Inhibitory Effect of Salvia miltiorrhiza Extract and Its Active Components on Cervical Intraepithelial Neoplastic Cells
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of Extracts
2.3. Detection Conditions of HPLC-MS
2.4. Cell Lines and Culture
2.5. Cell Viability Assay
2.6. Synergistic Cytotoxicity Effect of Drug Combination
2.7. Fluorescence Morphology Assay
2.8. Cell Cycle Detection by Flow Cytometry
2.9. Western Blot Analysis
2.10. Statistical Analyses
3. Results
3.1. Inhibitory Effect of the SME on H8 Cells
3.2. HPLC–MS Identification of the SME
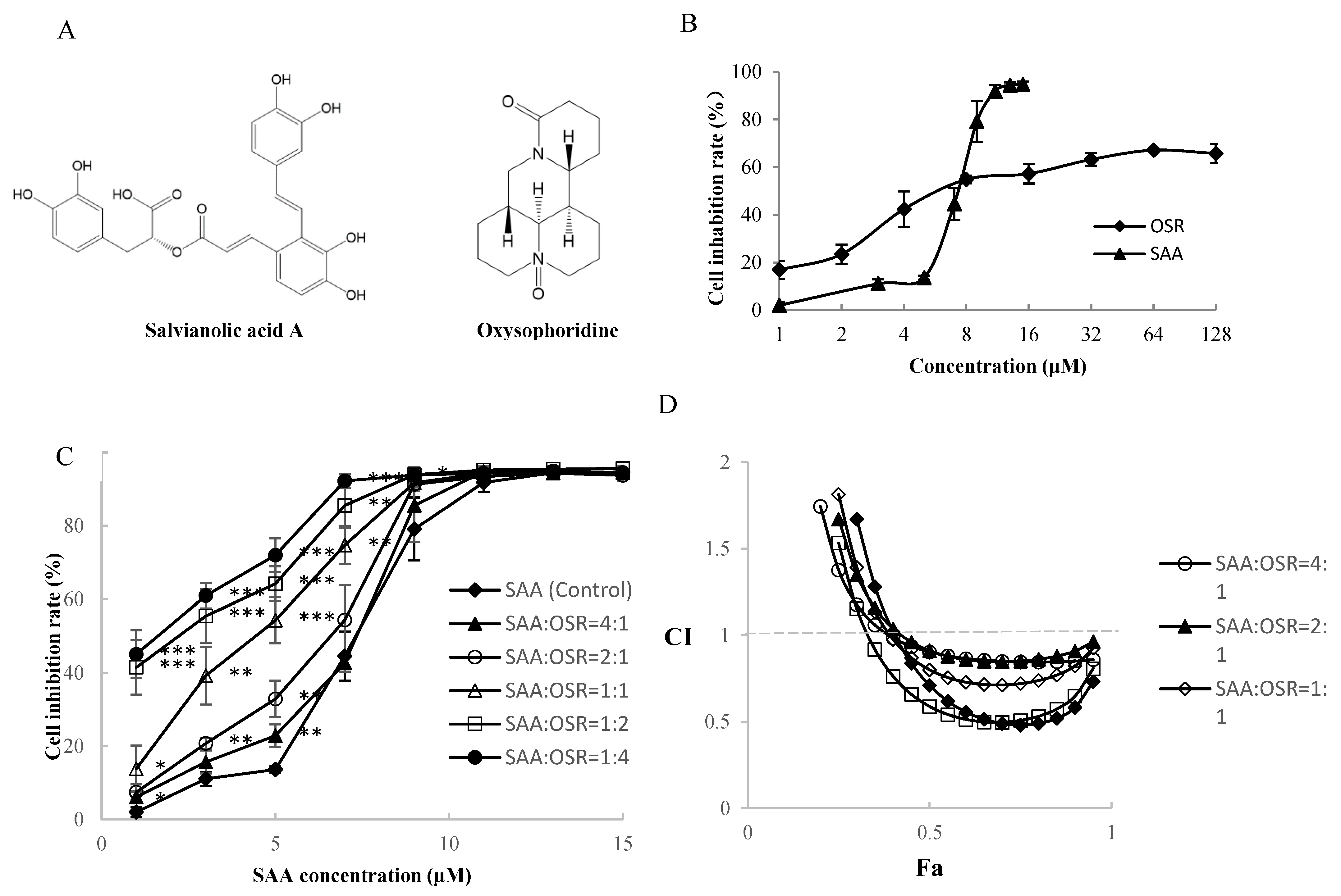
3.3. Inhibitory Effect of the Active Components of the SME on H8 Cells
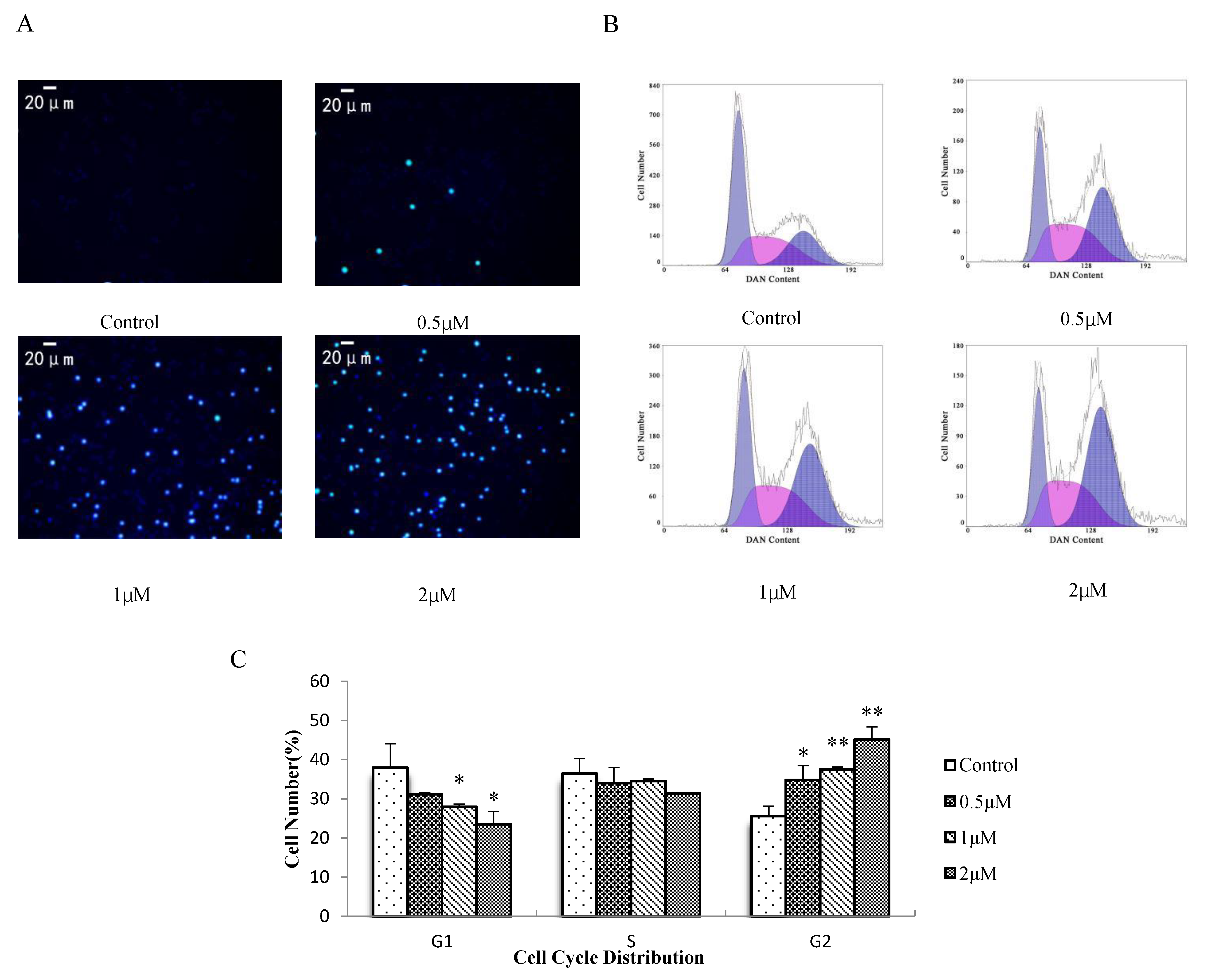
3.4. Effect of AO on H8 Cell Apoptosis and the Cell Cycle
3.5. Effect of AO on the Expression of Cell-Cycle-Related Proteins in H8 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- William, S.; Monica, A.; Amishi, B.; Linus, T.; Brandon, J.; Matthew, M.; Anuja, J.; Henry, C.; Linda, R.; Akila, N.; et al. Cervical Cancer: A Global Health Crisis. Cancer 2017, 123, 2404–2412. [Google Scholar]
- Hu, L.; Bell, D.; Antani, S.; Xue, Z.; Yu, K.; Horning, M.P.; Gachuhi, N.; Wilson, B.; Jaiswal, M.S.; Befano, B.; et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. JNCI J. Natl. Cancer Inst. 2019, 111, 923–932. [Google Scholar] [CrossRef]
- Monique, L.R.; Lui, S. From bad to worse II: Risk amplification of the HPV vaccine on Facebook. Vaccine 2021, 39, 303–308. [Google Scholar]
