Facile Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity
Abstract
:1. Introduction
2. Results and Discussion
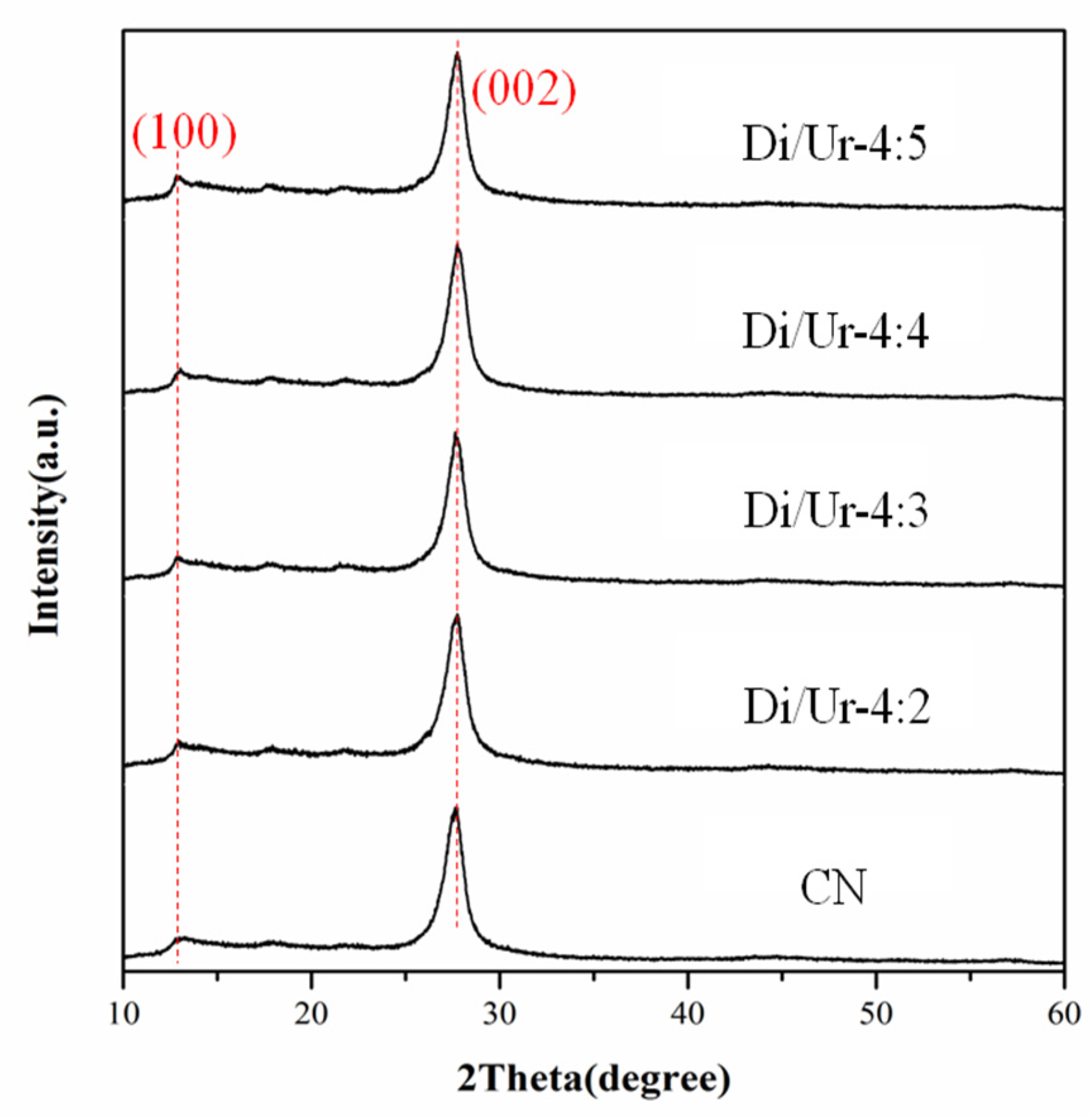
2.1. XRD Analysis
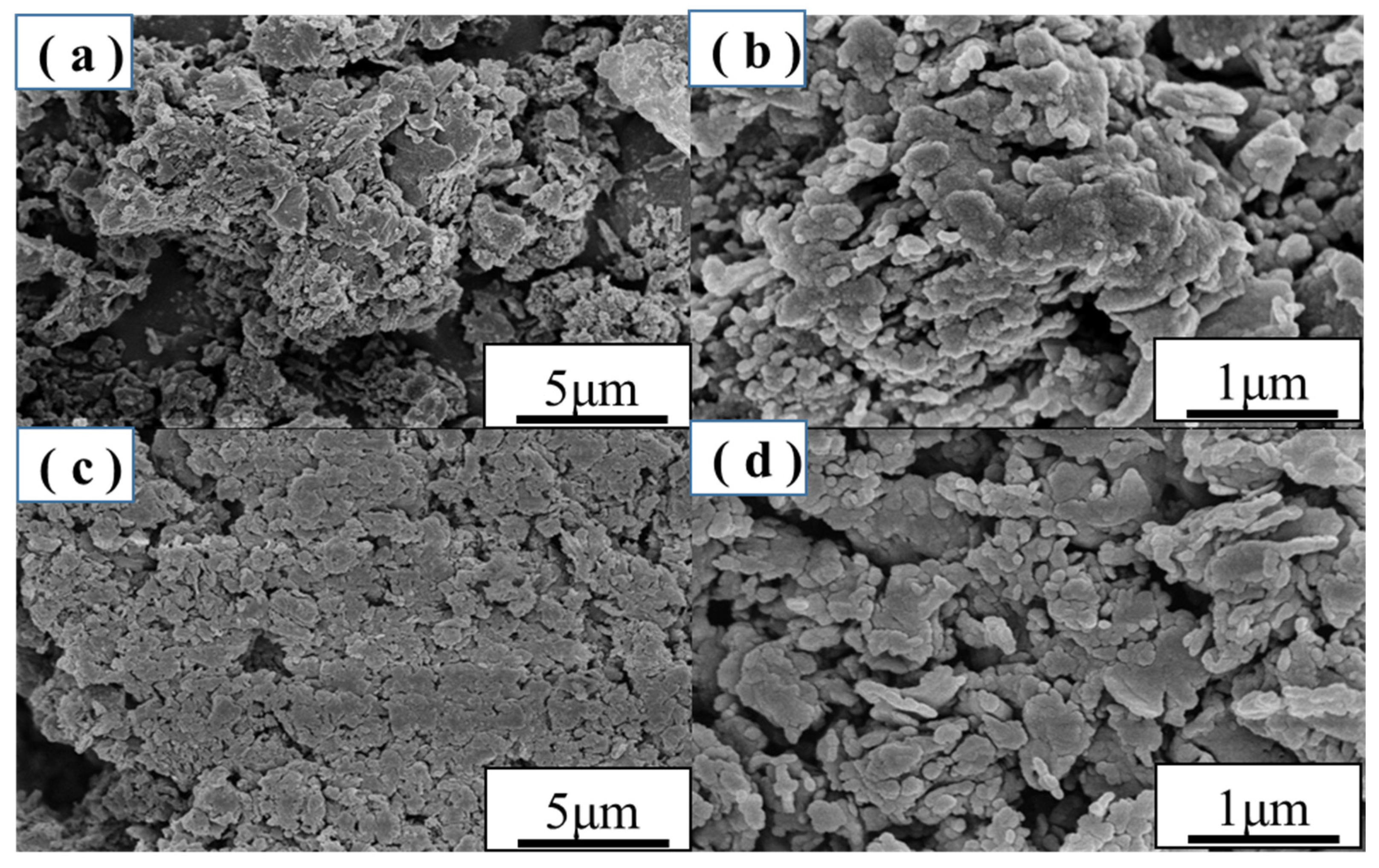
2.2. SEM Analysis
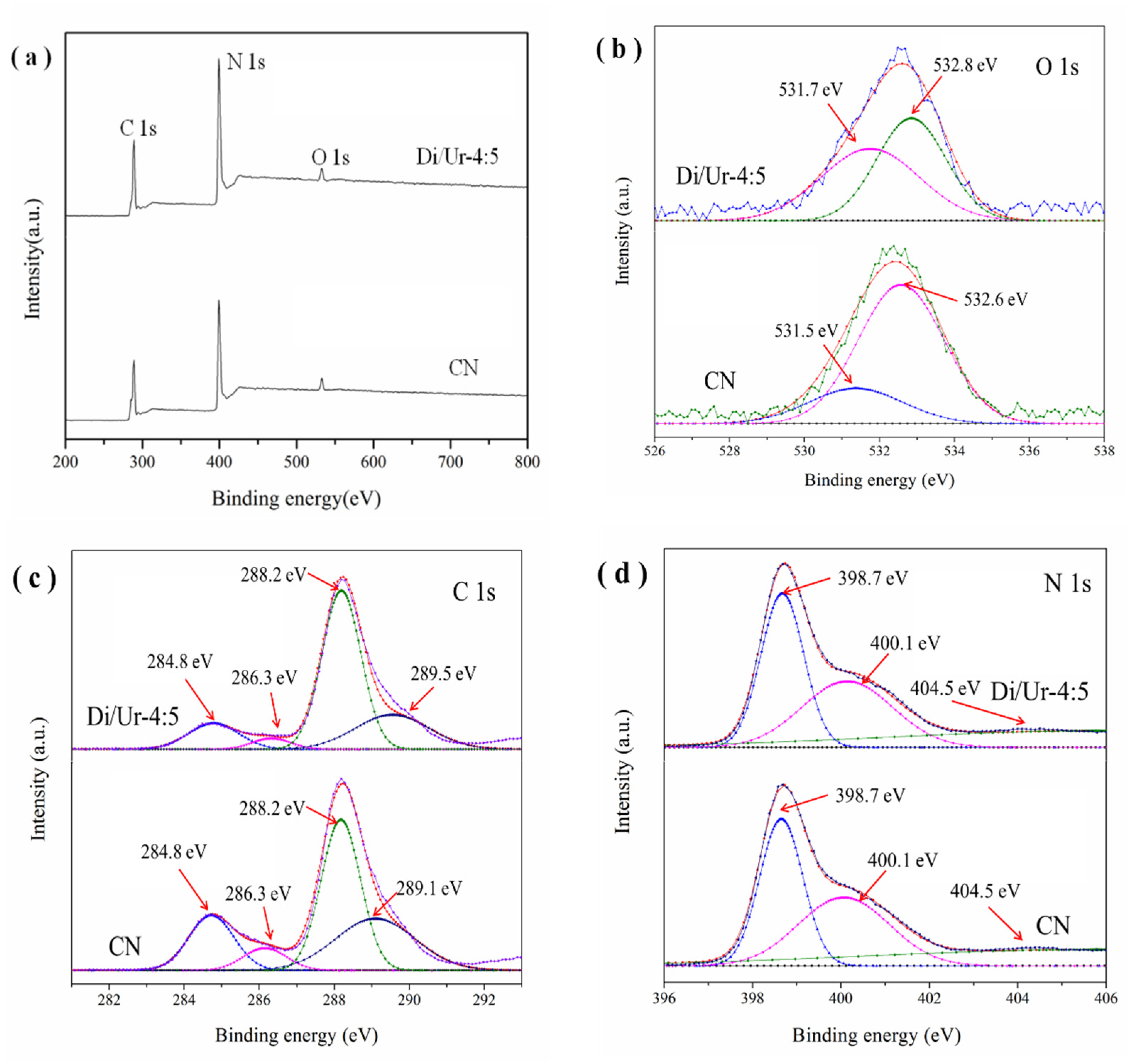
2.3. XPS Analysis
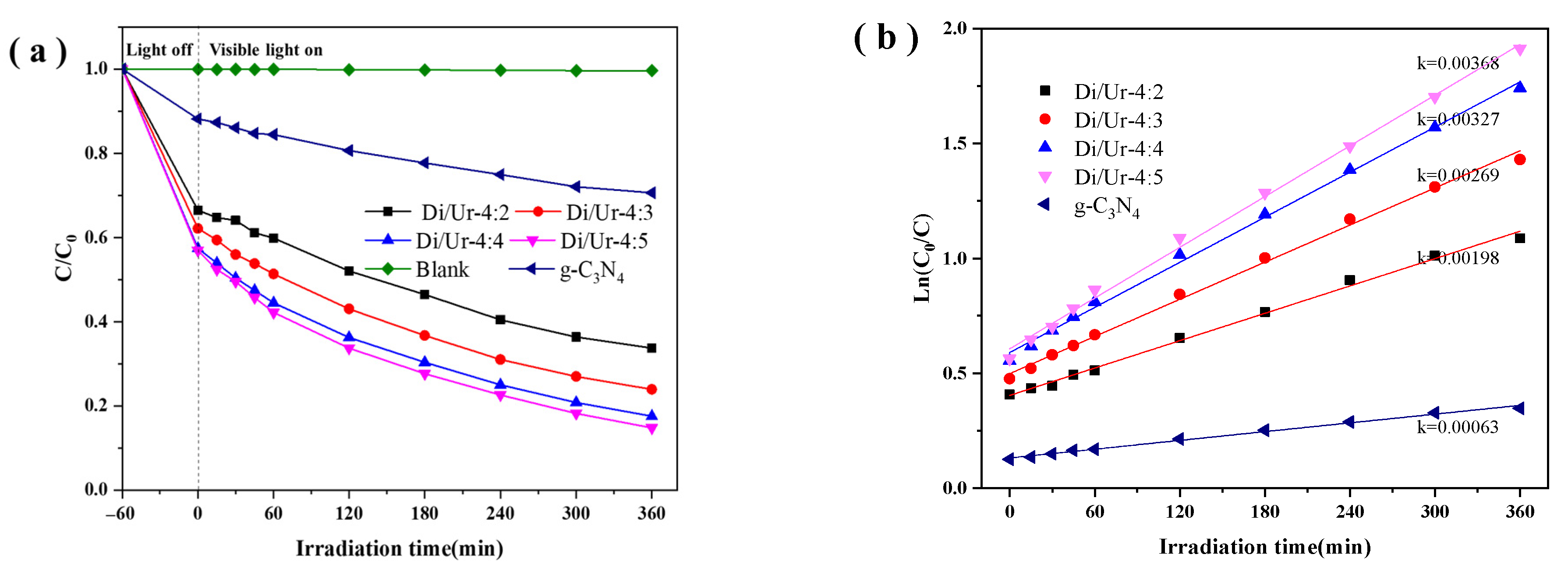
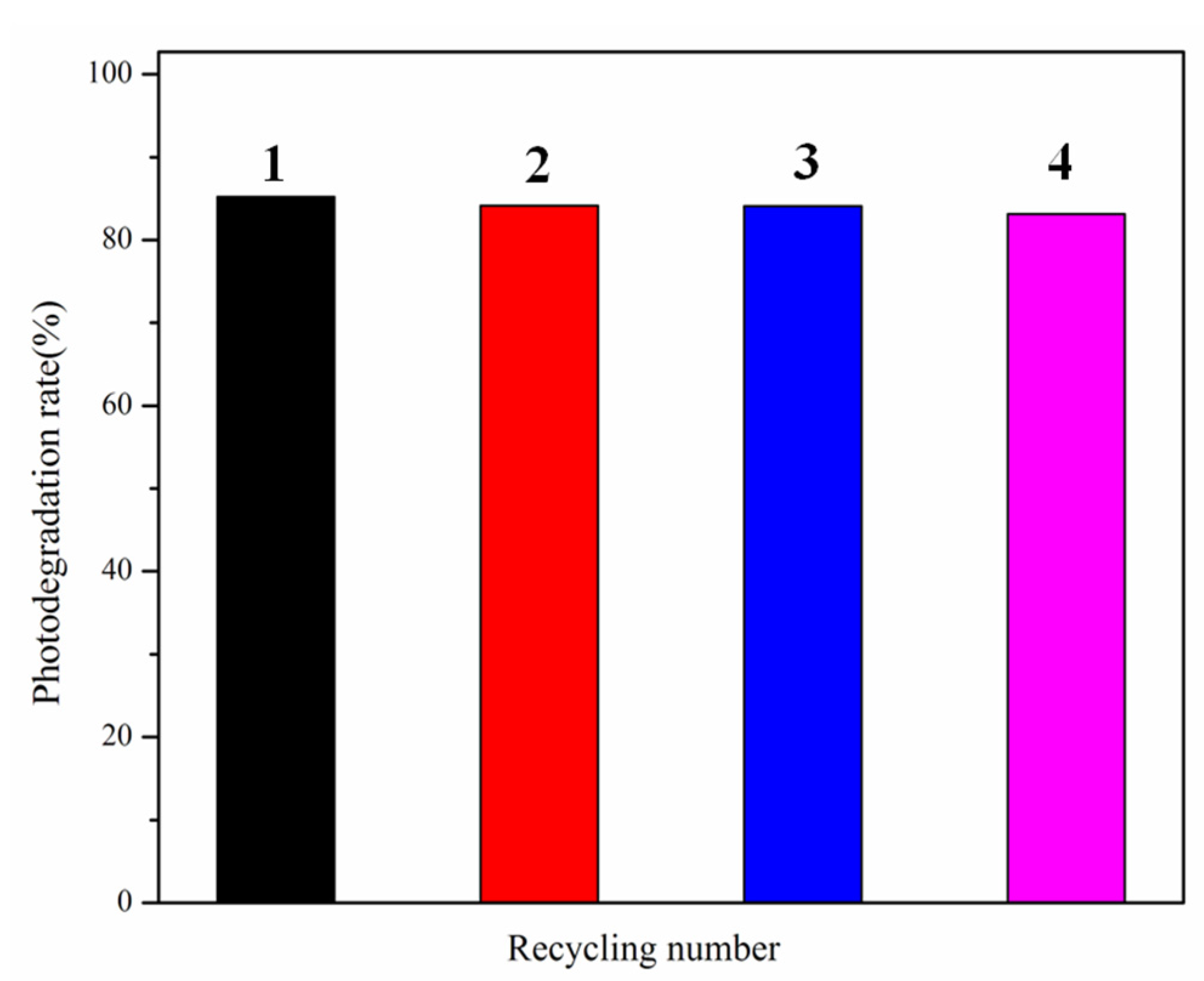
2.4. Photocatalytic Performance
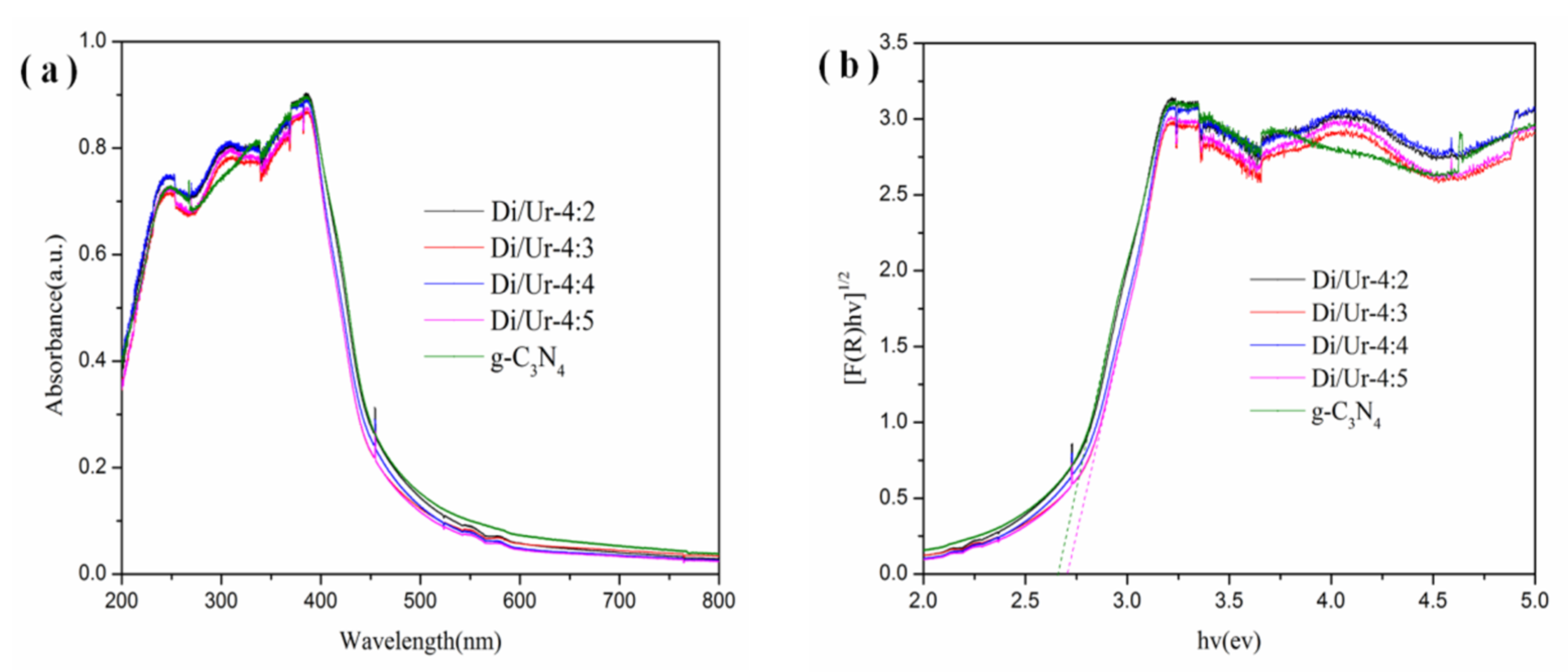
2.5. Optical Properties
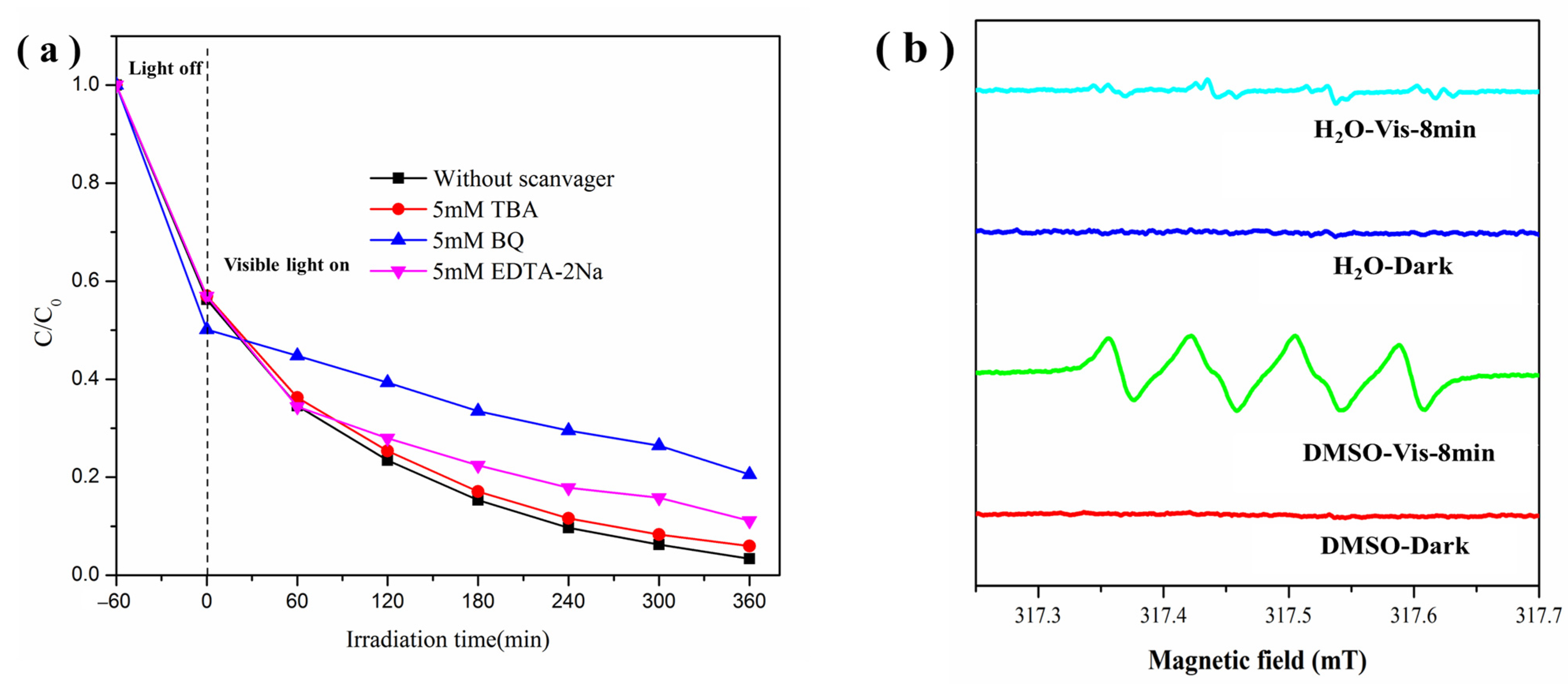
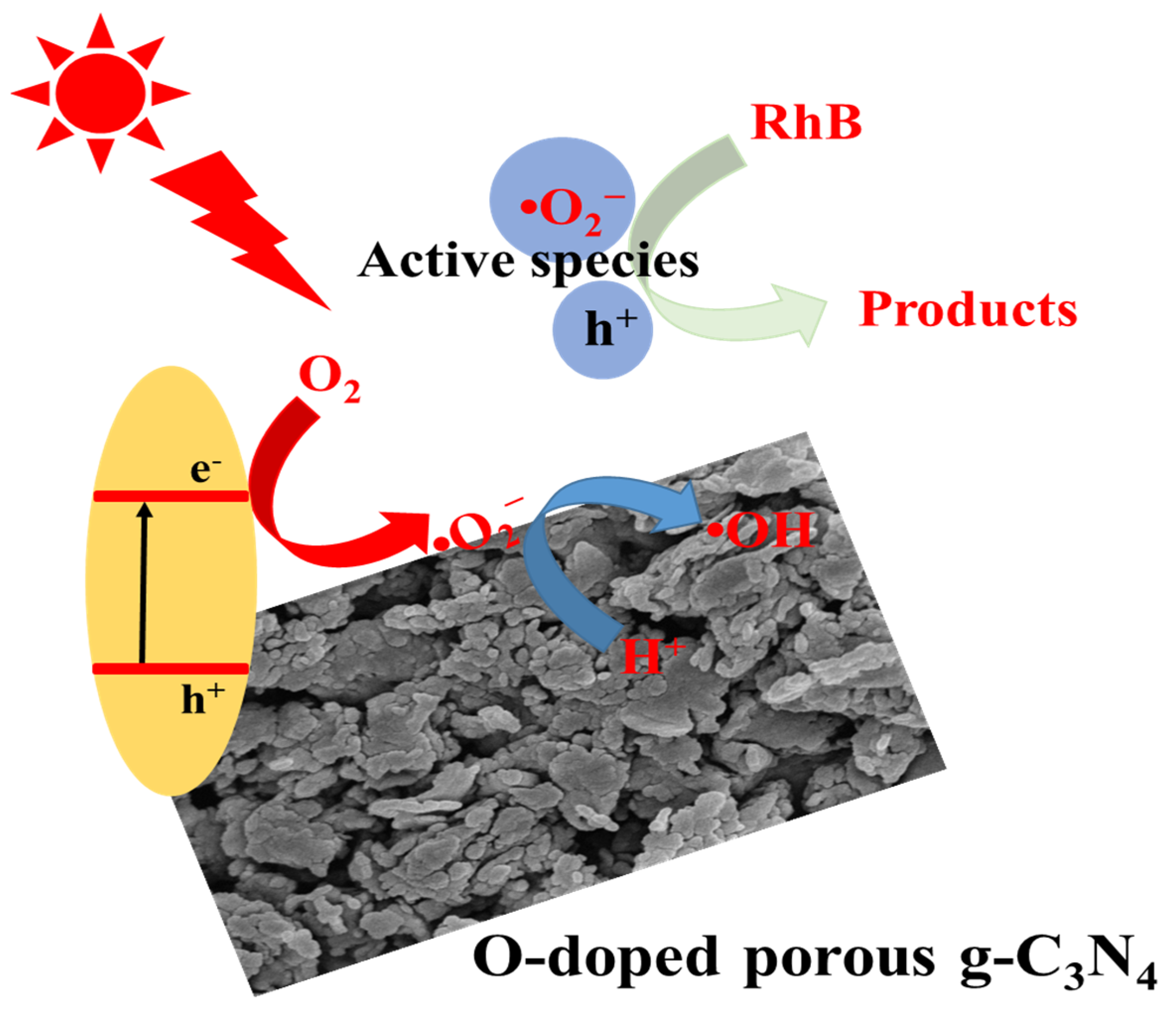
2.6. Possible Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Di/Ur Composites
3.3. Characterization
3.4. Photoactivity Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rayaroth, M.P.; Oh, D.; Lee, C.S.; Kang, Y.G.; Chang, Y.S. In situ chemical oxidation of contaminated groundwater using a sulfidized nanoscale zerovalent iron-persulfate system: Insights from a box-type study. Chemosphere 2020, 257, 127117. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Ali, N.; Khan, A.; Asiri, A.M.; Kamal, T. Catalytic potential of cobalt oxide and agar nanocomposite hydrogel for the chemical reduction of organic pollutants. Int. J. Biol. Macromol. 