A New Pt(II) Complex with Anionic s-Triazine Based NNO-Donor Ligand: Synthesis, X-ray Structure, Hirshfeld Analysis and DFT Studies
Abstract
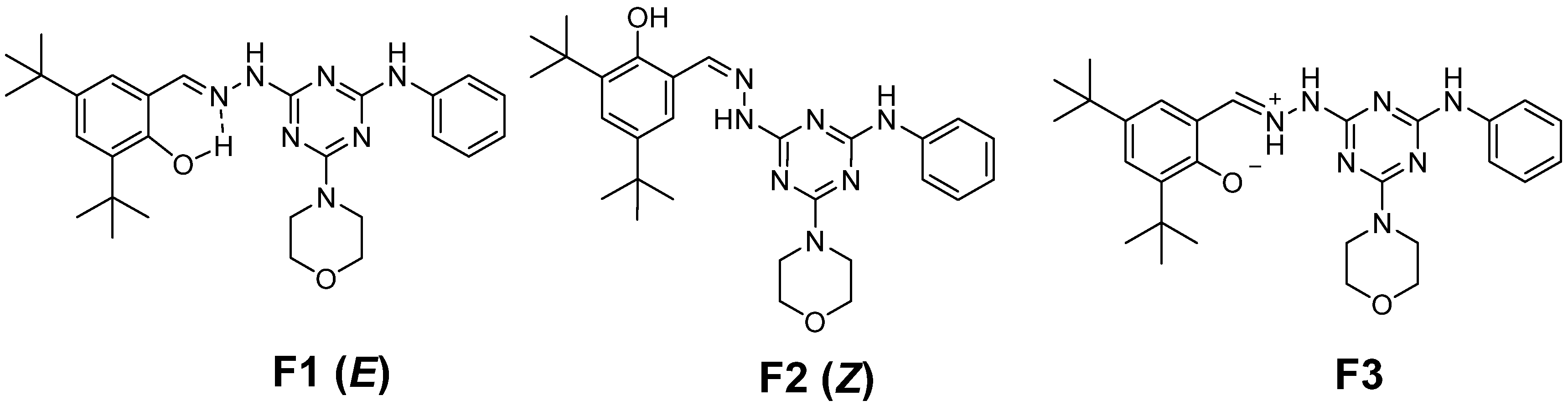
:1. Introduction
2. Results and Discussion
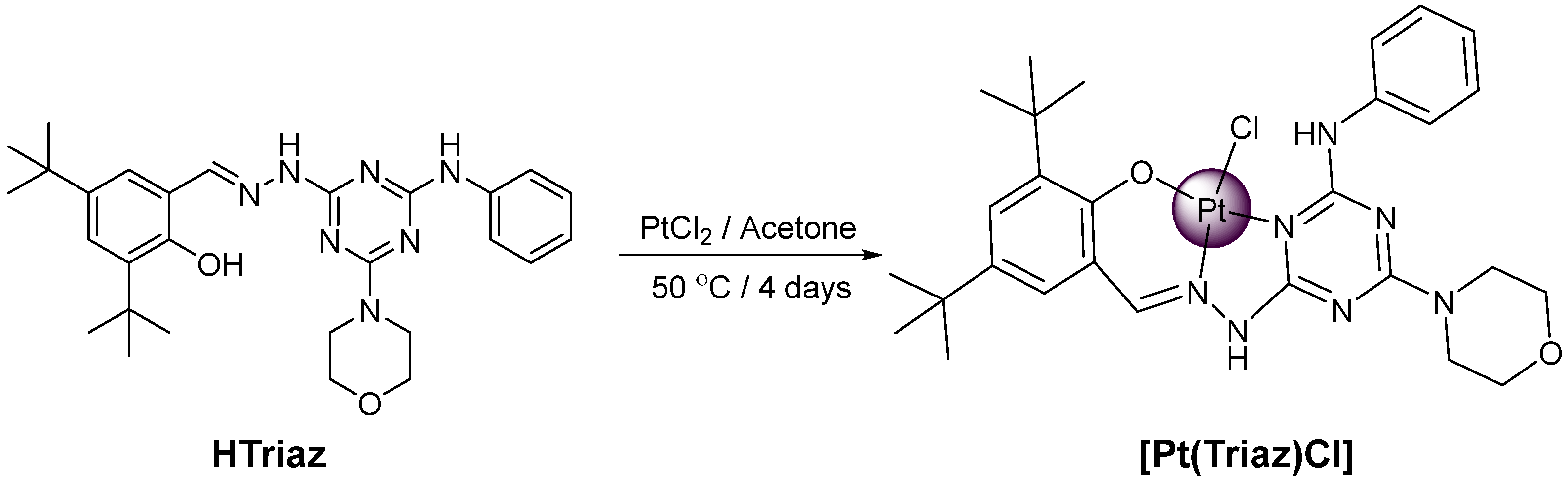
2.1. [Pt(Triaz)Cl] Complex Synthesis and Chracterization
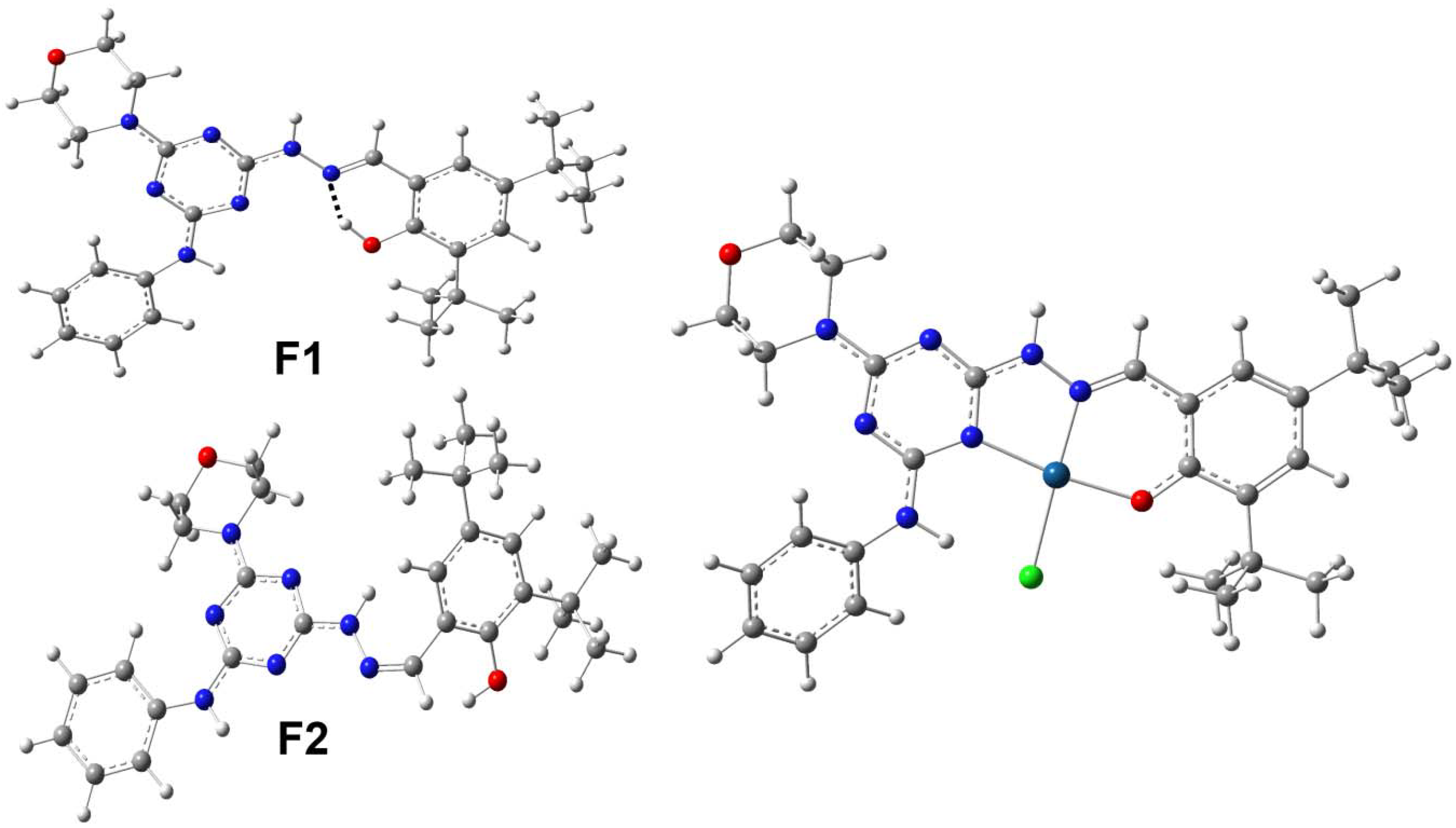
2.2. Crystal Structure Description
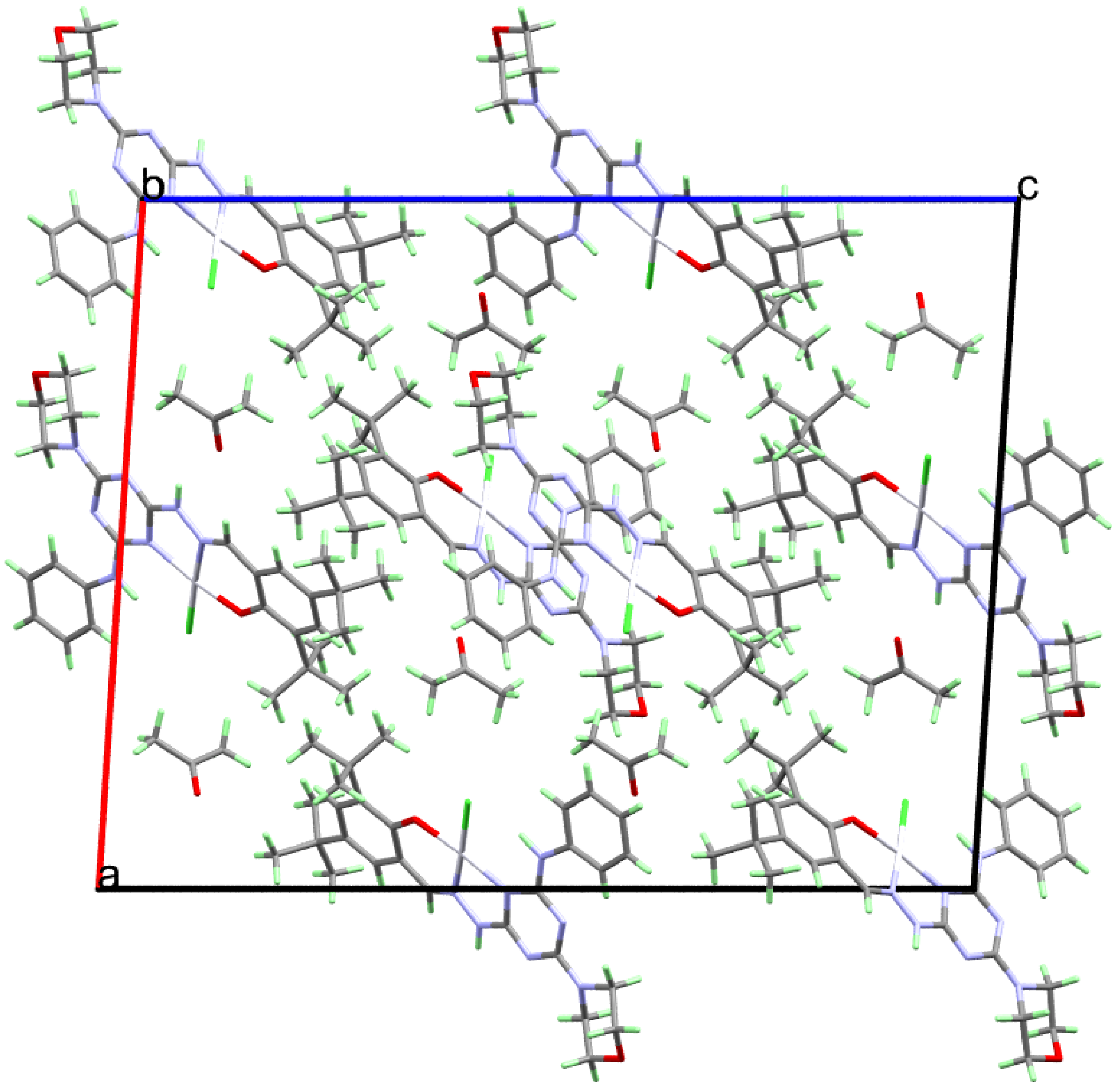
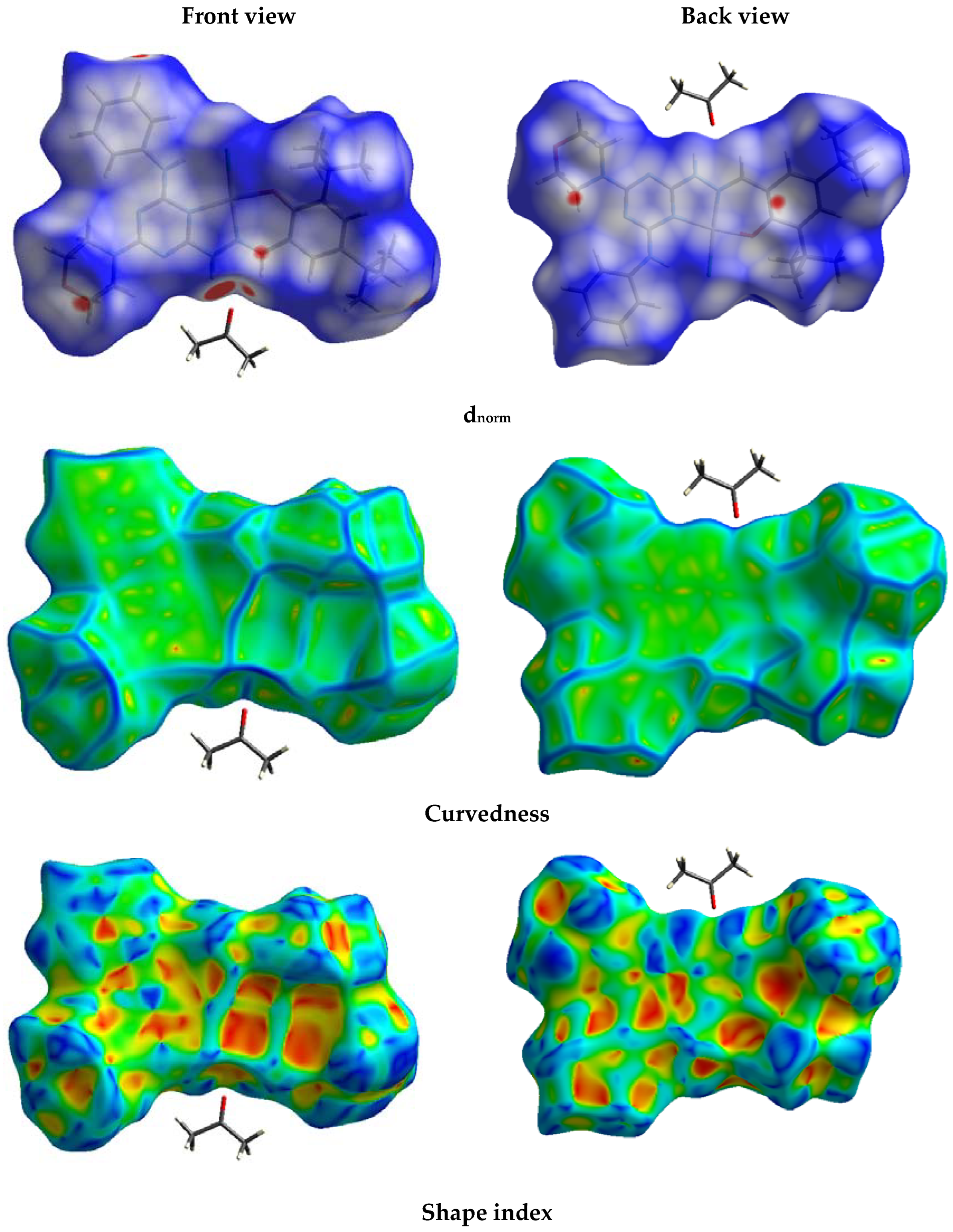
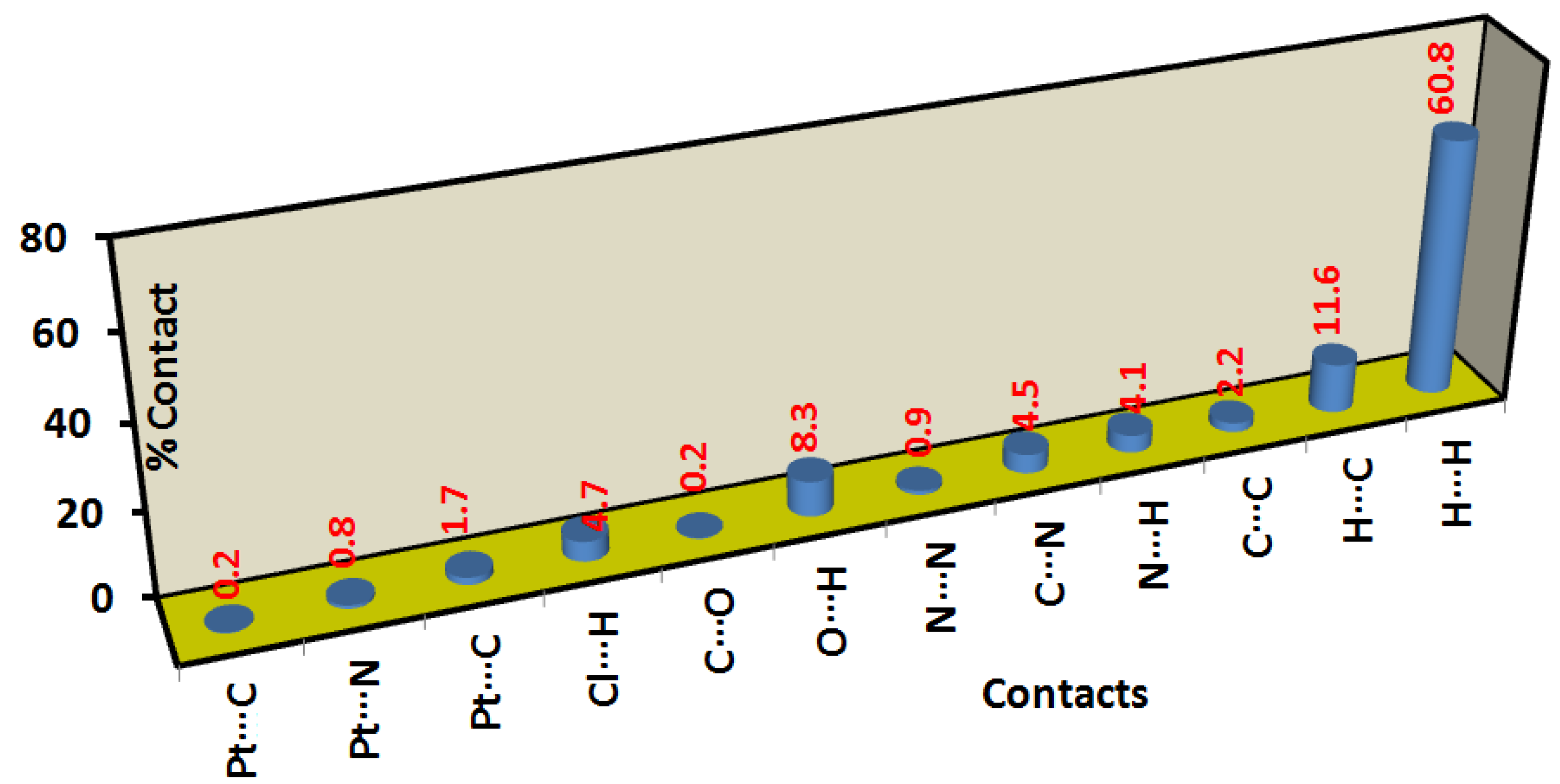
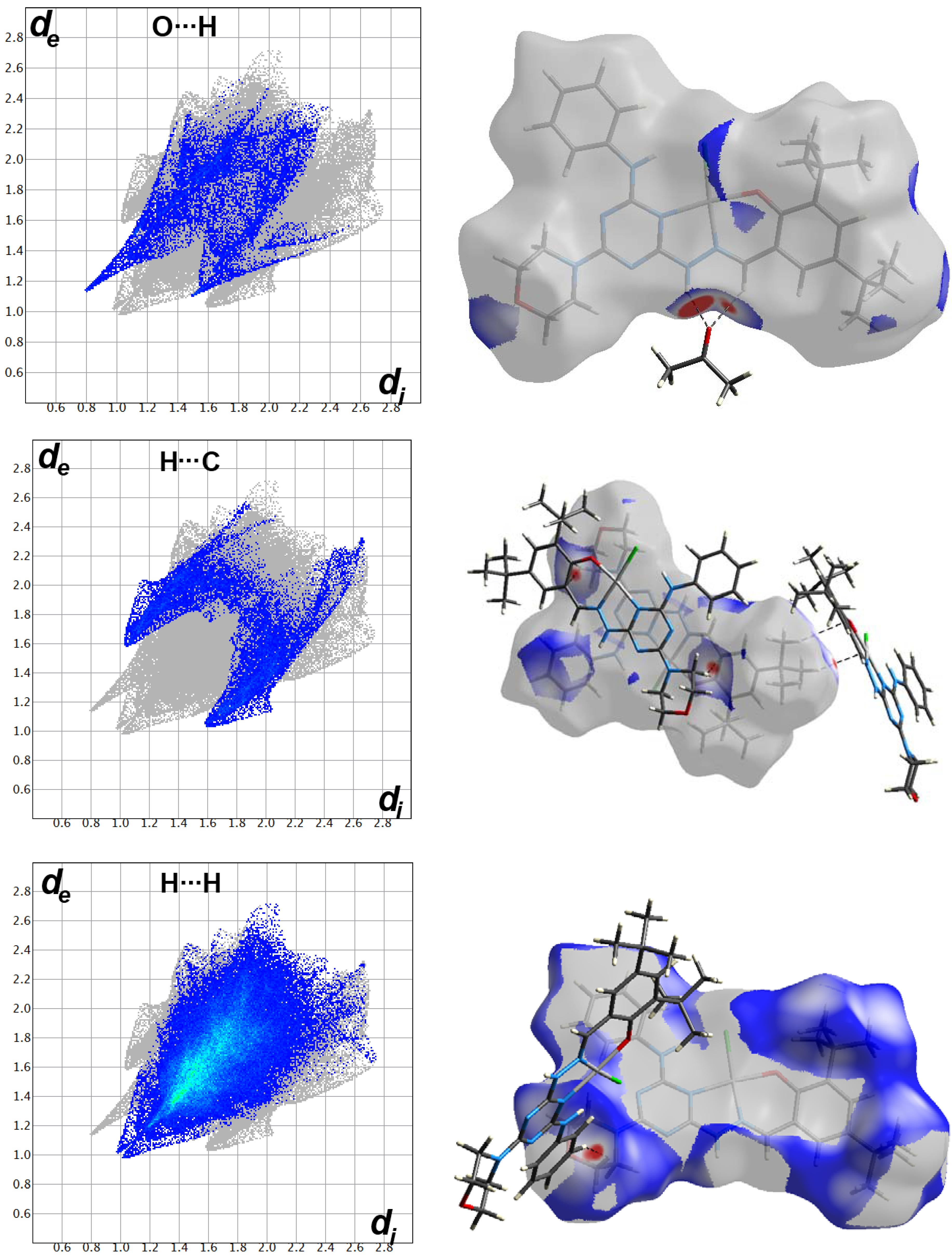
2.3. Analysis of Molecular Packing
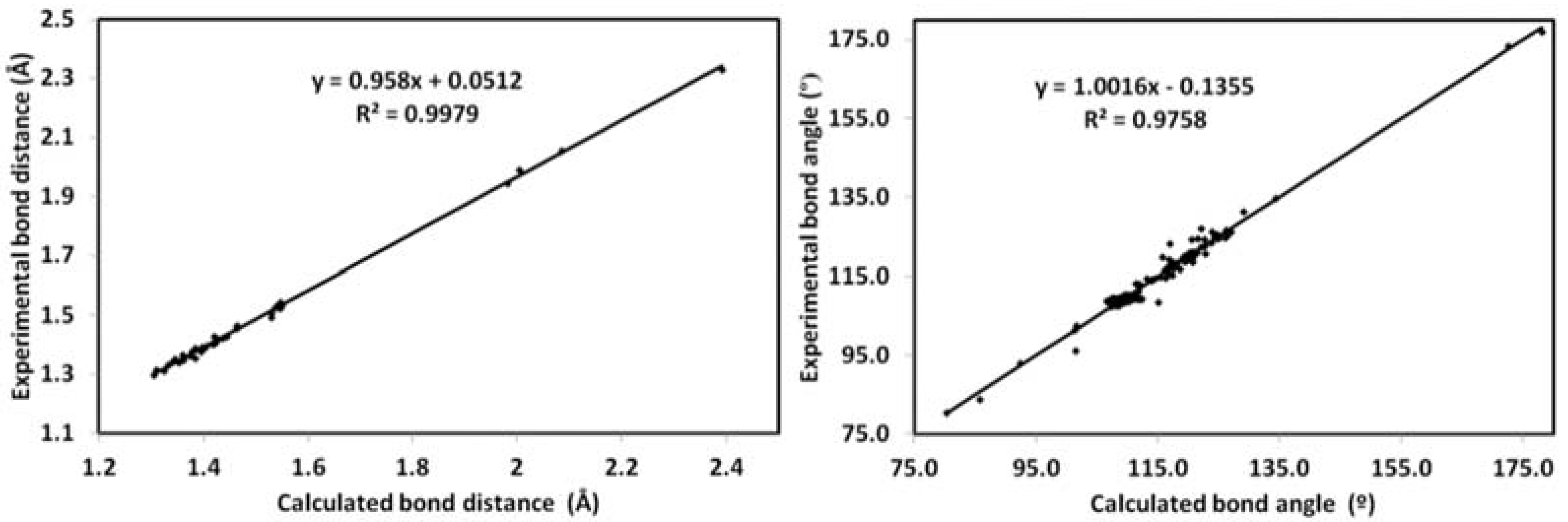
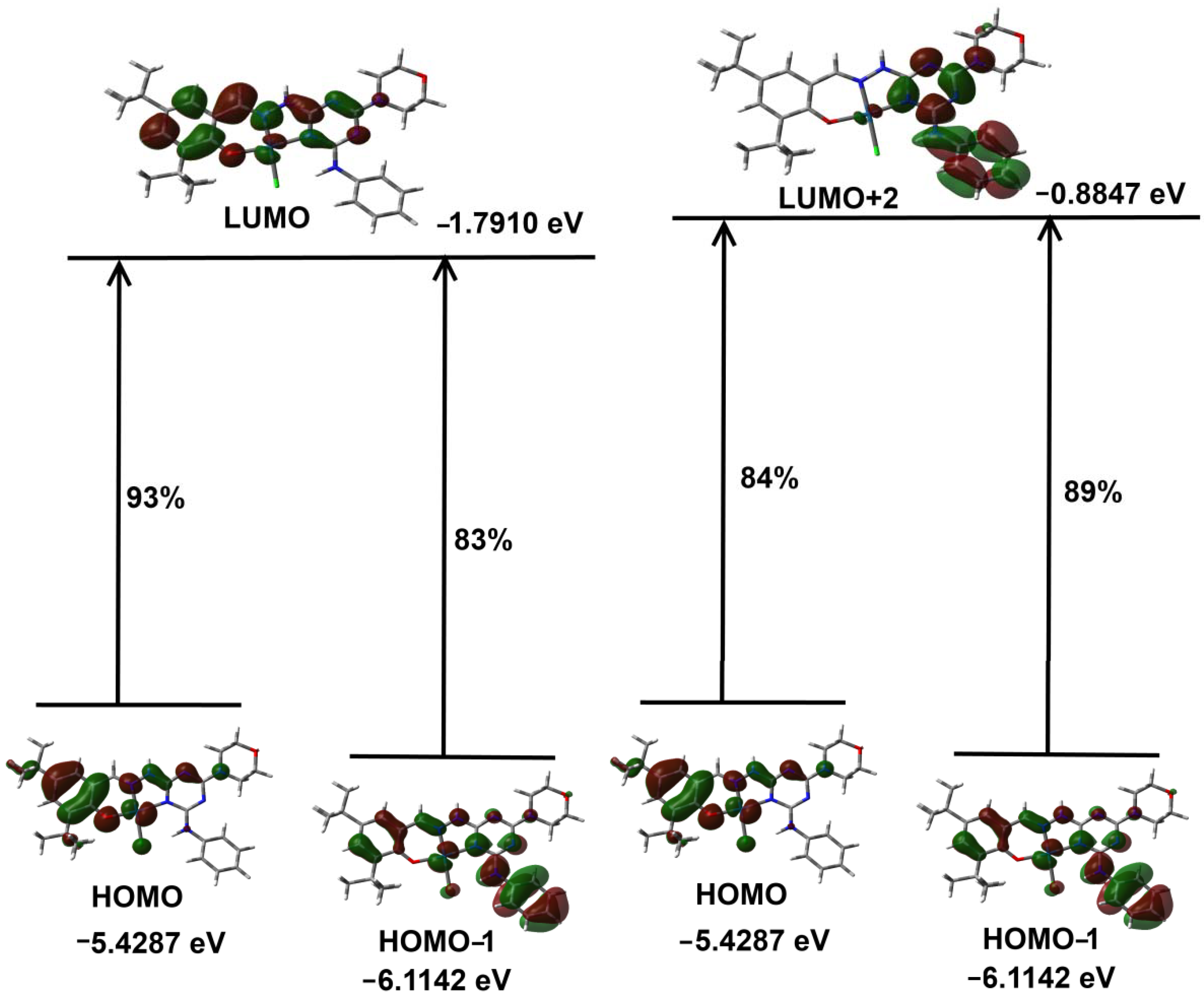
2.4. DFT Studies
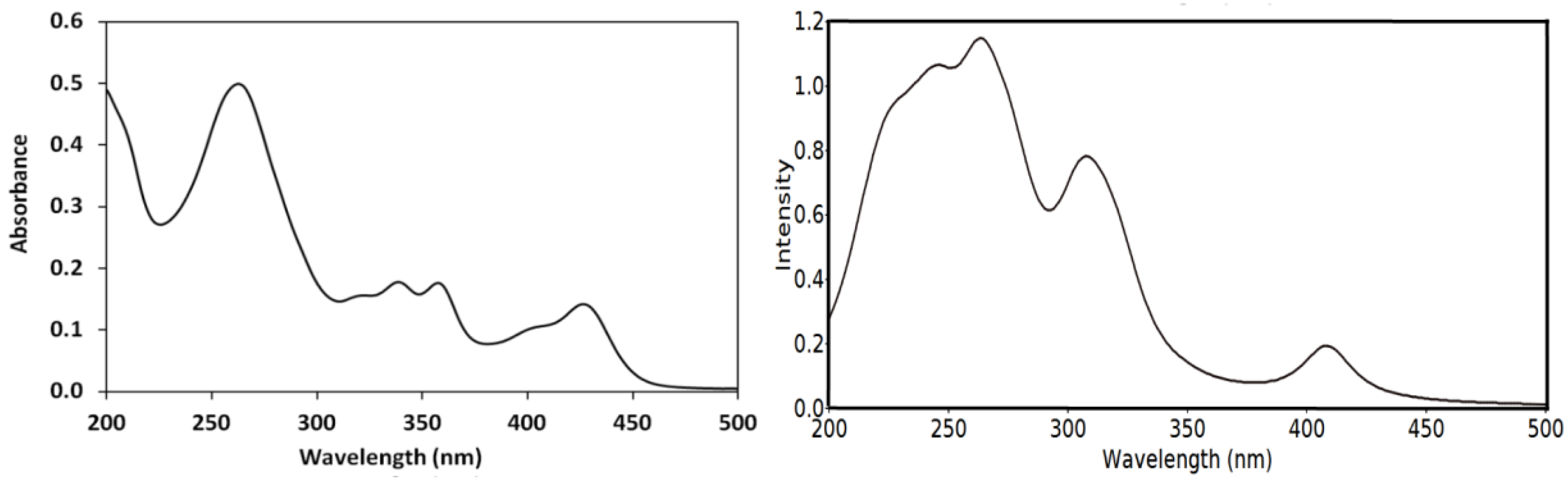
2.5. UV–Vis Spectra
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of the Ligand (HTriaz)
3.3. Synthesis of [Pt(Triaz)Cl] Complex
3.4. X-ray Structure Determinations
3.5. Hirshfeld and DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Barakat, A.; El-Faham, A.; Haukka, M.; Al-Majid, A.M.; Soliman, S.M. s-Triazine pincer ligands: Synthesis of their metal complexes, coordination behavior, and applications. Appl. Organomet. Chem. 2021, 35, e6317. [Google Scholar] [CrossRef]