- Rezqalla, J.; Alshatti, M.; Ibraheem, A.; Omar, D.; Houda, A.; Alhaqqan, S.; Alghurair, S.; Akhtar, S. Human Papillomavirus (HPV): Unawareness of causal role HPV infectionin cervical cancer, HPV vaccine availability and HPV vaccine uptakeamong female schoolteachers in a Middle Eastern country. J. Infect. Public Health 2021, 14, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, P.; Cui, Z.; Liang, Z.; Bin, X.; Lang, J.; Chen, C. Effect of laparoscopic versus abdominal radical hysterectomy on majorsurgical complications in women with stage IA-IIB cervical cancer in China, 2004–2015. Gynecol. Oncol. 2020, 156, 115–123. [Google Scholar] [CrossRef]
- Matsuo, K.; Matsuzaki, S.; Mandelbaum, R.; Matushima, K.; Klar, M.; Grubbs, B.; Roman, L.; Wright, J. Association between hospital surgical volume and perioperativeoutcomes of fertility-sparing trachelectomy for cervical cancer: Anational study in the United States. Gynecol. Oncol. 2020, 157, 173–180. [Google Scholar] [CrossRef]
- Asthana, S.; Labani, S. Adjunct screening of cervical or vaginal samples using care HPV testing with Pap and aided visual inspection for detecting high-grade cervicalintraepithelial neoplasia. Cancer Epidemiol. 2015, 39, 104–108. [Google Scholar] [CrossRef]
- Trimble, C.; Morrow, M.; Kraynyak, K.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- Lehtinen, M.; Paavonen, J.; Wheeler, C.; Jaisamrarn, U.; Garland, S.; Castellsague, X.; Skinner, S.; Apter, D.; Naud, P.; Salmeron, J.; et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012, 13, 89–99. [Google Scholar] [CrossRef]
- Mei, X.; Tan, J.; Xiao, N.; Fang, X.; Gu, S.; Li, J.; Wang, J. Simultaneous qualitative and quantitative determination of 17 bioactive components in the fibrous roots of Salvia miltiorrhiza Bunge by a combinatorial Ultra performance liquid chromatography- Ultraviolet characteristic spectra analysis. J. Appl. Res. Med. Aromat. Plants 2020, 20, 100291. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2018, 59, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, M.; Xu, S.W.; Liu, P.Q. Salvia miltirrhiza Burgr (Danshen): A golden herbal medicine in cardiovascular thercopeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar]