2020, 164, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yao, G.; Liu, M.; Zheng, S. In situ synthesis of magnetic MnFe2O4/diatomite nanocomposite adsorbent and its efficient removal of cationic dyes. J. Taiwan Inst. Chem. Eng. 2017, 71, 501–509. [Google Scholar] [CrossRef]
- Cao, H.; Wu, X.; Syed-Hassan, S.S.A.; Zhang, S.; Mood, S.H.; Milan, Y.J.; Garcia-Perez, M. Characteristics and mechanisms of phosphorous adsorption by rape straw-derived biochar functionalized with calcium from eggshell. Bioresour. Technol. 2020, 318, 124063. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, X.; Xu, Y.; Cheng, X.; Li, G.; Liang, H. Hybrid UF/NF process treating secondary effluent of wastewater treatment plants for potable water reuse: Adsorption vs. coagulation for removal improvements and membrane fouling alleviation. Environ. Res. 2020, 188, 109833. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cui, J.; Liu, C.; Yuan, F.; Guo, Y.; Deng, T. Separation of magnesium from high Mg/Li ratio brine by extraction with an organic system containing ionic liquid. Chem. Eng. Sci. 2021, 229, 116019. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, P.; He, Y.; Li, S.; Chen, K.; Yin, S. Purification of chlorine-containing wastewater using solvent extraction. J. Clean. Prod. 2020, 273, 122863. [Google Scholar] [CrossRef]
