinea Pigs From Ebola Virus Disease. J. Infect. Dis. 2018, 217, 451–455. [Google Scholar] [CrossRef]
- Cully, M. New tricks to treat Ebola. Nat. Rev. Drug Discov. 2016, 15, 675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.-H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef]
- Haynes, B.F. A New Vaccine to Battle Covid-19. N. Engl. J. Med. 2021, 384, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Amiji, M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: Impact on translational nanomedicine. Drug Deliv. Transl. Res. 2021, 11, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Nguyen, G.N.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; et al. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci. Adv. 2020, 6, eabc2315. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Love, K.T.; Chen, Y.; Eltoukhy, A.A.; Kastrup, C.; Sahay, G.; Jeon, A.; Dong, Y.; Whitehead, K.A.; Anderson, D.G. Rapid Discovery of Potent siRNA-Containing Lipid Nanoparticles Enabled by Controlled Microfluidic Formulation. J. Am. Chem. Soc. 2012, 134, 6948–6951. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.B.; Sun, L.; Chen, Z.J. Antiviral innate immunity pathways. Cell Res. 2006, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Srinivas, R.; Garu, A.; Moku, G.; Agawane, S.B.; Chaudhuri, A. A long-lasting dendritic cell DNA vaccination system using lysinylated amphiphiles with mannose-mimicking head-groups. Biomaterials 2012, 33, 6220–6229. [Google Scholar] [CrossRef]
- Sayour, E.J.; De Leon, G.; Pham, C.; Grippin, A.; Kemeny, H.; Chua, J.; Huang, J.; Sampson, J.H.; Sanchez-Perez, L.; Flores, C.; et al. Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. Oncoimmunology 2017, 6, e1256527. [Google Scholar] [CrossRef]
- Kapadia, C.H.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Nanoparticulate immunotherapy for cancer. J. Control. Release 2015, 219, 167–180. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).