- Kumar, A.; Mukherjee, P.S. Multicomponent Self-Assembly of PdII/PtII Interlocked Molecular Cages: Cage-to-Cage Conversion and Self-Sorting in Aqueous Medium. Chem. Eur. J. 2020, 26, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Motloch, P.; Hunter, C.A. Stimuli-Responsive Self-Sorting Hybrid Hydrogen-Bonded/Metal-Coordinated Cage. Chem. Eur. J. 2021, 27, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, M.; Que, W.; Stang, P.J. Self-assembly of metal-ion-responsive supramolecular coordination complexes and their photophysical properties. Dalton Trans. 2017, 46, 3120–3124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Yang, Y.; Yang, S.Q.; Han, K.L. Unveiling excited state energy transfer and charge transfer in a host/guest coordination cage. Phys. Chem. Chem. Phys. 2018, 20, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.L.K.; Leung, S.Y.L.; Yam, V.W.W. A rational molecular design of triazine-containing alkynylplatinum (II) terpyridine complexes and the formation of helical ribbons via Pt⋯ Pt, π–π stacking and hydrophobic–hydrophobic interactions. Chem. Commun. 2017, 53, 11349–11352. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Lin, T.; Wu, M.; Luo, H.K.; Huang, S.L.; Hor, T.A. Suite of Organoplatinum (II) triangular metallaprism: Aggregation-induced emission and coordination sequence induced emission tuning. J. Am. Chem. Soc. 2019, 141, 9448–9452. [Google Scholar] [CrossRef]