- Chambi-Rocha, A.; Cabrera-Dominguez, M.E.; Dominguez-Reyes, A. Breathing mode influence on craniofacial development and head posture. J. Pediatr. (Rio J.) 2018, 94, 123–130. [Google Scholar] [CrossRef]
- Yao, G.; Sun, Z.; Zheng, S. Synthesis and enhanced visible-light photocatalytic activity of wollastonite/g-C3N4 composite. Mater. Res. Bull. 2017, 86, 186–193. [Google Scholar] [CrossRef]
- Sun, J.X.; Yuan, Y.P.; Qiu, L.G.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef]
- Wang, D.; Sun, H.; Luo, Q.; Yang, X.; Yin, R. An efficient visible-light photocatalyst prepared from g-C3N4 and polyvinyl chloride. Appl. Catal. B Environ. 2014, 156–157, 323–330. [Google Scholar] [CrossRef]
- Li, F.T.; Zhao, Y.; Wang, Q.; Wang, X.J.; Hao, Y.J.; Liu, R.H.; Zhao, D. Enhanced visible-light photocatalytic activity of active Al2O3/g-C3N4 heterojunctions synthesized via surface hydroxyl modification. J. Hazard. Mater. 2015, 283, 371–381. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, F.; Zhu, Y.; Wei, F.; Liu, X.; Lian, C.; Wang, S. Magnetic core-shell CuFe2O4@C3N4 hybrids for visible light photocatalysis of Orange II. J. Hazard. Mater. 2015, 297, 224–233. [Google Scholar] [CrossRef]
- He, J.; Sun, H.; Indrawirawan, S.; Duan, X.; Tade, M.O.; Wang, S. Novel polyoxometalate@g-C3N4 hybrid photocatalysts for degradation of dyes and phenolics. J. Colloid Interface Sci. 2015, 456, 15–21. [Google Scholar] [CrossRef]