- Li, W.; Polachi, N.; Wang, X.; Chu, Y.; Wang, Y.; Tian, M.; Li, D.; Zhou, D.; Zhou, S.; Ju, A.; et al. A quality marker study on salvianolic acids for injection. Phytomedicine 2018, 44, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Rasul, A.; Sarfraz, A.; Sarfraz, I.; Hussain, G.; Anwar, H.; Riaz, A.; Liu, S.; Wei, W.; Li, J.; et al. Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int. J. Biol. Sci. 2019, 15, 2256–2264. [Google Scholar]
- Tang, X.-L.; Yan, L.; Zhu, L.; Jiao, D.-M.; Chen, J.; Chen, Q.-Y. Salvianolic acid A reverses cisplatin resistance in lung cancer A549 cells by targeting c-met and attenuating Akt/mTOR pathway. J. Pharmacol. Sci. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Wei, J.; Xie, G.; Ge, S.; Qiu, Y.; Liu, W.; Lu, A.; Chen, T.; Li, H.; Zhou, Z.; Jia, W. Metabolic Transformation of DMBA-Induced Carcinogenesis and Inhibitory Effect of Salvianolic Acid B and Breviscapine Treatment. J. Proteome Res. 2011, 11, 1302–1316. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Yu, Y.; Sun, S.; Zhang, Y.; Zhang, Y.; Yang, W.; Li, S.; Qiao, Y. Salvianolic Acid A, as a Novel ETA Receptor Antagonist, Shows Inhibitory Effects on Tumor in Vitro. Int. J. Mol. Sci. 2016, 17, 1244. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, C.; Zhang, L.; Li, Y.; Wang, S.; Wang, J.; Yuan, C.; Niu, J.; Wang, C.; Lu, G. Salvianolic acid A shows selective cytotoxicity against multidrug-resistant MCF-7 breast cancer cells. Anti-Cancer Drugs 2015, 26, 210–223. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Gu, X. Salvianolic Acid B a potential chemopreventive agent, for head and neck squamous cell cancer. J. Oncol. 2011, 2011, 534548. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Fei, W.; Zhou, J.; Zhang, L.; Chen, L.; Zhang, X.; Liang, X.; Xie, J.; Fang, Y.; Sui, X.; et al. Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 2016, 7, 61509–61519. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, J.; Jung, Y.L.; Hong, A.; Nam, S.-J.; Lim, B.-K.; So-Hee, K.; JiHye, L.; Lin, J.Y.; Aerum, H.; et al. Salvianolic Acid B Inhibits Hand-Foot-Mouth Disease Enterovirus 71 Replication through Enhancement of AKT Signaling Pathway. J. Microbiol. Biotechnol. 2020, 30, 38–43. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, P.; Dai, S.; Liu, Z.; Zheng, X.; Chen, T. Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell Mol. Neurobiol. 2013, 33, 921–928. [Google Scholar] [CrossRef]
- Zhai, X.J.; Xu, J.F. Simultaneous Determination of Hydrophilic and Lipophilic compounds in Radix salviae Miltiorrhizae by HPLC. Her. Med. 2009, 28, 1345–1348. [Google Scholar]
- Xie, L.; Li, J.; Zhang, Y.; Liu, B.; Peng, X.; Lin, Y.; Xu, W.; Hu, L. Inhibitors of differentiation-1 promotes nitrosopyrrolidine-induced transformation of HPV 16-immortalized cervical epithelial cell. Cancer Sci. 2014, 105, 506–511. [Google Scholar] [CrossRef]
- Lu, C.H.; Wang, H. eIF4E is a critical regulator of human papillomavirus (HPV)-immortalized cervical epithelial (H8) cell growth induced by nicotine. Toxicology 2019, 419, 1–10. [Google Scholar]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, L.; Li, X.; Song, W.; Liu, Y.; Huang, L. Nanoformulated Codelivery of Quercetin and Alantolactone Promotes an Antitumor Response through Synergistic Immunogenic Cell Death for Microsatellite-Stable Colorectal Cancer. ACS Nano 2019, 13, 12511–12524. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Zhu, Y.; Guan, Y.; Quan, W.; Li, Y.; Guo, C.; Weng, Y.; Cui, J.; Chen, L.; Ding, Y.; et al. Chemical Fingerprint and Metabolic Fingerprint of Danhong Injection by HPLC-UV and HPLC-MS. Asian J. Chem. 2013, 25, 6285–6292. [Google Scholar] [CrossRef]
- Song, Y.; Gao, H.; Zhang, S.; Zhang, Y.; Jin, X.; Sun, J. Prescription Optimization and Oral Bioavailability Study of Salvianolic Acid Extracts W/O/W Multiple Emulsion. Biol. Pharm. Bull. 2017, 40, 2081–2087. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Xiao, H.; Liu, J.; Liang, X. Identification of phenolic constituents in RadixSalvia miltiorrhizae by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 499–506. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.; Ahmed, F.; Maitra, A.; Prashant, C.; Dinda, A. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Cao, B.; Sun, B.; Cao, Y.; Yang, K.; Lin, Y.S. Highly-sensitive capture of circulating tumor cells using micro-ellipse flters. Sci. Rep. 2017, 7, 610. [Google Scholar] [CrossRef]
- Bianchi, A.; Lopez, S.; Altwerger, G.; Bellone, S.; Bonazzoli, E.; Zammataro, L.; Manzano, A.; Manara, P.; Perrone, E.; Zeybek, B.; et al. PARP-1 activity (PAR) determines the sensitivity of cervical cancer to olaparib. Gynecol. Oncol. 2019, 155, 144–150. [Google Scholar] [CrossRef]
- Qian, W.; Wang, Z.; Xu, T.; Li, D. Anti-apoptotic effects and mechanisms of salvianolic acid A on cardiomyocytes in ischemia-reperfusion injury. Histol. Histopathol. 2018, 34, 223–231. [Google Scholar]