- Marzo, T.; Cirri, D.; Ciofi, L.; Gabbiani, C.; Feis, A.; Di Pasquale, N.; Stefanini, M.; Biver, T.; Messori, L. Synthesis, characterization and DNA interactions of [Pt3(TPymT)Cl3], the trinuclear platinum (II) complex of the TPymT ligand. J. Inorg. Biochem. 2018, 183, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.M.; El Sayed, S.A.; Butler, I.S.; Mostafa, S.I. New Palladium (II), Platinum (II) and Silver (I) complexes of 2-amino-4, 6-dithio-1,3,5-triazine; synthesis, characterization and DNA binding properties. J. Mol. Struct. 2020, 1200, 127088. [Google Scholar] [CrossRef]
- Yetim, N.K.; Sarı, N. Novel dendrimers containing redox mediator: Enzyme immobilization and applications. J. Mol. Struct. 2019, 1191, 158–164. [Google Scholar] [CrossRef]
- Paul, L.E.; Therrien, B.; Furrer, J. The complex-in-a-complex cation [Pt (acac) 2-(p-cym)6Ru6(tpt)2(dhnq)3] 6+: Its stability towards biological ligands. Inorg. Chim. Acta 2018, 469, 1–10. [Google Scholar] [CrossRef]
- Asman, P.W. Kinetics and mechanistic study of polynuclear platinum (II) polypyridyl complexes; A paradigm shift in search of new anticancer agents. Inorg. Chim. Acta 2018, 469, 341–352. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Xu, Y.; Li, W.; Shen, W. Fine tuning phosphorescent properties of platinum complexes via different N-heterocyclic-based C-N-N ligands. J. Organomet. Chem. 2017, 836, 26–33. [Google Scholar] [CrossRef]
- Zheng, X.H.; Mu, G.; Zhong, Y.F.; Zhang, T.P.; Cao, Q.; Ji, L.N.; Zhao, Y.; Mao, Z.W. Trigeminal star-like platinum complexes induce cancer cell senescence through quadruplex-mediated telomere dysfunction. Chem. Comm. 2016, 52, 14101–14104. [Google Scholar] [CrossRef]
- Lasri, J.; Haukka, M.; Al-Rasheed, H.H.; Abutaha, N.; El-Faham, A.; Soliman, S.M. Synthesis, structure and in vitro anticancer activity of Pd (II) complex of pyrazolyl-s-triazine ligand; A new example of metal-mediated hydrolysis of s-triazine pincer ligand. Crystals 2021, 11, 119. [Google Scholar] [CrossRef]
- Lasri, J.; Al-Rasheed, H.H.; El-Faham, A.; Haukka, M.; Abutaha, N.; Soliman, S.M. Synthesis, structure and in vitro anticancer activity of Pd (II) complexes of mono-and bis-pyrazolyl-s-triazine ligands. Polyhedron 2020, 187, 114665. [Google Scholar] [CrossRef]