- Zhang, R.; Ran, T.; Cao, Y.; Ye, L.; Dong, F.; Zhang, Q.; Yuan, L.; Zhou, Y. Oxygen activation of noble-metal-free g-C3N4/α-Ni(OH)2 to control the toxic byproduct of photocatalytic nitric oxide removal. Chem. Eng. J. 2020, 382, 123029. [Google Scholar] [CrossRef]
- Cao, L.; Wang, R.; Wang, D. Synthesis and characterization of sulfur self-doped g-C3N4 with efficient visible-light photocatalytic activity. Mater. Lett. 2015, 149, 50–53. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Qian, X.; Dong, Y.; Xu, H.; Song, R.; Yan, C.; Zhu, H.; Zhong, Q.; Qian, G.; et al. One-pot synthesis of copper-doped graphitic carbon nitride nanosheet by heating Cu–melamine supramolecular network and its enhanced visible-light-driven photocatalysis. J. Solid State Chem. 2015, 228, 60–64. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Lu, G.; Liu, D.; Gui, J. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014, 311, 164–171. [Google Scholar] [CrossRef]
- Cui, J.; Liang, S.; Wang, X.; Zhang, J. First principle modeling of oxygen-doped monolayer graphitic carbon nitride. Mater. Chem. Phys. 2015, 161, 194–200. