Challenges of Measuring Soluble Mn(III) Species in Natural Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. LBB Method
2.3. TCPP Method
3. Results and Discussion
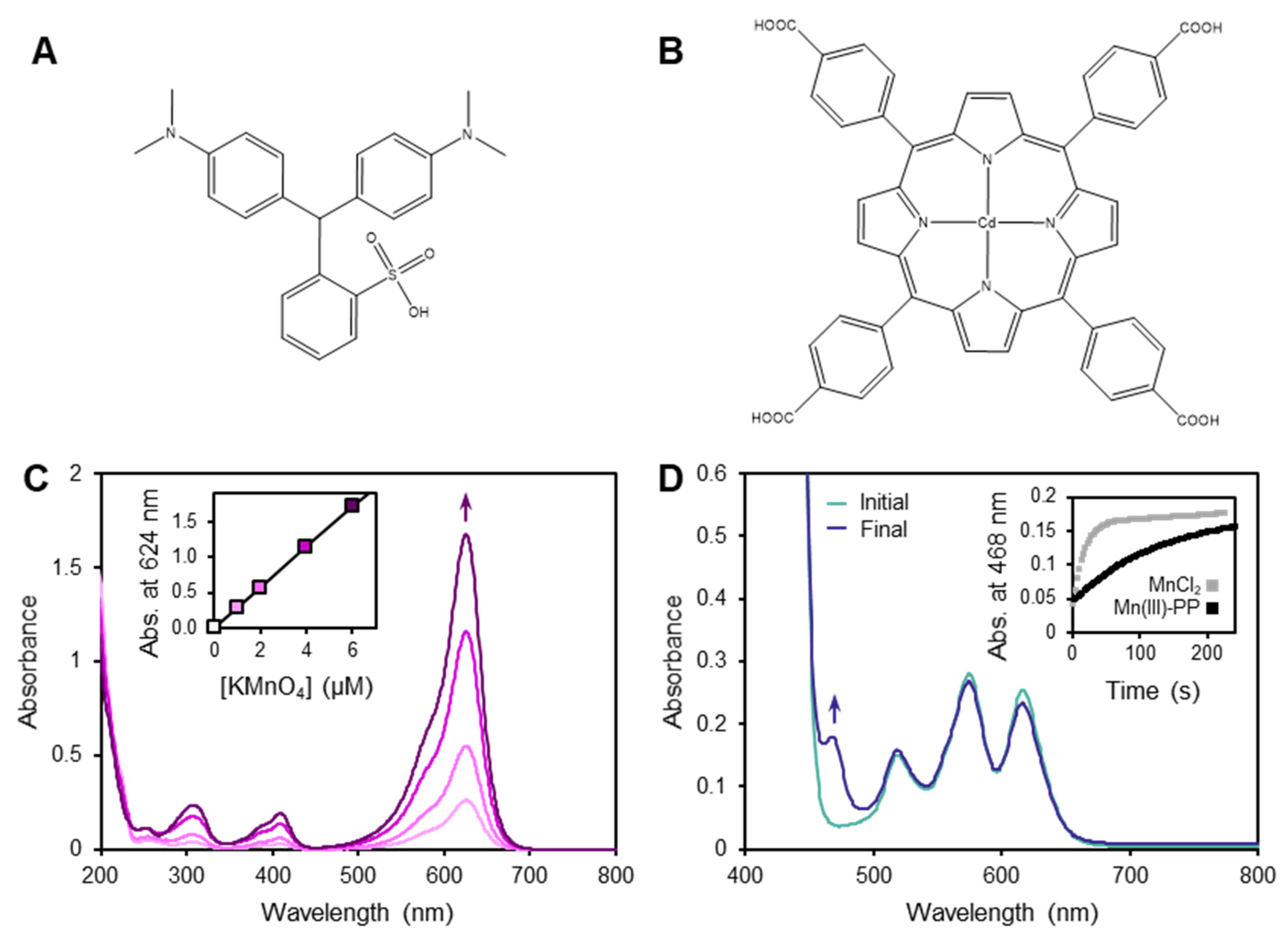
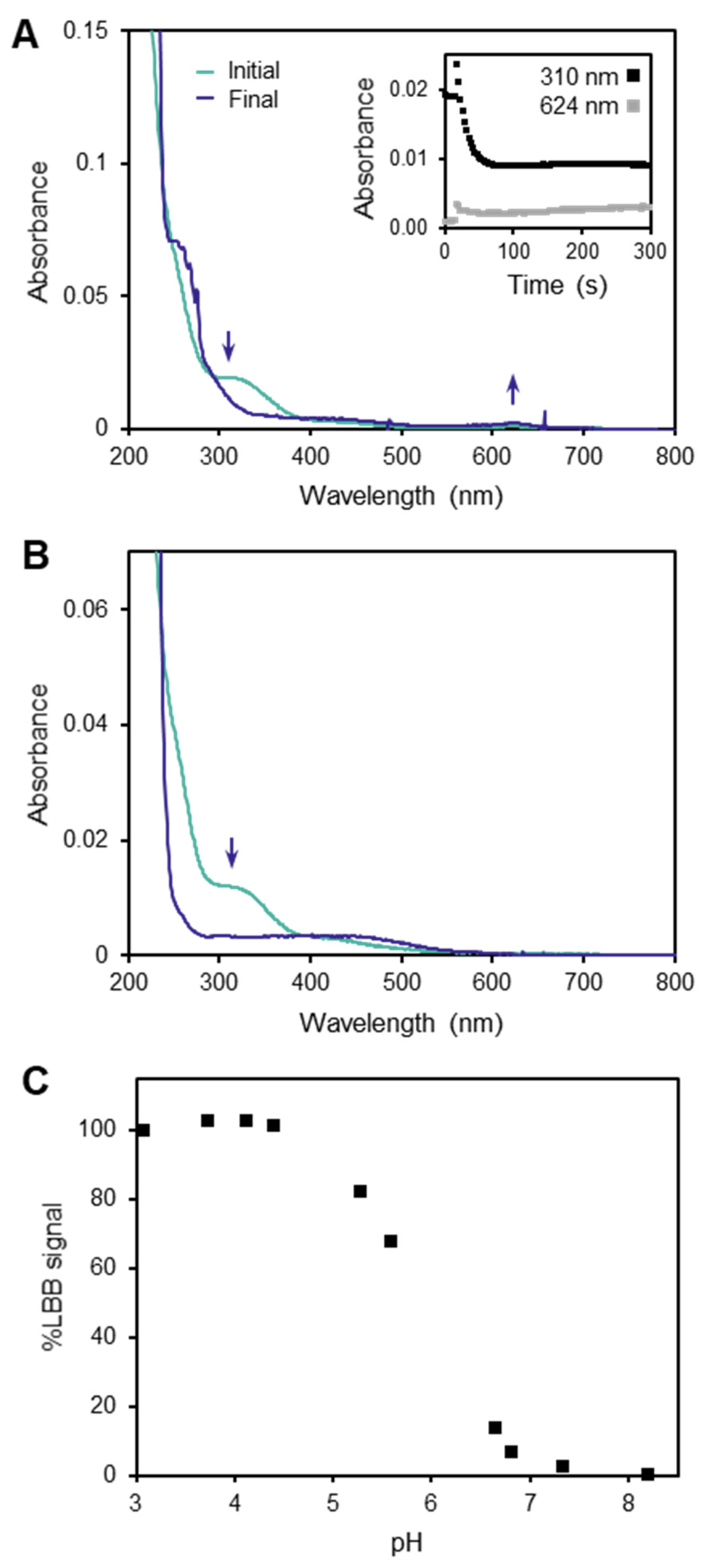
3.1. Limitations of the LBB Method
3.2. Behavior of the TCPP Method
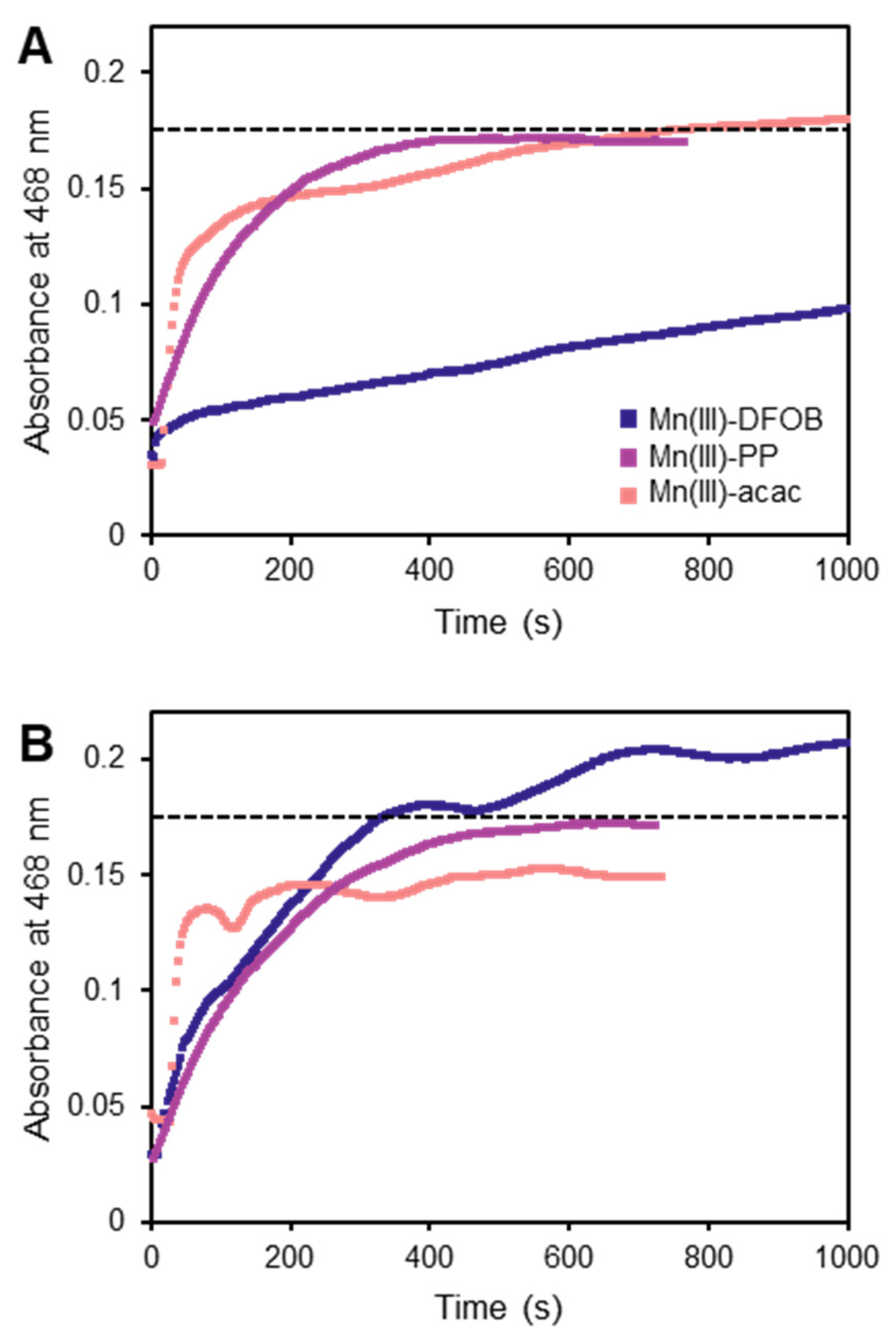
3.3. Ligand-Exchange Extractions Using DFOB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, J.J. Manganese in Natural Waters and Earth’s Crust: Its Availability to Organisms. Met. Ions Biol. Syst. 2000, 37, 1–34. [Google Scholar] [PubMed]
- Davies, G. Some Aspects of the Chemistry of Manganese(III) in Aqueous Solution. Coord. Chem. Rev. 1969, 4, 199–224. [Google Scholar] [CrossRef]
- Trouwborst, R.E. Soluble Mn(III) in Suboxic Zones. Science 2006, 313, 1955–1957. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.S.; Tebo, B.M.; Mucci, A.; Sundby, B.; Luther, G.W. Abundant Porewater Mn(III) Is a Major Component of the Sedimentary Redox System. Science 2013, 341, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Oldham, V.E.; Mucci, A.; Tebo, B.M.; Luther, G.W. Soluble Mn(III)–L Complexes Are Abundant in Oxygenated Waters and Stabilized by Humic Ligands. Geochim. Cosmochim. Acta 2017, 199, 238–246. [Google Scholar] [CrossRef]
- Qian, A.; Zhang, W.; Shi, C.; Pan, C.; Giammar, D.E.; Yuan, S.; Zhang, H.; Wang, Z. Geochemical Stability of Dissolved Mn(III) in the Presence of Pyrophosphate as a Model Ligand: Complexation and Disproportionation. Environ. Sci. Technol. 2019, 53, 5768–5777. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.; Harmon, M.E.; Mao, J.; Pett-Ridge, J.; Kleber, M. Long-Term Litter Decomposition Controlled by Manganese Redox Cycling. Proc. Natl. Acad. Sci. USA 2015, 112, E5253–E5260. [Google Scholar] [CrossRef]
