Conversion of Cellulose and Lignin Residues into Transparent UV-Blocking Composite Films
Abstract
:1. Introduction
2. Results and Discussion
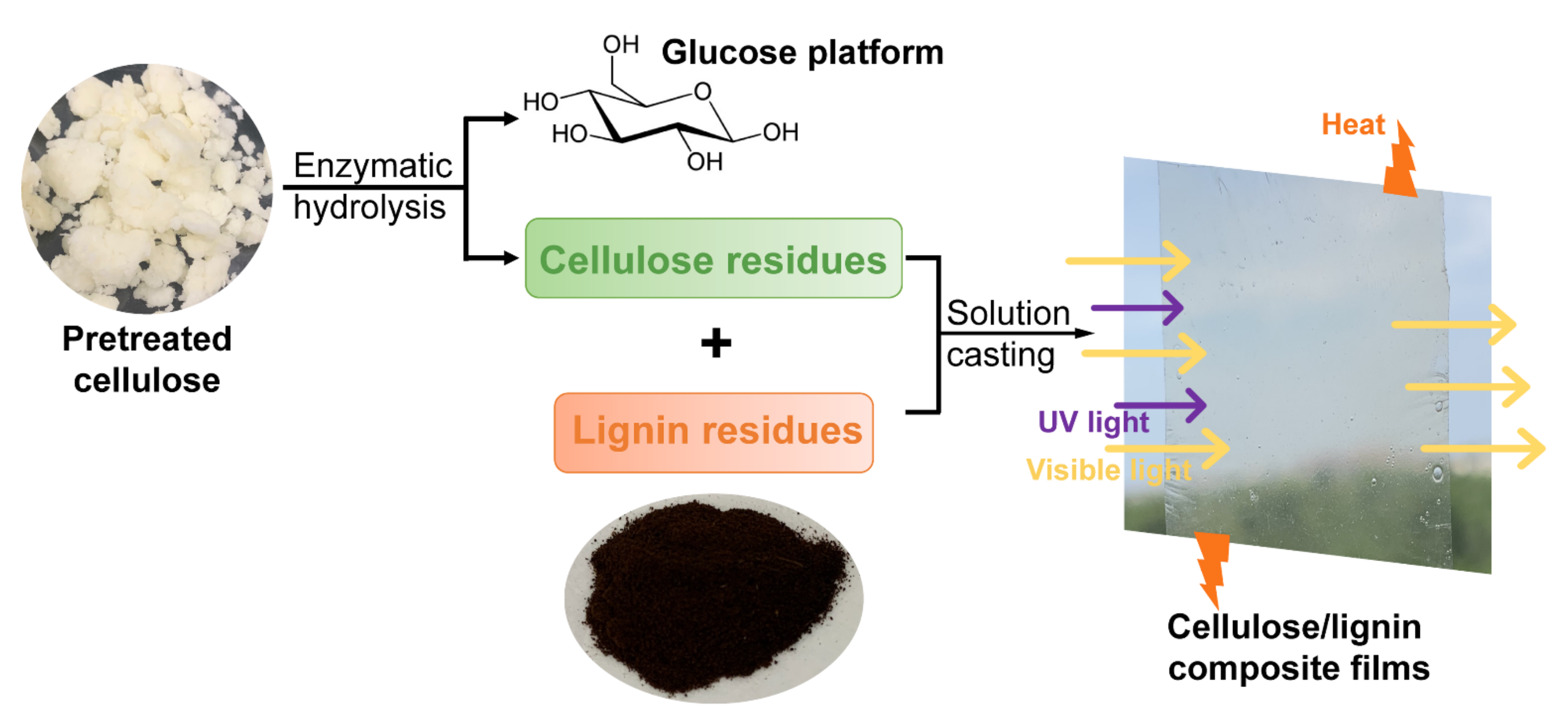
2.1. Cellulose/Lignin Composite Films Preparation
2.2. Lignin Characterization Using 31P-NMR
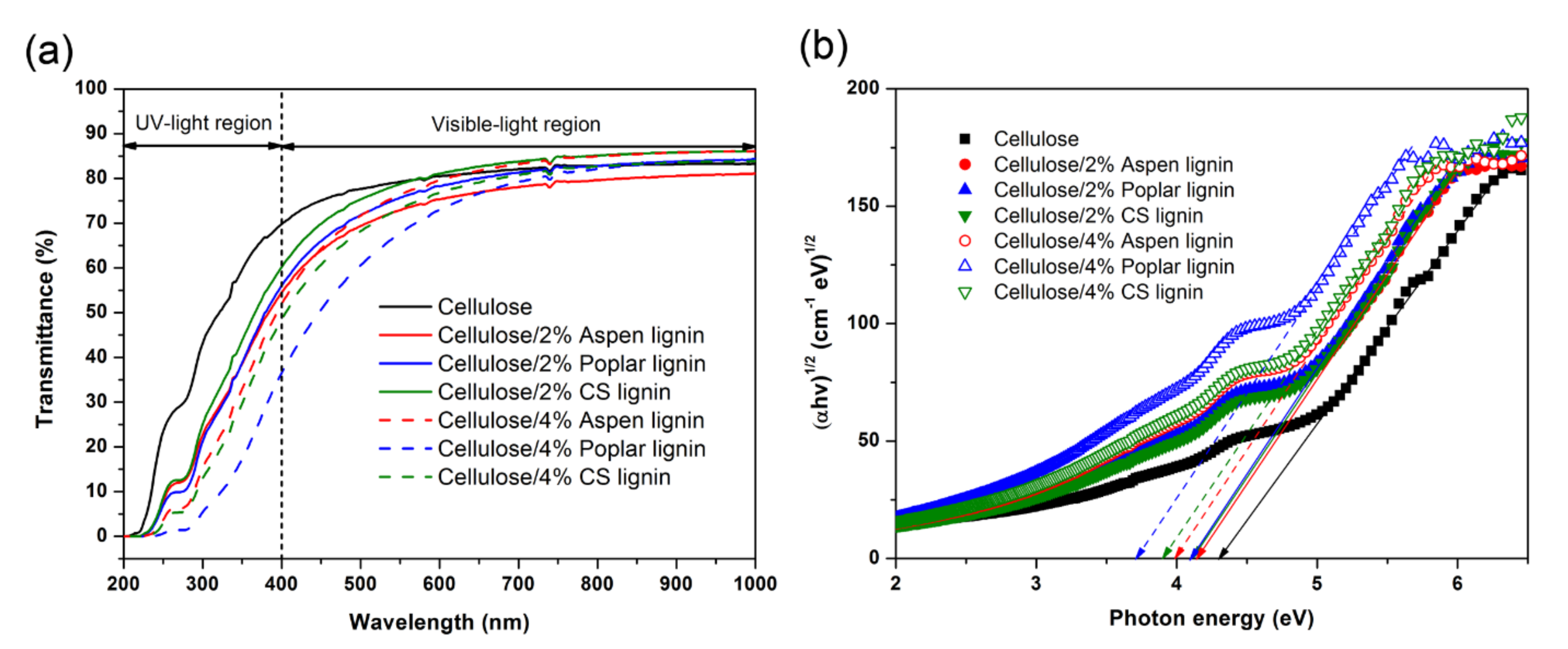
2.3. UV-Blocking Property of Cellulose/Lignin Composite Films
2.4. Mechanical Property of Cellulose/Lignin Composite Films
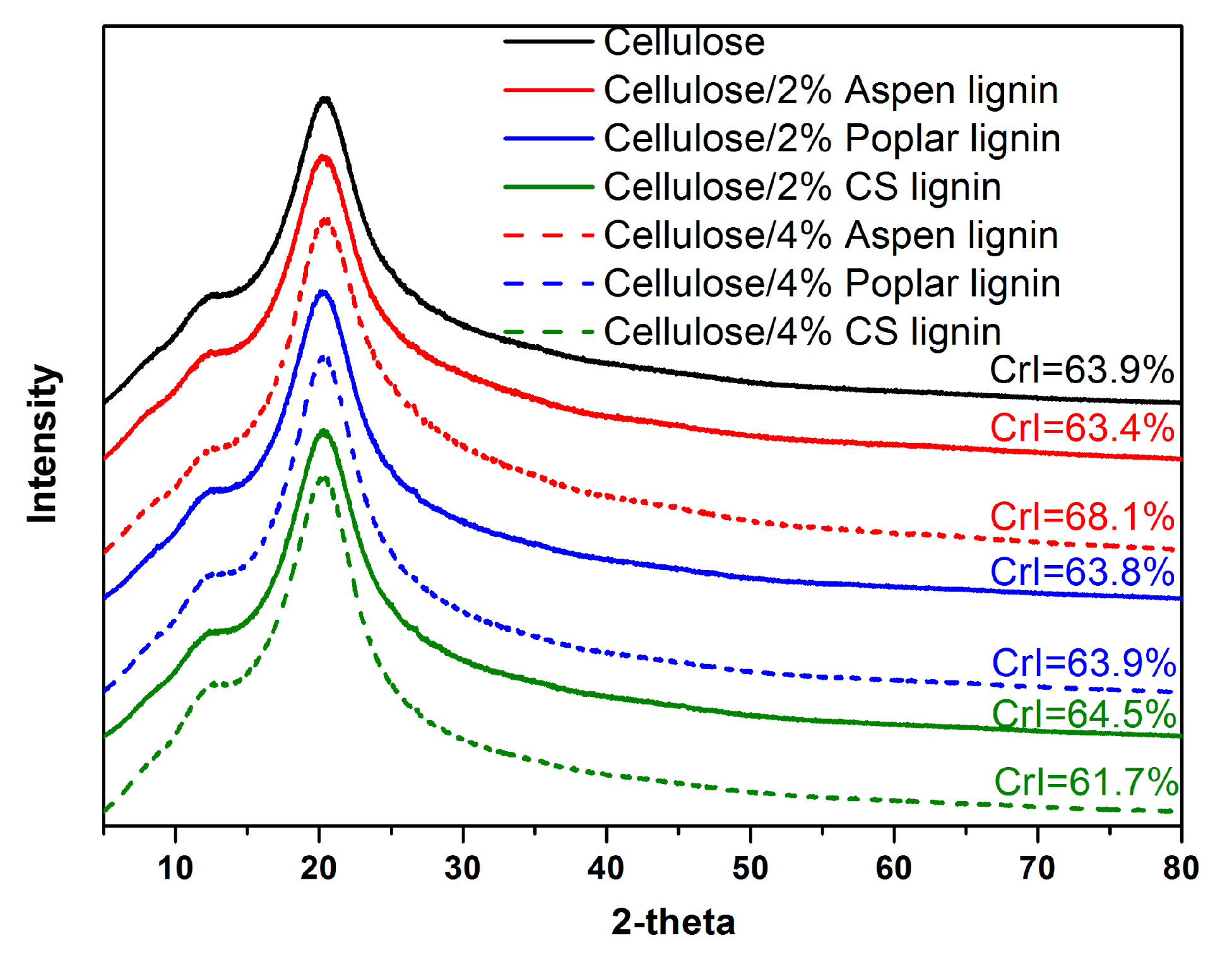
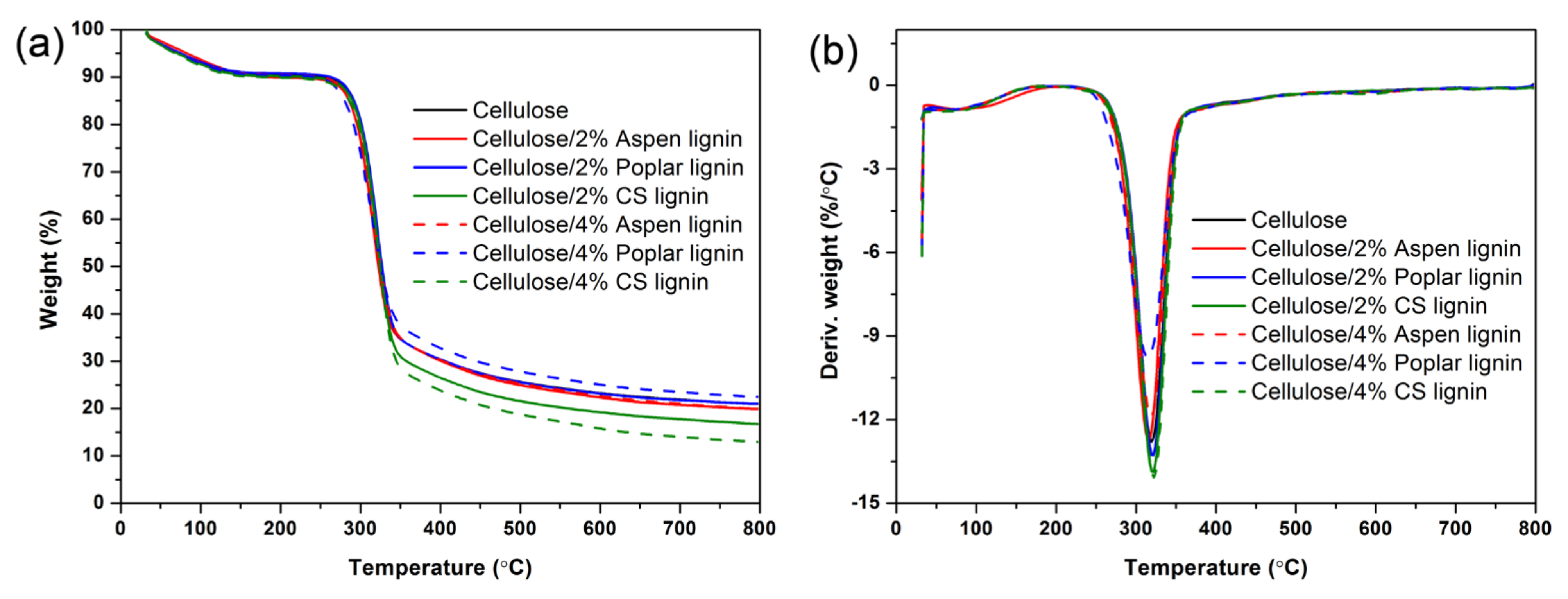
2.5. Crystalline Morphology and Thermal Stability Analysis
3. Materials and Methods
3.1. Materials
3.2. Cellulose and Lignin Residue Acquirement
3.3. Cellulose/Lignin Composite Film Preparation
3.4. UV-Blocking Assessment
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sust. Energ. Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R.P.; Lee, J.S.; Lu, C.; Saddler, J.N. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.H.P. New lignocellulose pretreatments using cellulose solvents: A review. J. Chem. Technol. Biotechnol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Rinaldi, R.; Weckhuysen, B.M. Unlocking the potential of a sleeping giant: Lignins as sustainable raw materials for renewable fuels, chemicals and materials. Green Chem. 2015, 17, 4860–4861. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almas, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.S.; Ok, Y.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Xu, R.; Du, H.; Wang, H.; Zhang, M.; Wu, M.; Liu, C.; Li, B. Valorization of enzymatic hydrolysis residues from corncob into lignin-containing cellulose nanofibrils and lignin nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 252. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, S.; Zhang, X.; Takabe, K.; Xu, F. Unraveling variations of crystalline cellulose induced by ionic liquid and their effects on enzymatic hydrolysis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose crystallinity—a key predictor of the enzymatic hydrolysis rate. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing recalcitrant cellulolytic enzyme lignin via lignin nanoparticles fabrication in an integrated biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Huang, C.; Ragauskas, A.J.; Wu, X.; Huang, Y.; Zhou, X.; He, J.; Yong, Q. Co-production of bio-ethanol, xylonic acid and slow-release nitrogen fertilizer from low-cost straw pulping solid residue. Bioresour. Technol. 2018, 250, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liu, W.; Song, X.; Qi, Y.; Zhang, C.; Gao, Z.; Jia, L. Antioxidant and anti-inflammation of enzymatic-hydrolysis residue polysaccharides by Lentinula edodes. Int. J. Biol. Macromol. 2018, 120, 811–822. [Google Scholar] [CrossRef]
- Yan, K.; Liu, F.; Chen, Q.; Ke, M.; Huang, X.; Hu, W.; Yu, H. Pyrolysis characteristics and kinetics of lignin derived from enzymatic hydrolysis residue of bamboo pretreated with white-rot fungus. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Chu, S.; Dauenhauer, P.J.; Huber, G.W. Kinetics and reaction chemistry for slow pyrolysis of enzymatic hydrolysis lignin and organosolv extracted lignin derived from maplewood. Green Chem. 2012, 14, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.M.; Djajadi, D.T.; Torri, C.; Rasmussen, H.B.; Madsen, R.B.; Venturini, E.; Glasius, M. Hydrothermal liquefaction of enzymatic hydrolysis lignin: Biomass pretreatment severity affects lignin valorization. ACS Sustain. Chem. Eng. 2018, 6, 5940–5949. [Google Scholar] [CrossRef]
- Long, L.; Shen, F.; Wang, F.; Tian, D.; Hu, J. Synthesis, characterization and enzymatic surface roughing of cellulose/xylan composite films. Carbohydr. Polym. 2019, 213, 121–127. [Google Scholar] [CrossRef]
- Hu, J.; Tian, D.; Renneckar, S.; Saddler, J.N. Enzyme mediated nanofibrillation of cellulose by the synergistic actions of an endoglucanase, lytic polysaccharide monooxygenase (LPMO) and xylanase. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. Simultaneous enhancements of UV-shielding properties and photostability of poly(vinyl alcohol) via incorporation of sepia eumelanin. ACS Sustain. Chem. Eng. 2016, 4, 2252–2258. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, L.; Jin, Y.Z.; Gao, C.; Ye, Z.Z.; Yang, Y.F.; Wang, Q.L. Transparent and flexible thin films of ZnO-polystyrene nanocomposite for UV-shielding applications. J. Mater. Chem. 2010, 20, 1594–1599. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Shen, F. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.; Long, L.; He, J.; Luo, L. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent cellulose/technical lignin composite films for advanced packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, Y.; Tian, D.; Shen, F.; Wan, X.; Xu, L.; Chen, Y.; Zhang, H.; Hu, J.; Shen, F. Fabrication and characterization of lignin–xylan hybrid nanospheres as pesticide carriers with enzyme-mediated release property. Soft Matter 2020, 16, 9083–9093. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Zou, T.; Riviere, G.; Sipponen, M.H.; Österberg, M. Strong, ductile, and waterproof cellulose nanofibril composite films with colloidal lignin particles. Biomacromolecules 2018, 20, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dong, H.; Zhang, Z.; Bian, H.; Yong, Q. Procuring the nano-scale lignin in prehydrolyzate as ingredient to prepare cellulose nanofibril composite film with multiple functions. Cellulose 2020, 27, 9355–9370. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, D.; Han, Y.; Lyu, S.; Lu, Y.; Li, G.; Jiang, F.; Wang, S. Effect of high residual lignin on the properties of cellulose nanofibrils/films. Cellulose 2018, 25, 6421–6431. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Liu, W.; Zhang, L.; Ouyang, H.; Hou, Q.; Fan, K.; Li, J.; Liu, P.; Liu, X. Fabricating cellulose nanofibril from licorice residues and its cellulose composite incorporated with natural nanoparticles. Carbohydr. Polym. 2020, 229, 115464. [Google Scholar] [CrossRef]
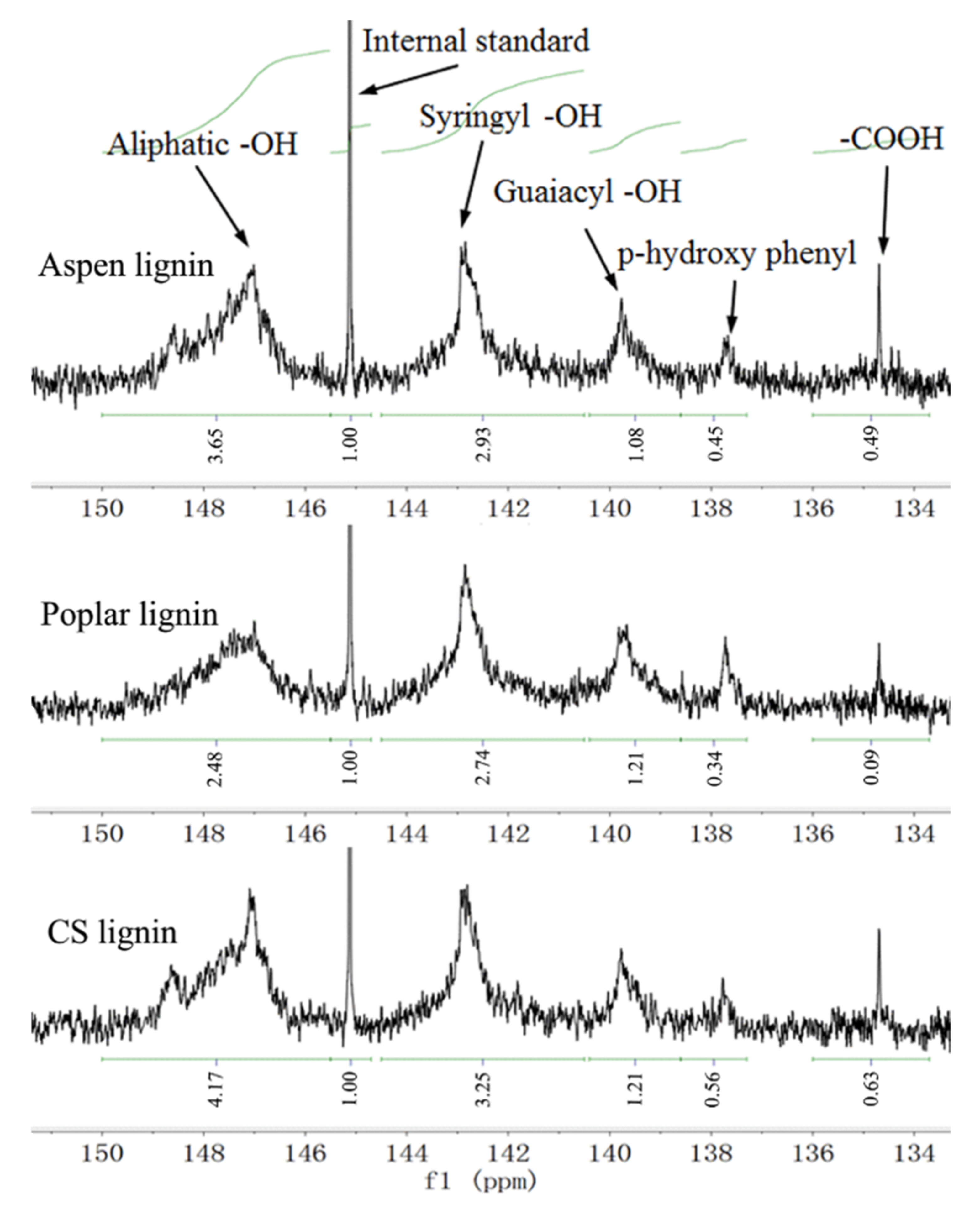

| -OH Content (mmol g−1) | Aspen Lignin | Poplar Lignin | CS Lignin |
|---|---|---|---|
| Aliphatic -OH | 2.23 | 1.52 | 2.55 |
| Syringyl phenolic -OH | 1.79 | 1.68 | 1.99 |
| Guaiacyl phenolic -OH | 0.66 | 0.74 | 0.74 |
| p-hydroxyphenyl -OH | 0.28 | 0.21 | 0.34 |
| Carboxylic acid -OH | 0.30 | 0.06 | 0.39 |
| Total phenolic -OH | 2.73 | 2.62 | 3.07 |
| Sample | Tonset (°C) | Tmax (°C) | Tcomp (°C) | Char (%) |
|---|---|---|---|---|
| Cellulose | 296.0 | 319.4 | 340.4 | 21.0 |
| Cellulose/2% Aspen lignin | 292.6 | 315.7 | 337.5 | 19.9 |