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Liu, F.; Yuan, X.; Guo, Y.; Zhang, S.; Guo, W.; Huo, M. Preparation and enhanced visible-light photocatalytic activity of silver deposited graphitic carbon nitride plasmonic photocatalyst. Appl. Catal. B Environ. 2013, 142–143, 828–837. [Google Scholar] [CrossRef]
- Han, T.; Li, X.; Li, Y.; Cao, W.; Wu, D.; Du, B.; Wei, Q. Gold nanoparticles enhanced electrochemiluminescence of graphite-like carbon nitride for the detection of Nuclear Matrix Protein 22. Sens. Actuators B Chem. 2014, 205, 176–183. [Google Scholar] [CrossRef]
- Cao, S.-W.; Yuan, Y.-P.; Barber, J.; Loo, S.C.J.; Xue, C. Noble-metal-free g-C3N4/Ni(dmgH)2 composite for efficient photocatalytic hydrogen evolution under visible light irradiation. Appl. Surf. Sci. 2014, 319, 344–349. [Google Scholar] [CrossRef]
- Jin, C.; Kang, J.; Li, Z.; Wang, M.; Wu, Z.; Xie, Y. Enhanced visible light photocatalytic degradation of tetracycline by MoS2/Ag/g-C3N4 Z-scheme composites with peroxymonosulfate. Appl. Surf. Sci. 2020, 514, 146076. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Cai, Q.; Shen, J.; Feng, Y.; Shen, Q.; Yang, H. Template-free preparation and characterization of nanoporous g-C3N4 with enhanced visible photocatalytic activity. J. Alloys Compd. 2015, 628, 372–378. [Google Scholar] [CrossRef]