- Oldham, V.E.; Jones, M.R.; Tebo, B.M.; Luther, G.W. Oxidative and Reductive Processes Contributing to Manganese Cycling at Oxic-Anoxic Interfaces. Mar. Chem. 2017, 195, 122–128. [Google Scholar] [CrossRef]
- Hu, E.; Zhang, Y.; Wu, S.; Wu, J.; Liang, L.; He, F. Role of Dissolved Mn(III) in Transformation of Organic Contaminants: Non-Oxidative versus Oxidative Mechanisms. Water Res. 2017, 111, 234–243. [Google Scholar] [CrossRef]
- Wang, X.; Yao, J.; Wang, S.; Pan, X.; Xiao, R.; Huang, Q.; Wang, Z.; Qu, R. Phototransformation of Estrogens Mediated by Mn(III), Not by Reactive Oxygen Species, in the Presence of Humic Acids. Chemosphere 2018, 201, 224–233. [Google Scholar] [CrossRef]
- Johnson, K.L.; McCann, C.M.; Wilkinson, J.-L.; Jones, M.; Tebo, B.M.; West, M.; Elgy, C.; Clarke, C.E.; Gowdy, C.; Hudson-Edwards, K.A. Dissolved Mn(III) in Water Treatment Works: Prevalence and Significance. Water Res. 2018, 140, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Nico, P.S.; Ying, S.; Regier, T.; Thieme, J.; Keiluweit, M. Manganese-Driven Carbon Oxidation at Oxic–Anoxic Interfaces. Environ. Sci. Technol. 2018, 52, 12349–12357. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, U.F.; Monteverde, D.R.; Magyar, J.S.; Valentine, J.S.; Fischer, W.W. How Manganese Empowered Life with Dioxygen (and Vice Versa). Free Radic. Biol. Med. 2019, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M. Review: Lignin Conversion by Manganese Peroxidase (MnP). Enzyme Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Klewicki, J.K.; Morgan, J.J. Kinetic Behavior of Mn(III) Complexes of Pyrophosphate, EDTA, and Citrate. Environ. Sci. Technol. 1998, 32, 2916–2922. [Google Scholar] [CrossRef]