- Soliman, S.M.; Albering, J.H.; Sholkamy, E.N.; El-Faham, A. Mono-and penta-nuclear self-assembled silver (I) complexes of pyrazolyl s-triazine ligand; synthesis, structure and antimicrobial studies. Appl. Organomet. Chem. 2020, 34, e5603. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A. Synthesis and structure diversity of high coordination number Cd (II) complexes of large s-triazine bis-Schiff base pincer chelate. Inorg. Chim. Acta 2019, 488, 131–140. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A.; Elsilk, S.E.; Farooq, M. Two heptacoordinated manganese (II) complexes of giant pentadentate s-triazine bis-Schiff base ligand: Synthesis, crystal structure, biological and DFT studies. Inorg. Chim. Acta 2018, 479, 275–285. [Google Scholar] [CrossRef]
- Soliman, S.M.; Lasri, J.; Haukka, M.; Elmarghany, A.; Al-Majid, A.M.; El-Faham, A.; Barakat, A. Synthesis, X-ray structure, Hirshfeld analysis, and DFT studies of a new Pd (II) complex with an anionic s-triazine NNO donor ligand. J. Mol. Struct. 2020, 1217, 128463. [Google Scholar] [CrossRef]
- Barakat, A.; El-Senduny, F.F.; Almarhoon, Z.; Al-Rasheed, H.H.; Badria, F.A.; Al-Majid, A.M.; Ghabbour, H.A.; El-Faham, A. Synthesis, X-ray crystal structures, and preliminary antiproliferative activities of new s-triazine-hydroxybenzylidene hydrazone derivatives. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Al-Rasheed, H.H.; Al Alshaikh, M.; Khaled, J.M.; Alharbi, N.S.; El-Faham, A. Ultrasonic irradiation: Synthesis, characterization, and preliminary antimicrobial activity of novel series of 4,6-disubstituted-1,3,5-triazine containing hydrazone derivatives. J. Chem. 2016, 2016, 3464758. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology, Volume 276, Macromolecular Crystallography, Part A; Carter, C.W., Sweet, J., Eds.; Academic Press: New York, NY, USA, 1997; pp. 307–326. [Google Scholar]
- Sheldrick, G.M. SADABS—Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Oxford, UK, 2020. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17 (2017) University of Western Australia. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 20 May 2021).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R., II; Keith, T.; Millam, J. GaussView; Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New model for calculation of solvation free energies: Correction of self-consistent reaction field continuum dielectric theory for short-range hydrogen-bonding effects. J. Phys. Chem. 1996, 100, 11775–11788. [Google Scholar] [CrossRef]
- Tannor, D.J.; Marten, B.; Murphy, R.; Friesner, R.A.; Sitkoff, D.; Nicholls, A.; Ringnalda, M.; Goddard, W.A.; Honig, B. Accurate first principles calculation of molecular charge distributions and solvation energies from ab initio quantum mechanics and continuum dielectric theory. J. Am. Chem. Soc. 1994, 116, 11875–11882. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 1–15. [Google Scholar] [CrossRef]
- Moser, A.; Range, K.; York, D.M. Accurate proton affinity and gas-phase basicity values for molecules important in biocatalysis. J. Phys. Chem. B 2010, 114, 13911–13921. [Google Scholar] [CrossRef] [Green Version]
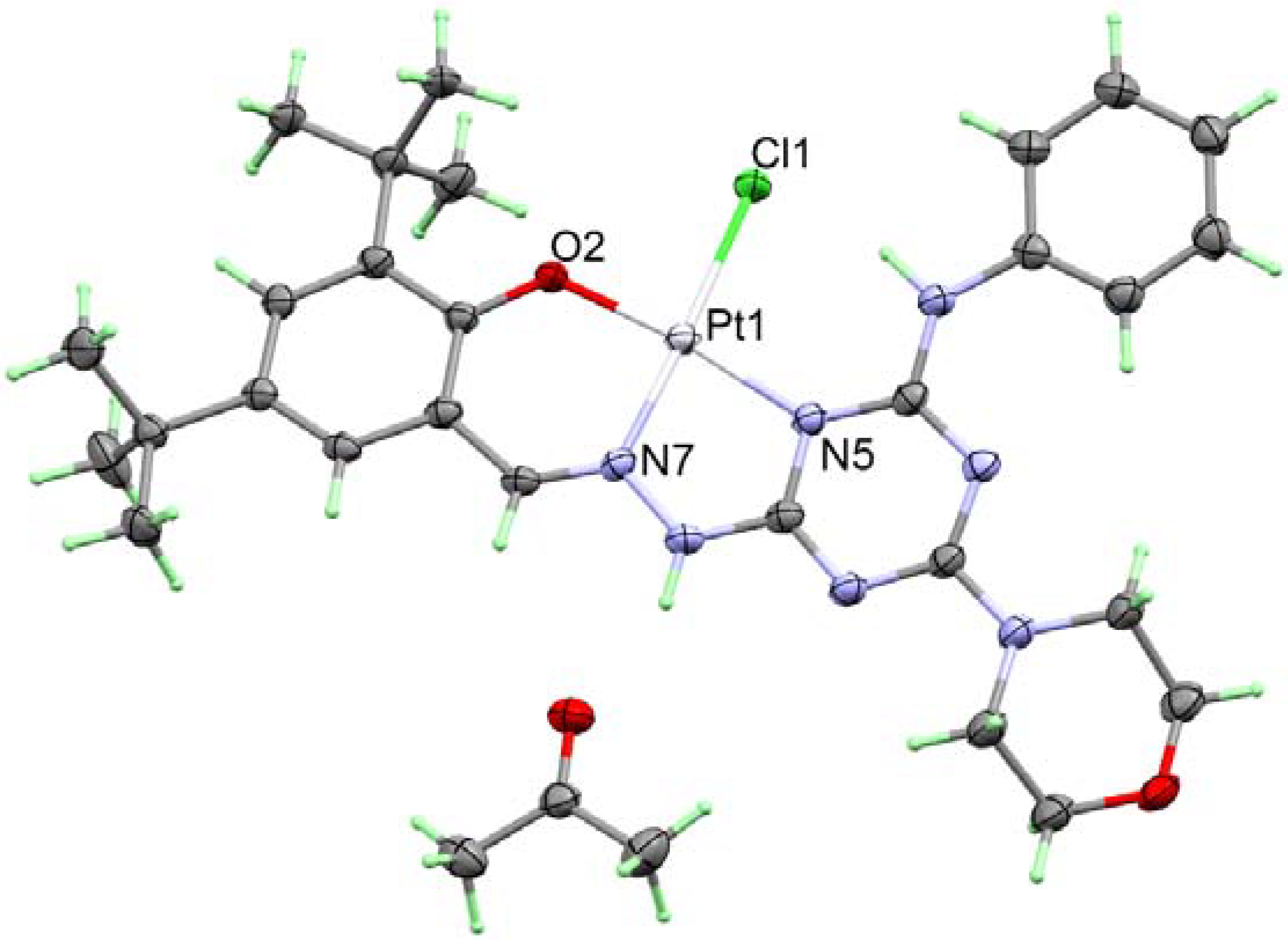
| Atoms | Distance | Atoms | Distance |
| Pt1-N7 | 1.945(4) | Pt1-N4 | 2.055(4) |
| Pt1-O2 | 1.991(4) | Pt1-Cl1 | 2.3308(13) |
| Atoms | Angle | Atoms | Angle |
| N7-Pt1-O2 | 93.09(16) | N7-Pt1-Cl1 | 177.01(13) |
| N7-Pt1-N4 | 80.42(18) | O2-Pt1-Cl1 | 83.91(11) |
| O2-Pt1-N4 | 173.35(16) | N4-Pt1-Cl1 | 102.58(13) |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| O3···H1 | 2.416 | H4A···C15 | 2.630 |
| O3···H6 | 1.934 | H4A ···C28 | 2.777 |
| H19B ···C16 | 2.785 | H11···C2 | 2.689 |
| H20B ···C14 | 2.656 | H11···H2B | 2.003 |
| Atom/Group | Optimized | X-ray |
|---|---|---|
| Pt | 0.4998 | 0.4857 |
| Cl | −0.4410 | −0.4402 |