- Gu, Q.; Liao, Y.; Yin, L.; Long, J.; Wang, X.; Xue, C. Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability. Appl. Catal. B Environ. 2015, 165, 503–510. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, G.; Zhang, X.; Zheng, S.; Frost, R.L. Enhanced visible-light photocatalytic activity of kaolinite/g-C3N4 composite synthesized via mechanochemical treatment. Appl. Clay Sci. 2016, 129, 7–14. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zong, R.; Zhu, Y. Enhancement of visible light photocatalytic activities via porous structure of g-C3N4. Appl. Catal. B Environ. 2014, 147, 229–235. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Dong, F.; Ho, W.K. Enhancing the photocatalytic activity of bulk g-C3N4 by introducing mesoporous structure and hybridizing with graphene. J. Colloid Interface Sci. 2014, 436, 29–36. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Zeng, M.; Li, J.; Xu, J.; Wang, X. Hierarchical nanocomposites of polyaniline nanorods arrays on graphitic carbon nitride sheets with synergistic effect for photocatalysis. Catal. Today 2014, 224, 114–121. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.; Li, X.; Lin, R.; Li, G. Synergistic collaboration of g-C3N4/SnO2 composites for enhanced visible-light photocatalytic activity. Chem. Eng. J. 2014, 246, 277–286. [Google Scholar] [CrossRef]