| Cellulose/2% Poplar lignin | 297.9 | 321.0 | 340.8 | 21.0 |
| Cellulose/2% CS lignin | 297.8 | 320.7 | 340.9 | 16.8 |
| Cellulose/4% Aspen lignin | 292.8 | 318.8 | 341.7 | 19.9 |
| Cellulose/4% Poplar lignin | 285.0 | 315.0 | 341.7 | 22.5 |
| Cellulose/4% CS lignin | 297.9 | 322.1 | 341.7 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Xu, L.; Dai, G.; Luo, L.; Yang, K.; Huang, C.; Tian, D.; Shen, F. Conversion of Cellulose and Lignin Residues into Transparent UV-Blocking Composite Films. Molecules 2022, 27, 1637. https://doi.org/10.3390/molecules27051637
Yang F, Xu L, Dai G, Luo L, Yang K, Huang C, Tian D, Shen F. Conversion of Cellulose and Lignin Residues into Transparent UV-Blocking Composite Films. Molecules. 2022; 27(5):1637. https://doi.org/10.3390/molecules27051637
Chicago/Turabian StyleYang, Fan, Lu Xu, Guodong Dai, Lin Luo, Kaifeng Yang, Churui Huang, Dong Tian, and Fei Shen. 2022. "Conversion of Cellulose and Lignin Residues into Transparent UV-Blocking Composite Films" Molecules 27, no. 5: 1637. https://doi.org/10.3390/molecules27051637
APA StyleYang, F., Xu, L., Dai, G., Luo, L., Yang, K., Huang, C., Tian, D., & Shen, F. (2022). Conversion of Cellulose and Lignin Residues into Transparent UV-Blocking Composite Films. Molecules, 27(5), 1637. https://doi.org/10.3390/molecules27051637