| Triaz | −0.0588 | −0.0455 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Soliman, S.M.; Lasri, J.; Eltayeb, N.E.; Haukka, M.; Barakat, A.; El-Faham, A. A New Pt(II) Complex with Anionic s-Triazine Based NNO-Donor Ligand: Synthesis, X-ray Structure, Hirshfeld Analysis and DFT Studies. Molecules 2022, 27, 1628. https://doi.org/10.3390/molecules27051628
Altowyan MS, Soliman SM, Lasri J, Eltayeb NE, Haukka M, Barakat A, El-Faham A. A New Pt(II) Complex with Anionic s-Triazine Based NNO-Donor Ligand: Synthesis, X-ray Structure, Hirshfeld Analysis and DFT Studies. Molecules. 2022; 27(5):1628. https://doi.org/10.3390/molecules27051628
Chicago/Turabian StyleAltowyan, Mezna Saleh, Saied M. Soliman, Jamal Lasri, Naser E. Eltayeb, Matti Haukka, Assem Barakat, and Ayman El-Faham. 2022. "A New Pt(II) Complex with Anionic s-Triazine Based NNO-Donor Ligand: Synthesis, X-ray Structure, Hirshfeld Analysis and DFT Studies" Molecules 27, no. 5: 1628. https://doi.org/10.3390/molecules27051628
APA StyleAltowyan, M. S., Soliman, S. M., Lasri, J., Eltayeb, N. E., Haukka, M., Barakat, A., & El-Faham, A. (2022). A New Pt(II) Complex with Anionic s-Triazine Based NNO-Donor Ligand: Synthesis, X-ray Structure, Hirshfeld Analysis and DFT Studies. Molecules, 27(5), 1628. https://doi.org/10.3390/molecules27051628