- Liang, Y.; Kang, L.; Qi, Z.; Gao, X.; Quan, H.; Lin, H. Salvia miltiorrhiza solution and its active compounds ameliorate human granulosa cell damage induced by H2O2. Exp. Ther. Med. 2020, 21, 64. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, J.H.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Wang, J.H.; Zhang, Z. Salvianolic Acid A Protects the Kidney against Oxidative Stress by Activating the Akt/GSK-3 β/Nrf2 Signaling Pathway and Inhibiting the NF-κ B Signaling Pathway in 5/6 Nephrectomized Rats. Oxid. Med. Cell Longev. 2019, 2019, 2853534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.F.; Chen, H.S.; Li, J.R.; Jiang, J.D.; Li, Z.R. Studies on polyphenolic chemical constitutents from root of Salvia miltiorrhiza. China J. Chin. Mater. Med. 2007, 32, 1886–1890. [Google Scholar]
- Yuan, C.P.; Hou, H.M.; Zeng, S. Metabolic stability of salvianolic acid B and dimethyl lithospermate B in human liver microsomes and metabolite identification. Chin. J. New Drugs 2016, 25, 1543–1549. [Google Scholar]
- Ceng, J.M.; Ge, T.T.; Zheng, Y.F. Study on the HPLC fingerprint of phenolic acids of Danshen Dripping Injection. Lishizhen Med. Mater. Res. 2013, 24, 979–982. [Google Scholar]
- Xia, H.; Sun, L.; Lou, H.; Rahman, M. Conversion of salvianolic acid B into salvianolic acid A in tissues of Radix Salviae Miltiorrhizae using high temperature, high pressure and high humidity. Phytomedicine 2014, 21, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Z.; Cheng, Y.; Dong, H.J.; Wang, X.; Li, J.; Gao, Q.S. Preparation of salvianolic acid A by the degradation reaction of salvianolic acid B in subcritical water integrated with pH-zone-refining counter-current chromatography. J. Chromatogr. A 2016, 1468, 42–48. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, L.; Liu, Y.B.; Peng, T. Oxysophoridine rescues spinal cord injury via anti inflammatory, anti oxidative stress and anti apoptosis effects. Mol. Med. Rep. 2018, 17, 2523–2528. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Y.; Jiang, N.; Chen, X.; Zhang, Y.; Zhang, K.; Wang, T.; Hao, Y.; Ma, L.; Zhao, C.; et al. Protective effect of oxysophoridine on cerebral ischemia/reperfusion injury in mice. Neural Regen. Res. 2013, 8, 1349–1359. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Liu, J.W.; Li, C.H.; Wu, H.Z.; Liu, Y.W. Kaempferol-7-O-β-D-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008, 264, 229–240. [Google Scholar] [CrossRef]
- Oshi, M.; Takahashi, H.; Tokumaru, Y.; Yan, L.; Rashid, O.M.; Matsuyama, R.; Endo, I.; Takabe, K. G2M Cell Cycle Pathway Score as a Prognostic Biomarker of Metastasis in Estrogen Receptor (ER)-Positive Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2921. [Google Scholar] [CrossRef] [Green Version]
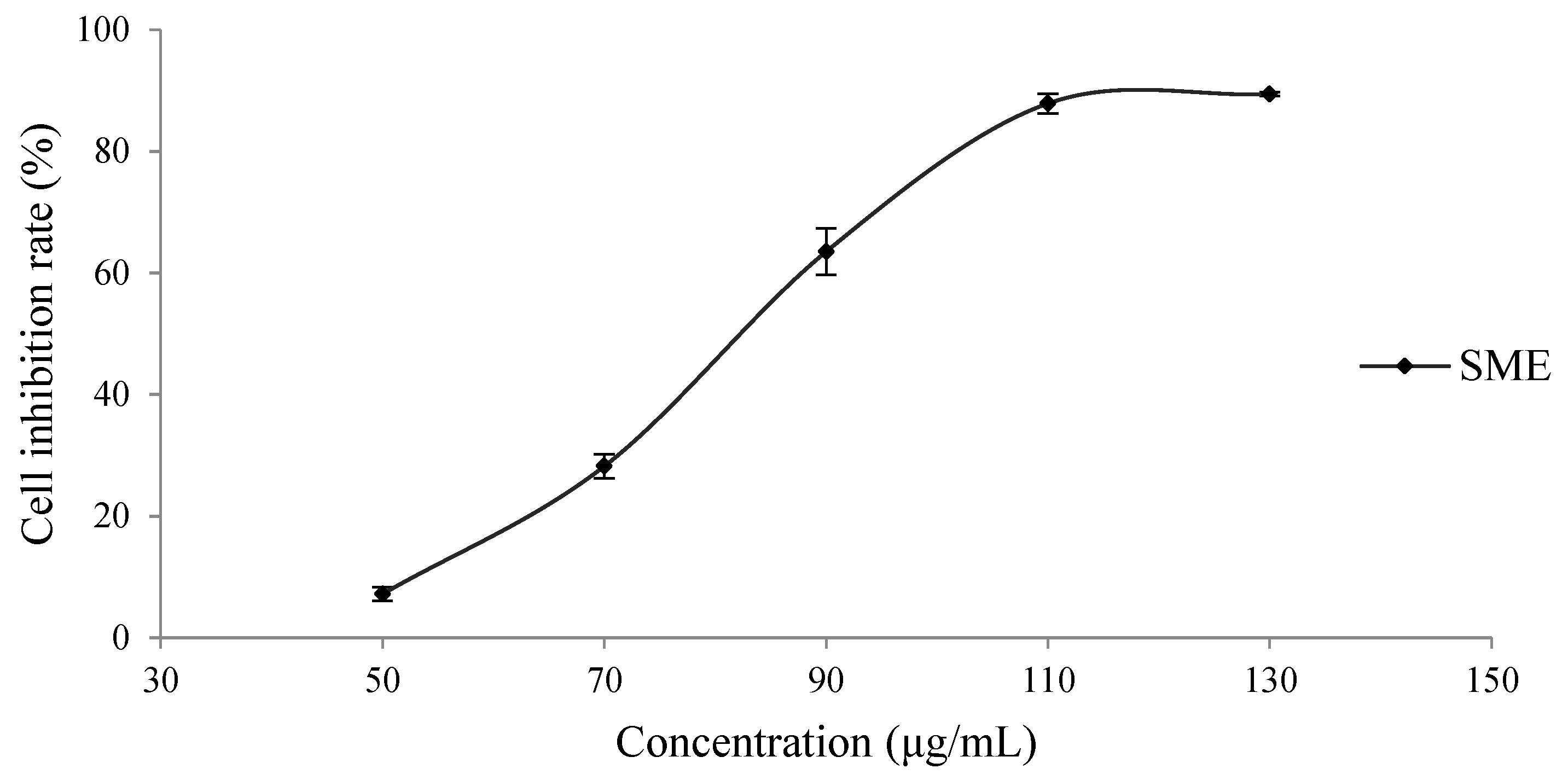
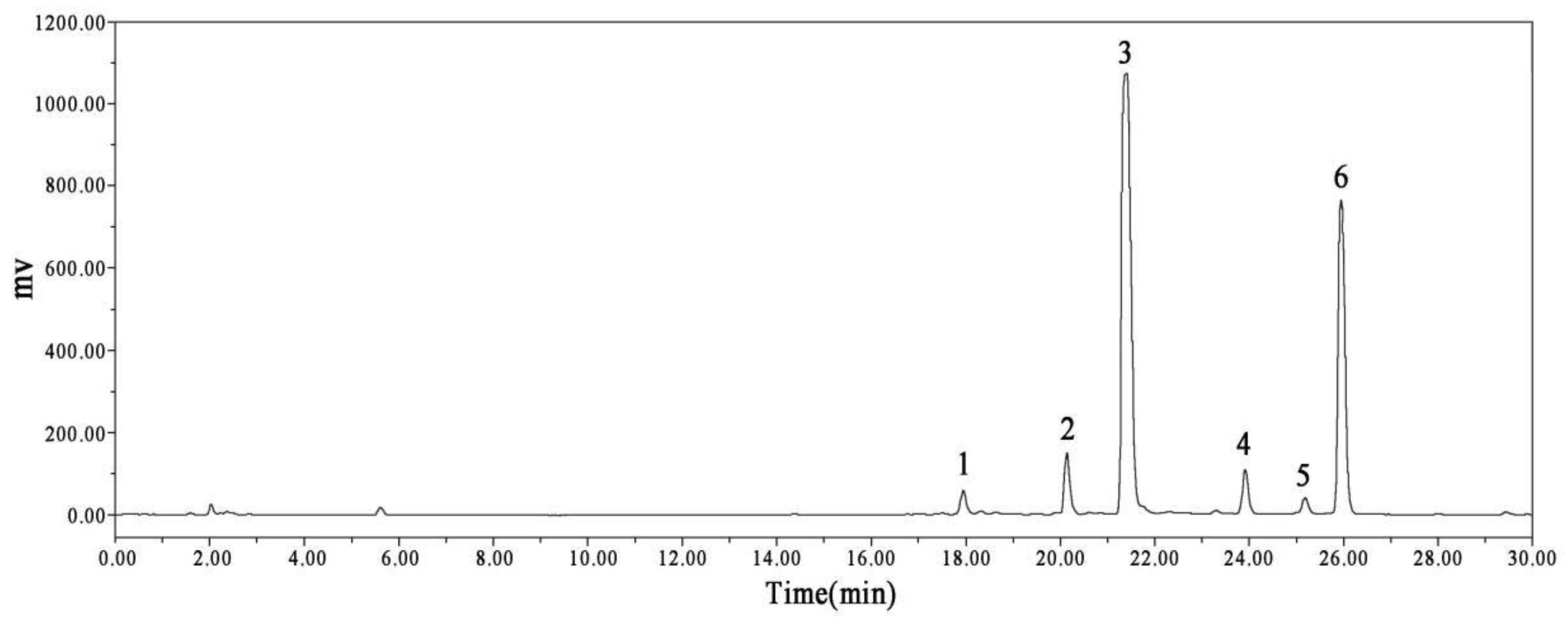

| Peak No. | Retention (min) | Molecular Formula | Measured Mass m/z (Error, ppm) | MS/MS Fragments Ions | Relative Quantification (%) (a) | Identification |
|---|---|---|---|---|---|---|
| 1 | 18.01 | C27H22O12 | 538.1018 (−2.5) | 538, 340, 295, and 185 | 1.96 | salvianolic acid H [31] |
| 2 | 20.20 | C26H22O10 | 493.1118 (0.3) | 493, 295, and 185 | 4.72 | salvianolic acid A [31] |
| 3 | 21.68 | C36H30O16 | 717.1461 (−5.0) | 717, 519, 339, 321, and 295 | 57.62 | salvianolic acid B [32] |
| 4 | 23.76 | C28H24O12 | 551.1171 (−4.9) | 551, 339, and 321 | 3.60 | monomethyl lithospermate [32] |
| 5 | 25.15 | C37H32O16 | 731.1618 (−4.9) | 731, 533, 339, 321, and 295 | 1.52 | 9‴-methyl lithospermate B [33] |