- Mei, R.; Ma, L.; An, L.; Wang, F.; Xi, J.; Sun, H.; Luo, Z.; Wu, Q. Layered Spongy-like O-Doped g-C3N4: An Efficient Non-Metal Oxygen Reduction Catalyst for Alkaline Fuel Cells. J. Electrochem. Soc. 2017, 164, F354–F363. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, F.; Cao, F.; Zhao, X.; Meng, S.; Cui, Y. Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis. Appl. Catal. B Environ. 2017, 206, 417–425. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Fang, W.; Liu, J.; Yu, L.; Jiang, Z.; Shangguan, W. Novel (Na, O) co-doped g-C3N4 with simultaneously enhanced absorption and narrowed bandgap for highly efficient hydrogen evolution. Appl. Catal. B Environ. 2017, 209, 631–636. [Google Scholar] [CrossRef]
- Wang, B.; de Godoi, F.C.; Sun, Z.; Zeng, Q.; Zheng, S.; Frost, R.L. Synthesis, characterization and activity of an immobilized photocatalyst: Natural porous diatomite supported titania nanoparticles. J. Colloid Interface Sci. 2015, 438, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Sun, H.; Suvorova, A.; Wang, S. One-pot hydrothermal synthesis of ZnO-reduced graphene oxide composites using Zn powders for enhanced photocatalysis. Chem. Eng. J. 2013, 229, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Hong, Z.; Chen, Y.; Lin, B.; Gao, B. Template-free synthesis of a novel porous g-C3N4 with 3D hierarchical structure for enhanced photocatalytic H2 evolution. Mater. Lett. 2014, 118, 208–211. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
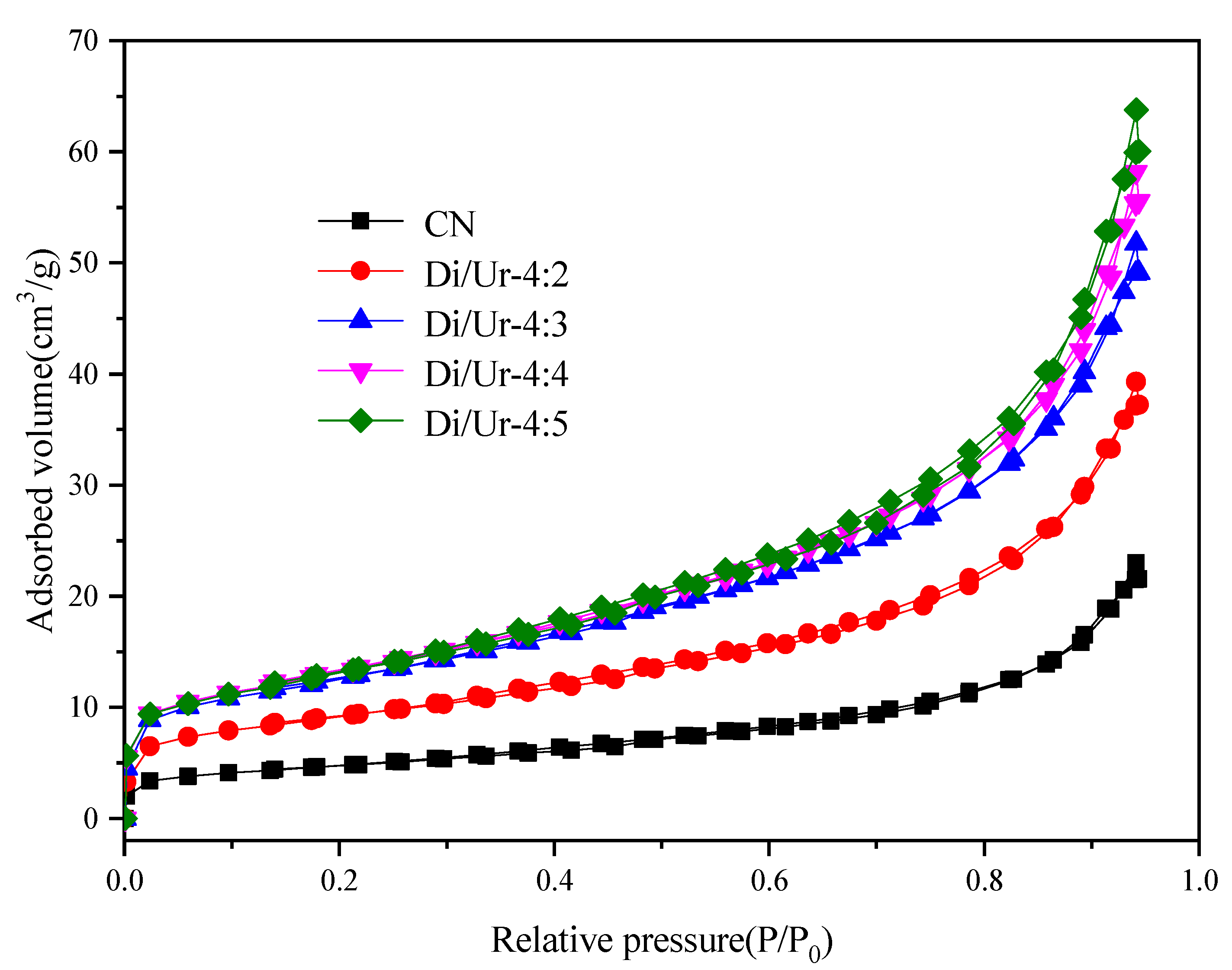


| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| CN | 17.115 | 0.038 | 8.315 |
| Di/Ur-4:2 | 32.483 | 0.065 | 7.490 |
| Di/Ur-4:3 | 44.694 | 0.085 | 7.167 |
| Di/Ur-4:4 | 46.919 | 0.096 | 7.675 |
| Di/Ur-4:5 | 48.002 | 0.107 | 8.220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Liu, Y.; Liu, J.; Xu, Y. Facile Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity. Molecules 2022, 27, 1754. https://doi.org/10.3390/molecules27061754
Yao G, Liu Y, Liu J, Xu Y. Facile Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity. Molecules. 2022; 27(6):1754. https://doi.org/10.3390/molecules27061754
Chicago/Turabian StyleYao, Guangyuan, Yuqiang Liu, Jingcai Liu, and Ya Xu. 2022. "Facile Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity" Molecules 27, no. 6: 1754. https://doi.org/10.3390/molecules27061754
APA StyleYao, G., Liu, Y., Liu, J., & Xu, Y. (2022). Facile Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity. Molecules, 27(6), 1754. https://doi.org/10.3390/molecules27061754