- Schroeder, K.A.; Hamm, R.E. Decomposition of the Ethylenediaminetetraacetate Complex of Manganese(III). Inorg. Chem. 1964, 3, 391–395. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Sposito, G. Siderophore−Manganese(III) Interactions. I. Air-Oxidation of Manganese(II) Promoted by Desferrioxamine B. Environ. Sci. Technol. 2005, 39, 6037–6044. [Google Scholar] [CrossRef]
- Morgan, J.J.; Stumm, W. Analytical Chemistry of Aqueous Manganese. J. Am. Water Works Assoc. 1965, 57, 107–119. [Google Scholar] [CrossRef]
- Krumbein, W.E.; Altmann, H.J. A New Method for the Detection and Enumeration of Manganese Oxidizing and Reducing Microorganisms. Helgoländ. Wiss. Meeresunters. 1973, 25, 347–356. [Google Scholar] [CrossRef]
- Okazaki, M.; Sugita, T.; Shimizu, M.; Ohode, Y.; Iwamoto, K.; de Vrind-de Jong, E.W.; de Vrind, J.P.; Corstjens, P.L. Partial Purification and Characterization of Manganese-Oxidizing Factors of Pseudomonas Fluorescens GB-1. Appl. Environ. Microbiol. 1997, 63, 4793–4799. [Google Scholar] [CrossRef]
- Boogerd, F.C.; de Vrind, J.P. Manganese Oxidation by Leptothrix Discophora. J. Bacteriol. 1987, 169, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.A.; Tebo, B.M. In Vitro Studies Indicate a Quinone Is Involved in Bacterial Mn(II) Oxidation. Arch. Microbiol. 2008, 189, 59–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Y.; Liang, X.; Zhao, H.; Yin, H.; Liu, M.; Liu, F.; Feng, X. Rapid Determination of the Mn Average Oxidation State of Mn Oxides with a Novel Two-Step Colorimetric Method. Anal. Methods 2017, 9, 103–109. [Google Scholar] [CrossRef]
- Jones, M.R.; Luther, G.W.; Mucci, A.; Tebo, B.M. Concentrations of Reactive Mn(III)-L and MnO2 in Estuarine and Marine Waters Determined Using Spectrophotometry and the Leuco Base, Leucoberbelin Blue. Talanta 2019, 200, 91–99. [Google Scholar] [CrossRef]
- Sañudo-Wilhelmy, S.A.; Rivera-Duarte, I.; Flegal, A.R. Distribution of Colloidal Trace Metals in the San Francisco Bay Estuary. Geochim. Cosmochim. Acta 1996, 60, 4933–4944. [Google Scholar] [CrossRef]