| 6 | 25.77 | C37H32O16 | 731.1618 (−5.1) | 731, 533, 507, 339, and 295 | 30.58 | 9‴-methyl lithospermate B/isomer [33] |
| SAA/OSR | 4:1 | 2:1 | 1:1 | 1:2 | 1:4 |
|---|---|---|---|---|---|
| CI | 0.9 | 0.91 | 0.8 | 0.59 | 0.71 |
| DRISAA | 1.22 | 1.32 | 1.76 | 3.1 | 3.73 |
| DRIOSR | 11.81 | 6.4 | 4.27 | 3.75 | 2.26 |
| IC50 of SAA (μM) | IC50 of OSR (μM) | |
|---|---|---|
| SAA | 5.74 ± 0.63 | |
| OSR | 13.70 ± 1.71 | |
| 4:1 | 4.83 ± 0.17 | 1.20 ± 0.04 |
| 2:1 | 4.50 ± 0.58 | 2.25 ± 0.29 |
| 1:1 | 3.26 ± 0.53 | 3.26 ± 0.53 |
| 1:2 | 1.87 ± 0.37 | 3.73 ± 0.74 |
| 1:4 | 1.51 ± 0.26 | 6.05 ± 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, X.; Kan, H.; Wu, Q.; Li, C.; Zheng, Y.; Peng, G. Inhibitory Effect of Salvia miltiorrhiza Extract and Its Active Components on Cervical Intraepithelial Neoplastic Cells. Molecules 2022, 27, 1582. https://doi.org/10.3390/molecules27051582
Leng X, Kan H, Wu Q, Li C, Zheng Y, Peng G. Inhibitory Effect of Salvia miltiorrhiza Extract and Its Active Components on Cervical Intraepithelial Neoplastic Cells. Molecules. 2022; 27(5):1582. https://doi.org/10.3390/molecules27051582
Chicago/Turabian StyleLeng, Xuejiao, Hongfei Kan, Qinhang Wu, Cunyu Li, Yunfeng Zheng, and Guoping Peng. 2022. "Inhibitory Effect of Salvia miltiorrhiza Extract and Its Active Components on Cervical Intraepithelial Neoplastic Cells" Molecules 27, no. 5: 1582. https://doi.org/10.3390/molecules27051582
APA StyleLeng, X., Kan, H., Wu, Q., Li, C., Zheng, Y., & Peng, G. (2022). Inhibitory Effect of Salvia miltiorrhiza Extract and Its Active Components on Cervical Intraepithelial Neoplastic Cells. Molecules, 27(5), 1582. https://doi.org/10.3390/molecules27051582