- Baalousha, M.; Stoll, S.; Motelica-Heino, M.; Guigues, N.; Braibant, G.; Huneau, F.; Le Coustumer, P. Suspended Particulate Matter Determines Physical Speciation of Fe, Mn, and Trace Metals in Surface Waters of Loire Watershed. Environ. Sci. Pollut. Res. 2019, 26, 5251–5266. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.A.; Zhou, M.; Song, Y.; Wysocki, V.H.; Dohnalkova, A.C.; Kovarik, L.; Paša-Tolić, L.; Tebo, B.M. Biogenic Manganese Oxide Nanoparticle Formation by a Multimeric Multicopper Oxidase Mnx. Nat. Commun. 2017, 8, 746. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Koh, H.; Satoh, K. Spectrophotometric Determination of Manganese Utilizing Metal Ion Substitution in the Cadmium-α,β,-γ,δ -Tetrakis(4-Carboxyphenyl)Porphine Complex. Anal. Chim. Acta 1982, 136, 347–352. [Google Scholar] [CrossRef]
- Madison, A.S.; Tebo, B.M.; Luther, G.W. Simultaneous Determination of Soluble Manganese(III), Manganese(II) and Total Manganese in Natural (Pore)Waters. Talanta 2011, 84, 374–381. [Google Scholar] [CrossRef]
- Acton, F.S. Numerical Methods That Work; Mathematical Association of America: Washington, DC, USA, 1990; ISBN 978-0-88385-450-1. [Google Scholar]
- De Groen, P.; De Moor, B. The Fit of a Sum of Exponentials to Noisy Data. J. Comput. Appl. Math. 1987, 20, 175–187. [Google Scholar] [CrossRef][Green Version]
- Luther, G.W.; Madison, A.S.; Mucci, A.; Sundby, B.; Oldham, V.E. A Kinetic Approach to Assess the Strengths of Ligands Bound to Soluble Mn(III). Mar. Chem. 2015, 173, 93–99. [Google Scholar] [CrossRef][Green Version]
- Jones, M.R.; Oldham, V.E.; Luther, G.W.; Mucci, A.; Tebo, B.M. Distribution of Desferrioxamine-B-Extractable Soluble Manganese(III) and Particulate MnO2 in the St. Lawrence Estuary, Canada. Mar. Chem. 2019, 208, 70–82. [Google Scholar] [CrossRef]
- Hering, J.G.; Morel, F.M.M. Slow Coordination Reactions in Seawater. Geochim. Cosmochim. Acta 1989, 53, 611–618. [Google Scholar] [CrossRef]
- Polonsky, A.B.; Grebneva, E.A. The Spatiotemporal Variability of PH in Waters of the Black Sea. Dokl. Earth Sci. 2019, 486, 669–674. [Google Scholar] [CrossRef]
- Springer, S.D.; Butler, A. Magnetic Susceptibility of Mn(III) Complexes of Hydroxamate Siderophores. J. Inorg. Biochem. 2015, 148, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Cavazos, A.R.; Glass, J.B. Simul-staining Manganese Oxides and Microbial Cells. Limnol. Oceanogr. Methods 2020, 18, 362–373. [Google Scholar] [CrossRef]
- Thibault de Chanvalon, A.; Luther, G.W. Mn Speciation at Nanomolar Concentrations with a Porphyrin Competitive Ligand and UV–Vis Measurements. Talanta 2019, 200, 15–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Lingappa, U.F.; Magyar, J.; Monteverde, D.; Valentine, J.S.; Cho, J.; Fischer, W. Challenges of Measuring Soluble Mn(III) Species in Natural Samples. Molecules 2022, 27, 1661. https://doi.org/10.3390/molecules27051661
Kim B, Lingappa UF, Magyar J, Monteverde D, Valentine JS, Cho J, Fischer W. Challenges of Measuring Soluble Mn(III) Species in Natural Samples. Molecules. 2022; 27(5):1661. https://doi.org/10.3390/molecules27051661
Chicago/Turabian StyleKim, Bohee, Usha Farey Lingappa, John Magyar, Danielle Monteverde, Joan Selverstone Valentine, Jaeheung Cho, and Woodward Fischer. 2022. "Challenges of Measuring Soluble Mn(III) Species in Natural Samples" Molecules 27, no. 5: 1661. https://doi.org/10.3390/molecules27051661
APA StyleKim, B., Lingappa, U. F., Magyar, J., Monteverde, D., Valentine, J. S., Cho, J., & Fischer, W. (2022). Challenges of Measuring Soluble Mn(III) Species in Natural Samples. Molecules, 27(5), 1661. https://doi.org/10.3390/molecules27051661





